Trans Isomeric Fatty Acids in Children and Young Adults with Type 1 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Trans Fatty Acids in Diabetic Young Adults
3.2. Trans Fatty Acids in Diabetic Children
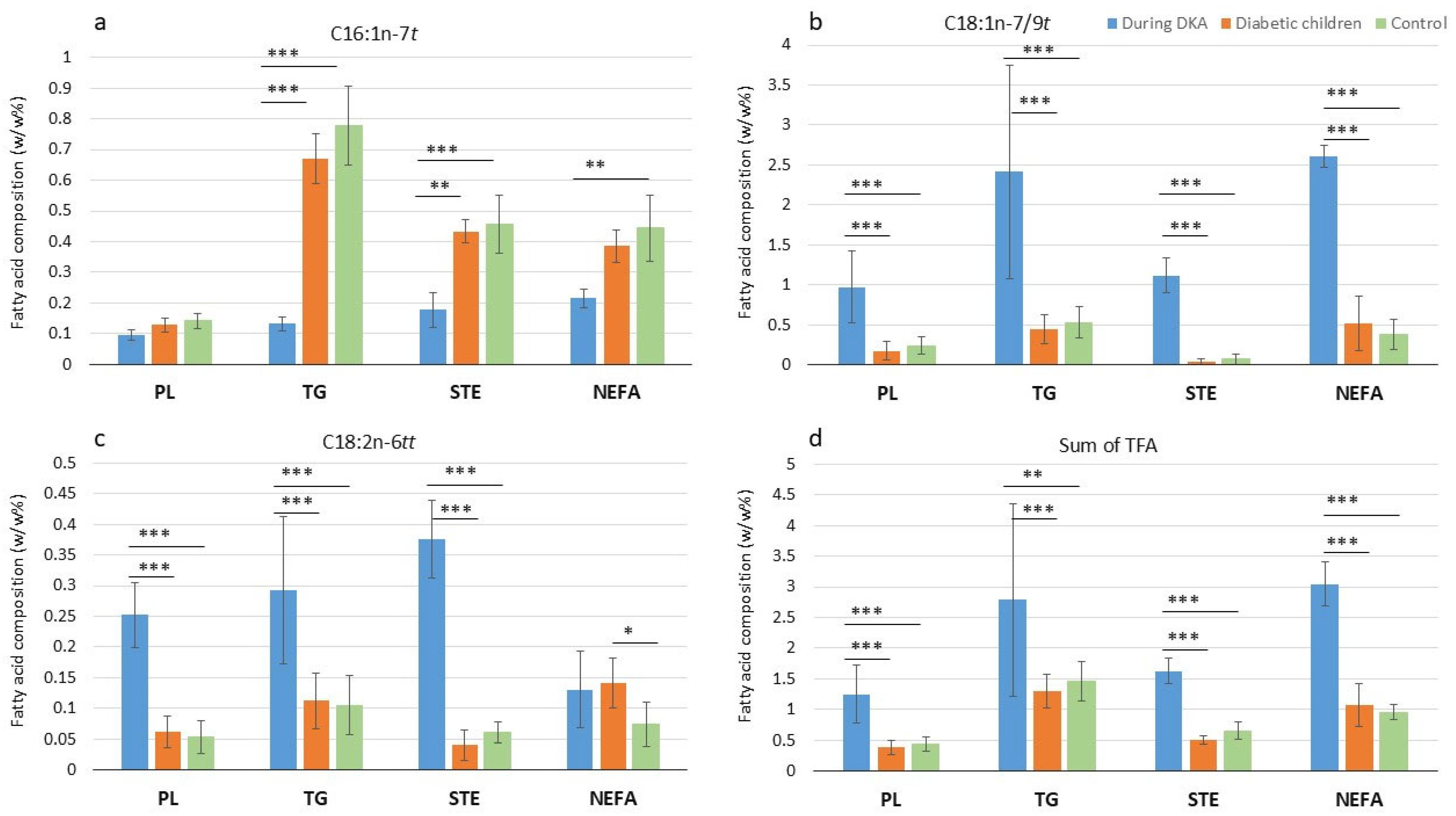
3.3. Trans Fatty Acids in Diabetic Children with and Without Diabetic Ketoacidosis
3.4. Correlation Between Trans Isomeric and Polyunsaturated Fatty Acids
3.5. Correlation Between Trans Isomeric Fatty Acids and Blood Lipids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- The, N.S.; King, I.B.; Couch, S.C.; Crandell, J.L.; Dabelea, D.; Liese, A.D.; Mayer-Davis, E.J. Plasma trans-palmitoleic acid is associated with cardio-metabolic risk factors in youth with type 1 diabetes. Diabetes Metab. 2018, 44, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Luongo, D.; Naviglio, D.; Bozzetto, L.; Mirabella, M.; Rivieccio, A.M.; Giacco, A.; Rivellese, A.A. Trans fatty acids consumption in type 1 diabetic patients: Evaluation by dietary records and measurement in serum phospholipids. Acta Diabetol. 2013, 50, 651–654. [Google Scholar] [CrossRef]
- Akmurzina, V.A.; Petryairina, E.E.; Saveliev, S.V.; Selishcheva, A.A. The profile of plasma non-esterified fatty acids in children with different terms of type 1 diabetes mellitus. Biomed. Khim 2016, 62, 206–211. [Google Scholar] [CrossRef]
- Mesa, A.; Cofan, M.; Esmatjes, E.; Perea, V.; Boswell, L.; Gimenez, M.; Sala-Vila, A.; Vinagre, I.; Vinals, C.; Chiva-Blanch, G.; et al. Biomarkers of fatty acid intake are independently associated with preclinical atherosclerosis in individuals with type 1 diabetes. Eur. J. Nutr. 2021, 60, 4595–4605. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Passi, S.J.; Misra, A. Overview of trans fatty acids: Biochemistry and health effects. Diabetes Metab. Syndr. 2011, 5, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Craig-Schmidt, M.C. World-wide consumption of trans fatty acids. Atheroscler. Suppl. 2006, 7, 1–4. [Google Scholar] [CrossRef]
- Authority, E.F.S. Scientific and technical assistance on trans fatty acids. EFSA Support. Publ. 2018, 15, EN-1433. [Google Scholar] [CrossRef]
- Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children; WHO Guideline; WHO: Geneva, Switzerland, 2023.
- Wanders, A.J.; Zock, P.L.; Brouwer, I.A. Trans Fat Intake and Its Dietary Sources in General Populations Worldwide: A Systematic Review. Nutrients 2017, 9, 840. [Google Scholar] [CrossRef]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D.; et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 2014, 348, g2272. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schunemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.G.; Pham, M.; Rizzo, N.O.; Cheng, A.M.; Maloney, E.; Kim, F. Trans fatty acids induce vascular inflammation and reduce vascular nitric oxide production in endothelial cells. PLoS ONE 2011, 6, e29600. [Google Scholar] [CrossRef] [PubMed]
- Verneque, B.J.F.; Machado, A.M.; de Abreu Silva, L.; Lopes, A.C.S.; Duarte, C.K. Ruminant and industrial trans-fatty acids consumption and cardiometabolic risk markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2050–2060. [Google Scholar] [CrossRef]
- Gayet-Boyer, C.; Tenenhaus-Aziza, F.; Prunet, C.; Marmonier, C.; Malpuech-Brugere, C.; Lamarche, B.; Chardigny, J.M. Is there a linear relationship between the dose of ruminant trans-fatty acids and cardiovascular risk markers in healthy subjects: Results from a systematic review and meta-regression of randomised clinical trials. Br. J. Nutr. 2014, 112, 1914–1922. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutr. 2009, 63 (Suppl. S2), S5–S21. [Google Scholar] [CrossRef] [PubMed]
- Tardy, A.L.; Morio, B.; Chardigny, J.M.; Malpuech-Brugere, C. Ruminant and industrial sources of trans-fat and cardiovascular and diabetic diseases. Nutr. Res. Rev. 2011, 24, 111–117. [Google Scholar] [CrossRef]
- Te Morenga, L.; Montez, J.M. Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0186672. [Google Scholar] [CrossRef]
- Szabo, E.; Boehm, G.; Beermann, C.; Weyermann, M.; Brenner, H.; Rothenbacher, D.; Decsi, T. trans Octadecenoic acid and trans octadecadienoic acid are inversely related to long-chain polyunsaturates in human milk: Results of a large birth cohort study. Am. J. Clin. Nutr. 2007, 85, 1320–1326. [Google Scholar] [CrossRef][Green Version]
- Elias, S.L.; Innis, S.M. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am. J. Clin. Nutr. 2001, 73, 807–814. [Google Scholar] [CrossRef]
- Franz, M.J.; MacLeod, J.; Evert, A.; Brown, C.; Gradwell, E.; Handu, D.; Reppert, A.; Robinson, M. Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Systematic Review of Evidence for Medical Nutrition Therapy Effectiveness and Recommendations for Integration into the Nutrition Care Process. J. Acad. Nutr. Diet. 2017, 117, 1659–1679. [Google Scholar] [CrossRef]
- Dyson, P.A.; Twenefour, D.; Breen, C.; Duncan, A.; Elvin, E.; Goff, L.; Hill, A.; Kalsi, P.; Marsland, N.; McArdle, P.; et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet. Med. 2018, 35, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.; Adams, L.; Anderson, J.; Maftei, O.; Couper, J.; Giles, L.; Pena, A.S. Australian children with type 1 diabetes consume high sodium and high saturated fat diets: Comparison with national and international guidelines. J. Paediatr. Child. Health 2019, 55, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Rovner, A.J.; Nansel, T.R. Are children with type 1 diabetes consuming a healthful diet?: A review of the current evidence and strategies for dietary change. Diabetes Educ. 2009, 35, 97–107. [Google Scholar] [CrossRef]
- Decsi, T.; Minda, H.; Hermann, R.; Kozari, A.; Erhardt, E.; Burus, I.; Molnar, S.; Soltesz, G. Polyunsaturated fatty acids in plasma and erythrocyte membrane lipids of diabetic children. Prostaglandins Leukot. Essent. Fatty Acids 2002, 67, 203–210. [Google Scholar] [CrossRef]
- Decsi, T.; Szabo, E.; Burus, I.; Marosvolgyi, T.; Kozari, A.; Erhardt, E.; Soltesz, G. Low contribution of n-3 polyunsaturated fatty acids to plasma and erythrocyte membrane lipids in diabetic young adults. Prostaglandins Leukot. Essent. Fatty Acids 2007, 76, 159–164. [Google Scholar] [CrossRef]
- Decsi, T.; Szabó, É.; Kozári, A.; Erhardt, É.; Marosvölgyi, T.; Soltész, G. Polyunsaturated fatty acids in plasma lipids of diabetic children during and after diabetic ketoacidosis. Acta Paediatrica 2005, 94, 850–855. [Google Scholar] [CrossRef]
- Mozzillo, E.; Zito, E.; Maffeis, C.; De Nitto, E.; Maltoni, G.; Marigliano, M.; Zucchini, S.; Franzese, A.; Valerio, G. Unhealthy lifestyle habits and diabetes-specific health-related quality of life in youths with type 1 diabetes. Acta Diabetol. 2017, 54, 1073–1080. [Google Scholar] [CrossRef]
- Krzyzowska, S.; Matejko, B.; Kiec-Wilk, B.; Wilk, M.; Malecki, M.; Klupa, T. Assessment of selected food intake frequency in patients with type 1 diabetes treated with personal insulin pumps. Rocz. Panstw. Zakl. Hig. 2019, 70, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Patton, S.R. Adherence to diet in youth with type 1 diabetes. J. Am. Diet. Assoc. 2011, 111, 550–555. [Google Scholar] [CrossRef]
- Helgeson, V.S.; Viccaro, L.; Becker, D.; Escobar, O.; Siminerio, L. Diet of Adolescents With and Without Diabetes. Diabetes Care 2006, 29, 982–987. [Google Scholar] [CrossRef]
- Marosvolgyi, T.; Mintal, K.; Farkas, N.; Sipos, Z.; Makszin, L.; Szabo, E.; Toth, A.; Kocsis, B.; Kovacs, K.; Hormay, E.; et al. Antibiotics and probiotics-induced effects on the total fatty acid composition of feces in a rat model. Sci. Rep. 2024, 14, 6542. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.; Li, X.; Li, J.; Zheng, T.; Xie, L.; Li, C.; Tang, Y.; Guo, K.; Huang, J.; et al. Distinct signatures of gut microbiota and metabolites in different types of diabetes: A population-based cross-sectional study. EClinicalMedicine 2023, 62, 102132. [Google Scholar] [CrossRef]
- Szustak, M.; Korkus, E.; Madaj, R.; Chworos, A.; Dabrowski, G.; Czaplicki, S.; Tabandeh, E.; Maciejewska, G.; Koziolkiewicz, M.; Konopka, I.; et al. Lysophosphatidylcholines Enriched with cis and trans Palmitoleic Acid Regulate Insulin Secretion via GPR119 Receptor. ACS Med. Chem. Lett. 2024, 15, 197–204. [Google Scholar] [CrossRef]
- Aronis, K.N.; Khan, S.M.; Mantzoros, C.S. Effects of trans fatty acids on glucose homeostasis: A meta-analysis of randomized, placebo-controlled clinical trials. Am. J. Clin. Nutr. 2012, 96, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Soltani, S.; Emadi, A.; Ghods, K.; Shab-Bidar, S. Dietary intake, biomarkers and supplementation of fatty acids and risk of coronary events: A systematic review and dose-response meta-analysis of randomized controlled trials and prospective observational studies. Crit. Rev. Food Sci. Nutr. 2024, 64, 12363–12382. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Uauy, R. WHO Scientific Update on health consequences of trans fatty acids: Introduction. Eur. J. Clin. Nutr. 2009, 63 (Suppl. S2), S1–S4. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.D. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Schwenke, D.C.; Foreyt, J.P.; Miller, E.R., 3rd; Reeves, R.S.; Vitolins, M.Z.; Oxidative Stress Subgroup of the Look, A.R.G. Plasma concentrations of trans fatty acids in persons with type 2 diabetes between September 2002 and April 2004. Am. J. Clin. Nutr. 2013, 97, 862–871. [Google Scholar] [CrossRef]
- Schlormann, W.; Kramer, R.; Lochner, A.; Rohrer, C.; Schleussner, E.; Jahreis, G.; Kuhnt, K. Foetal cord blood contains higher portions of n-3 and n-6 long-chain PUFA but lower portions of trans C18:1 isomers than maternal blood. Food Nutr. Res. 2015, 59, 29348. [Google Scholar] [CrossRef]
| Lipid Fraction | Trans Fatty Acids | Diabetic Adults (n = 34) | Controls (n = 36) | p |
|---|---|---|---|---|
| Plasma phospholipid | C16:1n-7t | 0.12 (0.05) | 0.16 (0.08) | ** |
| C18:1n-7/9t | 0.19 (0.16) | 0.21 (0.25) | ||
| C18:2n-6tt | 0.08 (0.04) | 0.08 (0.06) | ||
| Sum of trans | 0.43 (0.17) | 0.46 (0.33) | ||
| Plasma triacylglycerol | C16:1n-7t | 0.69 (0.19) | 0.79 (0.16) | * |
| C18:1n-7/9t | 0.52 (0.36) | 0.42 (0.48) | ||
| C18:2n-6tt | 0.12 (0.12) | 0.10 (0.06) | ||
| Sum of trans | 1.41 (0.61) | 1.37 (0.49) | ||
| Plasma sterol ester | C16:1n-7t | 0.42 (0.22) | 0.49 (0.23) | |
| C18:1n-7/9t | 0.06 (0.07) | 0.08 (0.16) | ||
| C18:2n-6tt | 0.06 (0.04) | 0.08 (0.08) | ||
| Sum of trans | 0.54 (0.34) | 0.64 (0.37) | * | |
| Plasma non-esterified fatty acid | C16:1n-7t | 0.56 (0.30) | 0.50 (0.44) | |
| C18:1n-7/9t | 0.27 (0.51) | 0.56 (0.59) | ||
| C18:2n-6tt | 0.08 (0.09) | 0.08 (0.05) | ||
| Sum of trans | 1.14 (0.62) | 1.36 (0.46) | * | |
| Erythrocyte membrane phosphatidylcholine | C16:1n-7t | 0.12 (0.04) | 0.12 (0.04) | |
| C18:1n-7/9t | 0.20 (0.17) | 0.18 (0.14) | ||
| C18:2n-6tt | 0.08 (0.02) | 0.07 (0.03) | ||
| Sum of trans | 0.42 (0.17) | 0.37 (0.16) | ||
| Erythrocyte membrane phosphatidylethanolamine | C16:1n-7t | 0.11 (0.04) | 0.13 (0.06) | ** |
| C18:1n-7/9t | 0.20 (0.12) | 0.17 (0.19) | ||
| C18:2n-6tt | 0.04 (0.02) | 0.04 (0.01) | ||
| Sum of trans | 0.36 (0.17) | 0.38 (0.22) |
| Lipid Fraction | Trans Fatty Acids | Diabetic Children (n = 40) | Controls (n = 40) | p |
|---|---|---|---|---|
| Plasma phospholipid | C16:1n-7t | 0.13 (0.05) | 0.14 (0.05) | |
| C18:1n-7/9t | 0.18 (0.22) | 0.24 (0.21) | ||
| C18:2n-6tt | 0.06 (0.05) | 0.05 (0.05) | ||
| Sum of trans | 0.39 (0.23) | 0.44 (0.24) | ||
| Plasma triacylglycerol | C16:1n-7t | 0.67 (0.16) | 0.78 (0.26) | * |
| C18:1n-7/9t | 0.45 (0.36) | 0.53 (0.39) | ||
| C18:2n-6tt | 0.11 (0.09) | 0.11 (0.10) | ||
| Sum of trans | 1.30 (0.56) | 1.46 (0.64) | ||
| Plasma sterol ester | C16:1n-7t | 0.43 (0.08) | 0.46 (0.19) | |
| C18:1n-7/9t | 0.04 (0.08) | 0.07 (0.13) | * | |
| C18:2n-6tt | 0.04 (0.05) | 0.06 (0.03) | ||
| Sum of trans | 0.51 (0.13) | 0.65 (0.29) | * | |
| Plasma non-esterified fatty acid | C16:1n-7t | 0.39 (0.11) | 0.44 (0.22) | * |
| C18:1n-7/9t | 0.52 (0.68) | 0.39 (0.38) | * | |
| C18:2n-6tt | 0.14 (0.08) | 0.08 (0.07) | ** | |
| Sum of trans | 1.08 (0.70) | 0.95 (0.24) | ||
| Erythrocyte membrane phosphatidylcholine | C16:1n-7t | 0.12 (0.05) | 0.12 (0.04) | |
| C18:1n-7/9t | 0.21 (0.17) | 0.18 (0.14) | ||
| C18:2n-6tt | 0.08 (0.05) | 0.07 (0.03) | ||
| Sum of trans | 0.41 (0.21) | 0.37 (0.16) | ||
| Erythrocyte membrane phosphatidylethanolamine | C16:1n-7t | 0.13 (0.10) | 0.14 (0.11) | |
| C18:1n-7/9t | 0.36 (0.26) | 0.23 (0.14) | * | |
| C18:2n-6tt | 0.04 (0.02) | 0.03 (0.02) | * | |
| Sum of trans | 0.52 (0.29) | 0.43 (0.23) | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, É.; Marosvölgyi, T.; Mihályi, K.; Lohner, S.; Decsi, T. Trans Isomeric Fatty Acids in Children and Young Adults with Type 1 Diabetes Mellitus. Nutrients 2025, 17, 1907. https://doi.org/10.3390/nu17111907
Szabó É, Marosvölgyi T, Mihályi K, Lohner S, Decsi T. Trans Isomeric Fatty Acids in Children and Young Adults with Type 1 Diabetes Mellitus. Nutrients. 2025; 17(11):1907. https://doi.org/10.3390/nu17111907
Chicago/Turabian StyleSzabó, Éva, Tamás Marosvölgyi, Krisztina Mihályi, Szimonetta Lohner, and Tamás Decsi. 2025. "Trans Isomeric Fatty Acids in Children and Young Adults with Type 1 Diabetes Mellitus" Nutrients 17, no. 11: 1907. https://doi.org/10.3390/nu17111907
APA StyleSzabó, É., Marosvölgyi, T., Mihályi, K., Lohner, S., & Decsi, T. (2025). Trans Isomeric Fatty Acids in Children and Young Adults with Type 1 Diabetes Mellitus. Nutrients, 17(11), 1907. https://doi.org/10.3390/nu17111907









