From Soil to Brain: Olive Oil Attributes, Consumer Choices, Intermittent Fasting, and Their Impact on Health
Abstract
1. Introduction
2. The Context of the Mediterranean Diet and Fasting
- Supporting satiety during fasting periods [19];
- Providing anti-inflammatory effects that complement fasting-induced reductions in pro-inflammatory cytokines [14];
- Minimally disrupting ketogenesis and glycemic control when consumed in small quantities during modified fasts [20];
- Offering gut and cardiovascular protection during refeeding phases [21].
3. Chrononutrition, EVOO Intake, and Health, from a Biochemical Perspective
4. Nutritional Strategies in the Context of Therapeutic Modulation
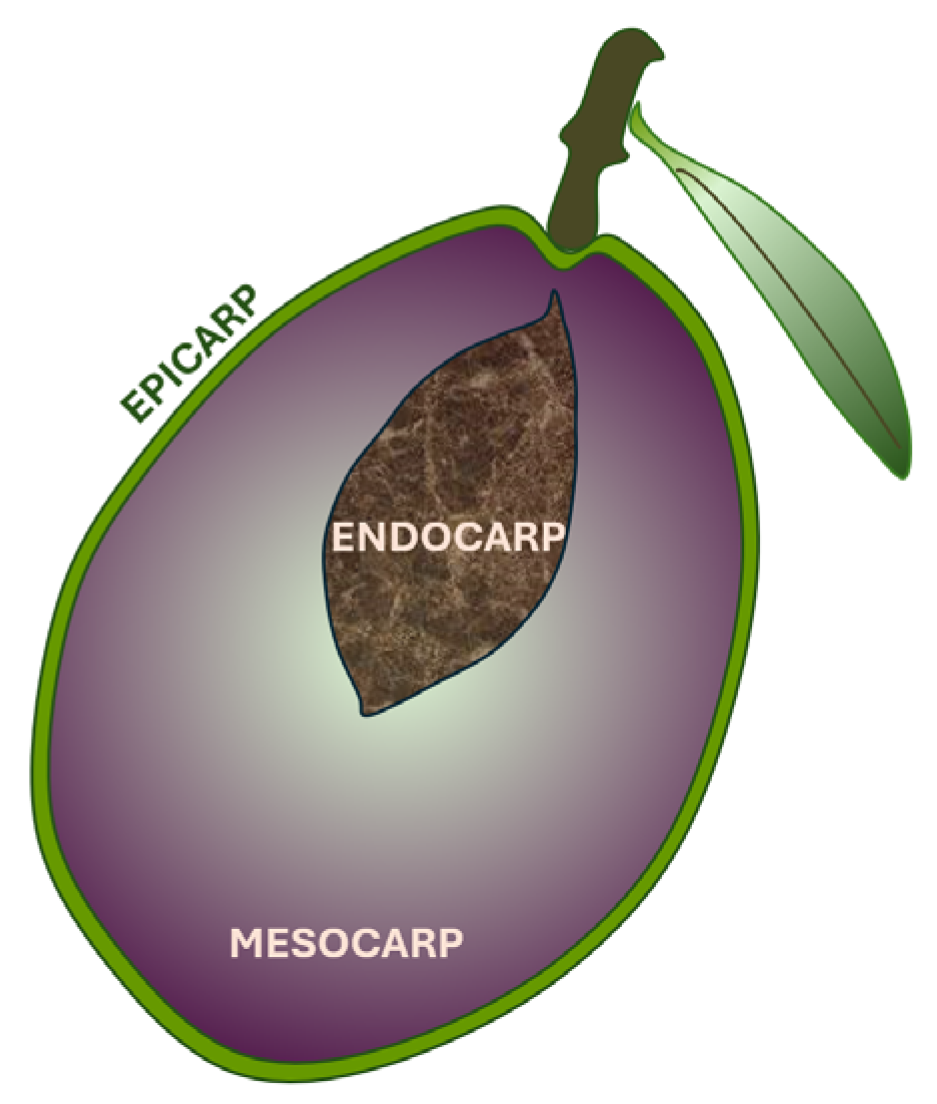
5. The Olive Fruit and the Types of Olive Oil
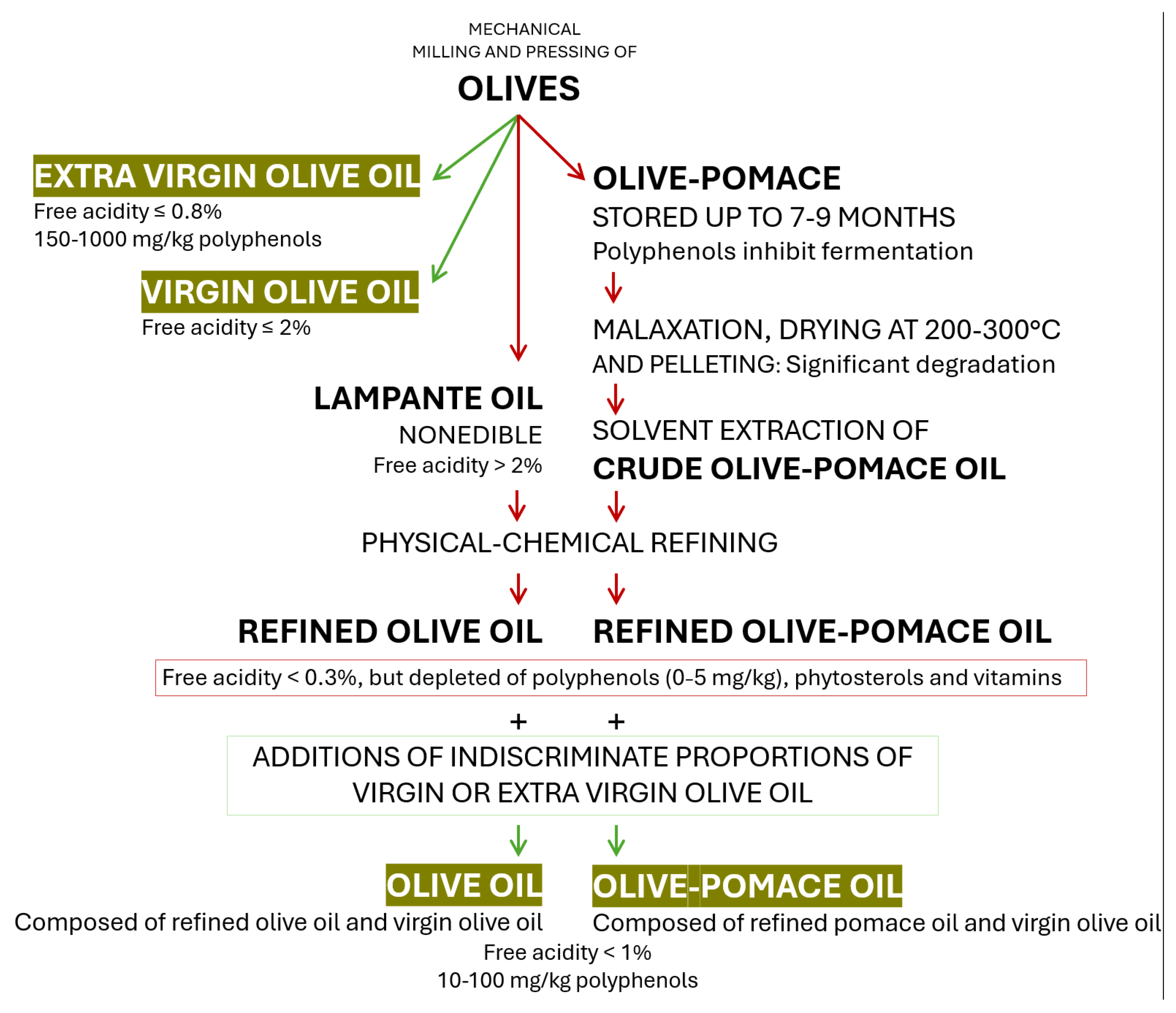
6. EVOO Constituents and Their Health Attributes
6.1. Legal and Regulatory Considerations
6.2. Age-Specific Considerations for Lipids Intake
6.3. Olive Oil in the Context of Mediterranean Diet
6.4. EVOO Composition and Organoleptic Attributes
6.5. Health Benefits of EVOO
6.5.1. Cardiovascular Health
6.5.2. Anti-Inflammatory Effects
6.5.3. Antioxidant Activities
6.5.4. Neuroprotective Potential
6.5.5. Metabolic Regulation and Longevity
6.5.6. Modulating Membrane Potential and Fluidity
6.5.7. Anticancer and Chemopreventive Effects
6.5.8. Gaps in Nutritional Research Related to EVOO
7. Factors That Influence the Quality of Olive Oil
8. EVOO Storage
9. Effects of Cooking on EVOO
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | amyloid-beta |
| AMPK | AMP-activated protein kinase |
| AP-1 | activator protein 1 |
| BCE | before current era |
| COX | cyclooxygenase |
| DNA | deoxyribonucleic acid |
| EFSA | European Food Safety Authority |
| EU | European Union |
| EVOO | extra-virgin olive oil |
| FAAH | fatty acid amide hydrolase |
| FDA | Food and Drug Administration |
| HDL | high-density lipoprotein |
| iNOS | inducible nitric oxide synthase |
| IOC | International Olive Council |
| Keap1 | Kelch-like ECH-associated protein 1 |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| LDL | low-density lipoprotein |
| LOPs | lipid oxidation products |
| LOX | lipoxygenase |
| MAO | monoamine oxidase |
| MD | Mediterranean diet |
| mTOR | mammalian target of rapamycin |
| MUFAs | monounsaturated fatty acids |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2/ARE | nuclear factor erythroid 2-related factor 2/antioxidant response element |
| NSAID | nonsteroidal anti-inflammatory drug |
| OLC | oleocanthal |
| OO | olive oil |
| PUFAs | polyunsaturated fatty acids |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SIRT | sirtuins |
| SFAs | saturated fatty acids |
| TFAs | trans-fatty acids |
| VLDL | very-low-density lipoprotein |
| VOO | virgin olive oil |
| WHO | World Health Organization |
Appendix A
Methodology
References
- Caramia, G.; Gori, A.; Valli, E.; Cerretani, L. Virgin olive oil in preventive medicine: From legend to epigenetics. Eur. J. Lipid Sci. Technol. 2012, 114, 375–388. [Google Scholar] [CrossRef]
- Grego, S. The Olive Tree: A Symbol. In Olive Cultivation; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Barazani, O.; Dag, A.; Dunseth, Z. The history of olive cultivation in the southern Levant. Front. Plant Sci. 2023, 14, 1131557. [Google Scholar] [CrossRef] [PubMed]
- Kaniewski, D.; Van Campo, E.; Boiy, T.; Terral, J.F.; Khadari, B.; Besnard, G. Primary domestication and early uses of the emblematic olive tree: Palaeobotanical, historical and molecular evidence from the Middle East. Biol. Rev. 2012, 87, 885–899. [Google Scholar] [CrossRef]
- IOC. International Olive Council, World Olive Oil Figures & EU Olive Oil Figures 2024. Available online: https://www.internationaloliveoil.org/world-market-of-olive-oil-and-table-olives-data-from-december-2024/ (accessed on 20 March 2025).
- Scotece, M.; Conde, J.; Abella, V.; Lopez, V.; Pino, J.; Lago, F.; Smith, I.I.I.A.B.; Gómez-Reino, J.J.; Gualillo, O. New drugs from ancient natural foods. Oleocanthal, the natural occurring spicy compound of olive oil: A brief history. Drug Discov. Today 2015, 4, 406–410. [Google Scholar] [CrossRef]
- UN Food and Agriculture Organization (FAOSTAT) Data 2021. Available online: https://data.un.org/ (accessed on 24 January 2025).
- Houmani, M.; Haidar, S.; Assi, R.; Hassan, H.F.; Rizk, R. Knowledge, perceptions, and practices regarding cooking and storage of olive oil: A consumer survey in Lebanon. J. Agric. Food Res. 2024, 18, 101279. [Google Scholar] [CrossRef]
- Tagliamonte, S.; De Luca, L.; Donato, A.; Paduano, A.; Balivo, A.; Genovese, A.; Romano, R.; Vitaglione, P.; Sacchi, R. A ‘Mediterranean ice-cream’: Sensory and nutritional aspects of replacing milk cream with extra virgin olive oil. J. Funct. Foods 2023, 102, 105470. [Google Scholar] [CrossRef]
- Lombardo, L.; Grasso, F.; Lanciano, F.; Loria, S.; Monetti, E. Broad-Spectrum Health Protection of Extra Virgin Olive Oil Compounds. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–77. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Ragusa, F.S.; Petralia, V.; Ciriminna, S.; Di Bella, G.; Schirò, P.; Sabico, S.; Al-Daghri, N.M.; Barbagallo, M. Mediterranean diet and spirituality/religion: Eating with meaning. Aging Clin. Exp. Res. 2024, 36, 223. [Google Scholar] [CrossRef]
- Purdel, C.; Margină, D.; Adam-Dima, I.; Ungurianu, A. The Beneficial Effects of Dietary Interventions on Gut Microbiota—An Up-to-Date Critical Review and Future Perspectives. Nutrients 2023, 15, 5005. [Google Scholar] [CrossRef]
- Ungurianu, A.; Margină, D.; Mihai, D.P.; Nicolae, A.C.; Drăgoi, C.M.; Grădinaru, D.; Zanfirescu, A. Caloric restriction mimetics: Pinostilbene versus resveratrol regarding SIRT1 and SIRT6 interaction. Adv. Med. Sci. 2025, 70, 44–50. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.M.; Drăgoi, C.M. Intermittent Fasting on Human Health and Disease. Nutrients 2023, 15, 4491. [Google Scholar] [CrossRef]
- Oliveras-López, M.J.; Molina, J.J.M.; Mir, M.V.; Rey, E.F.; Martín, F.; De la Serrana, H.L.G. Extra virgin olive oil (EVOO) consumption and antioxidant status in healthy institutionalized elderly humans. Arch. Gerontol. Geriatr. 2013, 57, 234–242. [Google Scholar] [CrossRef]
- Couto, S.; Cenit, M.C.; Montero, J.; Iguacel, I. The impact of Intermittent Fasting and Mediterranean Diet on older adults physical health and quality of life: A Randomized Clinical Trial. Nutr. Metab. Cardiovasc. Dis. 2025. accepted. [Google Scholar] [CrossRef]
- Arciero, P.J.; Poe, M.; Mohr, A.E.; Ives, S.J.; Arciero, A.; Sweazea, K.L.; Gumpricht, E.; Arciero, K.M. Intermittent fasting and protein pacing are superior to caloric restriction for weight and visceral fat loss. Obesity 2023, 31 (Suppl. S1), 139–149. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts, N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- de Oliveira Melo, N.C.; Cuevas-Sierra, A.; Souto, V.F.; Martínez, J.A. Biological Rhythms, Chrono-Nutrition, and Gut Microbiota: Epigenomics Insights for Precision Nutrition and Metabolic Health. Biomolecules 2024, 14, 559. [Google Scholar] [CrossRef]
- Patel, A.; Cheung, J. The effect of mediterranean diet and chrononutrition on sleep quality: A scoping review. Nutr. J. 2025, 24, 31. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Drăgoi, C.; Moroşan, E.; Dumitrescu, I.B.; Nicolae, A.C.; Arsene, A.L.; Drăgănescu, D.; Lupuliasa, D.; Ioniţă, A.C.; Stoian, A.P.; Nicolae, C.; et al. Insights into chrononutrition: The innermost interplay amongst nutrition, metabolism and the circadian clock, in the context of epigenetic reprogramming. Farmacia 2019, 67, 557–571. [Google Scholar] [CrossRef]
- Dragoi, C.M.; Yang, Z.; Fekry, B.; Brenna, A. Chronobiology in cardiometabolic health and disease. Front. Pharmacol. 2025, 15, 1544963. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Puppala, A.; Rankawat, S.; Ray, S.; Ray, S. Role of circadian rhythms in metabolic syndrome. In Metabolic Syndrome; Elsevier: Amsterdam, The Netherlands, 2024; pp. 199–218. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P. Chronobiology and obesity. Nutr. Hosp. 2013, 28 (Suppl. S5), 114–120. [Google Scholar] [CrossRef]
- Covas, M.I.; Ruiz-Gutiérrez, V.; De La Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor Components of Olive Oil: Evidence to Date of Health Benefits in Humans. Nutr. Rev. 2006, 64, 20–30. [Google Scholar] [CrossRef]
- Bailey, S.M.; Udoh, U.S.; Young, M.E. Circadian regulation of metabolism. J. Endocrinol. 2014, 222, 75–96. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Fernández del Río, L.; Gutiérrez-Casado, E.; Varela-López, A.; Villalba, J.M. Olive Oil and the Hallmarks of Aging. Molecules 2016, 21, 163. [Google Scholar] [CrossRef]
- Ahluwalia, M.K. Chrononutrition-When We Eat Is of the Essence in Tackling Obesity. Nutrients 2022, 14, 5080. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef]
- Moore, N.; Pollack, C.; Butkerait, P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther. Clin. Risk Manag. 2015, 11, 1061–1075. [Google Scholar] [CrossRef]
- Soydan, M.; Arabaci, G.; Utlu, N.; Halici, M.B.; Aktas Senocak, E.; Kiliçlioglu, M. Indomethacin-Induced Gastric Ulcer in Rats: Gastroprotectivity of Muscari neglectum in Water. Pharmaceuticals 2024, 18, 7. [Google Scholar] [CrossRef]
- Rallo, L.; Díez, C.M.; Morales-Sillero, A.; Miho, H.; Priego-Capote, F.; Rallo, P. Quality of olives: A focus on agricultural preharvest factors. Sci. Hortic. 2018, 233, 491–509. [Google Scholar] [CrossRef]
- Wiesman, Z. Desert Olive Oil Cultivation: Advanced Bio Technologies; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Clodoveo, M.L.; Camposeo, S.; Amirante, R.; Dugo, G.; Cicero, N.; Boskou, D. Research and Innovative Approaches to Obtain Virgin Olive Oils With a Higher Level of Bioactive Constituents. In Olive and Olive Oil Bioactive Constituents; Elsevier: Amsterdam, The Netherlands, 2015; pp. 179–215. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Gallina-Toschi, T.; Fernández-Gutiérrez, A. Analytical determination of polyphenols in olive oils. J. Sep. Sci. 2005, 28, 837–858. [Google Scholar] [CrossRef]
- Peri, C. (Ed.) The Extra-Virgin Olive Oil Handbook; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Le Clef, E.; Kemper, T. Sunflower Seed Preparation and Oil Extraction. In Sunflower: Chemistry, Production, Processing, and Utilization; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–226. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- European Parliament and Council Regulation EU 1308/2013. Available online: https://eur-lex.europa.eu/eli/reg/2013/1308/oj/eng (accessed on 20 February 2025).
- Skaltsounis, A.L.; Argyropoulou, A.; Aligiannis, N.; Xynos, N. Recovery of High Added Value Compounds from Olive Tree Products and Olive Processing Byproducts. In Olive and Olive Oil Bioactive Constituents; Elsevier: Amsterdam, The Netherlands, 2015; pp. 333–356. [Google Scholar] [CrossRef]
- Gullon, P.; Gullon, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- European Commission Regulation EU No 432/2012, Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32012R0432 (accessed on 20 February 2025).
- World Health Organization. Total Fat Intake for the Prevention of Unhealthy Weight Gain in Adults and Children WHO Guideline 2023. Available online: https://www.who.int/publications/i/item/9789240073654 (accessed on 20 February 2025).
- World Health Organization. Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children WHO Guideline 2023. Available online: https://www.who.int/publications/i/item/9789240073630 (accessed on 20 February 2025).
- Drăgoi, C.M.; Diaconu, C.C.; Nicolae, A.C.; Dumitrescu, I.B. Redox Homeostasis and Molecular Biomarkers in Precision Therapy for Cardiovascular Diseases. Antioxidants 2024, 13, 1163. [Google Scholar] [CrossRef]
- Diaconu, C.C. New Approaches and Perspectives for the Pharmacological Treatment of Arterial Hypertension. Farmacia 2018, 66, 408–415. [Google Scholar] [CrossRef]
- Riolo, R.; De Rosa, R.; Simonetta, I.; Tuttolomondo, A. Olive Oil in the Mediterranean Diet and Its Biochemical and Molecular Effects on Cardiovascular Health through an Analysis of Genetics and Epigenetics. Int. J. Mol. Sci. 2022, 23, 16002. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.J.; Wang, F.; Korat, A.A.; Eliassen, A.H.; Chavarro, J.; Grodstein, F.; Li, J.; Liang, L.; Willett, W.C.; Sun, Q.; et al. Optimal dietary patterns for healthy aging. Nat. Med. 2025, 31, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Mehany, T.; González-Sáiz, J.M.; Pizarro, C. Improving the Biostability of Extra Virgin Olive Oil with Olive Fruit Extract During Prolonged Deep Frying. Foods 2025, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Assmann, K.E.; Adjibade, M.; Andreeva, V.A.; Hercberg, S.; Galan, P.; Kesse-Guyot, E. Association Between Adherence to the Mediterranean Diet at Midlife and Healthy Aging in a Cohort of French Adults. J. Gerontol. Ser. A 2018, 73, 347–354. [Google Scholar] [CrossRef]
- Revelou, P.K.; Xagoraris, M.; Alexandropoulou, A.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Chemometric study of fatty acid composition of virgin olive oil from four widespread Greek cultivars. Molecules 2021, 26, 4151. [Google Scholar] [CrossRef]
- Abrante-Pascual, S.; Nieva-Echevarría, B.; Goicoechea-Oses, E. Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation. Foods 2024, 13, 4186. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Ausman, L.M.; Carrasco, W.; Jenner, J.L.; Gualtieri, L.J.; Goldin, B.R.; Ordovas, J.M.; Schaefer, E.J. Effects of canola, corn, and olive oils on fasting and postprandial plasma lipoproteins in humans as part of a National Cholesterol Education Program Step 2 diet. Arterioscler. Thromb. 1993, 13, 1533–1542. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Keast, R. Molecular Mechanisms of Inflammation. Anti-Inflammatory Benefits of Virgin Olive Oil and the Phenolic Compound Oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Theken, K.N.; Gong, L.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Ibuprofen pathways. Pharmacogenet. Genom. 2015, 25, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Abdallah, I.M.; Al-Shami, K.M.; Yang, E.; Wang, J.; Guillaume, C.; Kaddoumi, A. Oleuropein-Rich Olive Leaf Extract Attenuates Neuroinflammation in the Alzheimer’s Disease Mouse Model. ACS Chem. Neurosci. 2022, 13, 1002–1013. [Google Scholar] [CrossRef]
- Tarţa-Arsene, O. Dietary Omega-3 Fatty Acids Supplimentation for Attention Deficit with Hyperactivity Disorder in Epileptic Children. Farmacia 2017, 65, 550–556. [Google Scholar]
- Sharma, P.; Tandel, N.; Kumar, R.; Negi, S.; Sharma, P.; Devi, S.; Saxena, K.; Chaudhary, N.R.; Saini, S.; Kumar, R.; et al. Oleuropein activates autophagy to circumvent anti-plasmodial defense. iScience 2024, 27, 109463. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; López-Yerena, A.; Rinaldi de Alvarenga, J.F.; Romero del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernández, C.; Lahera, V.; Lago, F.; Pino, J.; Skaltsounis, L.; González-Gay, M.A.; Mobasheri, A.; Gómez, R.; Scotece, M.; et al. Natural Molecules for Healthy Lifestyles: Oleocanthal from Extra Virgin Olive Oil. J. Agric. Food Chem. 2019, 67, 3845–3853. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol.-Cell Physiol. 2002, 282, 227–241. [Google Scholar] [CrossRef]
- Correia, S.C.; Carvalho, C.; Cardoso, S.; XSantos, R.; IPlácido, A.; Candeias, E.; IDuarte, A.; IMoreira, P. Defective HIF Signaling Pathway and Brain Response to Hypoxia in Neurodegenerative Diseases: Not an “Iffy” Question! Curr. Pharm. Des. 2013, 19, 6809–6822. [Google Scholar] [CrossRef] [PubMed]
- González-Correa, J.A.; Muñoz-Marín, J.; Arrebola, M.M.; Guerrero, A.; Narbona, F.; López-Villodres, J.A.; De La Cruz, J.P. Dietary Virgin Olive Oil Reduces Oxidative Stress and Cellular Damage in Rat Brain Slices Subjected to Hypoxia–Reoxygenation. Lipids 2007, 42, 921–929. [Google Scholar] [CrossRef]
- Yoon, S.K. Oleuropein as an Antioxidant and Liver Protect. In The Liver; Elsevier: Amsterdam, The Netherlands, 2018; pp. 323–335. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Shahidi, F.; Anousakis, C. Antioxidants of Olive Oil, Olive Leaves, and their Bioactivity. In Olives and Olive Oil as Functional Foods; Wiley: Hoboken, NJ, USA, 2017; pp. 367–382. [Google Scholar] [CrossRef]
- Cuffaro, D.; Pinto, D.; Silva, A.M.; Bertolini, A.; Bertini, S.; Saba, A.; Macchia, M.; Rodrigues, F.; Digiacomo, M. Insights into the Antioxidant/Antiradical Effects and In Vitro Intestinal Permeation of Oleocanthal and Its Metabolites Tyrosol and Oleocanthalic Acid. Molecules 2023, 28, 5150. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.; Vale, N.; Silva, P. Neuroprotective Effects of Olive Oil: A Comprehensive Review of Antioxidant Properties. Antioxidants 2024, 13, 762. [Google Scholar] [CrossRef]
- Tang, J.; You, G.; Ruan, L.; Lu, Y.; Wen, B.; Wu, S. Antioxidant Behavior Affected by Polarity in the Olive Oil: Experimental and Molecular Simulation Investigations. ACS Omega 2021, 6, 7119–7126. [Google Scholar] [CrossRef]
- Gallardo-Fernandez, M.; Garcia, A.R.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Brito, M.A. In vitro study of the blood–brain barrier transport of bioactives from Mediterranean foods. Food Funct. 2024, 15, 3420–3432. [Google Scholar] [CrossRef]
- Butt, M.S.; Tariq, U.; Iahtisham-Ul-Haq; Naz, A.; Rizwan, M. Neuroprotective effects of oleuropein: Recent developments and contemporary research. J. Food Biochem. 2021, 45, e13967. [Google Scholar] [CrossRef]
- Nicolae, A.C.; Arsene, A.L.; Vuță, V.L.; Popa, D.E.; Sirbu, C.A.; Burcea Dragomiroiu, G.T.; Dumitrescu, I.B.; Velescu, B.Ș.; Gofiță, E.L.; Drăgoi, C.M. In vitro P-gp expression after administration of CNS active drugs. Farmacia 2016, 64, 844–850. [Google Scholar]
- Mohagheghi, F.; Bigdeli, M.R.; Rasoulian, B.; Hashemi, P.; Pour, M.R. The neuroprotective effect of olive leaf extract is related to improved blood–brain barrier permeability and brain edema in rat with experimental focal cerebral ischemia. Phytomedicine 2011, 18, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; El Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal Enhances Amyloid-β Clearance from the Brains of TgSwDI Mice and in Vitro across a Human Blood-Brain Barrier Model. ACS Chem. Neurosci. 2015, 6, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
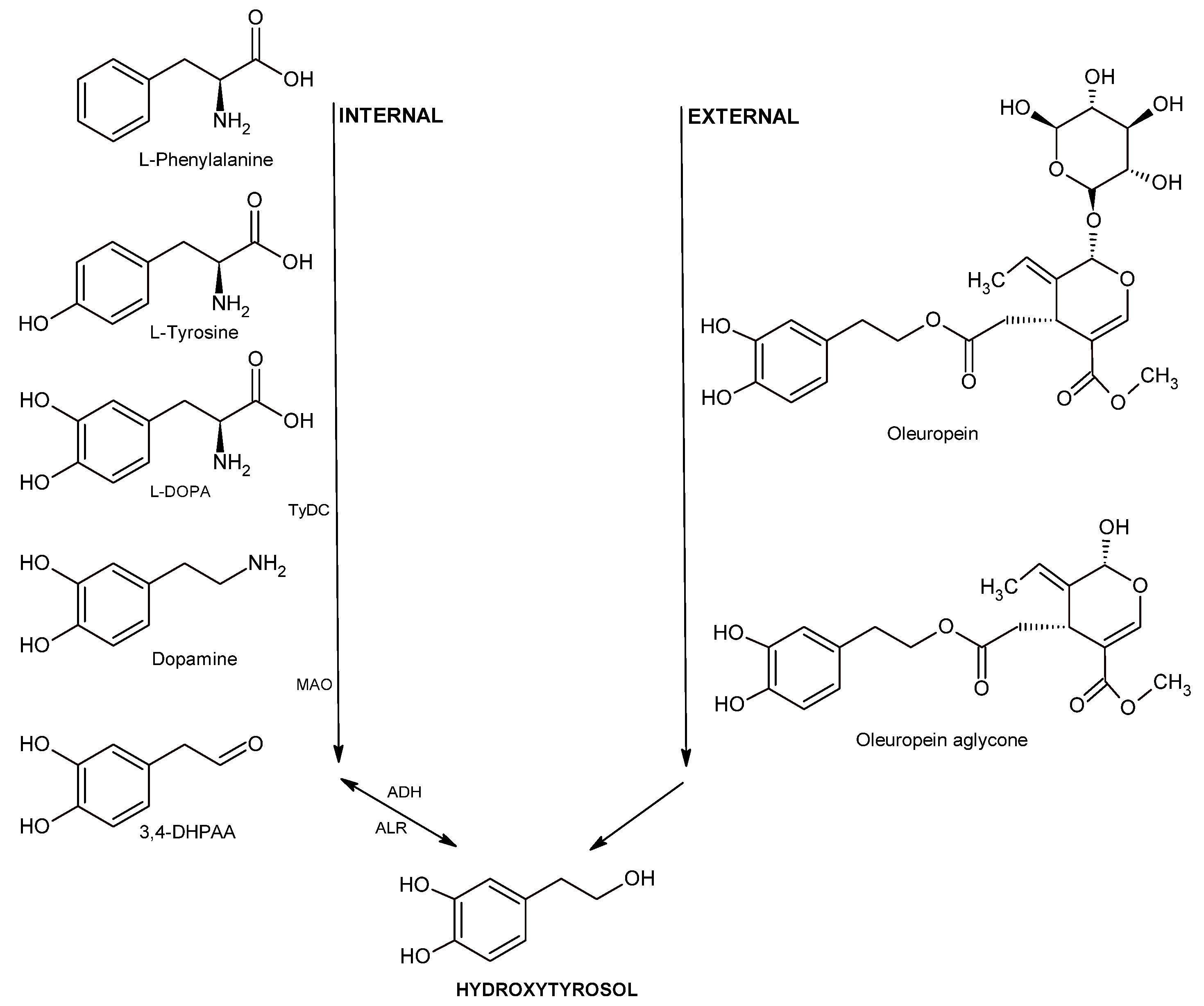
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Sánchez, R.; García-Vico, L.; Sanz, C.; Pérez, A.G. An Aromatic Aldehyde Synthase Controls the Synthesis of Hydroxytyrosol Derivatives Present in Virgin Olive Oil. Antioxidants 2019, 8, 352. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors Influencing Phenolic Compounds in Table Olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef]
- Chen, C.; Ai, Q.; Wei, Y. Potential role of hydroxytyrosol in neuroprotection. J. Funct. Foods. 2021, 82, 104506. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Blanco-Benítez, M.; Calderón-Fernández, A.; Canales-Cortés, S.; Alegre-Cortés, E.; Uribe-Carretero, E.; Paredes-Barquero, M.; Gimenez-Bejarano, A.; Duque González, G.; Gómez-Suaga, P.; Ortega-Vidal, J.; et al. Biological effects of olive oil phenolic compounds on mitochondria. Mol. Cell. Oncol. 2022, 9, 2044263. [Google Scholar] [CrossRef]
- Chen, S.; Cai, T.; Lu, J.; Le, J.; Zhang, J.; Yao, Q.; Chen, L. Hydroxytyrosol promotes random skin flap survival by activating SIRT1-mediated enhancement of autophagy. J. Funct. Foods 2024, 121, 106443. [Google Scholar] [CrossRef]
- Khalatbary, A.R. Olive oil phenols and neuroprotection. Nutr. Neurosci. 2013, 16, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Dragoi, C.M.; Mitrea, N.; Arsene, A.L.; Ilie, M.; Nicolae, A.C. Jurkat E6.1 cell line studies regarding the effects of some bio-indols on the membrane fluidity. Farmacia 2012, 60, 13–20. [Google Scholar]
- Dragoi, C.M.; Mitrea, N.; Arsene, A.L.; Nicolae, A.C.; Ilie, M. In vitro effects of some bio-indoles on the transmembrane potential of Jurkat E6.1 limphoblasts. Farmacia 2012, 60, 240–248. [Google Scholar]
- Al Rihani, S.B.; Darakjian, L.I.; Kaddoumi, A. Oleocanthal-Rich Extra-Virgin Olive Oil Restores the Blood–Brain Barrier Function through NLRP3 Inflammasome Inhibition Simultaneously with Autophagy Induction in TgSwDI Mice. ACS Chem. Neurosci. 2019, 10, 3543–3554. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R. Anti-cancer properties of olive oil secoiridoid phenols: A systematic review of: In vivo studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Negi, S.; Tandel, N.; Dalai, S.K.; Tyagi, R.K. Oleuropein: A viable therapeutic option for malaria and cancer. Drug Discov. Today 2025, 30, 104254. [Google Scholar] [CrossRef]
- Sirbu, C.A.; Georgescu, R.; Pleşa, F.C.; Paunescu, A.; Ţânţu, M.M.; Nicolae, A.C.; Caloianu, I.; Mitrica, M. Cannabis and Cannabinoids in Multiple Sclerosis: From Experimental Models to Clinical Practice—A Review. Am. J. Ther. 2023, 30, 220–231. [Google Scholar] [CrossRef]
- Carrera-González, M.P.; Ramírez-Expósito, M.J.; Mayas, M.D.; Martínez-Martos, J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci. Technol. 2013, 31, 92–99. [Google Scholar] [CrossRef]
- Tarun, M.T.I.; Elsayed, H.E.; Ebrahim, H.Y.; El Sayed, K.A. The Olive Oil Phenolic S-(-)-Oleocanthal Suppresses Colorectal Cancer Progression and Recurrence by Modulating SMYD2-EZH2 and c-MET Activation. Nutrients 2025, 17, 397. [Google Scholar] [CrossRef]
- Kusuma, I.Y.; Habibie, H.; Bahar, M.A.; Budán, F.; Csupor, D. Anticancer Effects of Secoiridoids—A Scoping Review of the Molecular Mechanisms behind the Chemopreventive Effects of the Olive Tree Components Oleocanthal, Oleacein, and Oleuropein. Nutrients 2024, 16, 2755. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A.; Gattullo, C.E. Ripening indices and harvesting times of different olive cultivars for continuous harvest. Sci. Hortic. 2013, 151, 1–10. [Google Scholar] [CrossRef]
- Miho, H.; Moral, J.; Barranco, D.; Ledesma-Escobar, C.A.; Priego-Capote, F.; Díez, C.M. Influence of genetic and interannual factors on the phenolic profiles of virgin olive oils. Food Chem. 2021, 342, 128357. [Google Scholar] [CrossRef]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean olive orchards under climate change: A review of future impacts and adaptation strategies. Agronomy 2021, 11, 56. [Google Scholar] [CrossRef]
- Barranco Navero, D. World Catalogue of Olive Varieties; International Olive Oil Council: Madrid, Spain, 2000. [Google Scholar]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Iqdiam, B.M.; Welt, B.A.; Goodrich-Schneider, R.; Sims, C.A.; Baker, G.L.; Marshall, M.R. Influence of headspace oxygen on quality and shelf life of extra virgin olive oil during storage. Food Packag. Shelf Life 2020, 23, 100433. [Google Scholar] [CrossRef]
- Sanmartin, C.; Venturi, F.; Sgherri, C.; Nari, A.; Macaluso, M.; Flamini, G.; Quartacci, M.F.; Taglieri, I.; Andrich, G.; Zinnai, A. The effects of packaging and storage temperature on the shelf-life of extra virgin olive oil. Heliyon 2018, 4, e00888. [Google Scholar] [CrossRef]
- Mousavi, S.; Mariotti, R.; Stanzione, V.; Pandolfi, S.; Mastio, V.; Baldoni, L.; Cultrera, N.G. Evolution of extra virgin olive oil quality under different storage conditions. Foods 2021, 10, 1945. [Google Scholar] [CrossRef] [PubMed]
- Majida, A.W.; Zeshan, A.; Mohamed, A.A.; Li, J.; Liu, Y.F. Enhancing extra virgin olive oil stability with chemically modified polysaccharides from Lycium barbarum L. leaves: Implications for heat resistance and shelf life. Heliyon 2025, 11, e42504. [Google Scholar] [CrossRef]
- Santos, C.S.P.; Cruz, R.; Cunha, S.C.; Casal, S. Effect of cooking on olive oil quality attributes. Food Res. Int. 2013, 54, 2016–2024. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; Rinaldi de Alvarenga, J.F.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Cooking with extra-virgin olive oil: A mixture of food components to prevent oxidation and degradation. Trends Food Sci. Technol. 2022, 123, 28–36. [Google Scholar] [CrossRef]
- Kishimoto, N. Microwave Heating Induces Oxidative Degradation of Extra Virgin Olive Oil. Food Sci. Technol. Res. 2019, 25, 75–79. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Study by 1H NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time. Food Chem. 2012, 134, 162–172. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target Ther. 2024, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Drăgoi, C.M.; Nicolae, A.C.; Ungurianu, A.; Margină, D.M.; Grădinaru, D.; Dumitrescu, I.B. Circadian Rhythms, Chrononutrition, Physical Training, and Redox Homeostasis—Molecular Mechanisms in Human Health. Cells 2024, 13, 138. [Google Scholar] [CrossRef]



| Health Claim/Recommendation | Conditions | Organization |
|---|---|---|
| Protection of LDL particles from oxidative damage | At least 5 mg hydroxytyrosol per 20 g olive oil; daily intake of 20 g | EFSA |
| Ensuring normal blood LDL cholesterol levels | Valid for foods high in unsaturated fats; oleic acid supports normal cholesterol levels | EFSA |
| Dietary fat intake recommendations | Total fat represents <30% of total energy intake; unsaturated fats preferred | WHO |
| Trans-fat intake recommendations | Trans-fats represent <1% of total energy intake (<2.2 g/day for 2000 kcal diet) | WHO |
| Major fraction 98–99% | saponifiable fraction mostly fatty acids in the form of TAGs, mainly triolein | ≈75% MUFAs | 55–83% ω-9 oleic acid <3.5% ω-7 palmitoleic acid <0.5% gadoleic acid, heptadecenoic acid | |
| <25% PUFAs | 3.5–21% ω-6 linoleic acid <1.5% ω-3 alpha-linolenic acid | |||
| <25% SFAs | 7.5–20% palmitic acid <5% stearic acid <1% lignoceric acid, arachidic acid <0.5% heptadecanoic acid, behenic acid <0.1% myristic acid | |||
| Minor fraction 1–2% | unsaponifiable fraction (nonpolar) | hydrocarbons | squalene (2–9 g/kg), β-carotene | EVOO has 20–30% more squalene compared to VOO. |
| tocopherols (lipophilic phenols) | 10–350 mg/kg | In refined OO the tocopherols are lost. Alpha-tocopherol can be added. | ||
| triterpenic alcohols and dialcohols | ||||
| phytosterols | 1–2.5 g/kg | But no cholesterol. | ||
| pigments | chlorophylls, pheophitins | |||
| hydrophilic fraction (polar) | phenolic compounds 120–600 mg/kg (1–3% of pulp) | secoiridoids 90% (almost exclusive to Olearaceae) | Oleuropein, oleacin, oleocanthal, ligstrozide. | |
| phenolic acids | Benzoic and cinnamic acids derivatives. | |||
| phenolic alcohols | Hydroxytyrosol tyrosol. | |||
| lignans | Pinoresinol. | |||
| flavonoids | Apigenin, luteolin. | |||
| hydroxy-isochromans | ||||
| volatile components | aldehydes, ketones and alcohols | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrescu, I.-B.; Drăgoi, C.M.; Nicolae, A.C. From Soil to Brain: Olive Oil Attributes, Consumer Choices, Intermittent Fasting, and Their Impact on Health. Nutrients 2025, 17, 1905. https://doi.org/10.3390/nu17111905
Dumitrescu I-B, Drăgoi CM, Nicolae AC. From Soil to Brain: Olive Oil Attributes, Consumer Choices, Intermittent Fasting, and Their Impact on Health. Nutrients. 2025; 17(11):1905. https://doi.org/10.3390/nu17111905
Chicago/Turabian StyleDumitrescu, Ion-Bogdan, Cristina Manuela Drăgoi, and Alina Crenguța Nicolae. 2025. "From Soil to Brain: Olive Oil Attributes, Consumer Choices, Intermittent Fasting, and Their Impact on Health" Nutrients 17, no. 11: 1905. https://doi.org/10.3390/nu17111905
APA StyleDumitrescu, I.-B., Drăgoi, C. M., & Nicolae, A. C. (2025). From Soil to Brain: Olive Oil Attributes, Consumer Choices, Intermittent Fasting, and Their Impact on Health. Nutrients, 17(11), 1905. https://doi.org/10.3390/nu17111905








