Do the Types of Dietary Carbohydrate and Protein Affect Postprandial Glycemia in Type 1 Diabetes?
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Meals
2.2. Glucose Monitoring
2.3. Statistical Analysis
3. Results
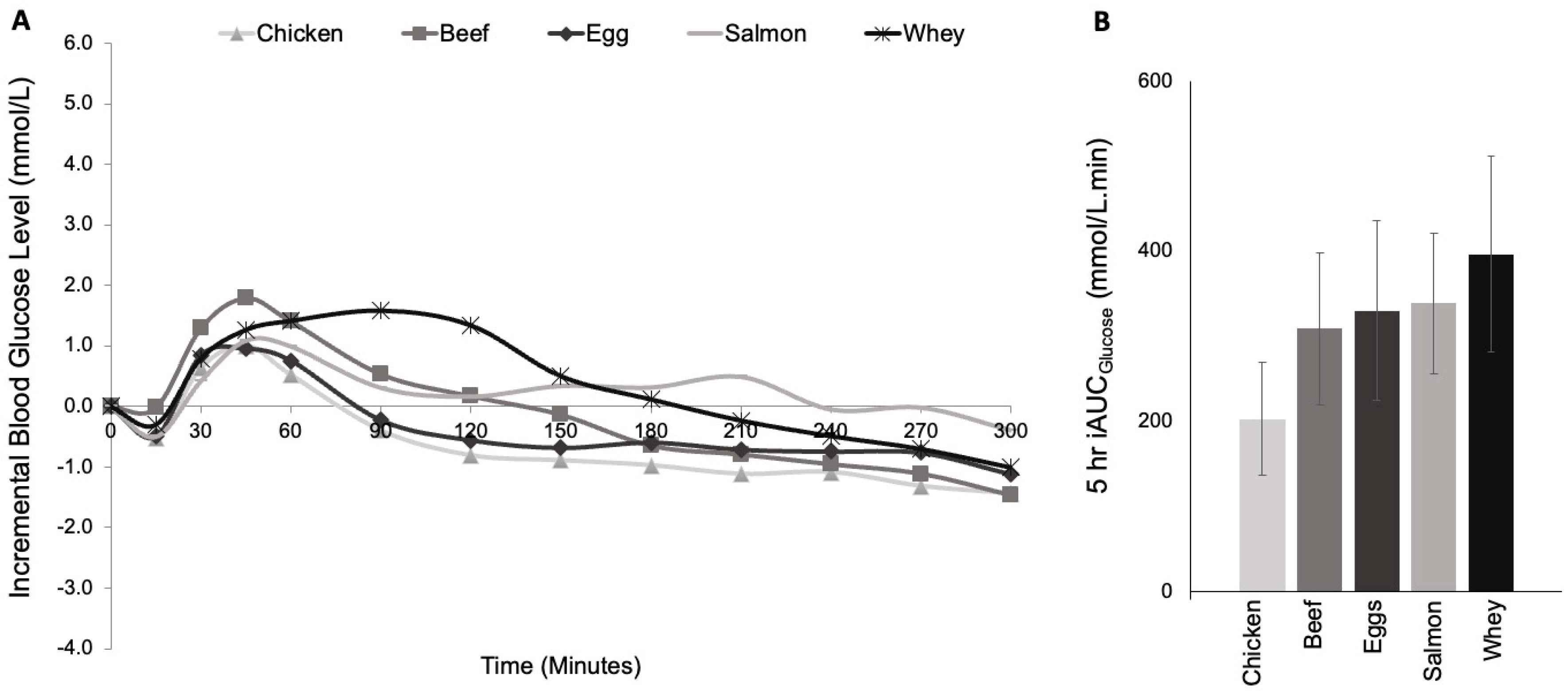
3.1. Study 1: Protein Type
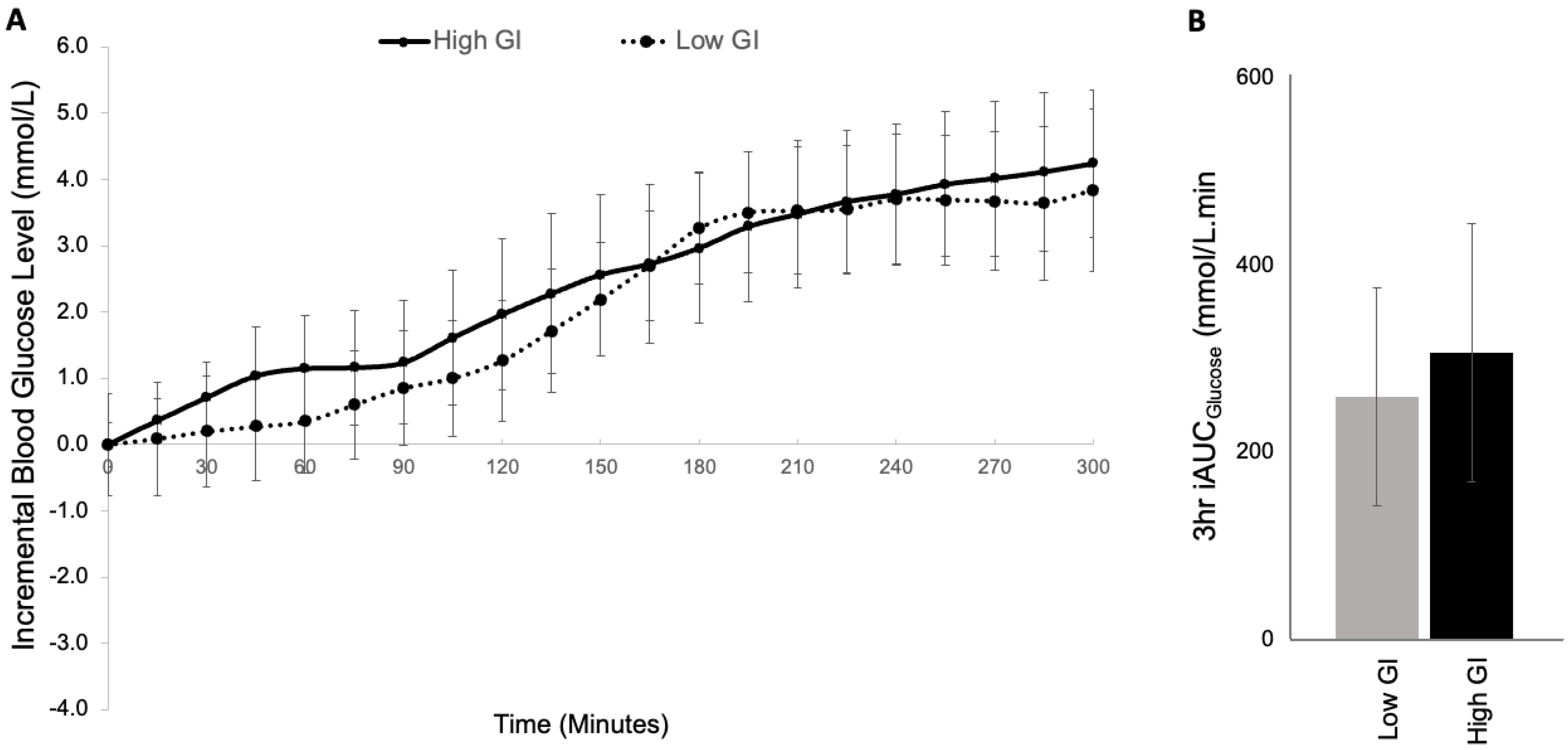
3.2. Study 2: GI Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48 (Suppl. S1), S86–S127. [Google Scholar]
- Annan, S.F.; Higgins, L.A.; Jelleryd, E.; Hannon, T.; Rose, S.; Salis, S.; Baptista, J.; Chinchilla, P.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1297–1321. [Google Scholar] [CrossRef]
- Bell, K.J.; Fio, C.Z.; Twigg, S.; Duke, S.-A.; Fulcher, G.; Alexander, K.; McGill, M.; Wong, J.; Brand-Miller, J.; Steil, G.M. and Insulin Requirements in Type 1 Diabetes: A Randomized Within-Subject Trial. Diabetes Care 2020, 43, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Toschi, E.; Steil, G.M.; Wolpert, H.A. Optimized Mealtime Insulin Dosing for Fat and Protein in Type 1 Diabetes: Application of a Model-Based Approach to Derive Insulin Doses for Open-Loop Diabetes Management. Diabetes Care 2016, 39, 1631–1634. [Google Scholar] [CrossRef]
- Pańkowska, E.; Błazik, M.; Groele, L. Does the fat-protein meal increase postprandial glucose level in type 1 diabetes patients on insulin pump: The conclusion of a randomized study. Diabetes Technol. Ther. 2012, 14, 16–22. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Papavasiliou, K.; Maouri, C.; Magriplis, E.; Pappas, S.; Bousboulas, S.; Koutsovasilis, A.; Pappas, M.; Sotiropoulos, A.; Kontogianni, M.D. Postprandial glucose response after the consumption of three mixed meals based on the carbohydrate counting method in adults with type 1 diabetes. A randomized crossover trial. Clin. Nutr. ESPEN 2019, 31, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.E.; Evans, M.; O’connell, S.M.; McElduff, P.; Lopez, P.E.; Jones, T.W.; Davis, E.A.; King, B.R. Both Dietary Protein and Fat Increase Postprandial Glucose Excursions in Children With Type 1 Diabetes, and the Effect Is Additive. Diabetes Care 2013, 36, 3897–3902. [Google Scholar] [CrossRef]
- Smith, T.A.; Smart, C.E.; Howley, P.P.; Lopez, P.E.; King, B.R. For a high fat- high protein breakfast, preprandial administration of 125% of the insulin dose improves postprandial glycaemic excursions in people with Type 1 diabetes using multiple daily injections: A cross-over trial. Diabet. Med. 2021, 38, e14512. [Google Scholar] [CrossRef]
- van der Hoogt, M.; van Dyk, J.; Dolman, R.; Pieters, M. Protein and fat meal content increase insulin requirement in children with type 1 diabetes—Role of duration of diabetes. J. Clin. Transl. Endocrinol. 2017, 10, 15–21. [Google Scholar] [CrossRef]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. protein and glycemic index on postprandial glucose control in type 1 diabetes: Implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef]
- Paterson, M.; Bell, K.J.; O’connell, S.M.; Smart, C.E.; Shafat, A.; King, B. The Role of Dietary Protein and Fat in Glycaemic Control in Type 1 Diabetes: Implications for Intensive Diabetes Management. Curr. Diabetes Rep. 2015, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- von Post-Skagegård, M.; Vessby, B.; Karlström, B. Glucose and insulin responses in healthy women after intake of composite meals containing cod-, milk-, and soy protein. Eur. J. Clin. Nutr. 2006, 60, 949–954. [Google Scholar] [CrossRef]
- Bell, K.J.; Petocz, P.; Colagiuri, S.; Brand-Miller, J.C. Algorithms to Improve the Prediction of Postprandial Insulinaemia in Response to Common Foods. Nutrients 2016, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Atkinson, F.; Petocz, P.; Willett, W.C.; Brand-Miller, J.C. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: Glycemic load compared with carbohydrate content alone. Am. J. Clin. Nutr. 2011, 93, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.C.; Stockmann, K.; Atkinson, F.; Petocz, P.; Denyer, G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: Analysis of a database of more than 1000 foods. Am. J. Clin. Nutr. 2009, 89, 97–105. [Google Scholar] [CrossRef]
- Thomas, D.; Elliott, E.J. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst. Rev. 2009, 2009, Cd006296. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.-L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef]
- Brand-Miller, J.; Hayne, S.; Petocz, P.; Colagiuri, S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003, 26, 2261–2267. [Google Scholar] [CrossRef]
- Metwally, M.; Cheung, T.O.; Smith, R.; Bell, K.J. Insulin pump dosing strategies for meals varying in fat, protein or glycaemic index or grazing-style meals in type 1 diabetes: A systematic review. Diabetes Res. Clin. Pract. 2021, 172, 108516. [Google Scholar] [CrossRef]
- Quarta, A.; Guarino, M.; Tripodi, R.; Giannini, C.; Chiarelli, F.; Blasetti, A. Diet and Glycemic Index in Children with Type 1 Diabetes. Nutrients 2023, 15, 3507. [Google Scholar] [CrossRef]
- Ryan, R.L.; King, B.R.; Anderson, D.G.; Attia, J.R.; Collins, C.E.; Smart, C.E. Influence of and optimal insulin therapy for a low-glycemic index meal in children with type 1 diabetes receiving intensive insulin therapy. Diabetes Care 2008, 31, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, P.; Wang, J.; Gregg, E.W.; Yang, W.; Gong, Q.; Li, H.; Li, H.; Jiang, Y.; An, Y.; et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet 2008, 371, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Kingwell, B.A.; Larsen, R.; Healy, G.N.; Cerin, E.; Hamilton, M.T.; Shaw, J.E.; Bertovic, D.A.; Zimmet, P.Z.; Salmon, J.; et al. Breaking Up Prolonged Sitting Reduces Postprandial Glucose and Insulin Responses. Diabetes Care 2012, 35, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, L.; Rabasa-Lhoret, R.; Poisson, D.; Ducros, F.; Chiasson, J.-L. Effects of different glycaemic index foods and dietary fibre intake on glycaemic control in type 1 diabetic patients on intensive insulin therapy. Diabet. Med. 1998, 15, 972–978. [Google Scholar] [CrossRef]
- Elleri, D.; Allen, J.M.; Harris, J.; Kumareswaran, K.; Nodale, M.; Leelarathna, L.; Acerini, C.L.; Haidar, A.; Wilinska, M.E.; Jackson, N.; et al. Absorption patterns of meals containing complex carbohydrates in type 1 diabetes. Diabetologia 2013, 56, 1108–1117. [Google Scholar] [CrossRef]
- MacDonald, K.; Lowe, J.M.; Barker, D.; Mensch, M.; Attia, J. Effect of popular takeaway foods on blood glucose levels in type 1 diabetes mellitus patients on intensive insulin therapy. Int. J. Clin. Pract. 2009, 63, 189–194. [Google Scholar] [CrossRef]
- Parillo, M.; Annuzzi, G.; Rivellese, A.A.; Bozzetto, L.; Alessandrini, R.; Riccardi, G.; Capaldo, B. Effects of meals with different glycaemic index on postprandial blood glucose response in patients with Type 1 diabetes treated with continuous subcutaneous insulin infusion. Diabet. Med. 2011, 28, 227–229. [Google Scholar] [CrossRef]
- Bozzetto, L.; Alderisio, A.; Giorgini, M.; Barone, F.; Giacco, A.; Riccardi, G.; Rivellese, A.A.; Annuzzi, G. Extra-Virgin Olive Oil Reduces Glycemic Response to a High–Glycemic Index Meal in Patients With Type 1 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 518–524. [Google Scholar] [CrossRef]
- Erdal, B.; Caferoglu, Z.; Hatipoglu, N. The comparison of two mealtime insulin dosing algorithms for high and low glycaemic index meals in adolescents with type 1 diabetes. Diabet. Med. 2021, 38, e14444. [Google Scholar] [CrossRef]
- Bergman, R.N.; Hope, I.D.; Yang, Y.J.; Watanabe, R.M.; Meador, M.A.; Youn, J.H.; Ader, M. Assessment of insulin sensitivity in vivo. Endocr. Rev. 1985, 5, 411–429. [Google Scholar] [CrossRef]
- Paterson, M.A.; Smart, C.E.M.; Lopez, P.E.; Howley, P.; McElduff, P.; Attia, J.; Morbey, C.; King, B.R. Increasing the protein quantity in a meal results in dose-dependent effects on postprandial glucose levels in individuals with Type 1 diabetes mellitus. Diabet. Med. 2017, 34, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Paterson, M.A.; Smart, C.E.M.; Lopez, P.E.; McElduff, P.; Attia, J.; Morbey, C.; King, B.R. Influence of dietary protein on postprandial blood glucose levels in individuals with Type 1 diabetes mellitus using intensive insulin therapy. Diabet. Med. 2016, 33, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, H.A.; Smith, S.A.; Atakov-Castillo, A.; Steil, G.M. Dietary Fat Acutely Increases Glucose Concentrations and Insulin Requirements in Patients With Type 1 Diabetes: Implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care 2013, 36, 810–816. [Google Scholar] [CrossRef] [PubMed]
| Demographic | Study 1: Protein Type (Mean ± SD) | Study 2: GI (Mean ± SD) |
|---|---|---|
| Total participants (n) | 16 | 8 |
| Women (n, %) | 12 (75) | 6 (75) |
| Age (years) | 35.5 ± 18.2 | 29.0 ± 6.0 |
| Body weight (kg) | 74.4 ± 9.9 | 78.7 ± 18.5 |
| BMI (kg/m2) | 26.1 ± 3.8 | 26.2 ± 7.2 |
| HbA1c (%,mmol/mol) | 7.4 ± 1.2 (57 ± 11) | 7.5 ± 0.7 (59 ± 8) |
| CRP (mg/L) | 3.4 ± 5.8 | 3.2 ± 4.5 |
| Total cholesterol (mmol/L) | 5.0 ± 1.3 | 4.9 ± 1.3 |
| Triglycerides (mmol/L) | 0.9 ± 0.6 | 1.2 ± 1.2 |
| HDL cholesterol (mmol/L) | 1.7 ± 0.3 | 1.5 ± 0.3 |
| LDL cholesterol (mmol/L) | 2.8 ± 0.9 | 2.8 ± 0.7 |
| C-Peptide (mmol/L) | Negative (<0.10) | Negative (<0.10) |
| T1D duration (years) | 16.8 ± 9.6 | 18.0 ± 6.8 |
| Total daily dose of insulin (insulin units/day) | 48.0 ± 16.6 | 53.0 ± 11.7 |
| Chicken | Egg | Beef | Salmon | Whey | Difference Between Groups (p Value) | |
|---|---|---|---|---|---|---|
| 5h iAUC (mmol·min/L) | 203 ± 66 | 263 ± 100 | 309 ± 89 | 338 ± 83 | 397 ± 115 | 0.347 |
| Early iAUC (0–2 h) (mmol/L) | 92 ± 26 | 115 ± 43 | 148 ± 44 | 116 ± 38 | 181 ± 42 | 0.292 |
| Late iAUC (2–5 h) (mmol·min/L) | 133 ± 54 | 180 ± 76 | 201 ± 66 | 257 ± 66 | 281 ± 96 | 0.314 |
| Fasting BGL (mmol/L) | 7.4 ± 0.4 | 8.0 ± 0.6 | 7.6 ± 0.5 | 7.9 ± 0.5 | 8.6 ± 0.5 | 0.198 |
| Incremental Mean BGL (mmol/L) | −0.5 ± 0.4 | −0.3 ± 0.6 | 0.0 ± 0.5 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.380 |
| SD BGL (mmol/L) | 1.6 ± 0.2 | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.5 ± 0.2 | 1.8 ± 0.1 | 0.480 |
| CV (mmol/L) | 25.3 ± 3.9 | 24.1 ± 2.7 | 23.4 ± 2.8 | 18.9 ± 2.4 | 21.7 ± 2.9 | 0.226 |
| J-Index (mmol/L) | 1.0 ± 0.4 | 2.4 ± 0.9 | 0.2 ± 0.1 | 2.2 ± 0.8 | 3.1 ± 1.1 | 0.028 * |
| Incremental Peak BGL (mmol/L) | 2.2 ± 0.4 | 2.5 ± 0.5 | 2.7 ± 0.5 | 2.3 ± 0.5 | 3.0 ± 0.5 | 0.455 |
| Time to Peak (minutes) | 86 ± 23 | 74 ± 19 | 93 ± 23 | 133 ± 25 | 103 ± 21 | 0.313 |
| Incremental Nadir BGL (mmol/L) | −2.5 ± 0.4 | −2.6 ± 0.6 | −2.2 ± 0.5 | −2.1 ± 0.4 | −2.3 ± 0.5 | 0.736 |
| Time to Nadir (minutes) | 118 ± 24 | 139 ± 25 | 148 ± 30 | 116 ± 28 | 142 ± 34 | 0.824 |
| Amplitude (mmol/L) | 4.7 ± 0.4 | 5.1 ± 0.4 | 4.9 ± 0.6 | 4.4 ± 0.5 | 5.3 ± 0.4 | 0.446 |
| Incidence of Hypoglycemia (>3.5 mmol/L) | 6/16 | 2/16 | 3/16 | 1/16 | 2/16 | 0.089 |
| Mixed-modelling analysis | Factors affecting ‘5h iAUC’: Protein Type: p = 0.091 Fasting Mean BGL: p = 0.005 * | |||||
| High GI Carbohydrate Meal | Low GI Carbohydrate Meal | p Value | |
|---|---|---|---|
| 1 h iAUC (mmol/L∙min) | 45 ± 19 | 28 ± 15 | 0.185 |
| 3 h iAUC (mmol/L∙min) | 307 ± 128 | 260 ± 117 | 0.538 |
| 5 h iAUC (mmol/L∙min) | 756 ± 188 | 692 ± 166 | 0.694 |
| Mean absolute fasting BGL (mmol/L) | 7.6 ± 0.4 | 7.9 ± 0.9 | 0.766 |
| Incremental mean BGL (mmol/L) | 2.1 ± 0.6 | 1.9 ± 0.5 | 0.646 |
| Incremental SD (mmol/L) | 2.0 ± 0.3 | 2.0 ± 0.3 | 0.881 |
| Incremental peak BGL (mmol/L) | 5.1 ± 0.8 | 4.9 ± 0.9 | 0.777 |
| Time to peak BGL (min) | 276 ± 18 | 263 ± 20 | 0.231 |
| Nadir BGL (mmol/L) | −1.3 ± 0.2 | −1.0 ± 0.2 | 0.250 |
| Time to Nadir BGL (min) | 4 ± 24 | 47 ± 22 | 0.100 |
| Coefficient of Variation (%) | 21 ± 4 | 20 ± 2 | 0.760 |
| J-index | 7.0 ± 2.5 | 6.4 ± 2.0 | 0.800 |
| Incremental amplitude (mmol/L) | 6.4 ± 0.8 | 5.9 ± 0.9 | 0.580 |
| Incidence of hypoglycemia (<3.5 mmol/L) | 0/8 | 0/8 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wainwright, A.; Fio, C.Z.; Brodie, S.; Alexander, K.; McGill, M.; Duke, S.-A.; Fulcher, G.; Twigg, S.; Wong, J.; et al. Do the Types of Dietary Carbohydrate and Protein Affect Postprandial Glycemia in Type 1 Diabetes? Nutrients 2025, 17, 1868. https://doi.org/10.3390/nu17111868
Li X, Wainwright A, Fio CZ, Brodie S, Alexander K, McGill M, Duke S-A, Fulcher G, Twigg S, Wong J, et al. Do the Types of Dietary Carbohydrate and Protein Affect Postprandial Glycemia in Type 1 Diabetes? Nutrients. 2025; 17(11):1868. https://doi.org/10.3390/nu17111868
Chicago/Turabian StyleLi, Xinyi, Alice Wainwright, Chantelle Z. Fio, Shannon Brodie, Kylie Alexander, Margaret McGill, Sally-Anne Duke, Gregory Fulcher, Stephen Twigg, Jencia Wong, and et al. 2025. "Do the Types of Dietary Carbohydrate and Protein Affect Postprandial Glycemia in Type 1 Diabetes?" Nutrients 17, no. 11: 1868. https://doi.org/10.3390/nu17111868
APA StyleLi, X., Wainwright, A., Fio, C. Z., Brodie, S., Alexander, K., McGill, M., Duke, S.-A., Fulcher, G., Twigg, S., Wong, J., Brand-Miller, J., Steil, G. M., & Bell, K. J. (2025). Do the Types of Dietary Carbohydrate and Protein Affect Postprandial Glycemia in Type 1 Diabetes? Nutrients, 17(11), 1868. https://doi.org/10.3390/nu17111868







