Vitamin D Levels in Patients Presenting to a Rheumatology Clinic in Germany: Associations with Patient Characteristics and Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Renal Status
2.3. Vitamin D Assay
2.4. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Vitamin D Levels and Status
3.3. Associations Between Vitamin D Levels and Patient Characteristics
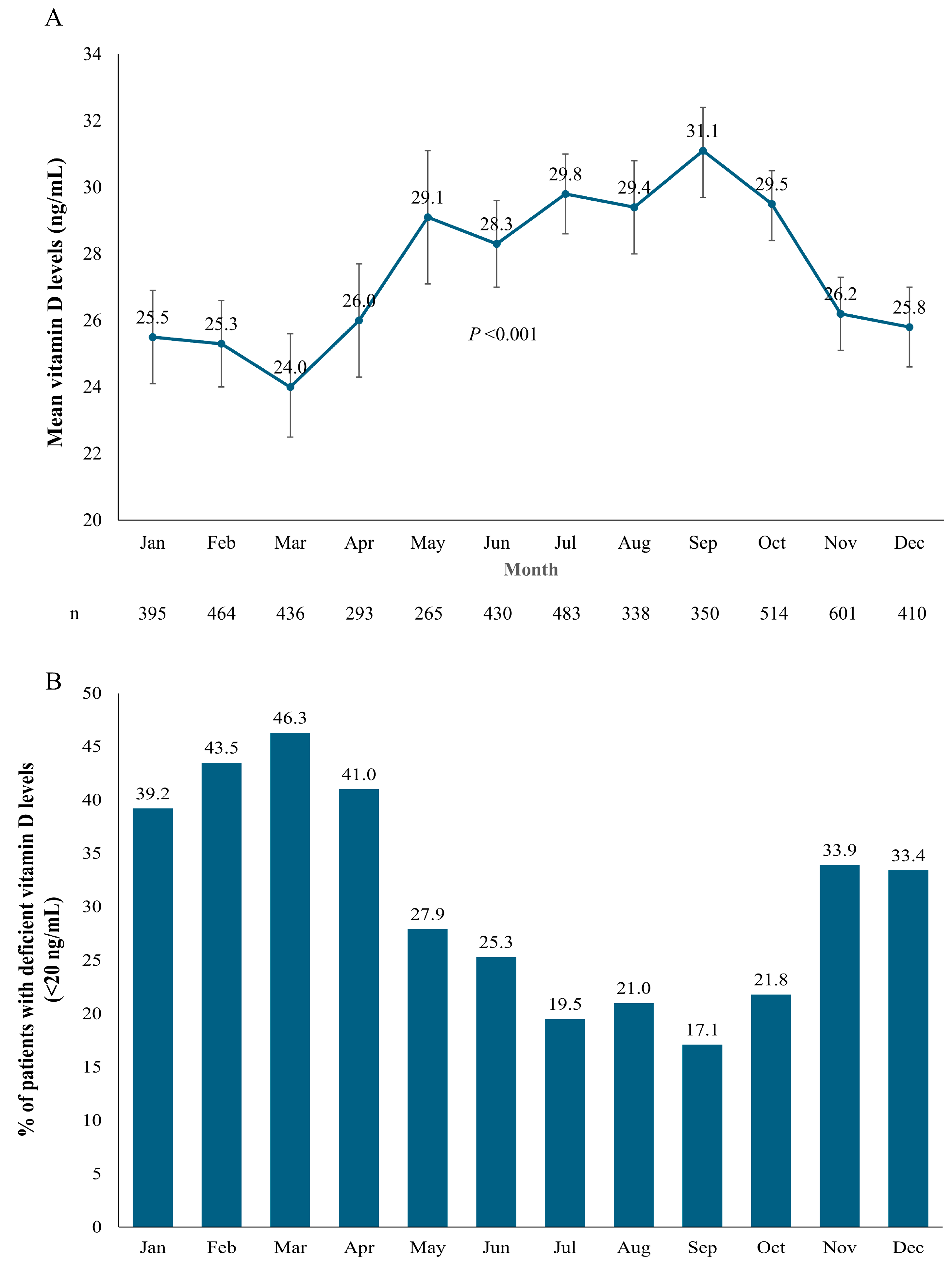
3.4. Seasonal Variations in Vitamin D Levels
3.5. Regression Analysis of Variables Influencing Vitamin D Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BMI | Body mass index |
| CKD | Chronic kidney disease |
| eGFR | Estimated glomerular filtration rate |
| EHR | Electronic health record |
| NS | Not significant |
| SD | Standard deviation |
References
- Rebelos, E.; Tentolouris, N.; Jude, E. The role of vitamin D in health and disease: A narrative review on the mechanisms linking vitamin D with disease and the effects of supplementation. Drugs 2023, 83, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Garnett, E.; Li, J.; Rajapakshe, D.; Tam, E.; Meng, Q.H.; Devaraj, S. Efficacy of two vitamin D immunoassays to detect 25-OH Vitamin D2 and D3. Pract. Lab. Med. 2019, 17, e00130. [Google Scholar] [CrossRef]
- Alonso, N.; Zelzer, S.; Eibinger, G.; Herrmann, M. Vitamin D metabolites: Analytical challenges and clinical relevance. Calcif. Tissue Int. 2022, 112, 158–177. [Google Scholar] [CrossRef]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus statement on vitamin D status assessment and supplementation: Whys, whens, and hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef]
- National Institutes of Health. Vitamin D Fact Sheet for Health Professionals. 2024. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 6 May 2025).
- Rupprecht, M.; Wagenpfeil, S.; Schöpe, J.; Vieth, R.; Vogt, T.; Reichrath, J. Meta-analysis of European clinical trials characterizing the healthy-adult serum 25-hydroxyvitamin D response to vitamin D supplementation. Nutrients 2023, 15, 3986. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Wetterslev, J.; Simonetti, R.G.; Bjelakovic, M.; Gluud, C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 2014, 2014, CD007470. [Google Scholar] [CrossRef]
- Rabenberg, M.; Scheidt-Nave, C.; Busch, M.A.; Rieckmann, N.; Hintzpeter, B.; Mensink, G.B.M. Vitamin D status among adults in Germany—Results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health 2015, 15, 641. [Google Scholar] [CrossRef]
- Rabenberg, M.; Scheidt-Nave, C.; Busch, M.A.; Thamm, M.; Rieckmann, N.; Durazo-Arvizu, R.A.; Dowling, K.G.; Škrabáková, Z.; Cashman, K.D.; Sempos, C.T.; et al. Implications of standardization of serum 25-hydroxyvitamin D data for the evaluation of vitamin D status in Germany, including a temporal analysis. BMC Public Health 2018, 18, 845. [Google Scholar] [CrossRef]
- Subramanian, A.; Burrowes, H.B.; Rumph, J.T.; Wilkerson, J.; Jackson, C.L.; Jukic, A.M.Z. Vitamin D levels in the United States: Temporal trends (2011–2018) and contemporary associations with sociodemographic characteristics (2017–2018). Nutrients 2024, 16, 3414. [Google Scholar] [CrossRef]
- CaMos Research Group; Greene-Finestone, L.S.; Berger, C.; De Groh, M.; Hanley, D.A.; Hidiroglou, N.; Sarafin, K.; Poliquin, S.; Krieger, J.; Richards, J.B.; et al. 25-hydroxyvitamin D in Canadian adults: Biological, environmental, and behavioral correlates. Osteoporos. Int. 2011, 22, 1389–1399. [CrossRef]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Tsiaras, W.; Weinstock, M. Factors influencing vitamin D status. Acta Derm. Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef] [PubMed]
- El-Gabalawy, H.; Guenther, L.C.; Bernstein, C.N. Epidemiology of immune-mediated inflammatory diseases: Incidence, prevalence, natural history, and comorbidities. J. Rheumatol. Suppl. 2010, 85, 2–10. [Google Scholar] [CrossRef]
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Charoenngam, N. Vitamin D and rheumatic diseases: A review of clinical evidence. Int. J. Mol. Sci. 2021, 22, 10659. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Roche Diagnostics. Elecsys Vitamin D Total III. 2025. Available online: https://elabdoc-prod.roche.com/eLD/api/downloads/8e91504f-e2dc-ef11-2891-005056a772fd?countryIsoCode=XG (accessed on 6 May 2025).
- Dai, Z.; McKenzie, J.E.; McDonald, S.; Baram, L.; Page, M.J.; Allman-Farinelli, M.; Raubenheimer, D.; Bero, L.A. Assessment of the methods used to develop vitamin D and calcium recommendations—A systematic review of bone health guidelines. Nutrients 2021, 13, 2423. [Google Scholar] [CrossRef]
- Heinz, T.; Hoxha, M.; Anderson, P.M.; Jakuscheit, A.; Weißenberger, M.; Lüdemann, M.; Rak, D.; Rudert, M.; Horas, K. Prevalence and associated risk factors for hypovitaminosis D in patients scheduled for primary total knee arthroplasty in Germany. Nutrients 2024, 16, 3991. [Google Scholar] [CrossRef]
- Zittermann, A.; Zelzer, S.; Herrmann, M.; Kleber, M.; Maerz, W.; Pilz, S. Association between magnesium and vitamin D status in adults with high prevalence of vitamin D deficiency and insufficiency. Eur. J. Nutr. 2025, 64, 48. [Google Scholar] [CrossRef] [PubMed]
- Mamilos, A.; Matzaroglou, C.; Maier, G.S.; Zawy Alsofy, S.; Drees, P.; Kafchitsas, K. Vitamin D deficiency in orthopedic patients in different latitudes—First study comparing German and Greek populations. Osteology 2023, 3, 11–20. [Google Scholar] [CrossRef]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D deficiency and insufficiency among US adults: Prevalence, predictors and clinical implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, A.; Oczkowicz, M. Sex differences in vitamin D metabolism, serum levels and action. Br. J. Nutr. 2022, 128, 2115–2130. [Google Scholar] [CrossRef]
- Geng, J.; Qiu, Y.; Li, Y.; Li, J.; Liao, R.; Du, H.; Jiang, L.; Wang, L.; Qin, Z.; Yang, Q.; et al. Associations between 25-hydroxyvitamin D, kidney function, and insulin resistance among adults in the United States of America. Front. Nutr. 2022, 8, 716878. [Google Scholar] [CrossRef]
- De Boer, I.H.; Sachs, M.C.; Chonchol, M.; Himmelfarb, J.; Hoofnagle, A.N.; Ix, J.H.; Kremsdorf, R.A.; Lin, Y.S.; Mehrotra, R.; Robinson-Cohen, C.; et al. Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: A participant-level analysis of 5 cohort studies and clinical trials. Am. J. Kidney Dis. 2014, 64, 187–197. [Google Scholar] [CrossRef]
- Wissenschaftlicher Dachverband Osteologie—DVO (Scientific Umbrella Association for Osteology). Prophylaxe, Diagnostik und Therapie der Osteoporose Bein Postmenopausalen Frauen und bei Männern ab Dem 50.Lebensjahr. 2023. Available online: https://leitlinien.dv-osteologie.org/wp-content/uploads/2024/02/DVO-Leitlinie-zur-Diagnostik-und-Therapie-der-Osteoporose-Version-2.1.-2023-002.pdf (accessed on 6 May 2024).
- German Nutrition Society. New reference values for vitamin D. Ann. Nutr. Metab. 2012, 60, 241–246. [Google Scholar] [CrossRef]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the prevention of disease: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef]
- Yeung, W.-C.G.; Toussaint, N.D.; Badve, S.V. Vitamin D rherapy in chronic kidney disease: A critical appraisal of clinical trial evidence. Clin. Kidney J. 2024, 17, sfae227. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Vervloet, M.; Cavalier, E.; Bacchetta, J.; De Borst, M.H.; Bover, J.; Cozzolino, M.; Ferreira, A.C.; Hansen, D.; Herrmann, M.; et al. The role of nutritional vitamin D in chronic kidney disease–mineral and bone disorder in children and adults with chronic kidney disease, on dialysis, and after kidney transplantation—A European consensus statement. Nephrol. Dial. Transplant. 2025, 40, 797–822. [Google Scholar] [CrossRef]
- Vervloet, M.G.; Hsu, S.; De Boer, I.H. Vitamin D supplementation in people with chronic kidney disease. Kidney Int. 2023, 104, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, M.B.; Russell, L.; Danila, M.I.; Fink, H.A.; Guyatt, G.; Cannon, M.; Caplan, L.; Gore, S.; Grossman, J.; Hansen, K.E.; et al. 2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2023, 75, 2088–2102. [Google Scholar] [CrossRef] [PubMed]
- Duru, N.; Van Der Goes, M.C.; Jacobs, J.W.G.; Andrews, T.; Boers, M.; Buttgereit, F.; Caeyers, N.; Cutolo, M.; Halliday, S.; Da Silva, J.A.P.; et al. EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann. Rheum. Dis. 2013, 72, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Meng, X.; Tian, Q.; Cao, W.; Fan, X.; Wu, L.; Song, M.; Meng, Q.; Wang, W.; Wang, Y. Vitamin D and multiple health outcomes: An umbrella review of observational studies, randomized controlled trials, and Mendelian randomization studies. Adv. Nutr. 2022, 13, 1044–1062. [Google Scholar] [CrossRef]
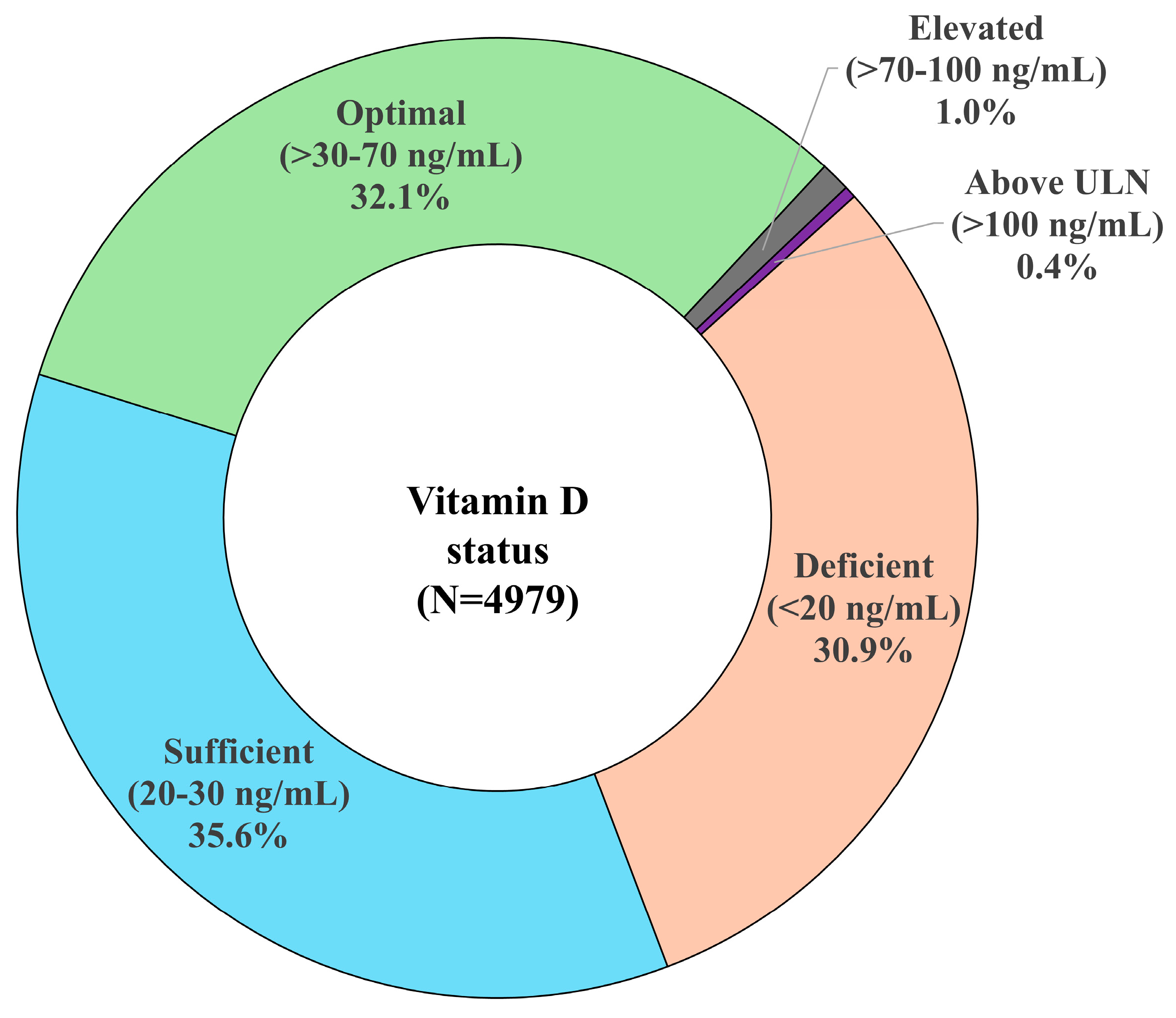

| Status Category | Vitamin D Level | |
|---|---|---|
| ng/mL | nmol/L | |
| Deficient | <20 | <50 |
| Sufficient | 20–30 | 50–75 |
| Optimal | >30–70 | >75–175 |
| Elevated | >70–100 | >175–250 |
| Above ULN | >100 | >250 |
| Variable | Clinic Cohort (N = 4979) |
|---|---|
| Sex, n (%) | |
| Females | 3230 (64.9%) |
| Males | 1749 (35.1%) |
| Age, mean (SD) [range] years | 53.6 (15.2) [11–94] |
| Body mass index, mean (SD) [range] kg/m2 | 27.2 (5.6) [15.4–67.1] |
| Renal status as assessed by eGFR a, n (%) | |
| ≥90 (normal or high kidney function) | 2559 (51.4%) |
| 60–89 (mildly decreased kidney function) | 2075 (41.7%) |
| 30–59 (moderately decreased kidney function) | 321 (6.4%) |
| 15–29 (severely decreased kidney function) | 20 (0.4%) |
| <15 (kidney failure) | 4 (0.1%) |
| Vitamin D, mean (SD) [range] ng/mL | 27.4 (14.0) [3–240] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feuchtenberger, M.; Kovacs, M.S.; Nigg, A.; Schäfer, A. Vitamin D Levels in Patients Presenting to a Rheumatology Clinic in Germany: Associations with Patient Characteristics and Season. Nutrients 2025, 17, 1893. https://doi.org/10.3390/nu17111893
Feuchtenberger M, Kovacs MS, Nigg A, Schäfer A. Vitamin D Levels in Patients Presenting to a Rheumatology Clinic in Germany: Associations with Patient Characteristics and Season. Nutrients. 2025; 17(11):1893. https://doi.org/10.3390/nu17111893
Chicago/Turabian StyleFeuchtenberger, Martin, Magdolna Szilvia Kovacs, Axel Nigg, and Arne Schäfer. 2025. "Vitamin D Levels in Patients Presenting to a Rheumatology Clinic in Germany: Associations with Patient Characteristics and Season" Nutrients 17, no. 11: 1893. https://doi.org/10.3390/nu17111893
APA StyleFeuchtenberger, M., Kovacs, M. S., Nigg, A., & Schäfer, A. (2025). Vitamin D Levels in Patients Presenting to a Rheumatology Clinic in Germany: Associations with Patient Characteristics and Season. Nutrients, 17(11), 1893. https://doi.org/10.3390/nu17111893







