Assessment of Vitamin D Metabolism Disorders in Hemodialysis Patients
Abstract
1. Introduction
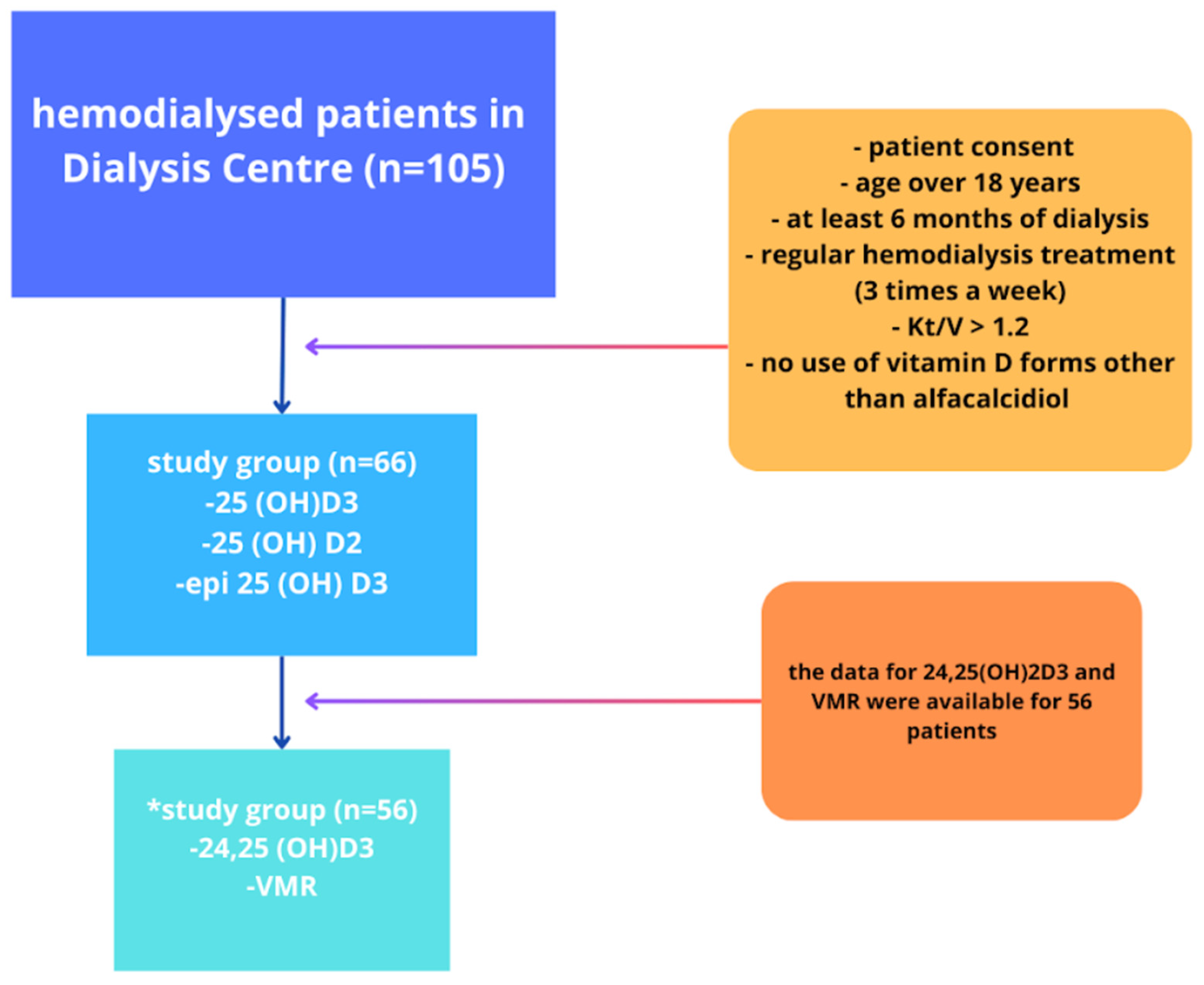
2. Materials and Methods
2.1. Sample Collection and Measurements of CBC, CRP, Phosphate, and Total Calcium
2.2. Sample Collection and Measurements of Vitamin D Metabolites Levels
2.3. Statistical Analysis
3. Results
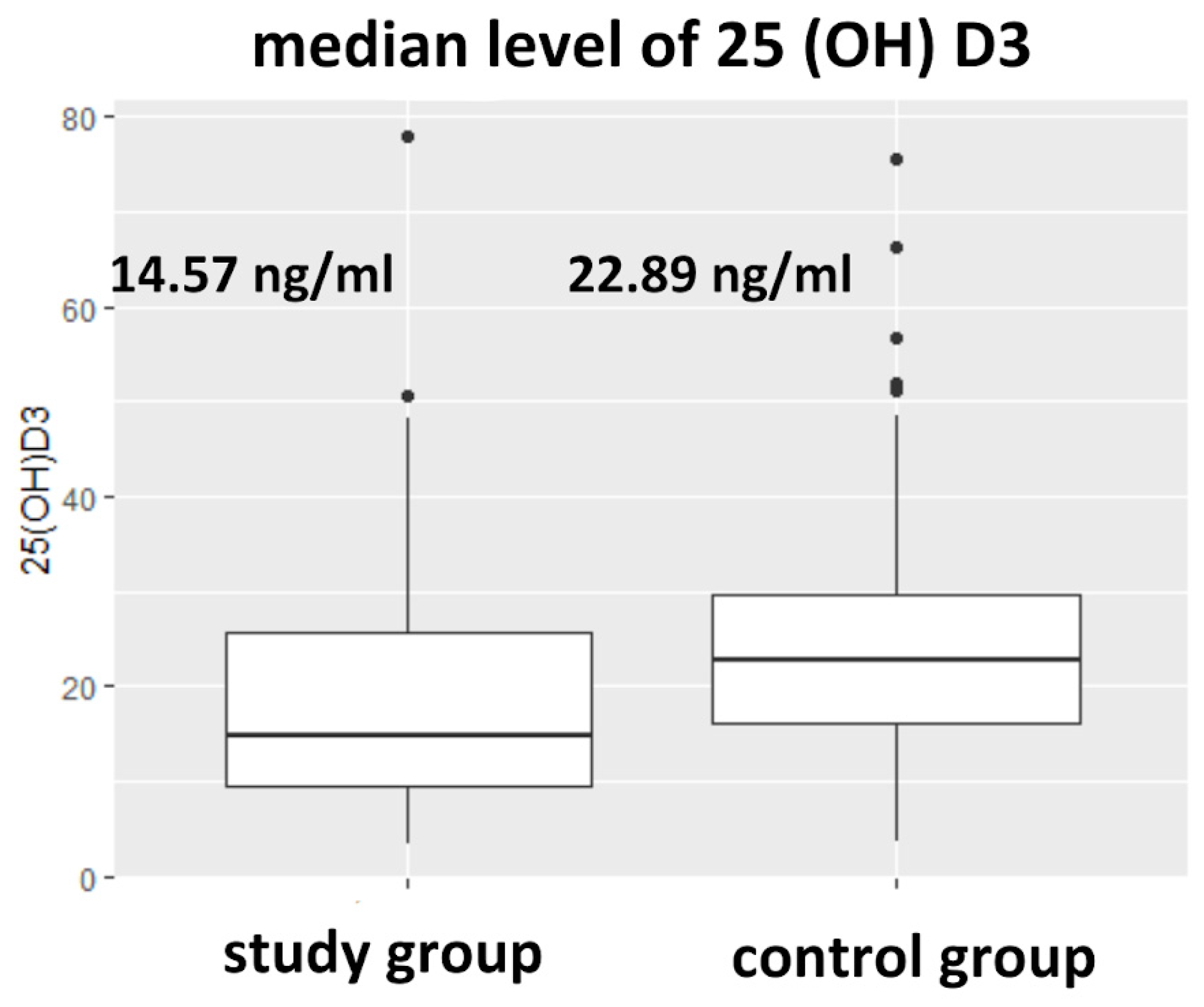
3.1. 25(OH)D3 Levels in Studied Groups
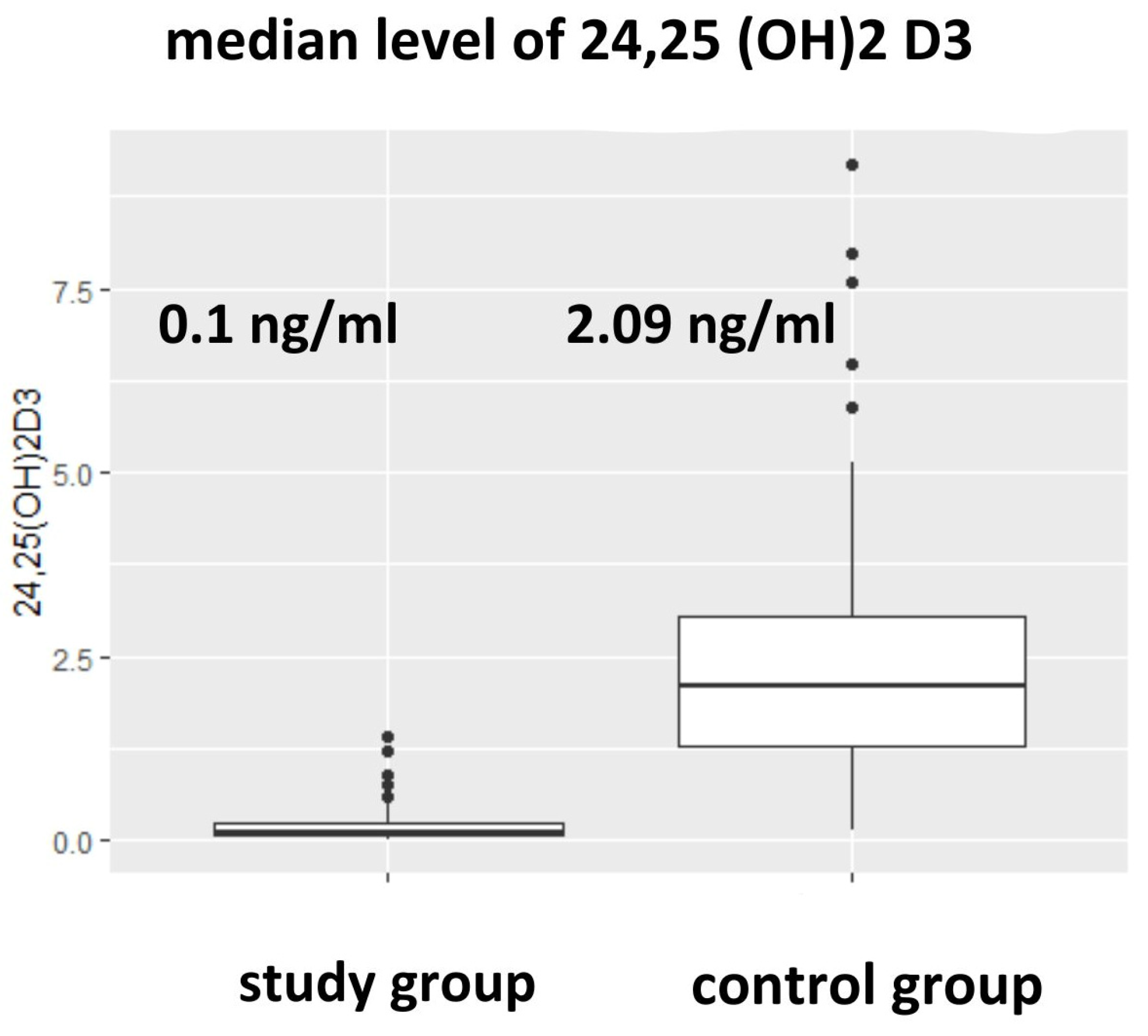
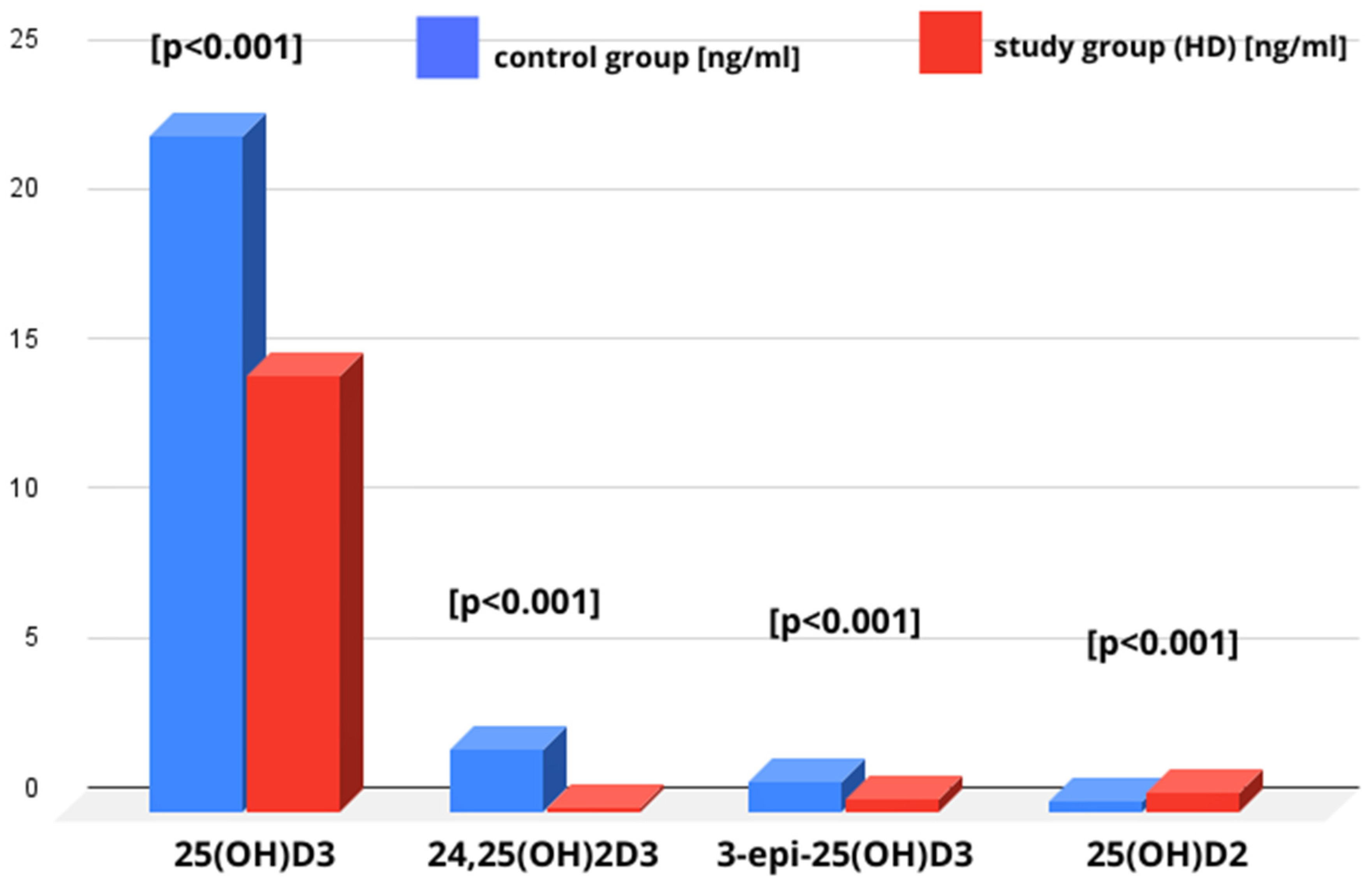
3.2. Vitamin D Metabolites and Vitamin D Metabolites Ratios (24,25(OH)2D3 to 25(OH)D3, epi-25(OH)D3/25(OH)D3) in Studied Groups
3.3. 25(OH)D2 Level in Studied Groups
3.4. 1,25(OH)2D3 in HD Patients Supplemented with Alphacalcidol
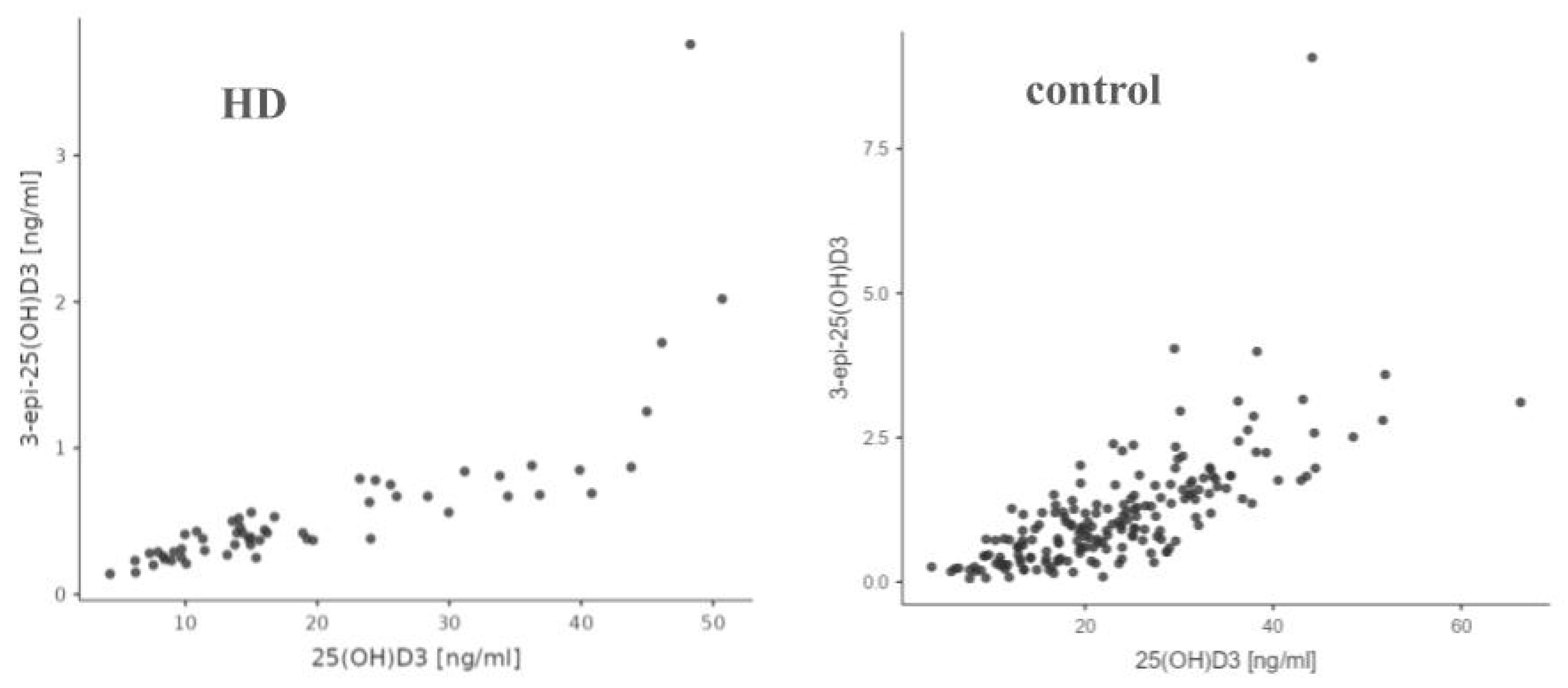
3.5. Correlations Between Studied Parameters
4. Discussion
4.1. 25(OH)D3
4.2. 24,25(OH)D3 and VMR
| Metabolite | Reference Value | Study Group [% n with Reference Range] | Control Group [% n with Reference Range] |
|---|---|---|---|
| 25(OH)D3 [ng/mL] | >30 [ng/mL] | 19.7% (n = 13/66) | 24.4% (n = 50/205) |
| epi-25(OH)D3 | no data existed | - | - |
| epi-25(OH)D3/25(OH)D3 | no data existed | - | - |
| 24,25(OH)2D3 [ng/mL] | >1.68 ng/mL (>4.2 nmol/L) | 0% (n = 0) | 62.0% (n = 127/205) |
| 24,25(OH)2D3/25(OH)D3 (VMR) | 4.4–14.3 [%] | 1.8% (n = 1/56) | 93.2% (n = 191/205) |
4.3. 25(OH)D2
4.4. 3-epi-25(OH)D3
4.5. 1,25(OH)D3
5. Conclusions
- The vitamin D3 reserves, assessed by 25(OH)D3 levels, were lower in the HD group than in the general population.
- Both functional deficiency and impaired vitamin D3 catabolism were present in the HD patients.
- The most sensitive parameter for assessing vitamin D3 deficiency was the VMR, which requires the measurement of 24,25(OH)D3.
- Alphacalcidol supplementation increases the concentration of 1,25(OH)2D3 without influencing 25(OH)D3.
- 25(OH)D2 is the only studied vitamin D metabolite that reached higher concentrations in the HD group than in the general population.
- This study demonstrated a statistically significant positive correlation between 25(OH)D3 and 24,25(OH)2D3 as well as 3-epi-25(OH)D3 in both the hemodialysis and control groups, indicating a strong relationship between these metabolites in vitamin D metabolism regardless of renal function status.
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic Kidney Disease |
| HD | Hemodialysis |
| VMR | Vitamin D Metabolite Ratio |
| SHPT | Secondary Hyperthyroidism |
| PTH | Parathormone |
| M-W | Mann–Whitney U |
| S-W | Shapiro–Wilk |
References
- Torres, P.A.U.; De Brauwere, D.P. Three feedback loops precisely regulating serum phosphate concentration. Kidney Int. 2011, 80, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Beggs, M.R.; Grimbly, C.; Alexander, R.T. Regulation of 1 and 24 hydroxylation of vitamin D metabolites in the proximal tubule. Exp. Biol. Med. 2022, 247, 1103–1111. [Google Scholar] [CrossRef]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M.; Zehnder, D.; Bland, R.; Stewart, P.M. 1alpha-Hydroxylase and the action of vitamin D. J. Mol. Endocrinol. 2000, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Htay, H.; Jha, V.; Wainstein, M.; Johnson, D.W. Epidemiology of haemodialysis outcomes. Nat. Rev. Nephrol. 2022, 18, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Hryciuk, M.; Heleniak, Z.; Ślizień, A.D. Management of chronic kidney disease–mineral and bone disorder. Ren. Dis. Transplant. Forum. 2023, 16, 65–80. Available online: https://journals.viamedica.pl/renal_disease_and_transplant/article/view/96819 (accessed on 23 December 2024).
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; E Kiely, M.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Dugar, A.; Hoofnagle, A.N.; Sanchez, A.P.; Ward, D.M.; Corey-Bloom, J.; Cheng, J.H.; Ix, J.H.; Ginsberg, C. The Vitamin D Metabolite Ratio (VMR) is a Biomarker of Vitamin D Status That is Not Affected by Acute Changes in Vitamin D Binding Protein. Clin. Chem. 2023, 69, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Sachs, M.C.; Chonchol, M.; Himmelfarb, J.; Hoofnagle, A.N.; Ix, J.H.; Kremsdorf, R.A.; Lin, Y.S.; Mehrotra, R.; Robinson-Cohen, C.; et al. Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: A participant-level analysis of 5 cohort studies and clinical trials. Am. J. Kidney Dis. 2014, 64, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Vitamin D Status Including 3-epi-25(OH)D3 Among Adult Patients with Thyroid Disorders During Summer Months|Kmieć|Endokrynologia Polska [Internet]. Available online: https://journals.viamedica.pl/endokrynologia_polska/article/view/57111 (accessed on 23 December 2024).
- Lensmeyer, G.; Poquette, M.; Wiebe, D.; Binkley, N. The C-3 epimer of 25-hydroxyvitamin D(3) is present in adult serum. J. Clin. Endocrinol. Metab. 2012, 97, 163–168. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef]
- Rola, R.; Kowalski, K.; Bieńkowski, T.; Studzińska, S. Improved sample preparation method for fast LC-MS/MS analysis of vitamin D metabolites in serum. J. Pharm. Biomed. Anal. 2020, 190, 113529. [Google Scholar] [CrossRef]
- Mieszkowski, J.; Stankiewicz, B.; Kochanowicz, A.; Niespodziński, B.; Kowalik, T.; Żmijewski, M.A.; Kowalski, K.; Rola, R.; Bieńkowski, T.; Antosiewicz, J. Ultra-Marathon-Induced Increase in Serum Levels of Vitamin D Metabolites: A Double-Blind Randomized Controlled Trial. Nutrients 2020, 12, 3629. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; E Powe, C.; Evans, M.K.; Wenger, J.; Ortiz, G.; Zonderman, A.B.; Suntharalingam, P.; Lucchesi, K.; Powe, N.R.; Karumanchi, S.A.; et al. 24,25-dihydroxyvitamin D3 and Vitamin D Status of Community Dwelling Black and White Americans. Clin. Chem. 2015, 61, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Chonchol, M. The Role of Phosphorus in the Development and Progression of Vascular Calcification. Am. J. Kidney Dis. 2011, 58, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Ureña-Torres, P.; Bover, J.; Luis Fernandez-Martín, J.; Cohen-Solal, M. Bone Fragility Fractures in CKD Patients. Calcif. Tissue Int. 2021, 108, 539–550. [Google Scholar] [CrossRef]
- Hollis, B.W. Editorial: The determination of circulating 25-hydroxyvitamin D: No easy task. J. Clin. Endocrinol. Metab. 2004, 89, 3149–3151. [Google Scholar] [CrossRef]
- Holick, M.F.; Biancuzzo, R.M.; Chen, T.C.; Klein, E.K.; Young, A.; Bibuld, D.; Reitz, R.; Salameh, W.; Ameri, A.; Tannenbaum, A.D. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J. Clin. Endocrinol. Metab. 2008, 93, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Chauhan, K.; Shahrokhi, M.; Huecker, M.R. Vitamin D. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441912/ (accessed on 23 December 2024).
- González-Gross, M.; Valtueña, J.; Breidenassel, C.; Moreno, L.A.; Ferrari, M.; Kersting, M.; De Henauw, S.; Gottrand, F.; Azzini, E.; Widhalm, K.; et al. Vitamin D status among adolescents in Europe: The Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br. J. Nutr. 2012, 107, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, R.L.; Sternberg, M.R.; Looker, A.C.; Yetley, E.A.; Lacher, D.A.; Sempos, C.T.; Taylor, C.L.; Durazo-Arvizu, R.A.; Maw, K.L.; Chaudhary-Webb, M.; et al. National Estimates of Serum Total 25-Hydroxyvitamin D and Metabolite Concentrations Measured by Liquid Chromatography-Tandem Mass Spectrometry in the US Population during 2007–2010. J. Nutr. 2016, 146, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Guessous, I.; McClellan, W.; Kleinbaum, D.; Vaccarino, V.; Zoller, O.; Theler, J.-M.; Paccaud, F.; Burnier, M.; Bochud, M.; Swiss Survey on Salt Group. Comparisons of serum vitamin D levels, status, and determinants in populations with and without chronic kidney disease not requiring renal dialysis: A 24-hour urine collection population-based study. J. Ren. Nutr. 2014, 24, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, C.R.; Levin, G.; Robinson-Cohen, C.; Hoofnagle, A.N.; Ruzinski, J.; Young, B.; Schwartz, S.M.; Himmelfarb, J.; Kestenbaum, B.; de Boer, I.H. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int. 2012, 82, 693–700. [Google Scholar] [CrossRef]
- Jean, G.; Charra, B.; Chazot, C. Vitamin D deficiency and associated factors in hemodialysis patients. J. Ren. Nutr. 2008, 18, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, Z.A.; Hamdan, Z.; Natour, N.; Barbar, M.; Rimawi, R.; Salaymeh, E. Prevalence of Vitamin D Deficiency among Hemodialysis Patients in Palestine: A Cross-Sectional Study. Int. J. Nephrol. 2021, 2021, 6684276. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Iguacel, C.; Gallar, P.; Qureshi, A.R.; Ortega, O.; Mon, C.; Ortiz, M.; Villarreal, I.; Garcia-Lacalle, C.; Olieta, A.; Sánchez, M.; et al. Vitamin D deficiency in dialysis patients: Effect of dialysis modality and implications on outcome. J. Ren. Nutr. 2010, 20, 359–367. [Google Scholar] [CrossRef]
- Kim, S.M.; Choi, H.J.; Lee, J.P.; Kim, D.K.; Oh, Y.K.; Kim, Y.S.; Lim, C.S. Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease. J. Ren. Nutr. 2014, 24, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, H.K.; Jain, D.; Mittal, A.; Pawar, S.; Ver, R. The prevalence of vitamin D deficiency in pre-dialysis patients with chronic kidney disease. Med. Stud. 2015, 31, 75–81. [Google Scholar] [CrossRef]
- Krause, R. Vitamin D and UV exposure in chronic kidney disease. Dermato-Endocrinology 2013, 5, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Ernst, J.B.; Becker, T.; Dreier, J.; Knabbe, C.; Gummert, J.F.; Kuhn, J. Measurement of Circulating 1,25-Dihydroxyvitamin D: Comparison of an Automated Method with a Liquid Chromatography Tandem Mass Spectrometry Method. Int. J. Anal. Chem. 2016, 2016, 8501435. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Chung, H.J.; Lê, H.G.; Na, B.K.; Cho, M.C. Serum 24,25-dihydroxyvitamin D level in general Korean population and its relationship with other vitamin D biomarkers. PLoS ONE 2021, 16, e0246541. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.C.Y.; Nicholls, H.; Piec, I.; Washbourne, C.J.; Dutton, J.J.; Jackson, S.; Greeves, J.; Fraser, W.D. Reference intervals for serum 24,25-dihydroxyvitamin D and the ratio with 25-hydroxyvitamin D established using a newly developed LC-MS/MS method. J. Nutr. Biochem. 2017, 46, 21–29. [Google Scholar] [CrossRef]
- Dirks, N.F.; Ackermans, M.T.; de Jonge, R.; Heijboer, A.C. Reference values for 24,25-dihydroxyvitamin D and the 25-hydroxyvitamin D/24,25-dihydroxyvitamin D ratio. Clin. Chem. Lab. Med. 2019, 57, e259–e261. [Google Scholar] [CrossRef]
- Tang, J.C.Y.; Jackson, S.; Walsh, N.P.; Greeves, J.; Fraser, W.D. The dynamic relationships between the active and catabolic vitamin D metabolites, their ratios, and associations with PTH. Sci. Rep. 2019, 9, 6974. [Google Scholar] [CrossRef] [PubMed]
- Graeff-Armas, L.A.; Kaufmann, M.; Lyden, E.; Jones, G. Serum 24,25-dihydroxyvitamin D3 response to native vitamin D2 and D3 Supplementation in patients with chronic kidney disease on hemodialysis. Clin. Nutr. Edinb. Scotl. 2018, 37, 1041–1045. [Google Scholar] [CrossRef]
- Weisman, Y.; Eisenberg, Z.; Leib, L.; Harell, A.; Shasha, S.M.; Edelstein, S. Serum concentrations of 24,25-dihydroxy vitamin D in different degrees of chronic renal failure. Br. Med. J. 1980, 281, 712–713. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, C.; de Boer, I.H. Impaired Vitamin D Metabolism in CKD. Semin. Nephrol. 2013, 33, 158–168. [Google Scholar] [CrossRef]
- Lee, S.; Chung, H.J.; Jung, S.; Jang, H.N.; Chang, S.-H.; Kim, H.-J.; Cho, M.-C. 24,25-Dihydroxy Vitamin D and Vitamin D Metabolite Ratio as Biomarkers of Vitamin D in Chronic Kidney Disease. Nutrients 2023, 15, 578. [Google Scholar] [CrossRef] [PubMed]
- Parviainen, M.T.; Savolainen, K.E.; Alhava, E.M.; Mäenpää, P.H. 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and total 24,25-dihydroxyvitamin D in human serum. Ann. Clin. Res. 1981, 13, 26–33. [Google Scholar]
- Bailey, D.; Veljkovic, K.; Yazdanpanah, M.; Adeli, K. Analytical measurement and clinical relevance of vitamin D(3) C3-epimer. Clin. Biochem. 2013, 46, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.M.; Nielson, C.M.; Shrestha, S.; Lee, C.G.; Barrett-Connor, E.; Jans, I.; Cauley, J.A.; Boonen, S.; Bouillon, R.; Vanderschueren, D.; et al. Higher 25(OH)D2 is associated with lower 25(OH)D3 and 1,25(OH)2D3. J. Clin. Endocrinol. Metab. 2014, 99, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Yang, Z.; Wang, J.; Duan, Y.; Zhao, L.; Yu, D.; Lai, J. Relationship between Serum 25OH-Vitamin D2 Level and Vitamin D Status of Children Aged 3-5 Years in China. Nutrients 2021, 13, 4135. [Google Scholar] [CrossRef]
- Nimitphong, H.; Saetung, S.; Chanprasertyotin, S.; Chailurkit, L.O.; Ongphiphadhanakul, B. Changes in circulating 25-hydroxyvitamin D according to vitamin D binding protein genotypes after vitamin D₃ or D₂supplementation. Nutr. J. 2013, 12, 39. [Google Scholar] [CrossRef]
- Tang, J.; Ying, B.; Yang, Y.; Xu, B.; Yu, L.; Jiang, W.; Pu, S. C3-Epimer of 25-Hydroxyvitamin D3 as a Superior Marker for Predicting the Severity of Chronic Kidney Disease in Rheumatoid Arthritis Patients. Oxid. Med. Cell Longev. 2022, 2022, 5268706. [Google Scholar] [CrossRef]
- Vitamin D Metabolism and Treatment in Chronic Kidney Disease [Internet]. Available online: https://www.medscape.org/viewarticle/571558_3 (accessed on 23 December 2024).
- Granado-Lorencio, F.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Donoso-Navarro, E.; Silvestre-Mardomingo, R. Serum levels of 3-epi-25-OH-D3 during hypervitaminosis D in clinical practice. J. Clin. Endocrinol. Metab. 2012, 97, E2266–E2270. [Google Scholar] [CrossRef]
- Arroyo, E.; A Leber, C.; Burney, H.N.; Li, Y.; Li, X.; Lu, T.-S.; Jones, G.; Kaufmann, M.; Ting, S.M.S.; Hiemstra, T.F.; et al. Epimeric vitamin D and cardiovascular structure and function in advanced CKD and after kidney transplantation. Nephrol. Dial. Transplant. 2024, 39, 264–276. [Google Scholar] [CrossRef]
- Souberbielle, J.-C.; Cavalier, E.; Delanaye, P.; Massart, C.; Brailly-Tabard, S.; Cormier, C.; Borderie, D.; Benachi, A.; Chanson, P. Serum calcitriol concentrations measured with a new direct automated assay in a large population of adult healthy subjects and in various clinical situations. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 451 Pt B, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Huish, S.A.; Jenkinson, C.; Dunn, J.A.; Meredith, D.J.; Bland, R.; Hewison, M. Low serum 1,25(OH)2D3 in end-stage renal disease: Is reduced 1α-hydroxylase the only problem? Endocr. Connect. 2021, 10, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Lotfollahi Haghi, L.; Ossareh, S.; Neyestani, T.R. SP392Evaluation of Serum Levels of 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D in Maintenance Hemodialysis Patients. Nephrol. Dial. Transplant. 2019, 34 (Suppl. 1), gfz103.SP392. [Google Scholar] [CrossRef]
- Moe, S.; Wazny, L.D.; Martin, J.E. Oral calcitriol versus oral alfacalcidol for the treatment of secondary hyperparathyroidism in patients receiving hemodialysis: A randomized, crossover trial. Can. J. Clin. Pharmacol. 2008, 15, e36–e43. [Google Scholar]
- Matuszkiewicz-Rowińska, J.; Kulicki, P.; Zebrowski, P.; Klatko, W.; Sokalski, A.; Niemczyk, S.; Wypych-Birecka, M.; Małyszko, J. Cholecalciferol vs. Small Doses of Alfacalcidol vs. Placebo in Chronic Kidney Disease Patients on Hemodialysis: A Randomized Parallel Group Study. Front. Med. 2021, 8, 81191. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Weekers, L.; Warling, X.; Moonen, M.; Smelten, N.; Médart, L.; Krzesinski, J.-M.; Cavalier, E. Cholecalciferol in haemodialysis patients: A randomized, double-blind, proof-of-concept and safety study. Nephrol. Dial. Transplant. 2013, 28, 1779–1786. [Google Scholar] [CrossRef] [PubMed]

| HD Group n = 66 | Reference Range | |
|---|---|---|
| males n(%) | 38 (57.6%) | |
| age [years] (average ± SD) (median) | 61.3 ± 16.4 67 | not applicable |
Hgb [g/dL] (median) (Q1;Q3) | 10.65 9.9; 11.6 | 12–15 |
| therapy with erythropoetin n(%) | 50 (75.8%) | not applicable |
| WBC [G/L] (median) (Q1;Q3) PLT [G/L] (median) (Q1;Q3) | 6.45 5.5; 7.98 206 172; 262 | 4–10 150–410 |
| residual diuresis (>500 mL/day) n(%) | 24 (36.3%) | not applicable |
| Kt/V (median) (Q1;Q3) | 1.63 1.46; 1.8 | >1.2 |
| CRP [mg/L] (median) (Q1;Q3) | 4 2; 9.8 | <5 |
| Pi [mg/dL] (median) (Q1;Q3) | 5.2 4.4; 6.3 | 2.5–4.5 |
| Ca [mg/dL] (median) (Q1;Q3) | 8.9 8.6; 9.5 | 8.5–10.2 |
| All Participants n = 272 | HD Group n = 66 | Control Group n = 206 | p-Value (Test M-W) | |
|---|---|---|---|---|
| males (n%) | 101 (37.1%) | 38 (57.6%) | 63 (30.6%) | 0.001 |
| age [years] (average ± SD median) | 60.9 ± 14.4 65 | 61.3 ± 16.4 67 | 60.8 ± 13.7 65 | 0.807 |
| 25(OH)D3 [ng/mL] (median) (Q1;Q3) | 20.80 13.55; 29.55 | 14.57 9.31; 25.27 | 22.89 16.17; 29.64 | 0.0001 |
| 25(OH)D2 [ng/mL] (median) (Q1;Q3) | 0.39 0.24; 0.66 | 0.61 0.46; 0.93 | 0.31 0.21; 0.53 | 0.0000 |
| epi-25(OH)D3 [ng/mL] (median) (Q1;Q3) | 0.75 0.39; 1.42 | 0.40 0.29; 0.67 | 0.96 0.52; 1.6 | 0.0000 |
| epi-25(OH)D3/25(OH)D3 | 3.72 [%] | 2.77 [%] | 4.59 [%] | 0.0000 |
| 24,25(OH)2D3 [ng/mL] (median) (Q1;Q3) | 1.50 0.73; 2.60 | 0.10 * 0.06; 0.31 | 2.09 1.30; 3.04 | 0.0000 |
| 24,25(OH)2D3/25(OH)D3 (VMR) (median) (Q1;Q3) | 8.24% 1.28%; 9.82% | 0.91% * 0.37%; 1.40% | 9.21% 5.23%; 10.22% | 0.0000 |
| 25(OH)D3 | Control Group [n]/% n = 206 | Study Group (HD) [n]/% n = 66 |
|---|---|---|
| deficiency < 20 ng/mL | 82/39.8% | 45/68.2% |
| insufficiency 20–30 ng/mL | 74/35.9% | 8/12.1% |
| sufficiency 30–50 ng/mL | 44/21.4% | 12/18.2% |
| high supply 50–100 ng/mL | 6/2.9% | 1/1.5% |
| toxicity > 100 ng/mL | 0 | 0 |
| Metabolite | Alphacalcidol Supply; n = 25 | Without Alphacalcidol; n = 41 | HD Group; n = 66 | p-Value |
|---|---|---|---|---|
| 1,25(OH)2D3 [pg/mL] | 30.4 | 16.2 | 21.4 | p < 0.01 |
| 25(OH)D3 [ng/mL] | 14.06 | 14.94 | 14.57 | p > 0.05 |
| 24,25(OH)2D3 [ng/mL] | 0.15 | 0.14 | 0.14 | p > 0.05 |
| VMR | 0.87% | 0.96% | 0.91% | p > 0.05 |
| 3-epi-25(OH)D3 | 0.42 | 0.38 | 0.40 | p > 0.05 |
| Metabolite | HD Group (R, p-Value) | Control Group (R, p-Value) | Interpretation |
|---|---|---|---|
| 24,25(OH)2D3 and 25(OH)D3 | R = 0.714 p < 0.001 | R = 0.885 p < 0.001 | Statistically significant positive correlation in both groups. |
| 25(OH)D2 and 25(OH)D3 | R = −0.179 p = 0.150 | R = −0.046 p = 0.513 | No statistically significant correlation in either group. |
| 3-epi-25(OH)D3 and 25(OH)D3 | R = 0.915 p < 0.001 | R = 0.776 p < 0.001 | Statistically significant positive correlation in both groups. |
| 24,25(OH)2D3 and 3-epi-25(OH)D3 | R = 0.692 p < 0.001 | R = 0.780 p < 0.001 | Statistically significant positive correlation in both groups. |
| 1,25(OH)2D3 and 25(OH)D3 | R = 0.383 p < 0.003 | lack of data for control group | Statistically significant positive correlation in HD group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hryciuk, M.; Heleniak, Z.; Małgorzewicz, S.; Kowalski, K.; Antosiewicz, J.; Koelmer, A.; Żmijewski, M.; Dębska-Ślizień, A. Assessment of Vitamin D Metabolism Disorders in Hemodialysis Patients. Nutrients 2025, 17, 774. https://doi.org/10.3390/nu17050774
Hryciuk M, Heleniak Z, Małgorzewicz S, Kowalski K, Antosiewicz J, Koelmer A, Żmijewski M, Dębska-Ślizień A. Assessment of Vitamin D Metabolism Disorders in Hemodialysis Patients. Nutrients. 2025; 17(5):774. https://doi.org/10.3390/nu17050774
Chicago/Turabian StyleHryciuk, Maksymilian, Zbigniew Heleniak, Sylwia Małgorzewicz, Konrad Kowalski, Jędrzej Antosiewicz, Anna Koelmer, Michał Żmijewski, and Alicja Dębska-Ślizień. 2025. "Assessment of Vitamin D Metabolism Disorders in Hemodialysis Patients" Nutrients 17, no. 5: 774. https://doi.org/10.3390/nu17050774
APA StyleHryciuk, M., Heleniak, Z., Małgorzewicz, S., Kowalski, K., Antosiewicz, J., Koelmer, A., Żmijewski, M., & Dębska-Ślizień, A. (2025). Assessment of Vitamin D Metabolism Disorders in Hemodialysis Patients. Nutrients, 17(5), 774. https://doi.org/10.3390/nu17050774








