Enhancing Resistance to Enterococcus faecalis: Immunobiotic Lactiplantibacillus plantarum Strains as a Strategy for Malnourished Hosts
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Survival of Lactic Acid Bacteria to Gastrointestinal Conditions In Vitro
2.3. Animals Feeding Procedures and Experimental Infection in Malnourished Mice
2.4. Counts of E. faecalis Colonies in Mouse Samples
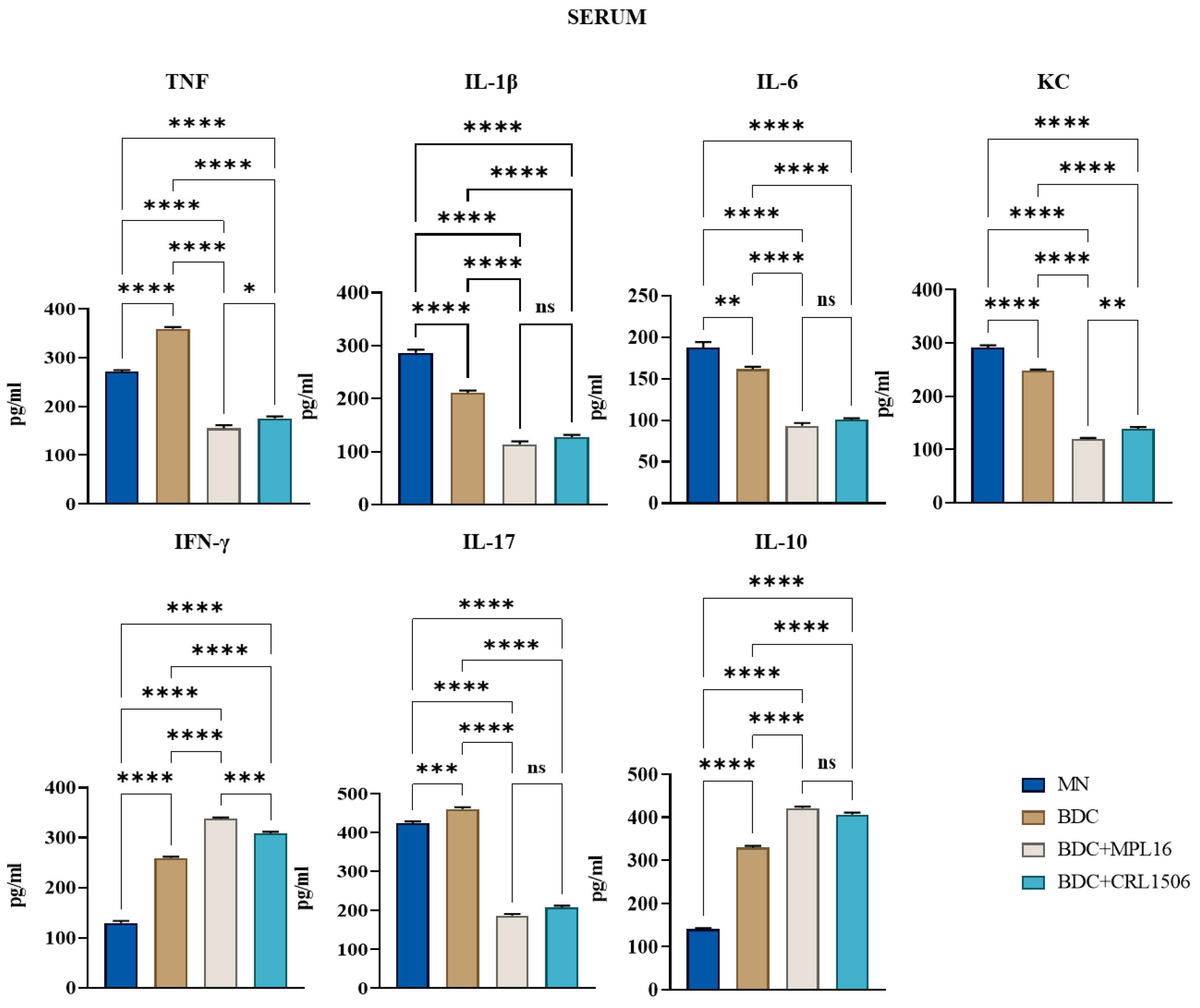
2.5. Determination of Cytokine Concentrations in Serum and Bowel Lavage
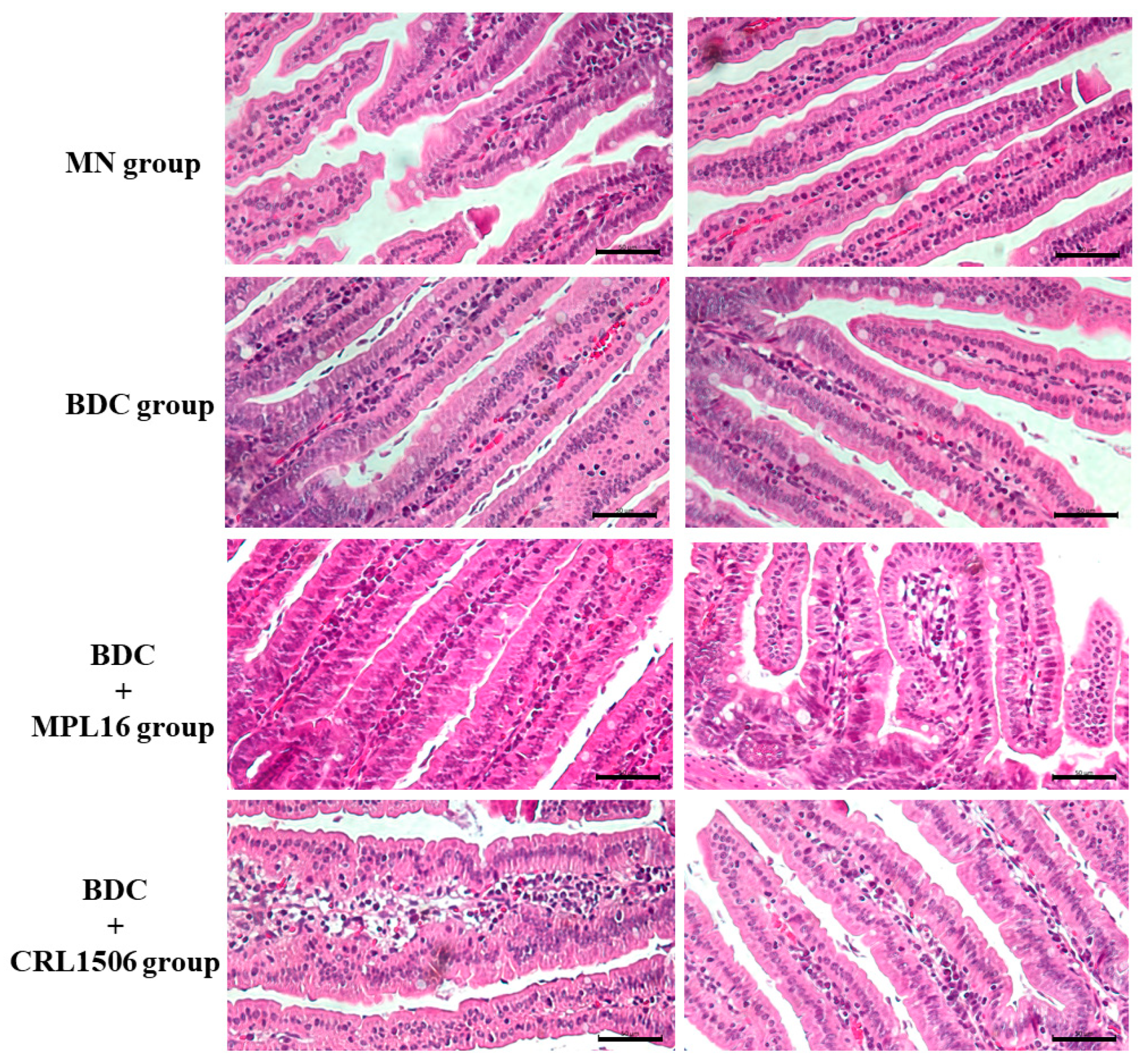
2.6. Histopathology
2.7. Statistical Analysis
2.8. Ethical Statement
3. Results
3.1. Survival to In Vitro Gastrointestinal Conditions
3.2. Effect of LAB on Resistance to Infection by E. faecalis
3.3. Effect of LAB on Serum Cytokine Levels After Infection with E. faecalis 102
3.4. Restoration of Small Intestine Histology During Re-Nutrition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuen, G.J.; Ausubel, F.M. Enterococcus infection biology: Lessons from invertebrate host models. J. Microbiol. 2014, 52, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Lindenstrauss, A.G.; Ehrmann, M.A.; Behr, J.; Landstorfer, R.; Haller, D.; Sartor, R.B.; Vogel, R.F. Transcriptome analysis of Enterococcus faecalis toward its adaption to surviving in the mouse intestinal tract. Arch. Microbiol. 2014, 196, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Medina-Contreras, O.; Geem, D.; Laur, O.; Williams, I.R.; Lira, S.A.; Nusrat, A.; Parkos, C.A.; Denning, T.L. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J. Clin. Investig. 2011, 121, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.L.; Jechorek, R.P.; Erlandsen, S.L. Evidence for the translocation of Enterococcus faecalis across the mouse intestinal tract. J. Infect. Dis. 1990, 162, 82–90. [Google Scholar] [CrossRef]
- Chakraborty, R.; Lam, V.; Kommineni, S.; Stromich, J.; Hayward, M.; Kristich, C.J.; Salzman, N.H. Ceftriaxone Administration Disrupts Intestinal Homeostasis, Mediating Noninflammatory Proliferation and Dissemination of Commensal Enterococci. Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef]
- Archambaud, C.; Derre-Bobillot, A.; Lapaque, N.; Rigottier-Gois, L.; Serror, P. Intestinal translocation of enterococci requires a threshold level of enterococcal overgrowth in the lumen. Sci. Rep. 2019, 9, 8926. [Google Scholar] [CrossRef]
- Geldart, K.G.; Kommineni, S.; Forbes, M.; Hayward, M.; Dunny, G.M.; Salzman, N.H.; Kaznessis, Y.N. Engineered E. coli Nissle 1917 for the reduction of vancomycin-resistant Enterococcus in the intestinal tract. Bioeng. Transl. Med. 2018, 3, 197–208. [Google Scholar] [CrossRef]
- McKenney, P.T.; Yan, J.; Vaubourgeix, J.; Becattini, S.; Lampen, N.; Motzer, A.; Larson, P.J.; Dannaoui, D.; Fujisawa, S.; Xavier, J.B.; et al. Intestinal Bile Acids Induce a Morphotype Switch in Vancomycin-Resistant Enterococcus that Facilitates Intestinal Colonization. Cell Host Microbe 2019, 25, 695–705.e695. [Google Scholar] [CrossRef]
- Repoila, F.; Le Bohec, F.; Guerin, C.; Lacoux, C.; Tiwari, S.; Jaiswal, A.K.; Santana, M.P.; Kennedy, S.P.; Quinquis, B.; Rainteau, D.; et al. Adaptation of the gut pathobiont Enterococcus faecalis to deoxycholate and taurocholate bile acids. Sci. Rep. 2022, 12, 8485. [Google Scholar] [CrossRef]
- Miyazaki, S.; Fujikawa, T.; Kobayashi, I.; Matsumoto, T.; Tateda, K.; Yamaguchi, K. Development of systemic bacteraemia after oral inoculation of vancomycin-resistant enterococci in mice. J. Med. Microbiol. 2001, 50, 695–701. [Google Scholar] [CrossRef][Green Version]
- Tuano, K.S.; Seth, N.; Chinen, J. Secondary immunodeficiencies: An overview. Ann. Allergy Asthma Immunol. 2021, 127, 617–626. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of Childhood Malnutrition on Host Defense and Infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef]
- Liu, J.; Bolick, D.T.; Kolling, G.L.; Fu, Z.; Guerrant, R.L. Protein Malnutrition Impairs Intestinal Epithelial Cell Turnover, a Potential Mechanism of Increased Cryptosporidiosis in a Murine Model. Infect. Immun. 2016, 84, 3542–3549. [Google Scholar] [CrossRef]
- Lykke, M.; Hother, A.L.; Hansen, C.F.; Friis, H.; Molgaard, C.; Michaelsen, K.F.; Briend, A.; Larsen, T.; Sangild, P.T.; Thymann, T. Malnutrition induces gut atrophy and increases hepatic fat infiltration: Studies in a pig model of childhood malnutrition. Am. J. Transl. Res. 2013, 5, 543–554. [Google Scholar] [PubMed]
- Kasai, A.; Gama, P.; Alvares, E.P. Protein restriction inhibits gastric cell proliferation during rat postnatal growth in parallel to ghrelin changes. Nutrition 2012, 28, 707–712. [Google Scholar] [CrossRef]
- Eyzaguirre-Velasquez, J.; Olavarria-Ramirez, L.; Gonzalez-Arancibia, C.; Diaz-Merino, C.; Ariz, R.; Lopez, S.; Quiroz, W.; Beltran, C.J.; Bravo, J.A.; Julio-Pieper, M. Protein Malnutrition During Juvenile Age Increases Ileal and Colonic Permeability in Rats. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 707–712. [Google Scholar] [CrossRef]
- Ling, C.; Versloot, C.J.; Arvidsson Kvissberg, M.E.; Hu, G.; Swain, N.; Horcas-Nieto, J.M.; Miraglia, E.; Thind, M.K.; Farooqui, A.; Gerding, A.; et al. Rebalancing of mitochondrial homeostasis through an NAD(+)-SIRT1 pathway preserves intestinal barrier function in severe malnutrition. EBioMedicine 2023, 96, 104809. [Google Scholar] [CrossRef]
- Amaral, J.F.; Foschetti, D.A.; Assis, F.A.; Menezes, J.S.; Vaz, N.M.; Faria, A.M. Immunoglobulin production is impaired in protein-deprived mice and can be restored by dietary protein supplementation. Braz. J. Med. Biol. Res. 2006, 39, 1581–1586. [Google Scholar] [CrossRef]
- Flo, J.; Elias, F.; Benedetti, R.; Massouh, E. Reversible effects on B and T cells of the gut-associated lymphoid tissues in rats malnourished during suckling: Impaired induction of the immune response to intra-Peyer patches immunization with cholera toxin. Clin. Immunol. Immunopathol. 1996, 80, 147–154. [Google Scholar] [CrossRef]
- Huus, K.E.; Hoang, T.T.; Creus-Cuadros, A.; Cirstea, M.; Vogt, S.L.; Knuff-Janzen, K.; Sansonetti, P.J.; Vonaesch, P.; Finlay, B.B. Cross-feeding between intestinal pathobionts promotes their overgrowth during undernutrition. Nat. Commun. 2021, 12, 6860. [Google Scholar] [CrossRef]
- Kau, A.L.; Planer, J.D.; Liu, J.; Rao, S.; Yatsunenko, T.; Trehan, I.; Manary, M.J.; Liu, T.C.; Stappenbeck, T.S.; Maleta, K.M.; et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci. Transl. Med. 2015, 7, 276ra224. [Google Scholar] [CrossRef]
- Oldenburg, C.E.; Hinterwirth, A.; Ourohire, M.; Dah, C.; Ouedraogo, M.; Sie, A.; Boudo, V.; Chen, C.; Ruder, K.; Zhong, L.; et al. Gut Resistome after Antibiotics among Children with Uncomplicated Severe Acute Malnutrition: A Randomized Controlled Trial. Am. J. Trop. Med. Hyg. 2022, 107, 59–64. [Google Scholar] [CrossRef]
- Villena, J.; Li, C.; Vizoso-Pinto, M.G.; Sacur, J.; Ren, L.; Kitazawa, H. Lactiplantibacillus plantarum as a Potential Adjuvant and Delivery System for the Development of SARS-CoV-2 Oral Vaccines. Microorganisms 2021, 9, 683. [Google Scholar] [CrossRef]
- Mizuno, H.; Arce, L.; Tomotsune, K.; Albarracin, L.; Funabashi, R.; Vera, D.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Sasaki, Y.; et al. Lipoteichoic Acid Is Involved in the Ability of the Immunobiotic Strain Lactobacillus plantarum CRL1506 to Modulate the Intestinal Antiviral Innate Immunity Triggered by TLR3 Activation. Front. Immunol. 2020, 11, 571. [Google Scholar] [CrossRef]
- Villena, J.; Chiba, E.; Vizoso-Pinto, M.G.; Tomosada, Y.; Takahashi, T.; Ishizuka, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H. Immunobiotic Lactobacillus rhamnosus strains differentially modulate antiviral immune response in porcine intestinal epithelial and antigen presenting cells. BMC Microbiol. 2014, 14, 126. [Google Scholar] [CrossRef]
- Albarracin, L.; Garcia-Castillo, V.; Masumizu, Y.; Indo, Y.; Islam, M.A.; Suda, Y.; Garcia-Cancino, A.; Aso, H.; Takahashi, H.; Kitazawa, H.; et al. Efficient Selection of New Immunobiotic Strains With Antiviral Effects in Local and Distal Mucosal Sites by Using Porcine Intestinal Epitheliocytes. Front. Immunol. 2020, 11, 543. [Google Scholar] [CrossRef]
- Salva, S.; Nunez, M.; Villena, J.; Ramon, A.; Font, G.; Alvarez, S. Development of a fermented goats’ milk containing Lactobacillus rhamnosus: In vivo study of health benefits. J. Sci. Food Agric. 2011, 91, 2355–2362. [Google Scholar] [CrossRef]
- Villena, J.; Salva, S.; Aguero, G.; Alvarez, S. Immunomodulatory and protective effect of probiotic Lactobacillus casei against Candida albicans infection in malnourished mice. Microbiol. Immunol. 2011, 55, 434–445. [Google Scholar] [CrossRef]
- Villena, J.; Racedo, S.; Aguero, G.; Bru, E.; Medina, M.; Alvarez, S. Lactobacillus casei improves resistance to pneumococcal respiratory infection in malnourished mice. J. Nutr. 2005, 135, 1462–1469. [Google Scholar] [CrossRef]
- Salva, S.; Villena, J.; Alvarez, S. Immunomodulatory activity of Lactobacillus rhamnosus strains isolated from goat milk: Impact on intestinal and respiratory infections. Int. J. Food Microbiol. 2010, 141, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Saavedra, L.; Hebert, E.M.; Suda, Y.; Masumizu, Y.; Albarracin, L.; Clua, P.; Ikeda-Ohtsubo, W.; Kitazawa, H. Draft Genome Sequence of Lactobacillus plantarum MPL16, a Wakame-Utilizing Immunobiotic Strain Isolated from Swine Feces. Genome Announc. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 1998, 84, 759–768. [Google Scholar] [CrossRef]
- Conway, P.L.; Gorbach, S.L.; Goldin, B.R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 1987, 70, 1–12. [Google Scholar] [CrossRef]
- Vizoso Pinto, M.G.; Franz, C.M.; Schillinger, U.; Holzapfel, W.H. Lactobacillus spp. with in vitro probiotic properties from human faeces and traditional fermented products. Int. J. Food Microbiol. 2006, 109, 205–214. [Google Scholar] [CrossRef]
- Muller, M.F.; Sacur, J.; Brancher, J.M.; Vera, M.D.; Arce, L.; Raya-Tonetti, M.F.; Kitazawa, H.; Villena, J.; Vizoso-Pinto, M.G. An experimental chimeric hepatitis E virus vaccine elicits both local and systemic immune responses. Front. Microbiol. 2024, 15, 1512018. [Google Scholar] [CrossRef]
- Villena, J.; Racedo, S.; Aguero, G.; Alvarez, S. Yoghurt accelerates the recovery of defence mechanisms against Streptococcus pneumoniae in protein-malnourished mice. Br. J. Nutr. 2006, 95, 591–602. [Google Scholar] [CrossRef]
- Kolling, Y.; Salva, S.; Villena, J.; Alvarez, S. Are the immunomodulatory properties of Lactobacillus rhamnosus CRL1505 peptidoglycan common for all Lactobacilli during respiratory infection in malnourished mice? PLoS ONE 2018, 13, e0194034. [Google Scholar] [CrossRef]
- Ge, X.; Ding, C.; Zhao, W.; Xu, L.; Tian, H.; Gong, J.; Zhu, M.; Li, J.; Li, N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017, 15, 13. [Google Scholar] [CrossRef]
- Thurstans, S.; Opondo, C.; Seal, A.; Wells, J.C.; Khara, T.; Dolan, C.; Briend, A.; Myatt, M.; Garenne, M.; Mertens, A.; et al. Understanding Sex Differences in Childhood Undernutrition: A Narrative Review. Nutrients 2022, 14, 948. [Google Scholar] [CrossRef]
- Correa-Martinez, C.L.; Schuler, F.; Kampmeier, S. Sex differences in vancomycin-resistant enterococci bloodstream infections-a systematic review and meta-analysis. Biol. Sex Differ. 2021, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Voskuijl, W.; Mouzaki, M.; Groen, A.K.; Alexander, J.; Bourdon, C.; Wang, A.; Versloot, C.J.; Di Giovanni, V.; Wanders, R.J.; et al. Impaired Bile Acid Homeostasis in Children with Severe Acute Malnutrition. PLoS ONE 2016, 11, e0155143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allori, C.; Aguero, G.; de Ruiz Holgado, A.P.; de Nader, O.M.; Perdigon, G. Gut mucosa morphology and microflora changes in malnourished mice after renutrition with milk and administration of Lactobacillus casei. J. Food Prot. 2000, 63, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Romano, C.; Pasini, E.; Testa, C.; Dioguardi, F.S. Qualitative Nitrogen Malnutrition Damages Gut and Alters Microbiome in Adult Mice. A Preliminary Histopathological Study. Nutrients 2021, 13, 1089. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Bolick, D.T.; Leng, J.; Medlock, G.L.; Kolling, G.L.; Papin, J.A.; Swann, J.R.; Guerrant, R.L. Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am. J. Clin. Nutr. 2016, 104, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Gauffin, C.P.; Aguero, G.; Perdigon, G. Adjuvant effects of Lactobacillus casei added to a renutrition diet in a malnourished mouse model. Biocell 2002, 26, 35–48. [Google Scholar] [CrossRef]
- Cano, P.G.; Aguero, G.; Perdigon, G. Immunological effects of yogurt addition to a re-nutrition diet in a malnutrition experimental model. J. Dairy Res. 2002, 69, 303–316. [Google Scholar] [CrossRef]
- Herrera, M.; Salva, S.; Villena, J.; Barbieri, N.; Marranzino, G.; Alvarez, S. Dietary supplementation with Lactobacilli improves emergency granulopoiesis in protein-malnourished mice and enhances respiratory innate immune response. PLoS ONE 2014, 9, e90227. [Google Scholar] [CrossRef]
- Doherty, J.F.; Golden, M.H.; Remick, D.G.; Griffin, G.E. Production of interleukin-6 and tumour necrosis factor-alpha in vitro is reduced in whole blood of severely malnourished children. Clin. Sci. 1994, 86, 347–351. [Google Scholar] [CrossRef]
- Manary, M.J.; Yarasheski, K.E.; Berger, R.; Abrams, E.T.; Hart, C.A.; Broadhead, R.L. Whole-body leucine kinetics and the acute phase response during acute infection in marasmic Malawian children. Pediatr. Res. 2004, 55, 940–946. [Google Scholar] [CrossRef]
- Fock, R.A.; Vinolo, M.A.; de Moura Sa Rocha, V.; de Sa Rocha, L.C.; Borelli, P. Protein-energy malnutrition decreases the expression of TLR-4/MD-2 and CD14 receptors in peritoneal macrophages and reduces the synthesis of TNF-alpha in response to lipopolysaccharide (LPS) in mice. Cytokine 2007, 40, 105–114. [Google Scholar] [CrossRef]
- de Oliveira, D.C.; Hastreiter, A.A.; Mello, A.S.; de Oliveira Beltran, J.S.; Oliveira Santos, E.W.; Borelli, P.; Fock, R.A. The effects of protein malnutrition on the TNF-RI and NF-kappaB expression via the TNF-alpha signaling pathway. Cytokine 2014, 69, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, Z.M.; Luz, R.A.; Victal, S.H.; Kurdian, B.; Fonseca, V.M.; Fitting, C.; Camara, F.P.; Haeffner-Cavaillon, N.; Cavaillon, J.M.; Gaspar Elsas, M.I.; et al. Increased production of tumor necrosis factor-alpha in whole blood cultures from children with primary malnutrition. Braz. J. Med. Biol. Res. 2005, 38, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Wlodarska, M.; Willing, B.P.; Vonaesch, P.; Han, J.; Reynolds, L.A.; Arrieta, M.C.; Uhrig, M.; Scholz, R.; Partida, O.; et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat. Commun. 2015, 6, 7806. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kim, S.C.; Sartor, R.B.; Haller, D. Enterococcus faecalis strains differentially regulate Alix/AIP1 protein expression and ERK 1/2 activation in intestinal epithelial cells in the context of chronic experimental colitis. J. Proteome Res. 2009, 8, 1183–1192. [Google Scholar] [CrossRef]
- Steck, N.; Hoffmann, M.; Sava, I.G.; Kim, S.C.; Hahne, H.; Tonkonogy, S.L.; Mair, K.; Krueger, D.; Pruteanu, M.; Shanahan, F.; et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 2011, 141, 959–971. [Google Scholar] [CrossRef]
- Patrick, D.M.; Leone, A.K.; Shellenberger, J.J.; Dudowicz, K.A.; King, J.M. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol. 2006, 6, 2. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef]
- Turner, J.R. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Onyeji, C.O.; Bui, K.Q.; Nicolau, D.P.; Nightingale, C.H.; Bow, L.; Quintiliani, R. Influence of adjunctive interferon-gamma on treatment of gentamicin- and vancomycin-resistant Enterococcus faecalis infection in mice. Int. J. Antimicrob. Agents 1999, 12, 301–309. [Google Scholar] [CrossRef]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.C.; Stappenbeck, T.S. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Albarracin, L.; Tomokiyo, M.; Valdez, J.C.; Sacur, J.; Vizoso-Pinto, M.G.; Andrade, B.G.N.; Cuadrat, R.R.C.; Kitazawa, H.; Villena, J. Immunobiotic Lactobacilli Improve Resistance of Respiratory Epithelial Cells to SARS-CoV-2 Infection. Pathogens 2021, 10, 1197. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera, M.D.; Arce, L.P.; Müller, M.F.; Raya Tonetti, F.; Ortiz Moyano, R.; Blanco, H.L.; Kitazawa, H.; Vizoso-Pinto, M.G.; Villena, J. Enhancing Resistance to Enterococcus faecalis: Immunobiotic Lactiplantibacillus plantarum Strains as a Strategy for Malnourished Hosts. Nutrients 2025, 17, 1770. https://doi.org/10.3390/nu17111770
Vera MD, Arce LP, Müller MF, Raya Tonetti F, Ortiz Moyano R, Blanco HL, Kitazawa H, Vizoso-Pinto MG, Villena J. Enhancing Resistance to Enterococcus faecalis: Immunobiotic Lactiplantibacillus plantarum Strains as a Strategy for Malnourished Hosts. Nutrients. 2025; 17(11):1770. https://doi.org/10.3390/nu17111770
Chicago/Turabian StyleVera, María Daniela, Lorena Paola Arce, Melisa Florencia Müller, Fernanda Raya Tonetti, Ramiro Ortiz Moyano, Héctor Luis Blanco, Haruki Kitazawa, María Guadalupe Vizoso-Pinto, and Julio Villena. 2025. "Enhancing Resistance to Enterococcus faecalis: Immunobiotic Lactiplantibacillus plantarum Strains as a Strategy for Malnourished Hosts" Nutrients 17, no. 11: 1770. https://doi.org/10.3390/nu17111770
APA StyleVera, M. D., Arce, L. P., Müller, M. F., Raya Tonetti, F., Ortiz Moyano, R., Blanco, H. L., Kitazawa, H., Vizoso-Pinto, M. G., & Villena, J. (2025). Enhancing Resistance to Enterococcus faecalis: Immunobiotic Lactiplantibacillus plantarum Strains as a Strategy for Malnourished Hosts. Nutrients, 17(11), 1770. https://doi.org/10.3390/nu17111770








