Weizmannia Coagulans BC99 Prevents Loperamide-Induced Functional Constipation in Mice Through Increased Intestinal Peristalsis and Modulation of Gut Microbiota Dysbiosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Experimental Design
2.2. Body Weight
2.3. Determination of Water Content of Defecation
2.4. Time to First Black Stool
2.5. Gastrointestinal Transit Rate
2.6. Microbiological Analysis
2.7. Short-Chain Fatty Acid (SCFA) Analysis
2.8. Serum Gastrointestinal Regulatory Peptides Analysis
2.9. Statistical Analysis
3. Results
3.1. Body Weight Change
3.2. Water Content of Defecation
3.3. The Time of the First Black Stool Defecation
3.4. Gastrointestinal Peristalsis
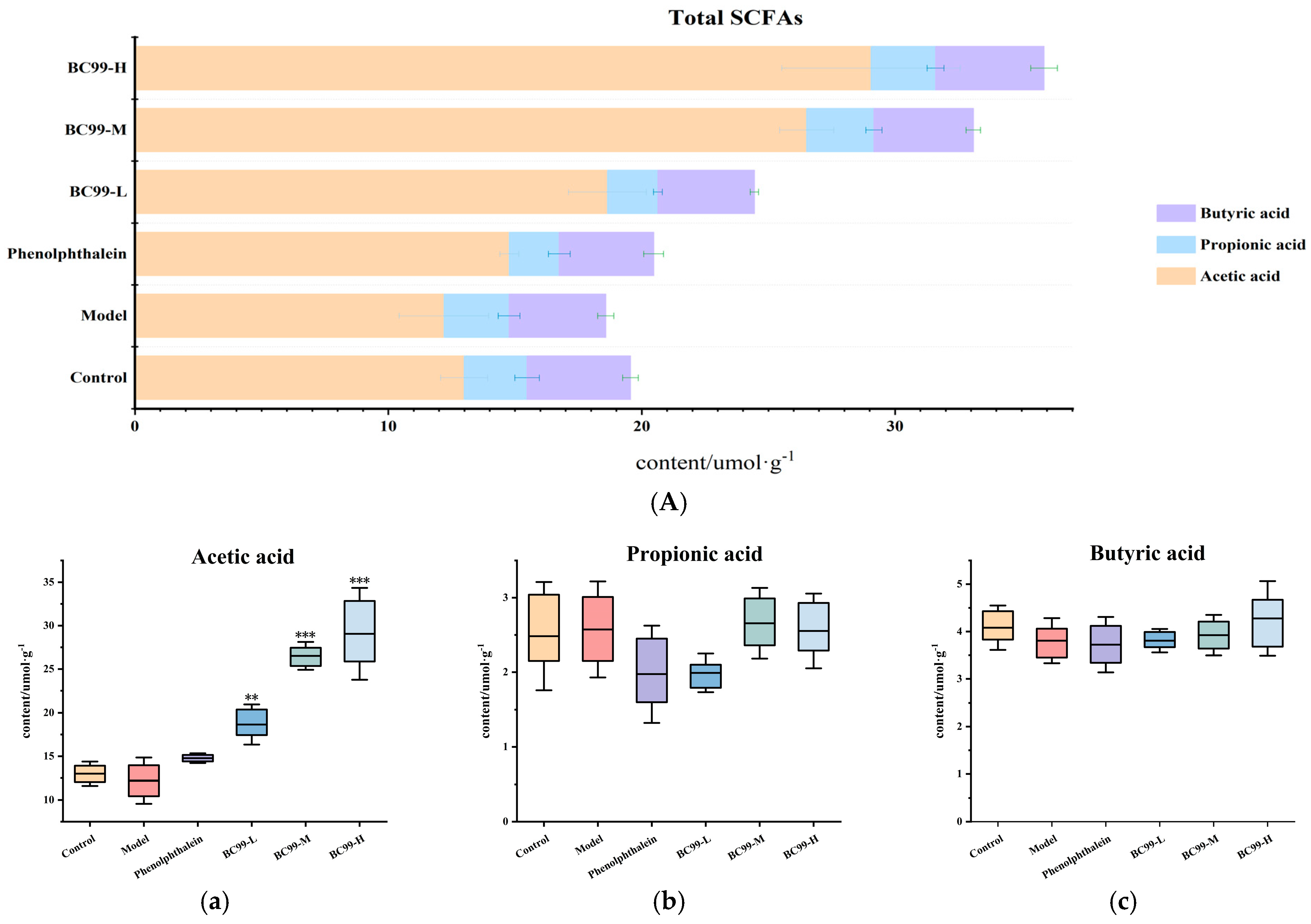
3.5. Effects of BC99 on SCFAs Levels
3.6. Effects of BC99 on Motilin and Somatostatin in Mice with Constipation
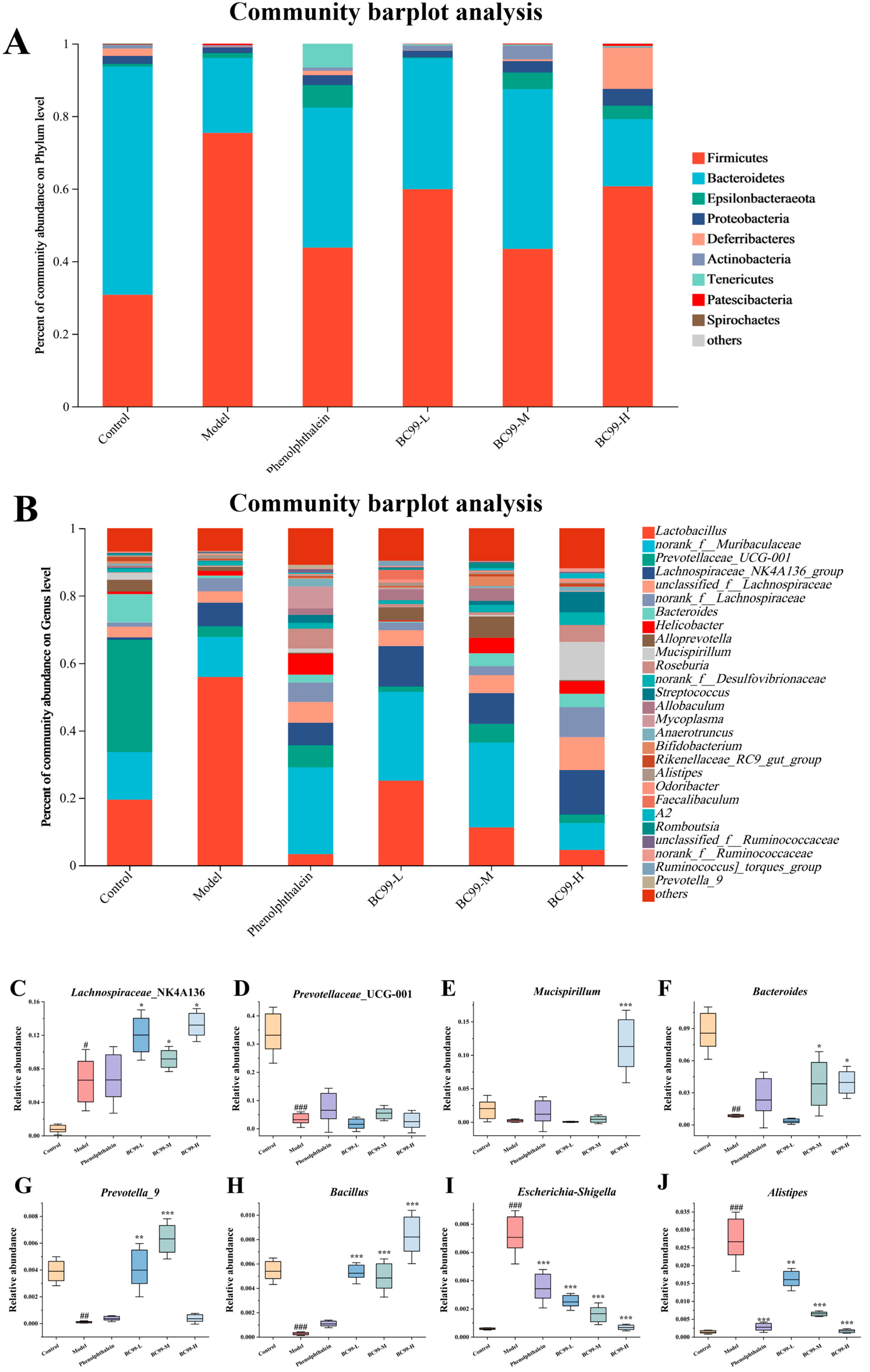
3.7. Effects of BC99 on Fecal Microbiota
3.8. Functional Characteristics of Fecal Microbiota
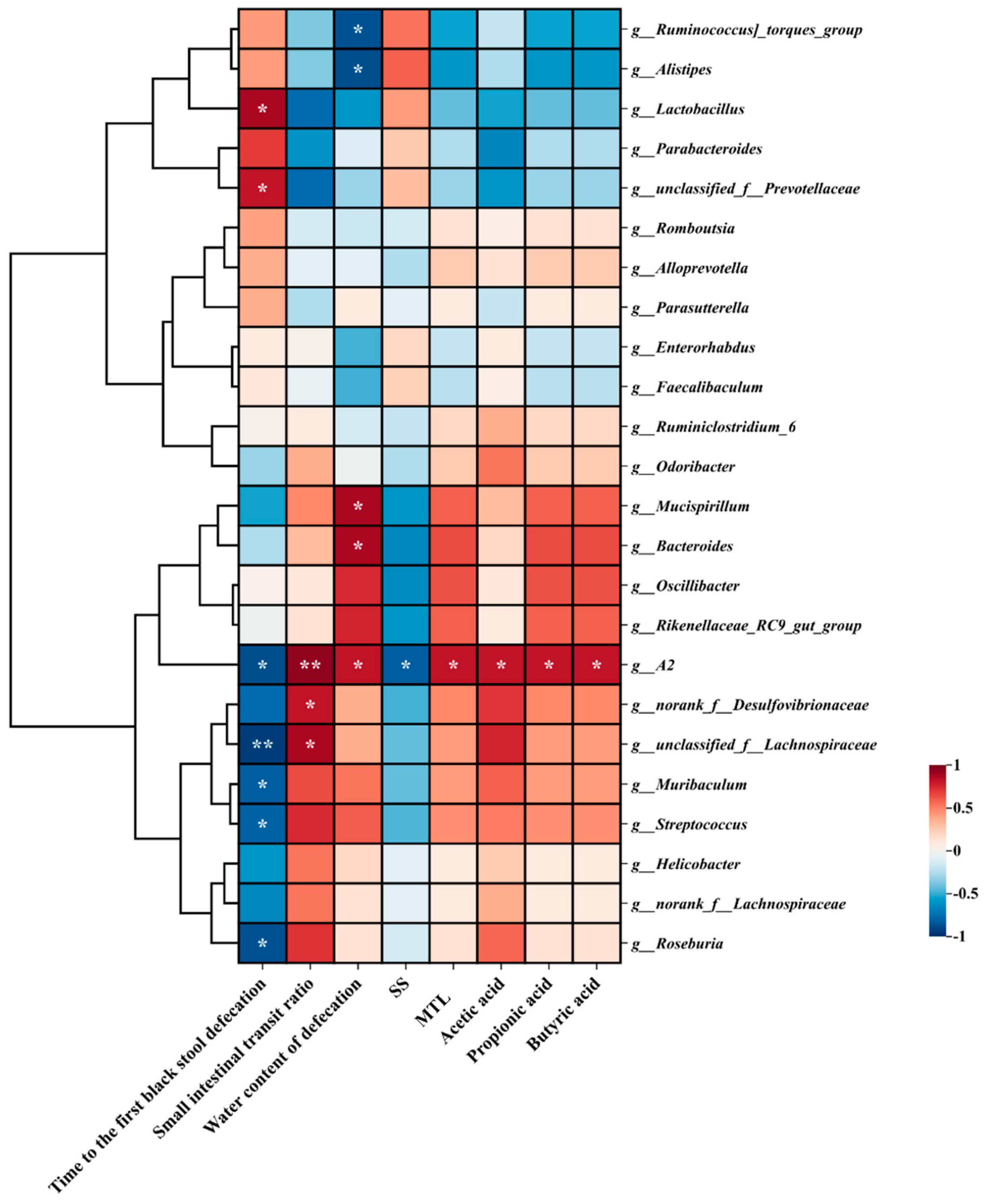
3.9. Correlation Analysis Between Vital Gut Genus and the Main Indicators of Constipation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, B.J.; Jung, H.K.; Jeong, Y.S.; Yang, S.J.; Hong, J.-H. Effect of microencapsulated Bacillus subtilis strain CBD2-fermented grain on loperamide-induced constipation in mice. Appl. Biol. Chem. 2016, 59, 451–462. [Google Scholar] [CrossRef]
- Huang, M.Q.; Wang, X.S.; Gan, Y.H.; Lu, S.Q.; Deng, Q.Q.; Zhu, Q.; Guo, J. Improvement effect and mechanism of lubricating granules on constipation in mice. China Pharm. 2024, 35, 160–165. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Eor, J.Y.; Tan, P.L.; Lim, S.M.; Choi, D.H.; Yoon, S.M.; Yang, S.Y.; Kim, S.H. Laxative effect of probiotic chocolate on loperamide-induced constipation in rats. Food Res. Int. 2019, 116, 1173–1182. [Google Scholar] [CrossRef]
- Chang, C.W.; Chen, M.J.; Shih, S.C.; Chang, C.W.; Chiau, J.C.; Lee, H.C.; Lin, Y.S.; Lin, W.C.; Wang, H.Y. Bacillus coagulans (PROBACI) in treating constipation-dominant functional bowel disorders. Medicine 2020, 99, e20098. [Google Scholar] [CrossRef]
- Mu, Y.; Cong, Y. Bacillus coagulans and its applications in medicine. Benef. Microbes 2019, 10, 679–688. [Google Scholar] [CrossRef]
- Dimidi, E.; Mark Scott, S.; Whelan, K. Probiotics and constipation: Mechanisms of action, evidence for effectiveness and utilisation by patients and healthcare professionals. Proc. Nutr. Soc. 2020, 79, 147–157. [Google Scholar] [CrossRef]
- Rogha, M.; Esfahani, M.Z.; Zargarzadeh, A.H. The efficacy of a synbiotic containing Bacillus Coagulans in treatment of irritable bowel syndrome: A randomized placebo-controlled trial. Gastroenterol. Hepatol. Bed Bench 2014, 7, 156–163. [Google Scholar]
- Jang, Y.J.; Moon, J.S.; Kim, J.E.; Kim, D.; Choi, H.S.; Oh, I. Blending Three Probiotics Alleviates Loperamide-Induced Constipation in Sprague-Dawley (SD)-Rats. Food Sci. Anim. Resour. 2024, 44, 119–131. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, G.H. Mechanism study and analysis of probiotic powder for the treatment and relief of constipation. Ind. Microbiol. 2023, 53, 1–3. [Google Scholar] [CrossRef]
- Jiang, T. Analysis of the Quantitative Effects of Microencapsulation Strategy on Bifidobacterium bifidum for Constipation Relief and Performance Enhancement. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2022. [Google Scholar]
- Zheng, W.; Zhao, Z.; Yang, Y.; Ding, L.; Yao, W. The synbiotic mixture of lactulose and Bacillus coagulans protects intestinal barrier dysfunction and apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. Biotechnol. 2023, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Li, Y.; He, Y.; Chen, F.; Mi, B.; Li, J.; Xie, J.; Ma, G.; Yang, J.; Xu, K.; et al. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: A double-blinded randomized placebo trial. Gut Microbes 2023, 15, 2197837. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.J.; Ganesan, R.; Jin, Y.J.; Park, H.J.; Min, B.H.; Jeong, M.K.; Yoon, S.J.; Choi, M.R.; Choi, J.; Moon, J.H.; et al. Multi-strain probiotics alleviate loperamide-induced constipation by adjusting the microbiome, serotonin, and short-chain fatty acids in rats. Front. Microbiol. 2023, 14, 1174968. [Google Scholar] [CrossRef]
- Parkar, N.; Spencer, N.J.; Wiklendt, L.; Olson, T.; Young, W.; Janssen, P.; McNabb, W.C.; Dalziel, J.E. Novel insights into mechanisms of inhibition of colonic motility by loperamide. Front. Neurosci. 2024, 18, 1424936. [Google Scholar] [CrossRef]
- Taheri, M.; Amiri-Farahani, L.; Haghani, S.; Shokrpour, M.; Shojaii, A. The effect of olive cream on pain and healing of caesarean section wounds: A randomised controlled clinical trial. J. Wound Care 2022, 31, 244–253. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, P.; Li, J.; Gao, H.; Tie, S.; Zhao, S.; Suo, H.; Gu, S. Weizmannia coagulans spores alleviates DSS-induced ulcerative colitis model by modulation of gut flora, metabolites and suppressing TLR4/MyD88/NF-κB pathway. Food Sci. Hum. Wellness 2024. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Chen, J.; Tang, X.; Pan, M.; Yuan, W.; Wang, H. Efficacy of Probiotic Compounds in Relieving Constipation and Their Colonization in Gut Microbiota. Molecules 2022, 27, 666. [Google Scholar] [CrossRef]
- Liu, W.Y.; Zhai, Q.X.; Tian, F.W.; Zhao, J.X.; Zhang, H.; Chen, W. Relieving effect of Bacillus coagulans B.C-39 complex microecological preparation on constipation in mice. Food Ferment. Ind. 2019, 45, 85–91. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, J.; Hong, Y.H.; Kim, Y.S.; Suh, H.J.; Ahn, Y. Coadministration of Lactulose with Probiotics Ameliorates Loperamide-Induced Constipation in Mice. Prev. Nutr. Food Sci. 2023, 28, 427–435. [Google Scholar] [CrossRef]
- Cao, J.; Liu, W.; Liliu, R.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. MLST analysis of genetic diversity of Bacillus coagulans strains to evaluate effects on constipation model. Food Sci. Hum. Wellness 2022, 11, 815–827. [Google Scholar] [CrossRef]
- Kang, Q.B.; Liu, J. Progress of traditional Chinese medicine research on the treatment of constipation based on intestinal microecology. China’s Naturop. 2023, 31, 113–116. [Google Scholar] [CrossRef]
- Wang, Y.F.; Cen, C.N.; Liu, F.Q.; Bao, W.C.; Fu, L.L.; Wang, Y.B. Advances in the study of probiotic properties and mechanism of Bacillus coagulans. Sci. Technol. Food Ind. 2023, 44, 458–464. [Google Scholar] [CrossRef]
- Inatomi, T.; Honma, M. Effects of probiotics on loperamide-induced constipation in rats. Sci. Rep. 2021, 11, 24098. [Google Scholar] [CrossRef]
- Nagamine, T. Effect of magnesium oxide with probiotics on bowel movements in elderly orthopedic patients with chronic constipation: A retrospective chart review. Biosci. Microbiota Food Health 2024, 43, 1–3. [Google Scholar] [CrossRef]
- Yan, J.; Wu, M.; Zhao, W.; Kwok, L.-Y.; Zhang, W. Effects of probiotics and its fermented milk on constipation: A systematic review. Food Sci. Hum. Wellness 2023, 12, 2124–2134. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, F.; Zhao, N.; Zhai, Q.; Zhang, H.; Chen, W. Effects of probiotics on d-galactose-induced oxidative stress in plasma: A meta-analysis of animal models. J. Funct. Foods 2017, 39, 44–49. [Google Scholar] [CrossRef]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Gélinas, P. Preventing constipation: A review of the laxative potential of food ingredients. Int. J. Food Sci. Technol. 2013, 48, 445–467. [Google Scholar] [CrossRef]
- van der Werf, M.J.; Venema, K. Bifidobacteria: Genetic modification and the study of their role in the colon. J. Agric. Food Chem. 2001, 49, 378–383. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes. Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Liu, X.; Wang, G.; Guo, X.; Liu, X.; Zhao, J.; Chen, W. The Different Ways Multi-Strain Probiotics with Different Ratios of Bifidobacterium and Lactobacillus Relieve Constipation Induced by Loperamide in Mice. Nutrients 2023, 15, 4230. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Chen, J.; Chen, X.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship Between Microbiota of the Colonic Mucosa vs. Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2016, 150, 367–379.e361. [Google Scholar] [CrossRef]
- Li, L.; Liu, B.; Cao, J.; Zhang, H.; Tian, F.; Yu, L.; Chen, W.; Zhai, Q. Different effects of Bacillus coagulans vegetative cells and spore isolates on constipation-induced gut microbiota dysbiosis in mice. Food Funct. 2022, 13, 9645–9657. [Google Scholar] [CrossRef]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile colitis: Pathogenesis and host defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 2011, 53, 994–1002. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes. Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Wintola, O.A.; Sunmonu, T.O.; Afolayan, A.J. The effect of Aloe ferox Mill. in the treatment of loperamide-induced constipation in Wistar rats. BMC Gastroenterol. 2010, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ji, L.; Wang, Y.; Zhang, Y.; Wang, H.; Wang, J.; Zhu, Q.; Xie, M.; Ou, W.; Liu, J.; et al. Acetate enables metabolic fitness and cognitive performance during sleep disruption. Cell Metab. 2024, 36, 1998–2014.e1915. [Google Scholar] [CrossRef]
- Zhou, X.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Cui, S. Exploring the Dose-Effect Relationship of Bifidobacterium longum in Relieving Loperamide Hydrochloride-Induced Constipation in Rats through Colon-Released Capsules. Int. J. Mol. Sci. 2023, 24, 6585. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front. Microbiol. 2016, 7, 1144. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, F.V.; Nielsen, H.; Mulvany, M.J.; Hessov, I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 1990, 31, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, L.; Xu, Q.; Yin, B.; Fang, D.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis Exerts Strain-Specific Effects on Constipation Induced by Loperamide in BALB/c Mice. Int. J. Mol. Sci. 2017, 18, 318. [Google Scholar] [CrossRef]
- Fukui, H.; Xu, X.; Miwa, H. Role of Gut Microbiota-Gut Hormone Axis in the Pathophysiology of Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2018, 24, 367–386. [Google Scholar] [CrossRef]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010, 138, 1772–1782. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wu, Y.; Liang, H.; Dong, Y.; Fang, S.; Jeong, P.-Y.; Kim, H.-R.; Gu, S. Weizmannia Coagulans BC99 Prevents Loperamide-Induced Functional Constipation in Mice Through Increased Intestinal Peristalsis and Modulation of Gut Microbiota Dysbiosis. Nutrients 2025, 17, 1729. https://doi.org/10.3390/nu17101729
Li C, Wu Y, Liang H, Dong Y, Fang S, Jeong P-Y, Kim H-R, Gu S. Weizmannia Coagulans BC99 Prevents Loperamide-Induced Functional Constipation in Mice Through Increased Intestinal Peristalsis and Modulation of Gut Microbiota Dysbiosis. Nutrients. 2025; 17(10):1729. https://doi.org/10.3390/nu17101729
Chicago/Turabian StyleLi, Cheng, Ying Wu, Hua Liang, Yao Dong, Shuguang Fang, Pan-Young Jeong, Hye-Rim Kim, and Shaobin Gu. 2025. "Weizmannia Coagulans BC99 Prevents Loperamide-Induced Functional Constipation in Mice Through Increased Intestinal Peristalsis and Modulation of Gut Microbiota Dysbiosis" Nutrients 17, no. 10: 1729. https://doi.org/10.3390/nu17101729
APA StyleLi, C., Wu, Y., Liang, H., Dong, Y., Fang, S., Jeong, P.-Y., Kim, H.-R., & Gu, S. (2025). Weizmannia Coagulans BC99 Prevents Loperamide-Induced Functional Constipation in Mice Through Increased Intestinal Peristalsis and Modulation of Gut Microbiota Dysbiosis. Nutrients, 17(10), 1729. https://doi.org/10.3390/nu17101729





