Recent Advances in the Therapeutic Potential of Bioactive Molecules from Plants of Andean Origin
Abstract
1. Introduction
2. Ancestral Edible Plants and Their Historical Significance
2.1. The Andean Region as a Center of Plant Diversity
2.2. Quinoa: The “Mother Grain” of the Andes
2.3. Amaranth: An Ancient Crop of the Americas
2.4. Lupinus: A Versatile Andean Legume
3. The Andean Environment and Its Impact on Phytochemistry
3.1. Unique Ecological Factors of the Andes Region
3.2. Adaptations of Andean Plants
3.3. Influence of Environment on Bioactive Compound Potency
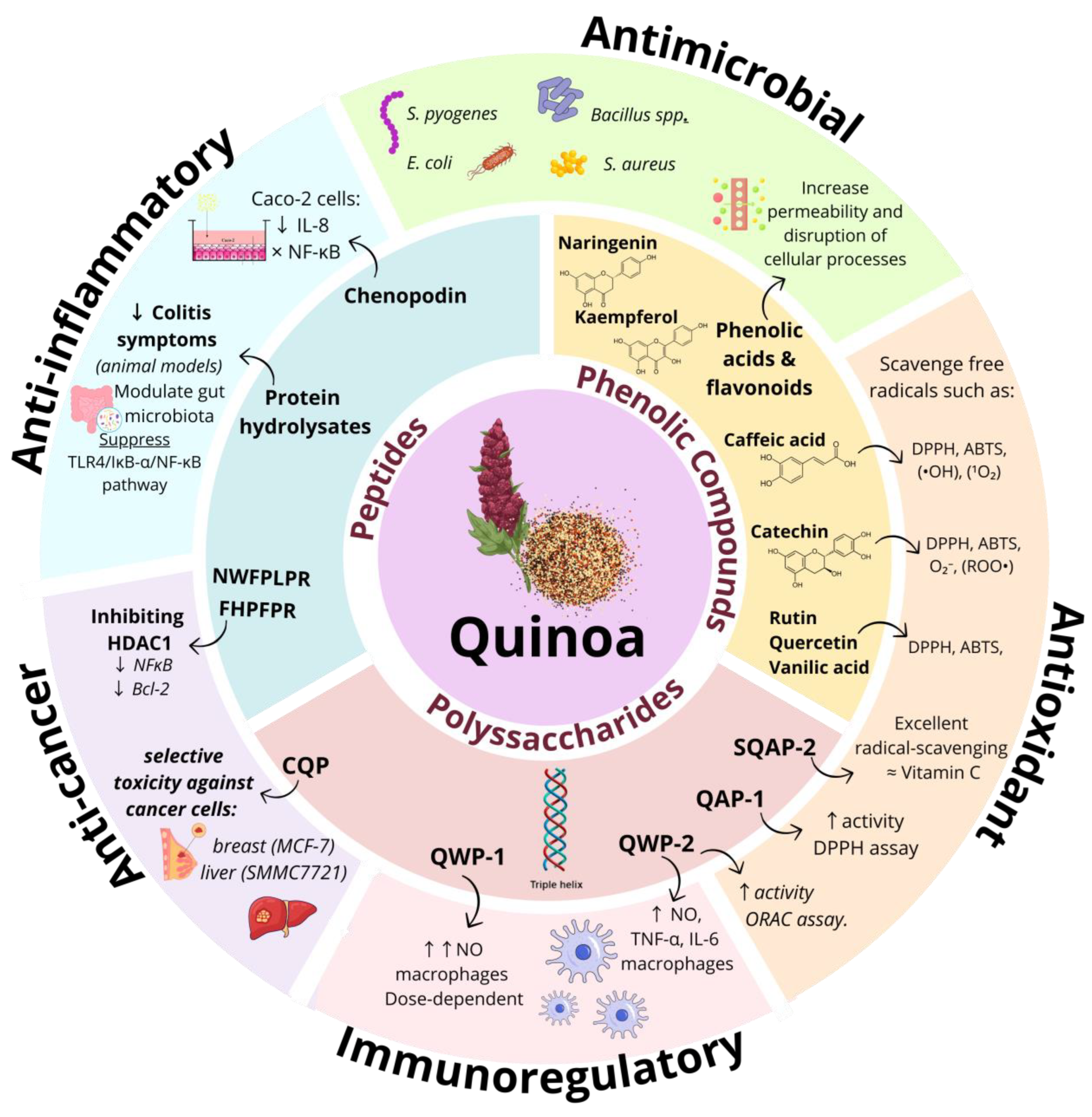
4. Bioactive Compounds in Quinoa (Chenopodium quinoa)
4.1. Phenolic Compounds
4.2. Polysaccharides
4.3. Peptides
5. Health Applications of Quinoa Bioactive Compounds
5.1. Antimicrobial Activity
5.2. Antioxidant Activity
5.3. Anticancer and Anti-Inflammatory Activity
5.4. Additional Health Benefits: Metabolic, Cardiovascular, Immune, and Digestive Health
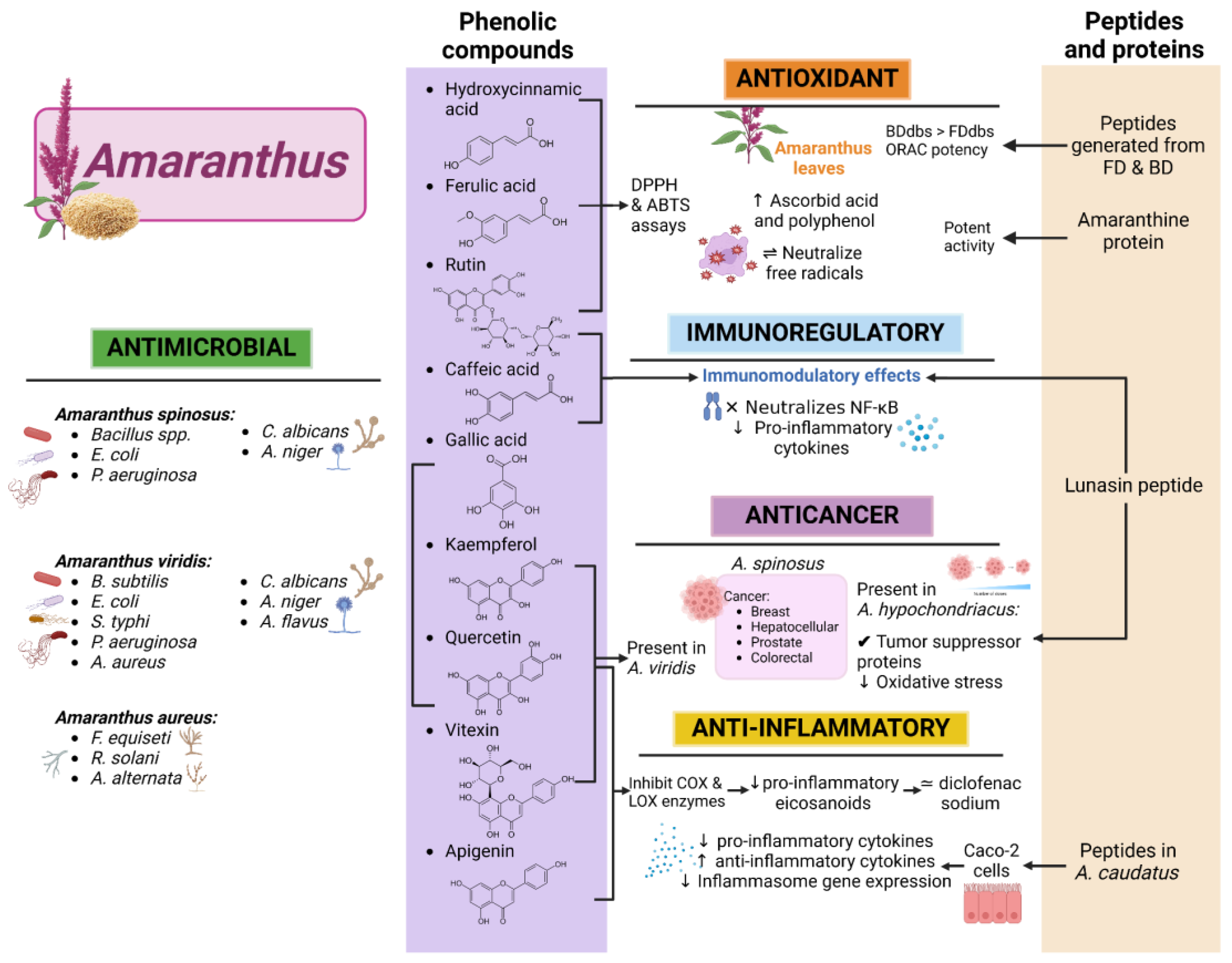
6. Bioactive Compounds in Amaranthus
6.1. Phenolic Compounds
6.2. Peptides
6.3. Polysaccharides
7. Health Applications of Amaranthus
7.1. Antimicrobial Activity
7.2. Antioxidant Activity
7.3. Anticancer and Anti-Inflammatory Activity
7.4. Additional Health Benefits: Metabolic, Cardiovascular, Immune, and Digestive Health
8. Bioactive Compounds in Lupinus
8.1. Polyphenols
8.2. Peptides
8.3. Polysasccharides
9. Health Applications of Lupinus
9.1. Antimicrobial Activity
9.2. Antioxidant Activity
9.3. Anticancer and Anti-Inflammatory Activity
9.4. Additional Health Benefits: Metabolic, Cardiovascular, Immune, and Digestive Health
10. Potential Therapeutic Use of the Andean Plants Based on Clinical Trials
10.1. C. quinoa
10.2. Amaranth
10.3. Lupinus
11. Concluding Remarks
- Standardization and bioavailability: The optimization of extraction techniques, formulation strategies, and dosage recommendations is necessary to ensure consistent bioactivity and efficacy [244].
- Clinical validation: Rigorous clinical trials are essential to substantiate their therapeutic properties and establish evidence-based applications [245].
- Sustainability and conservation: Agricultural and conservation strategies must prioritize biodiversity preservation [246] and the safeguarding of indigenous knowledge while fostering equitable economic opportunities for local communities.
Considerations on Antinutritional Factors
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-Fu | 5-Fluorouracil |
| 5637 | Urothelial cancer cell line (5637) |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay |
| ACE | Angiotensin-Converting Enzyme |
| ACO | Acyl-CoA Oxidase |
| AKT | Protein Kinase B |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransferase |
| ANGPTL4 | Angiopoietin-like 4 |
| apoA5 | Apolipoprotein A5 |
| AST | Aspartate Aminotransferase |
| BLAD | Banda de Lupinus alba doce |
| Bax | Pro-apoptotic protein (Bcl-2-associated X protein) |
| Bcl-2 | Anti-apoptotic protein |
| BCRP | Breast Cancer Resistance Protein |
| Beclin1 | Factor involved in autophagy |
| BMDCs | Bone Marrow-Derived Dendritic Cells |
| BMDMs | Bone Marrow-Derived Macrophages |
| BS | 11S Basic unit |
| CaCo-2 | Colon cancer cell line (Cancer coli cell line) |
| CCR2 | C-C Chemokine Receptor Type 2 |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| CCl3+ | Trichloromethyl radical |
| COX | Cyclooxygenase |
| COX-2 | Cyclooxygenase-2 |
| CPT-1a | Carnitine Palmitoyltransferase 1a |
| CQP | Quinoa’s Polysaccharides |
| CYP4A1 | Cytochrome P450 4A1 |
| DNMT1 | DNA Methyltransferase 1 |
| DNMT3a | DNA Methyltransferase 3 alpha |
| DNMT3b | DNA Methyltransferase 3 beta |
| DPP IV | Dipeptidyl Peptidase IV |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl assay |
| E2 | Estradiol |
| FABP5 | Fatty Acid-Binding Protein 5 |
| FAS | Fatty Acid Synthase |
| FINS | Fasting Insulin |
| GPAT | Glycerol-3-Phosphate Acyltransferase |
| GPETAFLR | Lupin-derived Peptide |
| GPx | Glutathione Peroxidase |
| GPT | Glutamic-pyruvic transaminase |
| G protein | Guanine nucleotide-binding protein |
| GSH | Glutathione |
| GSH-PX | Glutathione Peroxidase |
| HbA1c | Glycated Hemoglobin A1c |
| HDAC1 | Histone Deacetylase 1 |
| HDL | High-Density Lipoprotein |
| HepG2/C3A | Hepatocellular carcinoma cell line subclone |
| HL | Hepatic lipase |
| HMGCS1 | 3-Hydroxy-3-Methylglutaryl-CoA Synthase 1 |
| HMGCoAR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase |
| HNF-1α | Hepatocyte Nuclear Factor 1 alpha |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| HT29 | Colon adenocarcinoma cell line |
| IBD | Inflammatory Bowel Disease |
| IFN-γ | Interferon gamma |
| IL-1 | Interleukin 1 |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IL-13 | Interleukin 13 |
| IL-17 | Interleukin 17 |
| IL-2 | Interleukin 2 |
| IL-27p28 | Interleukin 27p28 |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| IL-9 | Interleukin 9 |
| ILC2s | Innate Lymphoid Cells type 2 |
| ILC3s | Innate Lymphoid Cells type 3 |
| LC3B | Microtubule-associated protein 1A/1B light chain 3B (autophagy marker) |
| LDL | Low-Density Lipoprotein |
| LDLR | Low-Density Lipoprotein Receptor |
| LDP | Lupin-Derived Peptides |
| LH | Luteinizing Hormone |
| LOX | Lipoxygenase enzyme |
| LPL | Lipoprotein Lipase |
| LPC | Lupin Protein Concentrate |
| LPH | Lupin Protein Hydrolysate |
| LPHs | Lupin Protein Hydrolysates |
| LPO | Lipid Peroxidation |
| LPS | Lipopolysaccharide |
| M11S | Methylated Derivative of 11S Globulin |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MDA | Malondialdehyde |
| MCF-10A | Non-cancerous mammary epithelial cell line |
| MCF-7 | Breast Cancer Cell Line |
| MIP-1α | Macrophage Inflammatory Protein-1 alpha |
| MoMFs | Monocyte-derived Macrophages |
| mTOR | Mechanistic Target of Rapamycin |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| NEFAs | Non-esterified fatty acids |
| NLRP3 | NOD-like Receptor Family Pyrin Domain Containing 3 |
| NO | Nitric Oxide |
| NP-SH | Non-protein sulfhydryl groups |
| NF-κB | Nuclear Factor Kappa B |
| ORAC | Oxygen Radical Absorbance Capacity Assay |
| P5 | Lupin peptide (LILPKHSDAD) |
| P5-met | Modified lupin peptide (LPKHSDAD) |
| PCK1 | Phosphoenolpyruvate Carboxykinase 1 |
| PCOS | Polycystic Ovary Syndrome |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin Type 9 |
| PI3K | Phosphoinositide 3-Kinase |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| PPAR-α | Peroxisome Proliferator-Activated Receptor Alpha |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor Gamma |
| PRF | Protein-Rich Fraction |
| p53 | Tumor suppressor protein |
| p62 | Sequestosome 1 |
| P-gp | P-glycoprotein |
| PYY | Peptide YY |
| QAP | Quinoa’s Alkali-Extractable Polysaccharides |
| QWP | Quinoa’s Water-Extractable Polysaccharides |
| RNase 7 | Ribonuclease 7 |
| ROS | Reactive Oxygen Species |
| SCD1 | Stearoyl-CoA Desaturase 1 |
| SH-SY5Y | Human neuroblastoma cell line |
| SiHa | Cervical cancer cell line |
| SK-BR-3 | HER2+ breast cancer cell line with nonfunctional p53 |
| SOD | Superoxide Dismutase |
| SREBP-1c | Sterol Regulatory Element-Binding Protein 1c |
| SREBP-2 | Sterol Regulatory Element-Binding Protein 2 |
| SQAP | Specific Quinoa’s Alkali-Extractable Polysaccharides |
| TC | Total cholesterol |
| T24 | Urothelial cancer cell line |
| TG | Triglycerides |
| Th1 | T helper 1 cells |
| Th17 | T helper 17 cells |
| Th2 | T helper 2 cells |
| THP-1 | Monocyte Cell Line |
| TNF | Tumor Necrosis Factor |
| TNF-α | Tumor Necrosis Factor Alpha |
| TLR | Toll-Like Receptor |
| TLR4 | Toll-Like Receptor 4 |
| Treg | Regulatory T Cells |
| ULK1 | Unc-51 Like Autophagy Activating Kinase 1 |
| Vero | African green monkey kidney cell line |
| γH2Ax | Phosphorylated H2A histone variant (DNA damage marker) |
References
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef]
- Ayoka, T.O.; Ezema, B.O.; Eze, C.N.; Nnadi, C.O. Antioxidants for the Prevention and Treatment of Non-communicable Diseases. JERP 2022, 7, 179–189. [Google Scholar] [CrossRef]
- Badr, M.M.; Valencia Quiroz, I. Antimicrobial Effect of Natural Products against Bacteria, Fungi, and Yeasts. In Biotechnology and Drug Development for Targeting Human Diseases; Valencia Quiroz, I., Ed.; Recent Advances in Biotechnology; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; Volume 9, pp. 137–164. ISBN 9789815223163. [Google Scholar]
- Suomela, J.-P.; Repo-Carrasco-Valencia, R.; Lutz, M. Andean native grains, quinoa, and lupin as sources of bioactive components. In Native Crops in Latin America: Biochemical, Processing, and Nutraceutical Aspects; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–34. ISBN 9781003087618. [Google Scholar]
- Niveyro, S.L.; Mortensen, A.G.; Fomsgaard, I.S.; Salvo, A. Erratum to: Differences among five amaranth varieties (Amaranthus spp.) regarding secondary metabolites and foliar herbivory by chewing insects in the field. Arthropod Plant Interact. 2013, 7, 247–248. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Zhu, F.; Li, G. Beverages developed from pseudocereals (quinoa, buckwheat, and amaranth): Nutritional and functional properties. Comp. Rev. Food Sci. Food Saf. 2025, 24, e70081. [Google Scholar] [CrossRef]
- Aguilar-Llanos, E.; Carrera-Pacheco, S.E.; González-Pastor, R.; Zúñiga-Miranda, J.; Rodríguez-Pólit, C.; Romero-Benavides, J.C.; Heredia-Moya, J. Synthesis and Evaluation of Biological Activities of Schiff Base Derivatives of 4-Aminoantipyrine and Cinnamaldehydes. Chem. Proc. 2022, 12, 43. [Google Scholar] [CrossRef]
- Ishaq, A.R.; El-Nashar, H.A.S.; Younis, T.; Mangat, M.A.; Shahzadi, M.; Ul Haq, A.S.; El-Shazly, M. Genus Lupinus (Fabaceae): A review of ethnobotanical, phytochemical and biological studies. J. Pharm. Pharmacol. 2022, 74, 1700–1717. [Google Scholar] [CrossRef]
- Raj, P.; Paliwal, A. Quinoa’s (Chenopodium quinoa Willd.) Nutraceutical Properties and Traditional Lore. Environ. Ecol. 2024, 42, 1670–1676. [Google Scholar] [CrossRef]
- Sidorova, Y.; Perova, I.; Paleeva, M.; Petrov, N. Main biologically active substances of amaranth grain. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2024; Volume 539, p. 02038. [Google Scholar] [CrossRef]
- Batool, Z.; Lone, J.F.; Singh, K.; Gairola, S. Nutraceutical and antioxidant potential of selected wild edible plants from the cold-arid desert of Ladakh, India. Not. Bot. Horti Agrobot. Cluj Napoca 2024, 52, 13286. [Google Scholar] [CrossRef]
- Urrialde, R.; Gómez Cifuentes, A.; Pintos, B.; Gómez-Garay, M.A.; Cifuentes, B. Bioactive compounds from plants. Development of new or novel food. Nutr. Hosp. 2022, 39, 8–11. [Google Scholar] [CrossRef]
- Robles-Botero, M.V.; Ronquillo-de Jesús, E.; Quiroz-Reyes, C.N.; Aguilar-Méndez, M.A. Caracterización e identificación de compuestos bioactivos con actividad antioxidante de la cáscara, pulpa y semilla del fruto de tejocote (Crataegus mexicana). TIP RECQB 2020, 23, 1–10. [Google Scholar] [CrossRef]
- Sar, T.; Kiraz, P.; Braho, V.; Harirchi, S.; Akbas, M.Y. Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020. Microorganisms 2023, 11, 2234. [Google Scholar] [CrossRef]
- González-Caro, S.; Duque, Á.; Feeley, K.J.; Cabrera, E.; Phillips, J.; Ramirez, S.; Yepes, A. The legacy of biogeographic history on the composition and structure of Andean forests. Ecology 2020, 101, e03131. [Google Scholar] [CrossRef]
- Staller, J.E. Andean Foodways: Interdisciplinary Approaches to Pre-Columbian, Colonial, and Contemporary Food and Culture. In Andean Foodways: Pre-Columbian, Colonial, and Contemporary Food and Culture; Staller, J.E., Ed.; The Latin American Studies Book Series; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–20. ISBN 978-3-030-51628-4. [Google Scholar]
- Repo-Carrasco-Valencia, R.A.-M.; Serna, L.A. Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Ciênc. Tecnol. Aliment. 2011, 31, 225–230. [Google Scholar] [CrossRef]
- Jerez, M.P.; Ortiz, J.; Castro, C.; Escobar, E.; Sanhueza, C.; Del-Saz, N.F.; Ribas-Carbo, M.; Coba de la Peña, T.; Ostria-Gallardo, E.; Fischer, S.; et al. Nitrogen sources differentially affect respiration, growth, and carbon allocation in Andean and Lowland ecotypes of Chenopodium quinoa Willd. Front. Plant Sci. 2023, 14, 1070472. [Google Scholar] [CrossRef] [PubMed]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Bioversity International; Food and Agriculture Organization of the United Nations; Fundación para la Promoción e Investigación de Productos Andinos; Instituto Nacional de Innovación Agropecuaria y Forestal; International Fund for Agricultural Development. Descriptores para Quinua (Chenopodium quinoa Willd.) y sus Parientes Silvestres; Bioversity International: Rome, Italy, 2013; ISBN 978-92-9043-927-1. [Google Scholar]
- Anaya, R.B.; De La Cruz, E.; Muñoz-Centeno, L.M.; Cóndor, R.; León, R.; Carhuaz, R. Food and Medicinal Uses of Ancestral Andean Grains in the Districts of Quinua and Acos Vinchos (Ayacucho-Peru). Agronomy 2022, 12, 1014. [Google Scholar] [CrossRef]
- Schnetzler, K.A. Food uses and Amaranth Product Research: A Comprehensive Review. In Amaranth Biology, Chemistry, and Technology; Paredes-López, O., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 155–184. ISBN 9781351069601. [Google Scholar]
- Fadeev, L. AMARANTH. GPMF 2024, 24, 13–19. [Google Scholar] [CrossRef]
- Parra-Gallardo, G.; Salas-Sanjuán, M.d.C.; del Moral, F.; Valenzuela, J.L. Characterising the Nutritional and Alkaloid Profiles of Tarwi (Lupinus mutabilis Sweet) Pods and Seeds at Different Stages of Ripening. Agriculture 2024, 14, 1812. [Google Scholar] [CrossRef]
- Knecht, K.T.; Sanchez, P.; Kinder, D.H. Lupine Seeds (Lupinus spp.). In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–402. ISBN 9780128185537. [Google Scholar]
- Kocjan Ačko, D.; Flajšman, M. Production and Utilization of Lupinus spp. In Production and Utilization of Legumes—Progress and Prospects; Hasanuzzaman, M., Ed.; IntechOpen: London, UK, 2023; ISBN 978-1-83768-646-9. [Google Scholar]
- Ashraf, M.V.; Khan, S.; Misri, S.; Gaira, K.S.; Rawat, S.; Rawat, B.; Khan, M.A.H.; Shah, A.A.; Asgher, M.; Ahmad, S. High-Altitude Medicinal Plants as Promising Source of Phytochemical Antioxidants to Combat Lifestyle-Associated Oxidative Stress-Induced Disorders. Pharmaceuticals 2024, 17, 975. [Google Scholar] [CrossRef]
- Berru, L.B.; Glorio-Paulet, P.; Basso, C.; Scarafoni, A.; Camarena, F.; Hidalgo, A.; Brandolini, A. Chemical Composition, Tocopherol and Carotenoid Content of Seeds from Different Andean Lupin (Lupinus mutabilis) Ecotypes. Plant Foods Hum. Nutr. 2021, 76, 98–104. [Google Scholar] [CrossRef]
- Blair, M.W.; Londoño, J.M.; Buitrago-Bitar, M.A.; Wu, X.; Brenner, D.M. Differentiation of Andean and Mesoamerican accessions in a proposed core collection of grain amaranths. Front. Plant Sci. 2023, 14, 1144681. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Terán, M.; Padilla-Arias, K.; Beltrán-Novoa, A.; González-Paramás, A.M.; Giampieri, F.; Battino, M.; Vásquez-Castillo, W.; Fernandez-Soto, P.; Tejera, E.; Alvarez-Suarez, J.M. Influence of Altitudes and Development Stages on the Chemical Composition, Antioxidant, and Antimicrobial Capacity of the Wild Andean Blueberry (Vaccinium floribundum Kunth). Molecules 2022, 27, 7525. [Google Scholar] [CrossRef] [PubMed]
- Torres-Miño, C.J.; Hernández Maqueda, R.; Moreno, Á.H. Adaptation Strategies and Microwave Drying of Amaranth Species with a High Nutritional Value to the Ecuadorian Andean Region. In Nutritional Value of Amaranth; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-083-3. [Google Scholar]
- Urrego-Pava, F.; Coy-Barrera, E. Isoflavone Content and Nutritional-Related Properties of Debittered Seeds from Two Andean Lupin (Lupinus mutabilis Sweet) Ecotypes Propagated in Two Soils. Foods 2023, 12, 1841. [Google Scholar] [CrossRef] [PubMed]
- Carvajal Acosta, A.N.; Formenti, L.; Godschalx, A.; Katsanis, A.; Schapheer, C.; Mooney, K.; Villagra, C.; Rasmann, S. Ecological convergence in phytochemistry and flower-insect visitor interactions along an Andean elevation gradient. Ecol. Evol. 2023, 13, e10418. [Google Scholar] [CrossRef]
- Chalampuente-Flores, D.; Mosquera-Losada, M.R.; Ron, A.M.D.; Tapia Bastidas, C.; Sørensen, M. Morphological and Ecogeographical Diversity of the Andean Lupine (Lupinus mutabilis Sweet) in the High Andean Region of Ecuador. Agronomy 2023, 13, 2064. [Google Scholar] [CrossRef]
- Antognoni, F.; Potente, G.; Biondi, S.; Mandrioli, R.; Marincich, L.; Ruiz, K.B. Free and Conjugated Phenolic Profiles and Antioxidant Activity in Quinoa Seeds and Their Relationship with Genotype and Environment. Plants 2021, 10, 1046. [Google Scholar] [CrossRef]
- Rodés-Bachs, C.; Van der Fels-Klerx, H.J. Impact of environmental factors on the presence of quinolizidine alkaloids in lupins: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2023, 40, 757–769. [Google Scholar] [CrossRef]
- Samsami, H.; Maali-Amiri, R. Global insights into intermediate metabolites: Signaling, metabolic divergence and stress response modulation in plants. Plant Physiol. Biochem. 2024, 213, 108862. [Google Scholar] [CrossRef]
- Pandya, A.; Thiele, B.; Köppchen, S.; Zurita-Silva, A.; Usadel, B.; Fiorani, F. Characterization of Bioactive Phenolic Compounds in Seeds of Chilean Quinoa (Chenopodium quinoa Willd.) Germplasm. Agronomy 2023, 13, 2170. [Google Scholar] [CrossRef]
- Zhang, L.; Dang, B.; Lan, Y.; Zheng, W.; Kuang, J.; Zhang, J.; Zhang, W. Metabolomics Characterization of Phenolic Compounds in Colored Quinoa and Their Relationship with In Vitro Antioxidant and Hypoglycemic Activities. Molecules 2024, 29, 1509. [Google Scholar] [CrossRef]
- Dostalíková, L.; Hlásná Čepková, P.; Janovská, D.; Jágr, M.; Svoboda, P.; Dvořáček, V.; Viehmannová, I. The impact of germination and thermal treatments on bioactive compounds of quinoa (Chenopodium quinoa Willd.) seeds. Eur. Food Res. Technol. 2024, 250, 1457–1471. [Google Scholar] [CrossRef]
- Kadri, M.; Salhi, N.; Chana, A. Algeria Phytochemical Analysis and Allelopathic Effects of Quinoa (Chenopodium quinoa Willd.) Grain Extract. AJPP 2023, 41, 246–257. [Google Scholar] [CrossRef]
- Peñarrieta, J.M.; Alvarado, J.A.; Akesson, B.; Bergenståhl, B. Total antioxidant capacity and content of flavonoids and other phenolic compounds in canihua (Chenopodium pallidicaule): An Andean pseudocereal. Mol. Nutr. Food Res. 2008, 52, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco-Valencia, R.; Acevedo de La Cruz, A.; Icochea Alvarez, J.C.; Kallio, H. Chemical and functional characterization of Kañiwa (Chenopodium pallidicaule) grain, extrudate and bran. Plant Foods Hum. Nutr. 2009, 64, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wang, X.; Wang, L.; Zhang, W.; Song, Y.; Zhao, S.; Yang, X.; Liu, X. Change of physiochemical characteristics, nutritional quality, and volatile compounds of Chenopodium quinoa Willd. during germination. Food Chem. 2024, 445, 138693. [Google Scholar] [CrossRef]
- Tan, M.; Chang, S.; Liu, J.; Li, H.; Xu, P.; Wang, P.; Wang, X.; Zhao, M.; Zhao, B.; Wang, L.; et al. Physicochemical Properties, Antioxidant and Antidiabetic Activities of Polysaccharides from Quinoa (Chenopodium quinoa Willd.) Seeds. Molecules 2020, 25, 3840. [Google Scholar] [CrossRef]
- Fan, S.; Li, J.; Bai, B. Purification, structural elucidation and in vivo immunity-enhancing activity of polysaccharides from quinoa (Chenopodium quinoa Willd.) seeds. Biosci. Biotechnol. Biochem. 2019, 83, 2334–2344. [Google Scholar] [CrossRef]
- Cao, R.-A.; Ma, N.; Palanisamy, S.; Talapphet, N.; Zhang, J.; Wang, C.; You, S. Structural Elucidation and Immunostimulatory Activities of Quinoa Non-starch Polysaccharide Before and After Deproteinization. J. Polym. Environ. 2022, 30, 2291–2303. [Google Scholar] [CrossRef]
- Wu, D.-T.; Li, J.; Wang, J.; Lei, J.; Gan, R.-Y.; Qin, P.; Hu, Y.-C.; Wu, X.-Y.; Zou, L. Comparison of soluble dietary fibers from various quinoa microgreens: Structural characteristics and bioactive properties. Food Res. Int. 2024, 181, 114108. [Google Scholar] [CrossRef]
- Yao, Y.; Shi, Z.; Ren, G. Antioxidant and immunoregulatory activity of polysaccharides from quinoa (Chenopodium quinoa Willd.). Int. J. Mol. Sci. 2014, 15, 19307–19318. [Google Scholar] [CrossRef]
- Salas-Veizaga, D.M.; Villagomez, R.; Linares-Pastén, J.A.; Carrasco, C.; Álvarez, M.T.; Adlercreutz, P.; Nordberg Karlsson, E. Extraction of Glucuronoarabinoxylan from Quinoa Stalks (Chenopodium quinoa Willd.) and Evaluation of Xylooligosaccharides Produced by GH10 and GH11 Xylanases. J. Agric. Food Chem. 2017, 65, 8663–8673. [Google Scholar] [CrossRef] [PubMed]
- Wefers, D.; Gmeiner, B.M.; Tyl, C.E.; Bunzel, M. Characterization of diferuloylated pectic polysaccharides from quinoa (Chenopodium quinoa WILLD.). Phytochemistry 2015, 116, 320–328. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B. Quinoa (Chenopodium quinoa Willd.) as source of bioactive compounds: A review. BCHD 2019, 2, 27. [Google Scholar] [CrossRef]
- Zhu, F. Quinoa protein. In Quinoa; Elsevier: Amsterdam, The Netherlands, 2023; pp. 117–149. ISBN 9780323999090. [Google Scholar]
- Reyes-Bautista, R.; Jesús Flores-Sierra, J.d.; Hernández-Mendoza, G.; Xoca-Orozco, L.Á. Biologically Active Peptides from Quinoa (Chenopodium quinoa Willd) Grain. In Potential Health Benefits of Biologically Active Peptides Derived from Underutilized Grains: Recent Advances in Their Isolation, Identification, Bioactivity and Molecular Analysis; Tovar-Pérez, E.G., Lugo-Radillo, A., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023; pp. 54–75. ISBN 9789815123340. [Google Scholar]
- Sen, A.; Sharma, G.; Tomer, N.; Shibu, B.S.; Moin, S. Isolation, purification, and characterization of bioactive peptide from Chenopodium quinoa seeds: Therapeutic and functional insights. JOAPR 2024, 12, 184–191. [Google Scholar] [CrossRef]
- Cheng, L.; De Leon-Rodriguez, L.M.; Gilbert, E.P.; Loo, T.; Petters, L.; Yang, Z. Self-assembly and hydrogelation of a potential bioactive peptide derived from quinoa proteins. Int. J. Biol. Macromol. 2024, 259, 129296. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, F.F.; Mudgil, P.; Jobe, A.; Antony, P.; Vijayan, R.; Gan, C.-Y.; Maqsood, S. Novel Plant-Protein (Quinoa) Derived Bioactive Peptides with Potential Anti-Hypercholesterolemic Activities: Identification, Characterization and Molecular Docking of Bioactive Peptides. Foods 2023, 12, 1327. [Google Scholar] [CrossRef]
- Tocmo, R.; Balagiannis, D.; Quinn, E.; Finnegan, D.; Collins, M.; Loscher, C.E. Protein hydrolysates from quinoa (Chenopodium quinoa Willd.) modulate macrophage polarization and the expression of surface antigen molecules. Sustain. Food Proteins 2024, 2, 282–296. [Google Scholar] [CrossRef]
- Mahdavi-Yekta, M.; Reihani, S.F.S.; Mohammadi, M. Antimicrobial Activity of Quinoa Protein Hydrolysate against Streptococcus pyogenes and Escherichia coli. J. Food Qual. 2023, 2023, 1–7. [Google Scholar] [CrossRef]
- Dong, S.; Yang, X.; Zhao, L.; Zhang, F.; Hou, Z.; Xue, P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind. Crops Prod. 2020, 149, 112350. [Google Scholar] [CrossRef]
- Romero-Benavides, J.C.; Guaraca-Pino, E.; Duarte-Casar, R.; Rojas-Le-Fort, M.; Bailon-Moscoso, N. Chenopodium quinoa Willd. and Amaranthus hybridus L.: Ancestral Andean Food Security and Modern Anticancer and Antimicrobial Activity. Pharmaceuticals 2023, 16, 1728. [Google Scholar] [CrossRef]
- Galindo-Luján, R.; Pont, L.; Sanz-Nebot, V.; Benavente, F. A Proteomics Data Mining Strategy for the Identification of Quinoa Grain Proteins with Potential Immunonutritional Bioactivities. Foods 2023, 12, 390. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, Y.J.; Kim, Y.H.; Yoon, K.S. Antioxidant and Antimicrobial Activities of Quinoa (Chenopodium quinoa Willd.) Seeds Cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Casalvara, R.F.A.; Ferreira, B.M.R.; Gonçalves, J.E.; Yamaguchi, N.U.; Bracht, A.; Bracht, L.; Comar, J.F.; de Sá-Nakanishi, A.B.; de Souza, C.G.M.; Castoldi, R.; et al. Biotechnological, Nutritional, and Therapeutic Applications of Quinoa (Chenopodium quinoa Willd.) and Its By-Products: A Review of the Past Five-Year Findings. Nutrients 2024, 16, 840. [Google Scholar] [CrossRef]
- Buitrago, D.; Buitrago-Villanueva, I.; Barbosa-Cornelio, R.; Coy-Barrera, E. Comparative Examination of Antioxidant Capacity and Fingerprinting of Unfractionated Extracts from Different Plant Parts of Quinoa (Chenopodium quinoa) Grown under Greenhouse Conditions. Antioxidants 2019, 8, 238. [Google Scholar] [CrossRef]
- Lopez-Moreno, M.; Sabater-Muñoz, B.; Iglesias-López, M.T.; Miguel-Castro, M.; Garcés-Rimón, M. Red Quinoa hydrolysates with antioxidant bioactive properties on oxidative stress-induced Saccharomyces cerevisiae. LWT 2023, 184, 115038. [Google Scholar] [CrossRef]
- Teng, C.; Qin, P.; Shi, Z.; Zhang, W.; Yang, X.; Yao, Y.; Ren, G. Structural characterization and antioxidant activity of alkali-extracted polysaccharides from quinoa. Food Hydrocoll. 2021, 113, 106392. [Google Scholar] [CrossRef]
- Abdelaleem, M.A.; Elbassiony, K.R.A. Evaluation of phytochemicals and antioxidant activity of gamma irradiated quinoa (Chenopodium quinoa). SciELO J. 2021, 81, 806–813. [Google Scholar] [CrossRef]
- Fan, X.; Guo, H.; Teng, C.; Zhang, B.; Blecker, C.; Ren, G. Anti-Colon Cancer Activity of Novel Peptides Isolated from In Vitro Digestion of Quinoa Protein in Caco-2 Cells. Foods 2022, 11, 194. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Zou, L.; Fu, C.; Li, P.; Zhao, G. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar] [CrossRef]
- Rueda, J.; Lobo, M.O.; Galdeano, C.M.; Samman, N.C. Quinoa protein hydrolysate as potential immunomodulators: Effects on cytokine production and macrophage activation. Res. Sq. 2024, 80, 1–7. [Google Scholar] [CrossRef]
- Marcos Pasero, H.; Bojarczuk, A.; Haros, C.M.; Laparra Llopis, J.M. Immunonutritional Benefits of Chenopodium quinoa’s Ingredients Preventing Obesity-Derived Metabolic Imbalances. Biol. Life Sci. Forum 2022, 17, 20. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Z.; Wu, T.; You, H.; Wang, W.; Liu, X.; Ding, L. Quinoa Peptides Alleviate Obesity in Mice Induced by a High-Fat Diet via Regulating of the PPAR-α/γ Signaling Pathway and Gut Microbiota. Mol. Nutr. Food Res. 2023, 67, e2300258. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ji, X.; Song, L.; Cao, Y.; Feng, J.; Zhang, R.; Tao, F.; Zhang, F.; Xue, P. Saponins from Chenopodium quinoa Willd. husks alleviated high-fat-diet-induced hyperlipidemia via modulating the gut microbiota and multiple metabolic pathways. J. Sci. Food Agric. 2024, 104, 2417–2428. [Google Scholar] [CrossRef]
- Zhong, L.; Lyu, W.; Lin, Z.; Lu, J.; Geng, Y.; Song, L.; Zhang, H. Quinoa ameliorates hepatic steatosis, oxidative stress, inflammation and regulates the gut microbiota in nonalcoholic fatty liver disease rats. Foods 2023, 12, 1780. [Google Scholar] [CrossRef]
- Dou, J.; Wu, Y.; Hu, R.; Liu, J.; Zhang, Y.; Zhen, X.; Wu, T.; Zhang, C.; Liu, Y.; Zheng, R.; et al. Quinoa ameliorates polycystic ovary syndrome via regulating gut microbiota through PI3K/AKT/mTOR pathway and autophagy. Nutr. Metab. 2024, 21, 80. [Google Scholar] [CrossRef]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In vitro study of the effect of quinoa and quinoa polysaccharides on human gut microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhang, X.; Shi, J.; Cui, K.; Yang, R.; Liu, F.; Shan, S.; Israr, G.; Li, Z. Terpenoids of quinoa bran suppresses colorectal cancer by inducing cell apoptosis. Food Biosci. 2023, 53, 102615. [Google Scholar] [CrossRef]
- Feng, M.; Fan, X.; Shi, J.; Shan, S.; Li, S.; He, S.; Ding, M.; Li, Z. Terpenoids from quinoa reverse drug resistance of colon cancer by upregulating miR-495-3p. J. Sci. Food Agric. 2024, 104, 8916–8927. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.L.; Cheng, D.M.; Esposito, D.; Shertel, T.; Poulev, A.; Plundrich, N.; Itenberg, D.; Dayan, N.; Lila, M.A.; Raskin, I. Compounds leached from quinoa seeds inhibit matrix metalloproteinase activity and intracellular reactive oxygen species. Int. J. Cosmet. Sci. 2015, 37, 212–221. [Google Scholar] [CrossRef]
- Capraro, J.; De Benedetti, S.; Di Dio, M.; Bona, E.; Abate, A.; Corsetto, P.A.; Scarafoni, A. Characterization of Chenopodin Isoforms from Quinoa Seeds and Assessment of Their Potential Anti-Inflammatory Activity in Caco-2 Cells. Biomolecules 2020, 10, 795. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Tuo, Y.; You, H.; Li, J.; Wang, L.; Liu, X.; Ding, L. Quinoa protein and its hydrolysate ameliorated DSS-induced colitis in mice by modulating intestinal microbiota and inhibiting inflammatory response. Int. J. Biol. Macromol. 2023, 253, 127588. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Lan, X.-Z.; Wu, Y.-Y.; Ou, Y.-W.; Chen, T.C.; Wu, W.-T. The antioxidant activity and nitric oxide production of extracts obtained from the leaves of Chenopodium quinoa Willd. Biomedicine 2017, 7, 24. [Google Scholar] [CrossRef]
- Alamri, E.; Amany, B.; Bayomy, H. Quinoa seeds (Chenopodium quinoa): Nutritional value and potential biological effects on hyperglycemic rats. J. King Saud Univ. Sci. 2023, 35, 102427. [Google Scholar] [CrossRef]
- Belguet, A.; Bouchareb, R.; Djoudi, M.; Guendouz, A. Agro-nutritionnel Characterisation of Quinoa (Chenopodium quinoa, Willd.). J. Agron. Technol. Eng. Manag. 2024, 7, 1043–1053. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Rashwan, A.K.; Osman, A.I. Potential food applications and biological activities of fermented quinoa: A review. Trends Food Sci. Technol. 2024, 144, 104339. [Google Scholar] [CrossRef]
- Huang, H.; Jia, C.; Chen, X.; Zhang, L.; Jiang, Y.; Meng, X.; Liu, X. Progress in research on the effects of quinoa (Chenopodium quinoa) bioactive compounds and products on intestinal flora. Front. Nutr. 2024, 11, 1308384. [Google Scholar] [CrossRef]
- Singhania, N.; Kumar, R.; Pramila; Bishnoi, S.; Ray, A.B.; Diwan, A. Bioactive properties and health benefits of amaranthus. In Harvesting Food from Weeds; Gupta, P., Chhikara, N., Panghal, A., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 351–383. ISBN 9781119791973. [Google Scholar]
- Sarker, U.; Oba, S.; Ullah, R.; Bari, A.; Ercisli, S.; Skrovankova, S.; Adamkova, A.; Zvonkova, M.; Mlcek, J. Nutritional and bioactive properties and antioxidant potential of Amaranthus tricolor, A. lividus, A viridis, and A. spinosus leafy vegetables. Heliyon 2024, 10, e30453. [Google Scholar] [CrossRef]
- Hutsko, K.; Petrina, R.O. Amaranthus as a source of polyphenolic compounds and flavonoids for use in medicine. Biotechnol. Acta 2024, 17, 46–48. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kim, M.J.; Kwon, Y.S.; Chen, W.; Yin, F. Analysis and phytochemical profile of Amaranthus tricolor L. extract with antioxidative and antimicrobial properties. Food Sci. Technol. 2023, 43, e004823. [Google Scholar] [CrossRef]
- Boro, S.; Bharadwaj, B.; Unni, B.; Kumar Rai, A.; Bhattacharjee, M. Phytochemical Analysis and Identification of Bio-active compounds in Ethanolic leaf extract of Amaranthus spinosus. Res. J. Pharm. Technol. 2023, 16, 3685–3690. [Google Scholar] [CrossRef]
- Kar, A.; Bhattacharjee, S. Bioactive polyphenolic compounds, water-soluble vitamins, in vitro anti-inflammatory, anti-diabetic and free radical scavenging properties of underutilized alternate crop Amaranthus spinosus L. from Gangetic plain of West Bengal. Food Biosci. 2022, 50, 102072. [Google Scholar] [CrossRef]
- Manyelo, T.G.; Sebola, N.A.; Hassan, Z.M.; Mabelebele, M. Characterization of the Phenolic Compounds in Different Plant Parts of Amaranthuscruentus Grown under Cultivated Conditions. Molecules 2020, 25, 4273. [Google Scholar] [CrossRef]
- Sandoval-Sicairos, E.; Montoya-Rodriguez, A.; Antunes-Ricardo, M.; Luna-Vital, D.; Milán-Carrillo, J.; Milán-Noris, A. Assessment of Anti-inflammatory and Antioxidant Compounds in Gastrointestinal Digests From Germinated Amaranth Protein Concentrate. Curr. Dev. Nutr. 2022, 6, 532. [Google Scholar] [CrossRef]
- Bang, J.-H.; Jo, I.-H.; Sebastin, R.; Jeong, W.T.; Oh, S.; Heo, T.-Y.; Sung, J.; Hyun, T.K.; So, Y.-S.; Yu, J.-K.; et al. Comparative analysis of polyphenolic compounds in different amaranthus species: Influence of genotypes and harvesting year. Antioxidants 2024, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Añón, M.C.; Quiroga, A.V.; Scilingo, A.A.; Tironi, V.A.; Sabbione, A.C.; Nardo, A.E.; Suárez, S.E.; Fillería, S.F.G. Action of amaranth peptides on the cardiovascular system. In Native Crops in Latin America: Biochemical, Processing, and Nutraceutical Aspects; CRC Press: Boca Raton, FL, USA, 2022; pp. 209–236. ISBN 9781003087618. [Google Scholar]
- Soares, R.A.M.; Mendonça, S.; de Castro, L.Í.A.; Menezes, A.C.C.C.C.; Arêas, J.A.G. Major peptides from amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef]
- Ayala-Niño, A.; Rodríguez-Serrano, G.M.; González-Olivares, L.G.; Contreras-López, E.; Regal-López, P.; Cepeda-Saez, A. Sequence Identification of Bioactive Peptides from Amaranth Seed Proteins (Amaranthus hypochondriacus spp.). Molecules 2019, 24, 3033. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, A.; Gómez-Favela, M.A.; Reyes-Moreno, C.; Milán-Carrillo, J.; González de Mejía, E. Identification of Bioactive Peptide Sequences from Amaranth (Amaranthus hypochondriacus) Seed Proteins and Their Potential Role in the Prevention of Chronic Diseases. Comp. Rev. Food Sci. Food Safety 2015, 14, 139–158. [Google Scholar] [CrossRef]
- Rang, Y.; Liu, H.; Cheng, X.; Li, W.; Shi, J.; Ou, G.; Huang, H.; Chen, C.; Xiao, X.; Liu, C. Structural characterization of pectic polysaccharides from Amaranth caudatus leaves and the promotion effect on hippocampal glucagon-like peptide-1 level. Int. J. Biol. Macromol. 2023, 242, 124967. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, C.; Cai, Y.; Tang, Y.; Sun, W.; Yao, H.; Zheng, T.; Chen, H.; Xiao, Y.; Shan, Z.; et al. Purification, characterization and antioxidant activities in vitro of polysaccharides from Amaranthus hybridus L. PeerJ 2020, 8, e9077. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, Y.; Tang, Z.; Jin, W.; Wang, Y.; Chen, H.; Yao, H.; Shan, Z.; Bu, T.; Wang, X. Extraction of polysaccharides from Amaranthus hybridus L. by hot water and analysis of their antioxidant activity. PeerJ 2019, 7, e7149. [Google Scholar] [CrossRef]
- Sleptsov, I.V.; Zhuravskay, A.N. Polysaccharides in tissues Amaranthus caudatus, Amaranthus retroflexus, Agastache rugosa and Thlaspi arvense in the conditions of central Yakutia. CPRM 2018, 4, 73–79. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Tsepaeva, O.V.; Mironova, L.G.; Vyshtakalyuk, A.B.; Mindubaev, A.Z.; Nizameev, I.R.; Kholin, K.V.; Pashagin, A.V. Isolation and Structural And Chemical Analysis of Pectinic Polysaccharides from Amaranthus cruentus. Chem. Nat. Compd. 2014, 50, 54–59. [Google Scholar] [CrossRef]
- Sarkar, R.; Nandan, C.K.; Mandal, S.; Patra, P.; Das, D.; Islam, S.S. Structural characterization of a heteropolysaccharide isolated from hot water extract of the stems of Amaranthus tricolor Linn. (Amaranthus gangeticus L.). Carbohydr. Res. 2009, 344, 2412–2416. [Google Scholar] [CrossRef] [PubMed]
- Gins, E.M.; Motyleva, S.M.; Gins, V.K.; Kulikov, I.M.; Gins, M.S. Comparative analysis of carbohydrate metabolites in amaranth leaves of different age. SABRAOJBG 2022, 54, 897–907. [Google Scholar] [CrossRef]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydr. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef]
- Anyanele, S.I.; Ajiwe, V.I.E.; Anyanele, W.C.; Umeokoli, B.O. Blessing Structural Elucidation and Antimicrobial Analysis of Bioactive Compounds from Amaranthus spinosus. AJACR 2023, 13, 31–39. [Google Scholar] [CrossRef]
- Fouad, M.S.; Ghareeb, M.A.; Hamed, A.A.; Aidy, E.A.; Tabudravu, J.; Sayed, A.M.; Tammam, M.A.; Mwaheb, M.A. Exploring the antioxidant, anticancer and antimicrobial potential of Amaranthus viridis L. collected from Fayoum depression: Phytochemical, and biological aspects. S. Afr. J. Bot. 2024, 166, 297–310. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Skwaryło-Bednarz, B.; Kowalski, R.; Yildirim, I.; Patkowska, E. Antifungal potency of amaranth leaf extract: An in vitro study. Plants 2023, 12, 1723. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Toxicity and Antimicrobial Activities of Amaranthus caudatus L. (Amaranthaceae) Harvested From Formulated Soils at Different Growth Stages. J. Evid. Based Integr. Med. 2020, 25, 2515690X20971578. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Bi, X.; Duo, K.; Sun, Q.; Yun, X.; Zhang, Y.; Fei, P.; Han, J. Antimicrobial Activity and Mechanism of Action of the Amaranthus tricolor Crude Extract against Staphylococcus aureus and Potential Application in Cooked Meat. Foods 2020, 9, 359. [Google Scholar] [CrossRef]
- Effoe, S.; Agban, A.; Hoekou, Y.; Dakey, K.A.; Kpabi, I.; Gbekley, H.E.; Pissang, P.; Tchacondo, T. Study of the antimicrobial potential of Amaranthus spinosus L. (Amaranthaceae) and Tridax procumbens L. (Asteraceae), two leafy vegetables from the maritime region of Togo. Int. J. Innov. Appl. Stud. 2020, 30, 239–245. [Google Scholar]
- Hipólito-Nolasco, C.; Ramírez-Isidro, O.; Núñez-Gaona, O.; Ávila-Alejandre, A.X.; Hernández-López, A.; García-Gómez, M.d.J. Antioxidant activity of peptides obtained by enzymatic hydrolysis from proteins of amaranth (Amaranthus hypochondriacus L.) stubble. Agrociencia 2022, 1–8. [Google Scholar] [CrossRef]
- Sattar, M.; Saeed, F.; Afzaal, M.; Rasheed, A.; Asif, A.; Sharif, S.; Hussain, M.; Asad Ur Rehman, H.; Raza, M.A.; Munir, H.; et al. An overview of the nutritional and therapeutic properties of amaranth. Int. J. Food Prop. 2024, 27, 263–272. [Google Scholar] [CrossRef]
- Rodríguez, M.; Tironi, V.A. Chemical and cell antioxidant activity of amaranth flour and beverage after simulated gastrointestinal digestion. Role of peptides. Food Res. Int. 2023, 173, 113410. [Google Scholar] [CrossRef]
- Gins, E.M. Amaranthus species assessment for morphological and biochemical parameters. SABRAOJBG 2024, 56, 1387–1399. [Google Scholar] [CrossRef]
- Johnmark, N.; Kinyi, H.W. Amaranth leaf extract protects against hydrogen peroxide induced oxidative stress in Drosophila melanogaster. BMC Res. Notes 2021, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Gupta, P. Evaluation of Bioactive Metabolites and Antioxidant-Rich Extracts of Amaranths with Possible Role in Pancreatic Lipase Interaction: In Silico and In Vitro Studies. Metabolites 2021, 11, 676. [Google Scholar] [CrossRef]
- Tripathi, G.K.; Hariwal, M. Anticancer activity of Amaranthus spinosus Linn. (Tanduliya): A Review. CUPMAP 2023, 6, 66–77. [Google Scholar] [CrossRef]
- Kumar, A.; Katiyar, A.; Gautam, V.; Singh, R.; Dubey, A. A Comprehensive Review on Anti-Cancer Properties of Amaranthus viridis. J. Res. Appl. Sci. Biotechnol. 2022, 1, 178–185. [Google Scholar] [CrossRef]
- Pooja, R.C.; Ajay, B.V.; Bharathi, D.R. Geomorphology, Phytochemistry, Ethnomedical and Pharmacological activities of Amaranthus viridis Linn. Asian J. Pharm. Res. 2023, 13, 41–44. [Google Scholar] [CrossRef]
- Schröter, D.; Neugart, S.; Schreiner, M.; Grune, T.; Rohn, S.; Ott, C. Amaranth’s 2-Caffeoylisocitric Acid-An Anti-Inflammatory Caffeic Acid Derivative That Impairs NF-κB Signaling in LPS-Challenged RAW 264.7 Macrophages. Nutrients 2019, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, A.; Rivero-Pino, F.; Villanueva, A.; Toscano, R.; Grao-Cruces, E.; Marquez-Paradas, E.; Martin, M.E.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Kiwicha (Amaranthus caudatus L.) protein hydrolysates reduce intestinal inflammation by modulating the NLRP3 inflammasome pathway. Food Funct. 2022, 13, 11604–11614. [Google Scholar] [CrossRef] [PubMed]
- Amornrit, W.; Santiyanont, R. Effect of Amaranthus on Advanced Glycation End-Products Induced Cytotoxicity and Proinflammatory Cytokine Gene Expression in SH-SY5Y Cells. Molecules 2015, 20, 17288–17308. [Google Scholar] [CrossRef]
- Peter, J.; Sabu, V.; Aswathy, I.S.; Krishnan, S.; Lal Preethi, S.S.; Simon, M.; Helen, A. Dietary amaranths modulate the immune response via balancing Th1/Th2 and Th17/Treg response in collagen-induced arthritis. Mol. Cell. Biochem. 2020, 472, 57–66. [Google Scholar] [CrossRef]
- Zambrana, S.; Lundqvist, L.C.E.; Veliz, V.; Catrina, S.-B.; Gonzales, E.; Östenson, C.-G. Amaranthus caudatus Stimulates Insulin Secretion in Goto-Kakizaki Rats, a Model of Diabetes Mellitus Type 2. Nutrients 2018, 10, 94. [Google Scholar] [CrossRef]
- Clemente, A.; Desai, P.V. Evaluation of the hematological, hypoglycemic, hypolipidemic and antioxidant properties of amaranthus tricolor leaf extract in rat. Trop. J. Pharm Res 2011, 10, 595–602. [Google Scholar] [CrossRef]
- Zeashan, H.; Amresh, G.; Singh, S.; Rao, C.V. Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem. Toxicol. 2008, 46, 3417–3421. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, M.S. The effectiveness of ethanolic extract of Amaranthus tricolor L.: A natural hepatoprotective agent. Am. J. Chin. Med. 2010, 38, 1051–1064. [Google Scholar] [CrossRef]
- Taniya, M.S.; Reshma, M.V.; Shanimol, P.S.; Krishnan, G.; Priya, S. Bioactive peptides from amaranth seed protein hydrolysates induced apoptosis and antimigratory effects in breast cancer cells. Food Biosci. 2020, 35, 100588. [Google Scholar] [CrossRef]
- Netshimbupfe, M.H.; Berner, J.; Van Der Kooy, F.; Oladimeji, O.; Gouws, C. The effect of environmental stressors on the anticancer potential of Amaranthus hypochondriacus aqueous extracts and fractions. Nat. Prod. Res. 2023, 39, 1729–1734. [Google Scholar] [CrossRef]
- Ndeda, V. The impact of Amaranthus diet on eicosanoid Profiles: Exploring the role in cancer treatment through cyclooxygenase (COX) and lipoxygenase (LOX) pathways: A mini-review. Int. J. Agric. Food Sci. 2023, 5, 153–161. [Google Scholar] [CrossRef]
- Kumar, V.; Monika, K.; Rathi, B.; Agarwal, R.; Khan, N.A.; Swain, S.R. Pharmacological evaluation of analgesic, anti-pyretic and anti-inflammatory activities of ethanolic root extract of Amaranthus caudatus. J. Drug Delivery Ther. 2023, 13, 11–16. [Google Scholar] [CrossRef]
- Ikram, M.; Haider, A.; Fatima, U. Pharmaceutical Activity of Medicinal Plant Amaranthus viridis Linn. Due to Its Chemical Constituents: A Review. BES 2023, 7, 143–148. [Google Scholar] [CrossRef]
- da Costa Ribeiro Quintans, I.L.A.; Pandolfi, V.; Gaudencio do Rêgo, T.; Neto, J.R.C.F.; AR Ramos, T.; Adhikary, D. Genome designing for nutritional quality in Amaranthus. In Compendium of Crop Genome Designing for Nutraceuticals; Kole, C., Ed.; Springer Nature: Singapore, 2023; pp. 1–33. ISBN 978-981-19-3627-2. [Google Scholar]
- Dania, O.E.; Dokunmu, T.M.; Adegboye, B.E.; Adeyemi, A.O.; Chibuzor, F.C.; Iweala, E.E.J. Pro-estrogenic and anti-inflammatory effects of Corchorus olitorius and Amaranthus hybridus leaves in DMBA-induced breast cancer. Phytomedicine Plus 2024, 4, 100567. [Google Scholar] [CrossRef]
- Malik, A.; Kasture, P.V.; Bhavana, N.V.; Rao, J.; Rao, G. The anticancer and anti-inflammatory effects of polyherbal drug AS20 on HeLa cells resistant to 5-Fluorouracil. J. Emerg. Investig. 2023, 6, 1–7. [Google Scholar] [CrossRef]
- Jimenez, M.D.; Lobo, M.; Sammán, N. 12th IFDC 2017 Special Issue—Influence of germination of quinoa (Chenopodium quinoa) and amaranth (Amaranthus) grains on nutritional and techno-functional properties of their flours. J. Food Compos. Anal. 2019, 84, 103290. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Iftikhar, M.; Khan, M. Amaranth. In Bioactive Factors and Processing Technology for Cereal Foods; Wang, J., Sun, B., Tsao, R., Eds.; Springer: Singapore, 2019; pp. 217–232. ISBN 978-981-13-6166-1. [Google Scholar]
- Martinez-Lopez, A.; Millan-Linares, M.C.; Rodriguez-Martin, N.M.; Millan, F.; Montserrat-de la Paz, S. Nutraceutical value of kiwicha (Amaranthus caudatus L.). J. Funct. Foods 2020, 65, 103735. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, K.; Dhaliwal, H.S. Nutritional facts, bio-active components and processing aspects of pseudocereals: A comprehensive review. Food Biosci. 2021, 42, 101170. [Google Scholar] [CrossRef]
- Upasana; Yadav, L. Pseudocereals: A Novel Path towards Healthy Eating. In Pseudocereals; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-180-7. [Google Scholar]
- Liberal, Â.; Calhelha, R.C.; Pereira, C.; Adega, F.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Abreu, R.M.V.; Ferreira, I.C.F.R. A comparison of the bioactivity and phytochemical profile of three different cultivars of globe amaranth: Red, white, and pink. Food Funct. 2016, 7, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, D.; Gupta, S. Pharmacokinetic study of amaranth extract in healthy humans: A randomized trial. Nutrition 2016, 32, 748–753. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Pasko, P.; Zagrodzki, P.; Gajdzik, E.; Wietecha-Posluszny, R.; Gorinstein, S. Selenium Supplementation of Amaranth Sprouts Influences Betacyanin Content and Improves Anti-Inflammatory Properties via NFκB in Murine RAW 264.7 Macrophages. Biol. Trace Elem. Res. 2016, 169, 320–330. [Google Scholar] [CrossRef]
- Martirosyan, D.M.; Miroshnichenko, L.A.; Kulakova, S.N.; Pogojeva, A.V.; Zoloedov, V.I. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis. 2007, 6, 1. [Google Scholar] [CrossRef][Green Version]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Liu, R.; Hernandez, M.; Draves, J.; Marcone, M.F.; Tsao, R. Assessing the fatty acid, carotenoid, and tocopherol compositions of amaranth and quinoa seeds grown in ontario and their overall contribution to nutritional quality. J. Agric. Food Chem. 2016, 64, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Cammilleri, G.; Calabrese, V.; Pantano, L.; Brunone, M.; Galluzzo, F.G.; Pulvirenti, A.; Fritsch, T.; Bongiorno, C.; Macaluso, A.; Ferrantelli, V. Polyphenols of white lupin (Lupinus albus L.) seeds cultivated in Southern Italy by a LC-HRMS method. Nat. Prod. Res. 2024, 38, 2864–2868. [Google Scholar] [CrossRef]
- Estivi, L.; Grassi, S.; Briceño-Berrú, L.; Glorio-Paulet, P.; Camarena, F.; Hidalgo, A.; Brandolini, A. Free Phenolic Compounds, Antioxidant Capacity and FT-NIR Survey of Debittered Lupinus mutabilis Seeds. Processes 2022, 10, 1637. [Google Scholar] [CrossRef]
- Zhong, L.; Wu, G.; Fang, Z.; Wahlqvist, M.L.; Hodgson, J.M.; Clarke, M.W.; Junaldi, E.; Johnson, S.K. Characterization of polyphenols in Australian sweet lupin (Lupinus angustifolius) seed coat by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2019, 116, 1153–1162. [Google Scholar] [CrossRef]
- Dalaram, I.S. Evaluation of total polyphenol content and antioxidant capacity of different verity lupin seeds. Slovak J. Food Sci./Potravinarstvo 2017, 11, 26–34. [Google Scholar] [CrossRef]
- Buszewski, B.; Rafińska, K.; Cvetanović, A.; Walczak, J.; Krakowska, A.; Rudnicka, J.; Zeković, Z. Phytochemical analysis and biological activity of Lupinus luteus seeds extracts obtained by supercritical fluid extraction. Phytochem. Lett. 2019, 30, 338–348. [Google Scholar] [CrossRef]
- Boinik, V.V.; Akritidu, K.P.; Demeshko, O.V. Phenolic Compounds from Roots of Lupinus polyphyllus. Chem. Nat. Compd. 2015, 51, 352. [Google Scholar] [CrossRef][Green Version]
- Silva, J.O.; Santos, D.N.S.; Cosenza, G.P.; Melo, J.C.S.; Monteiro, M.R.P.; Araújo, R.L.B. Determinação da atividade antioxidante de polifenóis extraíveis, macromoleculares e identificação de lupanina em tremoço branco (Lupinus albus). Sci. Elec. Arch. 2019, 12, 12–19. [Google Scholar] [CrossRef]
- Purohit, P.; Rawat, H.; Verma, N.; Mishra, S.; Nautiyal, A.; Anshul; Bhatt, S.; Bisht, N.; Aggarwal, K.; Bora, A.; et al. Analytical approach to assess anti-nutritional factors of grains and oilseeds: A comprehensive review. J. Agric. Food Res. 2023, 14, 100877. [Google Scholar] [CrossRef]
- Guo, X.; Shang, W.; Strappe, P.; Zhou, Z.; Blanchard, C. Peptides derived from lupin proteins confer potent protection against oxidative stress. J. Sci. Food Agric. 2018, 98, 5225–5234. [Google Scholar] [CrossRef] [PubMed]
- Garmidolova, A.; Desseva, I.; Mihaylova, D.; Lante, A. Bioactive Peptides from Lupinus spp. Seed Proteins-State-of-the-Art and Perspectives. Appl. Sci. 2022, 12, 3766. [Google Scholar] [CrossRef]
- Santos-Sánchez, G.; Cruz-Chamorro, I.; Álvarez-Ríos, A.I.; Álvarez-Sánchez, N.; Rodríguez-Ortiz, B.; Álvarez-López, A.I.; Fernández-Pachón, M.-S.; Pedroche, J.; Millán, F.; Millán-Linares, M.D.C.; et al. Bioactive Peptides from Lupin (Lupinus angustifolius) Prevent the Early Stages of Atherosclerosis in Western Diet-Fed ApoE-/- Mice. J. Agric. Food Chem. 2022, 70, 8243–8253. [Google Scholar] [CrossRef]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Obeme-Nmom, J.I.; Agboinghale, P.E.; Aguchem, R.N.; Nechi, R.N.; Lammi, C. Lupin-Derived Bioactive Peptides: Intestinal Transport, Bioavailability and Health Benefits. Nutrients 2021, 13, 3266. [Google Scholar] [CrossRef]
- Lemus-Conejo, A.; Grao-Cruces, E.; Toscano, R.; Varela, L.M.; Claro, C.; Pedroche, J.; Millan, F.; Millan-Linares, M.C.; Montserrat-de la Paz, S. A lupine (Lupinus angustifolious L.) peptide prevents non-alcoholic fatty liver disease in high-fat-diet-induced obese mice. Food Funct. 2020, 11, 2943–2952. [Google Scholar] [CrossRef]
- Zhou, Y.; Sarker, U.; Neumann, G.; Ludewig, U. The LaCEP1 peptide modulates cluster root morphology in Lupinus albus. Physiol. Plant. 2019, 166, 525–537. [Google Scholar] [CrossRef]
- Keller, J.; Marmit, S.P.; Bunzel, M. Structural Characterization of Dietary Fiber from Different Lupin Species (Lupinus sp.). J. Agric. Food Chem. 2022, 70, 8430–8440. [Google Scholar] [CrossRef]
- Iida, M.; Tsuda, S.; Kikuchi, M.; Samoto, M.; Adachi, N.; Nakamura, A. Extraction of water-soluble polysaccharides from lupin beans and their function of protein dispersion and stabilization under acidic conditions. Int. J. Biol. Macromol. 2024, 278, 134664. [Google Scholar] [CrossRef]
- Malekipoor, R.; Johnson, S.K.; Bhattarai, R.R. Lupin kernel fibre: Nutritional composition, processing methods, physicochemical properties, consumer acceptability and health effects of its enriched products. Nutrients 2022, 14, 2845. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.S.; Gladstones, J.S. Variability in the total and component galactosyl sucrose oligosaccharides of Lupinus species. Aust. J. Agric. Res. 1986, 37, 157. [Google Scholar] [CrossRef]
- Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Antioxidant activities and characterisation of polysaccharides isolated from the seeds of Lupinus angustifolius. Ind. Crops Prod. 2015, 74, 950–956. [Google Scholar] [CrossRef]
- Sosnina, N.A.; Mironov, V.F.; Karaseva, A.N.; Minzanova, S.T.; Karlin, V.V.; Enikeev, K.M.; Konovalov, A.I.; Lapin, A.A.; Kononov, A.S.; Takunov, I.P. Isolation, chemical composition, and structural features of polysaccharides fromLupinus species. Chem. Nat. Compd. 2000, 36, 40–43. [Google Scholar] [CrossRef]
- Bosmediano, S.G.; Villacís-Chiriboga, J.; Quimbita Yupangui, Y.; Drummond e Silva, F.G.; Nájera, J.R. Assessment of the antioxidant and antimicrobial activity of hydrolysates from lupine (Lupinus mutabilis) flour. J. Eng. Exact Sci. 2024, 10, 18729. [Google Scholar] [CrossRef]
- Romeo, F.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and Antimicrobial Activity of Alkaloid Extracts from Seeds of Different Genotypes of Lupinus spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef]
- Ohadoma, S.C.; Nnatuanya, I.; Amazu, L.U.; Okolo, C.E. Antimicrobial activity of the leaf extract and fractions of Lupinus arboreus. J. Med. Plants Res. 2014, 8, 386–391. [Google Scholar] [CrossRef]
- Ayoub, S. Chemical composition and antimicrobial activity of Sudanese Lupinus termis L. root extracts. Pharma Innov. J. 2015, 4, 1–4. [Google Scholar]
- Abdel-Shafi, S.; El-Nemr, M.; Enan, G.; Osman, A.; Sitohy, B.; Sitohy, M. Isolation and Characterization of Antibacterial Conglutinins from Lupine Seeds. Molecules 2022, 28, 35. [Google Scholar] [CrossRef]
- Enan, G.; Abdel-Shafi, S.; El-Nemr, M.; Shehab, W.; Osman, A.; Sitohy, M.; Sitohy, B. Controlling bacterial biofilm formation by native and methylated lupine 11S globulins. Front. Microbiol. 2023, 14, 1259334. [Google Scholar] [CrossRef]
- Use of Antimicrobial Polypeptide from Lupinus alba to Preserve Food. Available online: https://typeset.io/papers/use-of-antimicrobial-polypeptide-from-lupinus-alba-to-3gqdao8705 (accessed on 19 February 2025).
- Muhammed, A.; Asere, T.G.; Diriba, T.F. Photocatalytic and Antimicrobial Properties of ZnO and Mg-Doped ZnO Nanoparticles Synthesized Using Lupinus albus Leaf Extract. ACS Omega 2024, 9, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Kamolvit, W.; Nilsén, V.; Zambrana, S.; Mohanty, S.; Gonzales, E.; Östenson, C.-G.; Brauner, A. Lupinus mutabilis Edible Beans Protect against Bacterial Infection in Uroepithelial Cells. Evid. Based Complement. Alternat. Med. 2018, 2018, 1098015. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Rani, J. Lupinus A multipurpose crop: Potentialities and improvements. Annu. Res. Rev. Biol. 2025, 40, 87–100. [Google Scholar] [CrossRef]
- Estivi, L.; Brandolini, A.; Gasparini, A.; Hidalgo, A. Lupin as a source of bioactive antioxidant compounds for food products. Molecules 2023, 28, 7529. [Google Scholar] [CrossRef] [PubMed]
- Kamran, F.; Phillips, M.; Harman, D.G.; Reddy, N. Antioxidant activities of lupin (Lupinus angustifolius) protein hydrolysates and their potential for nutraceutical and functional foods. Food Chem. Adv. 2023, 2, 100297. [Google Scholar] [CrossRef]
- Chirinos, R.; Villasante-Bravo, N.; Aguilar-Galvez, A.; Figueroa-Merma, A.; Carpentier, S.; Pedreschi, R.; Campos, D. Antioxidant, antihypertensive and antidiabetic potential of peptidic fractions obtained from tarwi (Lupinus mutabilis ) protein hydrolysate and identification of promising multifunctional bioactive peptides. Int. J. Food Sci. Technol. 2022, 57, 7402–7411. [Google Scholar] [CrossRef]
- Intiquilla, A.; Jiménez-Aliaga, K.; Zavaleta, A.I.; Hernández-Ledesma, B. Production of Antioxidant Hydrolyzates from a Lupinus mutabilis (Tarwi) Protein Concentrate with Alcalase: Optimization by Response Surface Methodology. Nat. Prod. Commun. 2018, 13, 751–756. [Google Scholar] [CrossRef]
- Araceli Guzmán-Ortiz, F.; Baruchs Muñoz-Llandes, C.; Martínez-Villaluenga, C. Time maters: Exploring the dynamics of bioactive compounds content, bioaccessibility and antioxidant activity during Lupinus angustifolius germination. Food Res. Int. 2024, 187, 114426. [Google Scholar] [CrossRef]
- Intiquilla, A.; Jiménez-Aliaga, K.; Iris Zavaleta, A.; Gamboa, A.; Caro, N.; Diaz, M.; Gotteland, M.; Abugoch, L.; Tapia, C. Nanoencapsulation of antioxidant peptides from Lupinus mutabilis in chitosan nanoparticles obtained by ionic gelling and spray freeze drying intended for colonic delivery. Food Biosci. 2022, 50, 102055. [Google Scholar] [CrossRef]
- Yildirim, B.A.; Ertekin, A.; Kordali, S.; Yildirim, S. Antidiabetic and antioxidant effects of Lupinus albus L. seed roasting ethanol extract in streptozotocin diabetic rats. World J. Adv. Res. Rev. 2020, 7, 007–016. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.-E.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Villanueva, A.; Pedroche, J.; Millan, F.; Martin, M.E.; Millan-Linares, M.C. Antioxidant and Anti-Inflammatory Properties of Bioavailable Protein Hydrolysates from Lupin-Derived Agri-Waste. Biomolecules 2021, 11, 1458. [Google Scholar] [CrossRef]
- Ohadoma, S.C.; Akah, P.A.; Okolo, C.E. Isolation and Characterization of Flavonol Glycosides from Leaves Extract of Lupinus arboreus Sims. UK J. Pharm. Biosci. 2016, 4, 6. [Google Scholar] [CrossRef]
- Mota, J.; Casimiro, S.; Fernandes, J.; Hartmann, R.M.; Schemitt, E.; Picada, J.; Costa, L.; Marroni, N.; Raymundo, A.; Lima, A.; et al. Lupin Protein Concentrate as a Novel Functional Food Additive That Can Reduce Colitis-Induced Inflammation and Oxidative Stress. Nutrients 2022, 14, 2102. [Google Scholar] [CrossRef] [PubMed]
- Stapel, J.; Oppermann, C.; Richter, D.U.; Ruth, W.; Briese, V. Anti-carcinogenic effects of ethanolic extracts from root and shoot of Lupinus angustifolius on breast carcinoma cell lines MCF-7 and BT20. J. Med. Plants Res. 2015, 9, 561–568. [Google Scholar] [CrossRef]
- Mazumder, K.; Aktar, A.; Ramasamy, S.; Biswas, B.; Kerr, P.G.; Blanchard, C. Attenuating Colorectal Cancer Using Nine Cultivars of Australian Lupin Seeds: Apoptosis Induction Triggered by Mitochondrial Reactive Oxygen Species Generation and Caspases-3/7 Activation. Cells 2023, 12, 2557. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Ren, G.; Wu, C.; Qin, P.; Yao, Y. Peptides from Extruded Lupin (Lupinus albus L.) Regulate Inflammatory Activity via the p38 MAPK Signal Transduction Pathway in RAW 264.7 Cells. J. Agric. Food Chem. 2020, 68, 11702–11709. [Google Scholar] [CrossRef]
- Suvarna, C.M.; Raju, R.S.; Malothu, N.; Guntupalli, C. Investigation of Lupinus angustifolius Extracts against High-Fructose Diet-Induced Metabolic Syndrome in Male Wistar Rats Articles. Pharmacogn. Res. 2024, 16, 391–400. [Google Scholar] [CrossRef]
- Pereira, A.; Ramos, F.; Sanches Silva, A. Lupin (Lupinus albus L.) Seeds: Balancing the Good and the Bad and Addressing Future Challenges. Molecules 2022, 27, 8557. [Google Scholar] [CrossRef]
- Santos-Sánchez, G.; Cruz-Chamorro, I.; Bollati, C.; Bartolomei, M.; Pedroche, J.; Millán, F.; Millán-Linares, M.D.C.; Capriotti, A.L.; Cerrato, A.; Laganà, A.; et al. A Lupinus angustifolius protein hydrolysate exerts hypocholesterolemic effects in Western diet-fed ApoE-/- mice through the modulation of LDLR and PCSK9 pathways. Food Funct. 2022, 13, 4158–4170. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, G.; Cruz-Chamorro, I.; Álvarez-Ríos, A.I.; Fernández-Santos, J.M.; Vázquez-Román, M.V.; Rodríguez-Ortiz, B.; Álvarez-Sánchez, N.; Álvarez-López, A.I.; Millán-Linares, M.D.C.; Millán, F.; et al. Lupinus angustifolius Protein Hydrolysates Reduce Abdominal Adiposity and Ameliorate Metabolic Associated Fatty Liver Disease (MAFLD) in Western Diet Fed-ApoE-/- Mice. Antioxidants 2021, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.I.; Alanazi, F.K. The beneficial roles of Lupineus luteus and lifestyle changes in management of metabolic syndrome: A case study. Saudi Pharm. J. 2015, 23, 712–715. [Google Scholar] [CrossRef]
- Bhieldeen, M.N.; Moustafa, Y.M.; Tawfik, A.A.; Elsayed, S.M.; El-shaarawy, F.F. Effect of Lupinus albus Conglutin Gamma Protein on Experimentally Induced Diabetes in Rats. Open Access Maced. J. Med. Sci. 2023, 11, 281–289. [Google Scholar] [CrossRef]
- Ward, N.C.; Mori, T.A.; Beilin, L.J.; Johnson, S.; Williams, C.; Gan, S.K.; Puddey, I.B.; Woodman, R.; Phillips, M.; Connolly, E.; et al. The effect of regular consumption of lupin-containing foods on glycaemic control and blood pressure in people with type 2 diabetes mellitus. Food Funct. 2020, 11, 741–747. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Álvarez-Sánchez, N.; Millán-Linares, M.D.C.; Yust, M.D.M.; Pedroche, J.; Millán, F.; Lardone, P.J.; Carrera-Sánchez, C.; Guerrero, J.M.; Carrillo-Vico, A. Lupine protein hydrolysates decrease the inflammatory response and improve the oxidative status in human peripheral lymphocytes. Food Res. Int. 2019, 126, 108585. [Google Scholar] [CrossRef]
- Zambrana, S.; Lundqvist, L.C.E.; Mamani, O.; Catrina, S.-B.; Gonzales, E.; Östenson, C.-G. Lupinus mutabilis Extract Exerts an Anti-Diabetic Effect by Improving Insulin Release in Type 2 Diabetic Goto-Kakizaki Rats. Nutrients 2018, 10, 933. [Google Scholar] [CrossRef]
- Bettzieche, A.; Brandsch, C.; Weisse, K.; Hirche, F.; Eder, K.; Stangl, G.I. Lupin protein influences the expression of hepatic genes involved in fatty acid synthesis and triacylglycerol hydrolysis of adult rats. Br. J. Nutr. 2008, 99, 952–962. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Bollati, C.; Li, J.; Bartolomei, M.; Ranaldi, G.; Ferruzza, S.; Fassi, E.M.A.; Grazioso, G.; Sambuy, Y.; et al. Trans-Epithelial Transport, Metabolism, and Biological Activity Assessment of the Multi-Target Lupin Peptide LILPKHSDAD (P5) and Its Metabolite LPKHSDAD (P5-Met). Nutrients 2021, 13, 863. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Lemus-Conejo, A.; Toscano, R.; Pedroche, J.; Millan, F.; Millan-Linares, M.C. GPETAFLR, an octapeptide isolated from Lupinus angustifolius L. protein hydrolysate, promotes the skewing to the M2 phenotype in human primary monocytes. Food Funct. 2019, 10, 3303–3311. [Google Scholar] [CrossRef]
- Andor, B.; Danciu, C.; Alexa, E.; Zupko, I.; Hogea, E.; Cioca, A.; Coricovac, D.; Pinzaru, I.; Pătrașcu, J.M.; Mioc, M.; et al. Germinated and Ungerminated Seeds Extract from Two Lupinus Species: Biological Compounds Characterization and In Vitro and In Vivo Evaluations. Evid. Based Complement. Alternat. Med. 2016, 2016, 7638542. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Feliu, J.; García-Costela, M.; Moreno-SanJuan, S.; Puentes-Pardo, J.D.; Arrabal, S.R.; González-Novoa, P.; Núñez, M.I.; Carazo, Á.; Jimenez-Lopez, J.C.; León, J. Narrow Leafed Lupin (Lupinus angustifolius L.) β-Conglutin Seed Proteins as a New Natural Cytotoxic Agents against Breast Cancer Cells. Nutrients 2023, 15, 523. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Zou, M.; Zeng, H.; Wu, S.; Liang, Y.; Wang, D.; Yang, Y.; Qiu, Z.; Zhou, Q. Quinoa is more effective than other whole grains in the management of impaired glucose tolerance: A randomized controlled trial. Food Funct. 2025, 16, 763–773. [Google Scholar] [CrossRef]
- Zeng, H.; Cai, X.; Qiu, Z.; Liang, Y.; Huang, L. Glucolipid metabolism improvement in impaired glucose tolerance subjects consuming a Quinoa-based diet: A randomized parallel clinical trial. Front. Physiol. 2023, 14, 1179587. [Google Scholar] [CrossRef]
- Li, L.; Lietz, G.; Bal, W.; Watson, A.; Morfey, B.; Seal, C. Effects of Quinoa (Chenopodium quinoa Willd.) Consumption on Markers of CVD Risk. Nutrients 2018, 10, 777. [Google Scholar] [CrossRef]
- Abellán Ruiz, M.S.; Barnuevo Espinosa, M.D.; García Santamaría, C.; Contreras Fernández, C.J.; Aldeguer García, M.; Soto Méndez, F.; Guillén Guillén, I.; Luque Rubia, A.J.; Quinde Ràzuri, F.J.; Martínez Garrido, A.; et al. Effect of quinua (Chenopodium quinoa) consumption as a coadjuvant in nutritional intervention in prediabetic subjects. Nutr. Hosp. 2017, 34, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.G.; Ovídio, P.P.; Padovan, G.J.; Jordão Junior, A.A.; Marchini, J.S.; Navarro, A.M. Metabolic parameters of postmenopausal women after quinoa or corn flakes intake--a prospective and double-blind study. Int. J. Food Sci. Nutr. 2014, 65, 380–385. [Google Scholar] [CrossRef]
- Pourshahidi, L.K.; Caballero, E.; Osses, A.; Hyland, B.W.; Ternan, N.G.; Gill, C.I.R. Modest improvement in CVD risk markers in older adults following quinoa (Chenopodium quinoa Willd.) consumption: A randomized-controlled crossover study with a novel food product. Eur. J. Nutr. 2020, 59, 3313–3323. [Google Scholar] [CrossRef]
- Navarro-Perez, D.; Radcliffe, J.; Tierney, A.; Jois, M. Quinoa Seed Lowers Serum Triglycerides in Overweight and Obese Subjects: A Dose-Response Randomized Controlled Clinical Trial. Curr. Dev. Nutr. 2017, 1, e001321. [Google Scholar] [CrossRef]
- Espada, M.V.; De la Cruz, C.R.; Jeri, C.; Garcia-Tejedor, A.; Laparra, J.M. Chenopodium quinoa’s ingredients contribute to the gut microbiota’s metabolic adaptations on carbohydrate metabolism. Plant Foods Hum. Nutr. 2024, 80, 18. [Google Scholar] [CrossRef]
- Li, L.; Houghton, D.; Lietz, G.; Watson, A.; Stewart, C.J.; Bal, W.; Seal, C.J. Impact of Daily Consumption of Whole-Grain Quinoa-Enriched Bread on Gut Microbiome in Males. Nutrients 2022, 14, 4888. [Google Scholar] [CrossRef]
- Canaviri-Paz, P.; Oscarsson, E.; Kjellström, A.; Olsson, H.; Jois, C.; Håkansson, Å. Effects on Microbiota Composition after Consumption of Quinoa Beverage Fermented by a Novel Xylose-Metabolizing L. plantarum Strain. Nutrients 2021, 13, 3318. [Google Scholar] [CrossRef] [PubMed]
- Jamka, M.; Morawska, A.; Krzyżanowska-Jankowska, P.; Bajerska, J.; Przysławski, J.; Walkowiak, J.; Lisowska, A. Comparison of the Effect of Amaranth Oil vs. Rapeseed Oil on Selected Atherosclerosis Markers in Overweight and Obese Subjects: A Randomized Double-Blind Cross-Over Trial. Int. J. Environ. Res. Public Health 2021, 18, 8540. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, L.A.; Zoloedov, V.I.; Volynkina, A.P.; Kulakova, S.N. Influence with amaranth and sunflower oils used in dietary therapy of patients with diebetes mellitus 2 types on parameters of carbohydrate and lipid metabolism. Vopr Pitan. 2008, 77, 53–57. [Google Scholar]
- Moszak, M.; Zawada, A.; Juchacz, A.; Grzymisławski, M.; Bogdański, P. Comparison of the effect of rapeseed oil or amaranth seed oil supplementation on weight loss, body composition, and changes in the metabolic profile of obese patients following 3-week body mass reduction program: A randomized clinical trial. Lipids Health Dis. 2020, 19, 143. [Google Scholar] [CrossRef]
- Dus-Zuchowska, M.; Walkowiak, J.; Morawska, A.; Krzyzanowska-Jankowska, P.; Miskiewicz-Chotnicka, A.; Przyslawski, J.; Lisowska, A. Amaranth Oil Increases Total and LDL Cholesterol Levels without Influencing Early Markers of Atherosclerosis in an Overweight and Obese Population: A Randomized Double-Blind Cross-Over Study in Comparison with Rapeseed Oil Supplementation. Nutrients 2019, 11, 3069. [Google Scholar] [CrossRef]
- Orsango, A.Z.; Loha, E.; Lindtjørn, B.; Engebretsen, I.M.S. Efficacy of processed amaranth-containing bread compared to maize bread on hemoglobin, anemia and iron deficiency anemia prevalence among two-to-five year-old anemic children in Southern Ethiopia: A cluster randomized controlled trial. PLoS ONE 2020, 15, e0239192. [Google Scholar] [CrossRef]
- Liubertas, T.; Kairaitis, R.; Stasiule, L.; Capkauskiene, S.; Stasiulis, A.; Viskelis, P.; Viškelis, J.; Urbonaviciene, D. The influence of amaranth (Amaranthus hypochondriacus) dietary nitrates on the aerobic capacity of physically active young persons. J. Int. Soc. Sports Nutr. 2020, 17, 37. [Google Scholar] [CrossRef]
- Yelisyeyeva, O.; Semen, K.; Zarkovic, N.; Kaminskyy, D.; Lutsyk, O.; Rybalchenko, V. Activation of aerobic metabolism by Amaranth oil improves heart rate variability both in athletes and patients with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2012, 118, 47–57. [Google Scholar] [CrossRef]
- Antoun, M.D.; Taha, O.M. Studies on Sudanese medicinal plants. II. Evaluation of an extract of Lupinus termis seeds in chronic eczema. J. Nat. Prod. 1981, 44, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Egaña, J.I.; Uauy, R.; Cassorla, X.; Barrera, G.; Yañez, E. Sweet lupin protein quality in young men. J. Nutr. 1992, 122, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Petterson, D.S.; Sandström, B.; Cederblad, A. Absorption of zinc from lupin (Lupinus angustifolius)-based foods. Br. J. Nutr. 1994, 72, 865–871. [Google Scholar] [CrossRef]
- Hall, R.S.; Johnson, S.K.; Baxter, A.L.; Ball, M.J. Lupin kernel fibre-enriched foods beneficially modify serum lipids in men. Eur. J. Clin. Nutr. 2005, 59, 325–333. [Google Scholar] [CrossRef]
- Hall, R.S.; Thomas, S.J.; Johnson, S.K. Australian sweet lupin flour addition reduces the glycaemic index of a white bread breakfast without affecting palatability in healthy human volunteers. Asia Pac. J. Clin. Nutr. 2005, 14, 91–97. [Google Scholar]
- Smith, S.C.; Choy, R.; Johnson, S.K.; Hall, R.S.; Wildeboer-Veloo, A.C.M.; Welling, G.W. Lupin kernel fiber consumption modifies fecal microbiota in healthy men as determined by rRNA gene fluorescent in situ hybridization. Eur. J. Nutr. 2006, 45, 335–341. [Google Scholar] [CrossRef]
- Fiocchi, A.; Sarratud, P.; Terracciano, L.; Vacca, E.; Bernardini, R.; Fuggetta, D.; Ballabio, C.; Duranti, M.; Magni, C.; Restani, P. Assessment of the tolerance to lupine-enriched pasta in peanut-allergic children. Clin. Exp. Allergy 2009, 39, 1045–1051. [Google Scholar] [CrossRef]
- Lee, Y.P.; Mori, T.A.; Puddey, I.B.; Sipsas, S.; Ackland, T.R.; Beilin, L.J.; Hodgson, J.M. Effects of lupin kernel flour-enriched bread on blood pressure: A controlled intervention study. Am. J. Clin. Nutr. 2009, 89, 766–772. [Google Scholar] [CrossRef]
- Weisse, K.; Brandsch, C.; Zernsdorf, B.; Nkengfack Nembongwe, G.S.; Hofmann, K.; Eder, K.; Stangl, G.I. Lupin protein compared to casein lowers the LDL cholesterol:HDL cholesterol-ratio of hypercholesterolemic adults. Eur. J. Nutr. 2010, 49, 65–71. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Lee, Y.P.; Puddey, I.B.; Sipsas, S.; Ackland, T.R.; Beilin, L.J.; Belski, R.; Mori, T.A. Effects of increasing dietary protein and fibre intake with lupin on body weight and composition and blood lipids in overweight men and women. Int. J. Obes. 2010, 34, 1086–1094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, X.; Croft, K.D.; Lee, Y.P.; Mori, T.A.; Puddey, I.B.; Sipsas, S.; Barden, A.; Swinny, E.; Hodgson, J.M. The effects of a lupin-enriched diet on oxidative stress and factors influencing vascular function in overweight subjects. Antioxid. Redox Signal. 2010, 13, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Belski, R.; Mori, T.A.; Puddey, I.B.; Sipsas, S.; Woodman, R.J.; Ackland, T.R.; Beilin, L.J.; Dove, E.R.; Carlyon, N.B.; Jayaseena, V.; et al. Effects of lupin-enriched foods on body composition and cardiovascular disease risk factors: A 12-month randomized controlled weight loss trial. Int. J. Obes. 2011, 35, 810–819. [Google Scholar] [CrossRef]
- Dove, E.R.; Mori, T.A.; Chew, G.T.; Barden, A.E.; Woodman, R.J.; Puddey, I.B.; Sipsas, S.; Hodgson, J.M. Lupin and soya reduce glycaemia acutely in type 2 diabetes. Br. J. Nutr. 2011, 106, 1045–1051. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Triolo, M.; Bosisio, R.; Bondioli, A.; Calabresi, L.; De Vergori, V.; Gomaraschi, M.; Mombelli, G.; Pazzucconi, F.; Zacherl, C.; et al. Hypocholesterolaemic effects of lupin protein and pea protein/fibre combinations in moderately hypercholesterolaemic individuals. Br. J. Nutr. 2012, 107, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Fornasini, M.; Castro, J.; Villacrés, E.; Narváez, L.; Villamar, M.P.; Baldeón, M.E. Hypoglycemic effect of Lupinus mutabilis in healthy volunteers and subjects with dysglycemia. Nutr. Hosp. 2012, 27, 425–433. [Google Scholar]
- Bähr, M.; Fechner, A.; Krämer, J.; Kiehntopf, M.; Jahreis, G. Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: A randomized, controlled crossover study. Nutr. J. 2013, 12, 107. [Google Scholar] [CrossRef]
- Fechner, A.; Kiehntopf, M.; Jahreis, G. The formation of short-chain fatty acids is positively associated with the blood lipid-lowering effect of lupin kernel fiber in moderately hypercholesterolemic adults. J. Nutr. 2014, 144, 599–607. [Google Scholar] [CrossRef]
- Castañeda, E.L.J.; Arias, J. Challenges in Standardizing and Commercializing Products Derived from Ancestral Plants. J. Ethnobiol. Ethnomed. 2022, 18, 34. [Google Scholar]
- Calderón, A.I.G.M.; Muñoz, E. Clinical Trials on Ancestral Plant Extracts: Current Status and Future Directions. Phytother. Res. 2021, 35, 1245–1256. [Google Scholar]
- Montoya, M.S.F.; Ramírez, C. Sustainability in the Cultivation of Ancestral Plants: Strategies and Challenges. Agric. Ecosyst. Environ. 2022, 324, 107673. [Google Scholar]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Jan, N.; Hussain, S.Z.; Naseer, B.; Bhat, T.A. Amaranth and quinoa as potential nutraceuticals: A review of anti-nutritional factors, health benefits and their applications in food, medicinal and cosmetic sectors. Food Chem. X 2023, 18, 100687. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Larenas, F.E.; Linnemann, A.R.; Nout, M.J.R.; Koziol, M.; van Boekel, M.A.J.S. Lupinus mutabilis: Composition, Uses, Toxicology, and Debittering. Crit. Rev. Food Sci. Nutr. 2016, 56, 1454–1487. [Google Scholar] [CrossRef]
- Gabur, I.; Simioniuc, D.P. Pearl lupin (Lupinus mutabilis). In Neglected and Underutilized Crops; Elsevier: Amsterdam, The Netherlands, 2023; pp. 413–436. ISBN 9780323905374. [Google Scholar]


| Health Benefit | Origin | Evidence/Mechanism of Action | Model Used to Determination | References |
|---|---|---|---|---|
| Immunomodulatory | Protein hydrolysate and peptide fraction of C. quinoa cv. INTA Hornilloa | The protein hydrolysate elicited a balanced immunomodulatory response by increasing pro-inflammatory mediators (↑ IFN-γ, ↑ TNF-α) while regulating anti-inflammatory pathways (↑ IL-10, ↓ IL-6). The peptide fraction strongly stimulated phagocytosis, potentially boosting the innate immune response. | In vivo: BALB/c mice; peritoneal and splenic macrophages isolated from BALB/c mice. | [71] |
| Anti-inflammatory and Immunomodulatory | Protein-rich fraction (PRF) and PRF treated with food-grade enzymes from C. quinoa | The fractions reduced inflammation by inhibiting pro-inflammatory cytokines (↓ IL-6, ↓ TNF-α, ↓ IL-12p40, ↓ IL-27p28), chemokines (↓ MCP-1, ↓ MIP-1α), and nitric oxide (↓ NO) in M1 macrophages. They promoted M2 polarization by increasing IL-10 levels and arginase activity, and modulated the Toll-like receptor. | In vitro: Primary bone marrow-derived macrophages (BMDMs) and dendritic cells (BMDCs) from BALB/c mice; murine cell lines J774A.1 and JAWS II. | [58] |
| Hepatoprotective and Obesity prevention | Protein-rich fraction (PRF) and oil fraction from seeds of C. quinoa | The protein-rich fraction promoted monocyte migration to the liver, where they differentiated into monocyte-derived macrophages (MoMFs) that facilitated hepatic triglyceride mobilization. Innate lymphoid cells (ILC2s/ILC3s) were modulated, reducing hepatic triglyceride content and inflammation. | In vivo: Rag2−/− mice (lacking mature T and B lymphocytes, but retaining innate immune cells) and Rag2−/− IL2−/− mice (further deficient in IL-2, affecting ILC survival and function). | [72] |
| Anti-obesity and Gut microbiota modulation | Quinoa peptides from seeds of C. quinoa Willd | Upregulation of hepatic PPAR-α (↑ FABP5, ↑ HMGCS1) to enhance lipid oxidation and downregulating PPAR-γ (↓ ANGPTL4, ↓ PCK1) to suppress adipogenesis. Reduced systemic inflammation (↓ TNF-α, ↓ IL-6, ↓ IL-1β) and restored gut microbiota diversity by normalizing the Firmicutes/Bacteroidetes ratio (↑ Muribaculaceae, ↓ Desulfobacterota). | In vivo: Murine obesity model in male C57BL/6 mice. | [73] |
| Hypolipidemic and Hepatoprotective | Husk of C. quinoa containing triterpenoid saponins | Saponins lowered serum total cholesterol, triglycerides, and low-density lipoprotein cholesterol while mitigating liver injury (↓ ALT, ↓ AST, ↓ total bile acids) and systemic inflammation (↓ TNF-α, ↓ IL-6). They enhanced hepatic fatty acid β-oxidation and bile acid metabolism, and modulated gut microbiota by decreasing the Firmicutes/Bacteroidetes ratio and increasing beneficial taxa. | In vivo: Male Sprague Dawley (SD) rats with induced hypercholesterolemia. | [74] |
| Hepatoprotective and Microbiota Modulation | C. quinoa Willd. whole grain | Hepatoprotective effects by reducing hepatic lipid accumulation (↓ NEFAs, ↓ TG, ↓ TC) and improving liver function (↓ ALT, ↓ ALP, ↑ A/G ratio) to mitigate steatosis. It also enhanced antioxidant defenses (↑ SOD, ↑ GSH-PX, ↓ MDA) and normalized systemic inflammation, while restoring intestinal microbiota homeostasis (↑ Akkermansia, ↑ Blautia; ↓ Clostridium, ↓ Turicibacter). | In vivo: Male Sprague Dawley (SD) rats fed a high-fat diet. | [75] |
| Polycystic ovary syndrome (PCOS) and Gut Microbiota Modulation. | Extract from dried and ground seeds of C. quinoa Willd | Quinoa supplementation improved PCOS by ameliorating hyperandrogenemia and enhancing hormonal profiles (↓ T, ↓ LH, ↓ LH/FSH, ↓ FINS, ↓ HOMA-IR; ↑ E2). It activated the PI3K/AKT/mTOR pathway (↑ PI3K, ↑ AKT, ↑ mTOR) and modulated autophagy markers (↑ Bcl-2, ↑ p62; ↓ Beclin1, ↓ULK1, ↓LC3B), while reinforcing the intestinal barrier. | In vivo: Letrozole-induced PCOS model in Sprague Dawley rats. | [76] |
| Gut microbiota modulation (Prebiotic Effects) | Polysaccharide extract from C. quinoa Willd | Quinoa polysaccharides exhibited prebiotic effects by modulating gut microbiota composition (↑ Bifidobacterium, ↑ Collinsella, ↑ Prevotella, ↑ Bacteroides; ↓ Clostridia) and increasing short-chain fatty acids (↑ butyrate, ↑ propionate), stimulating GLP-1 and PYY secretion. | In vitro: Fermentation model using human fecal microbiota. | [77] |
| Anticancer | Extract obtained from C. quinoa bran | The extract upregulated apoptotic biomarkers (↑ Caspase-3, -8, -9; ↑ Bax), decreased mitochondrial membrane potential, and downregulated the anti-apoptotic protein (↓ Bcl-2), leading to reduced cell proliferation and colony formation. Also reduced tumor volume and weight in DLD-1 xenograft nude mice with minimal systemic toxicity. | In vitro: DLD-1 and HCT-8 colorectal cancer cell lines. In vivo: Xenograft model in BALB/c nude mice. | [78] |
| Terpenoid compounds obtained from C. quinoa bran | The extract down regulated drug resistance proteins (↓ P-gp, ↓ MRP1, ↓ BCRP) and upregulated miR-495-3p, which in turn decreased DNMTs (↓ DNMT1, ↓ DNMT3a, ↓ DNMT3b) expression. This reactivation enhanced chemotherapy efficacy (↑ 5-Fluorouracil (5-Fu) accumulation) and induced apoptosis (↑ Caspase-3, -8, -9). | In vitro: HCT-8 (5-Fu sensitive) and HCT-8/Fu (5-Fu resistant) colon cancer cell lines. In vivo: Xenograft in BALB/c nude mice. | [79] | |
| Skin anti-aging | Leachate and fractions from seeds of C. quinoa | Quinoa leachate and its fractions demonstrated robust collagen protection via the downregulation of MMP-1 mRNA expression and inhibition of MMP-9 enzymatic activity, along with antioxidant effects (ROS scavenging). Additionally, it exhibited anti-pigmentation effects by suppressing tyrosinase activity (↓ melanin synthesis). | In vitro: Human dermal fibroblasts (HDF), murine macrophages (RAW 264.7), murine hepatocytes (H4IIE), and human colon adenocarcinoma cell line (Caco-2). | [80] |
| Health Benefit | Origin | Evidence/Mechanism of Action | Model Used to Determination | References |
|---|---|---|---|---|
| Anti-inflammatory | Extract from the leaves of A. hybridus cv. Kongei and cv. IP-7. | The extract inhibited nuclear factor kappa B (NF-κB) signaling in macrophages, thereby reducing pro-inflammatory cytokine expression (↓ TNF-α, ↓ IL-6). The active compound was identified as 2-caffeoylisocitric acid. | In vitro: Murine macrophage cell line RAW 264.7. | [124] |
| Protein hydrolysates from A. caudatus L. | Protein hydrolysates modulated the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome pathway, reduced intestinal inflammation, and increased anti-inflammatory cytokines. | In vitro: Human colon adenocarcinoma cell line (Caco-2). | [125] | |
| Extract from the leaves of A. lividus and A. tricolor. | The extract downregulated pro-inflammatory cytokines (↓ TNF-α, ↓ IL-1, ↓ IL-6) in neuronal cells, thereby reducing oxidative stress and neuroinflammation induced by advanced glycation end products. | In vitro: Human neuroblastoma cell line (SH-SY5Y). | [126] | |
| Immunomodulatory | Boiled leaves of A. cruentus, A. viridis, and A. hybridus. | Food supplementation with boiled leaves restored the balance between Th1/Th2 and Th17/Treg responses by enhancing lymphocyte activation, increasing IL-4 and IL-10 secretion, and reducing IFN-γ and IL-17 levels. | In vivo: Female Wistar rats immunized with type II collagen. | [127] |
| Antidiabetic | Ethanolic hydroalcoholic extract from seeds of A. caudatus. | The extract improved glycemic control by increasing insulin production in pancreatic islets through the activation of L-type calcium channels, PKA, PKC, and G protein-coupled exocytosis, resulting in better glucose tolerance and reduced HbA1c levels. | In vivo: Type 2 diabetic Goto-Kakizaki rats and healthy Wistar rats. In vitro: Pancreatic islets from both models. | [128] |
| Aqueous extract of fresh leaves of A. tricolor. | The extract lowered blood glucose levels, improved lipid profiles (↓ cholesterol, ↓ LDL, ↑ HDL), and protected pancreatic β-cells against oxidative stress. | In vivo: Albino Wistar rats with alloxan-induced diabetes. | [129] | |
| Hepatoprotective | Ethanolic extract of whole plant of A. spinosus | The extract neutralized trichloromethyl radicals (CCl3+), reduced oxidative damage markers (↓ MDA, ↓ lipid hydroperoxides), and increased antioxidants (↑ GSH, ↑ SOD, ↑ CAT). It also normalized liver enzymes (↓ AST, ↓ ALT, ↓ ALP). | In vivo: Sprague Dawley rats intoxicated with carbon tetrachloride. | [130] |
| Ethanolic extract of leaves A. tricolor | The extract scavenged free radicals, inhibited lipid peroxidation (↓ MDA), increased endogenous antioxidants (↑ NP-SH, ↑ total proteins), normalized liver enzymes (↓ GOT, ↓ GPT), and reduced necrosis and inflammation. It preserved metabolic enzymatic activity, evidenced by decreasing pentobarbital-induced sleep time. | In vivo: Wistar rats intoxicated with carbon tetrachloride. | [131] | |
| Anticancer | Protein hydrolysates from seeds of A. caudatus. | The hydrolysates induced apoptosis, evidenced by DNA fragmentation indicating nuclear condensation and chromatin degradation, caspase-3 activation, loss of membrane integrity, and phosphatidylserine translocation, and inhibited cell migration and viability in a breast adenocarcinoma cell line. | In vitro: Human breast adenocarcinoma cell line (MDA-MB-231). | [132] |
| Methanol and aqueous extract form A. hypochondriacus, A. caudatus, A. cruentus, and A. spinosus. | The extract demonstrated greater anticancer activity by decreasing cell proliferation (↓ cell viability in an MTT assay). Notably, the aqueous extract from A. hypochondriacus showed higher cytotoxicity compared to the other species. | In vitro: Small-cell lung cancer cell line H69V, hepatocarcinoma cell line HepG2/C3A, and non-cancerous Vero cell line. | [133] |
| Health Benefit | Origin | Evidence/Mechanism of Action | Model Used to Determination | References |
|---|---|---|---|---|
| Anti-inflammatory | Protein hydrolysates from seeds of L. angustifolius | Inhibits the activation of the Th1 pathway (↓ IL-2, ↓ IL-12, ↓ IFN-γ, ↓ TNF), reduced the pro-inflammatory mediators of the Th17 (↓ IL-17) and Th9 (↓ IL-9) pathways to limit inflammation, and favored an anti-inflammatory profile through a relative increase in IL-4, IL-13, and IL-10 (↑ IL-4/IL-12, ↑ IL-13/IFN-γ, and ↑ IL-10/IFN-γ ratios). | In vitro: Human peripheral blood mononuclear cells (PBMCs). | [203] |
| Protein hydrolysates derived from Lupin flour. | Lupinus peptides crossed the in vitro intestinal barrier in a co-culture system of Caco-2 cells (intestinal epithelium model) and THP-1 cells (immune response model), resulting in reduced mRNA expression and secretion of pro-inflammatory cytokines (↓ TNF-α, ↓ IL-1β, ↓ IL-6) and decreased oxidative stress markers (↓ ROS, ↓ nitrites). | In vitro: Human colon adenocarcinoma cell line (Caco-2) and THP-1 macrophages. | [190] | |
| Protein concentrate from seeds of L. albus cv Amiga | The lupin protein concentrates reduced gelatinolytic activity (↓ MMP-9) to limit tissue breakdown and downregulated pro-inflammatory mediators (↓ TNF-α, ↓ COX-2) to reduce mucosal damage while enhancing antioxidant defenses (↑ SOD, ↑ GPx) and reducing lipid peroxidation (↓ LPO) to alleviate oxidative stress. | In vitro: Human colon adenocarcinoma cell line (HT29). In vivo: CD-1 mice (TNBS-induced colitis) and Wistar rats (acetic acid-induced colitis). | [192] | |
| Anti-diabetic | Ethanolic extract from seeds of L. mutabilis | Enhanced insulin secretion from pancreatic islets by modulating ion channels and signaling pathways (↑ action potential of L-type calcium channels, ↑ activity of PKA, PKC, and ↑ G protein-dependent exocytosis). Improvement in glycemic control as decreasing fasting glucose, reducing HbA1c. | In vivo: Goto-Kakizaki (GK) type 2 diabetic rats and healthy Wistar rats. In vitro: Pancreatic islets isolated from both models. | [204] |
| Protein extracts from seeds of L. albus | The extracts reduced hepatic lipogenesis by downregulating SREBP-1c (↓ FAS, ↓ SCD1, ↓ GPAT) to suppress hepatic lipogenesis and upregulated enzymes involved in triglyceride hydrolysis (↑ LPL, ↑ HL, ↑ apoA5), thereby enhancing LDL clearance without significantly affecting PPAR-α–dependent genes (ACO, CPT-1a, CYP4A1) or insulin and glucagon concentrations. | In vivo: Sprague Dawley (SD) rats with induced hypercholesterolemia. | [205] | |
| Peptides P5 (LILPKHSDAD) and its derivative P5-met (LPKHSDAD) from lupin protein. | P5 and P5-met displayed hypocholesterolemic effects by reducing factors that promote LDLR degradation (↓ PCSK9, ↓ HNF-1α), increasing SREBP-2 and LDLR expression, and lowering the activity of HMGCoAR (a key enzyme in cholesterol synthesis), thereby enhancing LDL clearance (↑ LDL uptake). | In vitro: Human colon adenocarcinoma cell line (Caco-2) and human hepatocarcinoma cell line (HepG2). | [206] | |
| Peptide (GPETAFLR) from seeds of L. angustifolius L. | The GPETAFLR exhibited anti-inflammatory effects by reducing the production of pro-inflammatory mediators (↓ TNF-α, ↓ IL-1β, ↓ IL-6), as well as CCR2/CCL2 expression and the proportion of classical monocytes (CD14++CD16−), while increasing IL-10, favoring the presence of non-classical monocytes (CD14+CD16++). | In vitro: Primary human monocytes isolated from healthy donors. | [207] | |
| Anticancer and anti-inflammatory | Ethanolic extracts (germinated and non-germinated) from seeds of L. albus and L. angustifolius. | Germinated seed extracts demonstrated greater anticancer and anti-inflammatory potential because of their increased content of genistein and cinnamic acid derivatives (↑ genistein, ↑ ferulic, ↑ rosmarinic, and ↑ caffeic) compared to non-germinated seeds. | In vitro: Breast cancer cell lines (MCF7, MDA-MB-231), ovarian cancer cell line (A2780), cervical cancer cell line (SiHa). In vivo: Ear inflammation model in female SKH-1 mice. | [208] |
| Anticancer | Isoforms of β-conglutin from seeds of L. angustifolius L. | The β-conglutins isoforms exhibited anticancer activity by decreasing cell proliferation, reducing oxidative stress (↓ ROS) and genotoxic damage (↓ γH2Ax), and inducing caspase-independent apoptosis (↓ caspase 3 activation). They regulated the SIRT1/FoxO1 pathway differently depending on the cell subtype: in MDA-MB-231 (p53 gain-of-function mutant), they promoted autophagy (↑ LC3B, ↓ p62), whereas in MCF-7 and SK-BR-3, they blocked this route and inactivate tumor stem cell traits. | In vitro Breast cancer cell lines MCF-7 (wild-type p53), SK-BR-3 (HER2+, nonfunctional p53), MDA-MB-231 (triple-negative, mutated p53), and non-cancerous mammary epithelial cell line (MCF-10A). | [209] |
| Antibacterial Activity | Extract from seeds of L. mutabilis | The extract exhibited anti-infective effects by modulating the host response, decreasing the expression of uroplakin1a (a key component of the uroepithelial cell surface that serves as a receptor for bacterial adhesion). Simultaneously, it increased the expression of the antimicrobial peptide RNase 7, a crucial component of the innate immune response in the urinary tract. Additionally, it inhibited biofilm formation. | In vitro: Human uroepithelial cell lines T24 and 5637 under normoglycemic and hyperglycemic conditions. | [180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba-Ostria, C.; Guamán-Bautista, J.; Tosi-Vélez, A.A.; Puente-Pineda, J.A.; Cedeño-Zambrano, M.A.; Teran, E.; Guamán, L.P. Recent Advances in the Therapeutic Potential of Bioactive Molecules from Plants of Andean Origin. Nutrients 2025, 17, 1749. https://doi.org/10.3390/nu17111749
Barba-Ostria C, Guamán-Bautista J, Tosi-Vélez AA, Puente-Pineda JA, Cedeño-Zambrano MA, Teran E, Guamán LP. Recent Advances in the Therapeutic Potential of Bioactive Molecules from Plants of Andean Origin. Nutrients. 2025; 17(11):1749. https://doi.org/10.3390/nu17111749
Chicago/Turabian StyleBarba-Ostria, Carlos, Jéssica Guamán-Bautista, Augusto A. Tosi-Vélez, Juan A. Puente-Pineda, Melanie A. Cedeño-Zambrano, Enrique Teran, and Linda P. Guamán. 2025. "Recent Advances in the Therapeutic Potential of Bioactive Molecules from Plants of Andean Origin" Nutrients 17, no. 11: 1749. https://doi.org/10.3390/nu17111749
APA StyleBarba-Ostria, C., Guamán-Bautista, J., Tosi-Vélez, A. A., Puente-Pineda, J. A., Cedeño-Zambrano, M. A., Teran, E., & Guamán, L. P. (2025). Recent Advances in the Therapeutic Potential of Bioactive Molecules from Plants of Andean Origin. Nutrients, 17(11), 1749. https://doi.org/10.3390/nu17111749











