Associations of Multimarkers of Metabolic Malnutrition and Inflammation with All-Cause Mortality by Multimorbidity Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Assessment of Exposures, Additional Risk Markers and Multimorbidity Status
2.3. Outcome Ascertainment
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Associations of MVX, IVX, and MMX with All-Cause Mortality Risk by Multimorbidity Status
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MVX | Metabolic Vulnerability Index |
| IVX | Inflammation Vulnerability Index |
| MMX | Metabolic Malnutrition Index |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| PREVEND | Prevention of Renal and Vascular End-stage Disease |
| NMR | Nuclear Magnetic Resonance |
| S-HDL-P | Small High-Density Lipoprotein Particles |
| CVD | Cardiovascular Disease |
| CKD | Chronic Kidney Disease |
| CRD | Chronic Respiratory Disease |
| T2D | Type 2 Diabetes |
| BMI | Body Mass Index |
| FPG | Fasting Plasma Glucose |
| EDTA | Ethylenediaminetetraacetic Acid |
| eGFR | Estimated Glomerular Filtration Rate |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| IDMS | Isotope Dilution Mass Spectrometry |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| RHR | Ratio of Hazard Ratios |
| CRP | C-Reactive Protein |
References
- Vetrano, D.L.; Calderon-Larranaga, A.; Marengoni, A.; Onder, G.; Bauer, J.M.; Cesari, M.; Ferrucci, L.; Fratiglioni, L. An International Perspective on Chronic Multimorbidity: Approaching the Elephant in the Room. J. Gerontol. Ser. A 2018, 73, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Mulder, I.; Nissinen, A.; Giampaoli, S.; Feskens, E.J.; Kromhout, D. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: The FINE study (Finland, Italy, Netherlands, Elderly). J. Clin. Epidemiol. 2001, 54, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Vogeli, C.; Shields, A.E.; Lee, T.A.; Gibson, T.B.; Marder, W.D.; Weiss, K.B.; Blumenthal, D. Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. J. Gen. Intern. Med. 2007, 22 (Suppl. 3), 391–395. [Google Scholar] [CrossRef] [PubMed]
- Palladino, R.; Tayu Lee, J.; Ashworth, M.; Triassi, M.; Millett, C. Associations between multimorbidity, healthcare utilisation and health status: Evidence from 16 European countries. Age Ageing 2016, 45, 431–435. [Google Scholar] [CrossRef]
- Feng, X.; Sarma, H.; Seubsman, S.A.; Sleigh, A.; Kelly, M. The Impact of Multimorbidity on All-Cause Mortality: A Longitudinal Study of 87,151 Thai Adults. Int. J. Public. Health 2023, 68, 1606137. [Google Scholar] [CrossRef]
- Willadsen, T.G.; Siersma, V.; Nicolaisdottir, D.R.; Koster-Rasmussen, R.; Jarbol, D.E.; Reventlow, S.; Mercer, S.W.; Olivarius, N.F. Multimorbidity and mortality: A 15-year longitudinal registry-based nationwide Danish population study. J. Comorb. 2018, 8, 2235042X18804063. [Google Scholar] [CrossRef]
- Otvos, J.D.; Shalaurova, I.; May, H.T.; Muhlestein, J.B.; Wilkins, J.T.; McGarrah, R.W., 3rd; Kraus, W.E. Multimarkers of metabolic malnutrition and inflammation and their association with mortality risk in cardiac catheterisation patients: A prospective, longitudinal, observational, cohort study. Lancet Healthy Longev. 2023, 4, e72–e82. [Google Scholar] [CrossRef]
- Conners, K.M.; Shearer, J.J.; Joo, J.; Park, H.; Manemann, S.M.; Remaley, A.T.; Otvos, J.D.; Connelly, M.A.; Sampson, M.; Bielinski, S.J.; et al. The Metabolic Vulnerability Index: A Novel Marker for Mortality Prediction in Heart Failure. JACC Heart Fail. 2024, 12, 290–300. [Google Scholar] [CrossRef]
- Yuxiu, Y.; Ma, X.; Gao, F.; Liu, T.; Deng, J.; Wang, Z. Combined effect of inflammation and malnutrition for long-term prognosis in patients with acute coronary syndrome undergoing percutaneous coronary intervention: A cohort study. BMC Cardiovasc. Disord. 2024, 24, 306. [Google Scholar] [CrossRef]
- Ruan, G.T.; Ge, Y.Z.; Xie, H.L.; Hu, C.L.; Zhang, Q.; Zhang, X.; Tang, M.; Song, M.M.; Zhang, X.W.; Liu, T.; et al. Association Between Systemic Inflammation and Malnutrition with Survival in Patients with Cancer Sarcopenia-A Prospective Multicenter Study. Front. Nutr. 2021, 8, 811288. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; Brantsma, A.H.; de Zeeuw, D.; Bakker, S.J.; de Jong, P.E.; Gansevoort, R.T.; Group, P.S. Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am. J. Epidemiol. 2008, 168, 897–905. [Google Scholar] [CrossRef]
- Sokooti, S.; Flores-Guerrero, J.L.; Kieneker, L.M.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. HDL Particle Subspecies and Their Association with Incident Type 2 Diabetes: The PREVEND Study. J. Clin. Endocrinol. Metab. 2021, 106, 1761–1772. [Google Scholar] [CrossRef]
- Wicks, T.R.; Shalaurova, I.; Wolska, A.; Browne, R.W.; Weinstock-Guttman, B.; Zivadinov, R.; Remaley, A.T.; Otvos, J.D.; Ramanathan, M. Endogenous Ketone Bodies Are Associated with Metabolic Vulnerability and Disability in Multiple Sclerosis. Nutrients 2025, 17, 640. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Diseases, G.B.D.; Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Ho, I.S.; Azcoaga-Lorenzo, A.; Akbari, A.; Black, C.; Davies, J.; Hodgins, P.; Khunti, K.; Kadam, U.; Lyons, R.A.; McCowan, C.; et al. Examining variation in the measurement of multimorbidity in research: A systematic review of 566 studies. Lancet Public Health 2021, 6, e587–e597. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Groenwold, R.H.; Klungel, O.H.; Grobbee, D.E.; Hoes, A.W. Selection of confounding variables should not be based on observed associations with exposure. Eur. J. Epidemiol. 2011, 26, 589–593. [Google Scholar] [CrossRef]
- Wicks, T.R.; Shalaurova, I.; Browne, R.W.; Wolska, A.; Weinstock-Guttman, B.; Zivadinov, R.; Remaley, A.T.; Otvos, J.D.; Ramanathan, M. Nuclear-Magnetic-Resonance-Spectroscopy-Derived Serum Biomarkers of Metabolic Vulnerability Are Associated with Disability and Neurodegeneration in Multiple Sclerosis. Nutrients 2024, 16, 2866. [Google Scholar] [CrossRef]
- Razzaq, R.; Nguyen, M.; Connelly, M.A.; Baral, A.; Khan, H.; Garg, S.; Ang, A.; Kim, A.; Roache, G.; Patidar, K.R.; et al. Liver Transplantation and Metabolic Dysfunction Associated Steatotic Liver Disease Is Associated with Markers of Metabolic Risk and Inflammation. Dig. Dis. Sci. 2025. ahead of print. [Google Scholar] [CrossRef]
- Harris, T.B.; Ferrucci, L.; Tracy, R.P.; Corti, M.C.; Wacholder, S.; Ettinger, W.H., Jr.; Heimovitz, H.; Cohen, H.J.; Wallace, R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999, 106, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Gruppen, E.G.; Kunutsor, S.K.; Kieneker, L.M.; van der Vegt, B.; Connelly, M.A.; de Bock, G.H.; Gansevoort, R.T.; Bakker, S.J.L.; Dullaart, R.P.F. GlycA, a novel pro-inflammatory glycoprotein biomarker is associated with mortality: Results from the PREVEND study and meta-analysis. J. Intern. Med. 2019, 286, 596–609. [Google Scholar] [CrossRef]
- Knappe-Drzikova, B.; Maasberg, S.; Vonderbeck, D.; Krafft, T.A.; Knuppel, S.; Sturm, A.; Muller-Nordhorn, J.; Wiedenmann, B.; Pape, U.F. Malnutrition predicts long-term survival in hospitalized patients with gastroenterological and hepatological diseases. Clin. Nutr. ESPEN 2019, 30, 26–34. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, Y.; Duan, C.; Gao, C.; Wang, Y.; Ni, H.; Zhou, L.; Xiang, Y.; Li, M.; Xu, Z. The nutritional metabolic risk index as a predictor of all-cause and cardiovascular mortality: A national cohort study. Clin. Nutr. ESPEN 2024, 63, 391–399. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; Horwich, T.; Fonarow, G.C. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J. Am. Coll. Cardiol. 2004, 43, 1439–1444. [Google Scholar] [CrossRef]
- Toma, L.; Stancu, C.S.; Sima, A.V. Endothelial Dysfunction in Diabetes Is Aggravated by Glycated Lipoproteins; Novel Molecular Therapies. Biomedicines 2020, 9, 18. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Organ System Crosstalk in Cardiometabolic Disease in the Age of Multimorbidity. Front. Cardiovasc. Med. 2020, 7, 64. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Frailty, sarcopenia, cachexia and malnutrition as comorbid conditions and their associations with mortality: A prospective study from UK Biobank. J. Public Health 2022, 44, e172–e180. [Google Scholar] [CrossRef]
| Baseline Disease Status | |||||
|---|---|---|---|---|---|
| Variable | Overall (N = 6054) Mean (SD) or Median (IQR) | No Disease (N = 3265) Mean (SD) or Median (IQR) | One Disease (N = 1695) Mean (SD) or Median (IQR) | Multimorbidity (N = 1094) Mean (SD) or Median (IQR) | p-Value |
| MVX | 44.9 (9.0) | 43.7 (8.9) | 45.5 (8.7) | 48.0 (9.0) | <0.001 |
| IVX | 39.3 (10.5) | 37.5 (10.2) | 39.9 (10.3) | 43.5 (10.5) | <0.001 |
| MMX | 54.3 (6.4) | 54.2 (6.4) | 54.4 (6.2) | 54.4 (6.6) | 0.47 |
| GlycA (µmol/L) | 373 (337, 416) | 358 (325, 399) | 383 (348, 422) | 402 (365, 443) | <0.001 |
| Small HDL particles (µmol/L) | 15.90 (2.43) | 15.93 (2.40) | 16.01 (2.45) | 15.65 (2.47) | <0.001 |
| Leucine (µmol/L) | 104.18 (27.02) | 100.94 (25.14) | 105.31 (27.80) | 112.09 (29.36) | <0.001 |
| Valine (µmol/L) | 201.29 (38.13) | 194.44 (35.69) | 205.03 (39.04) | 215.95 (38.84) | <0.001 |
| Isoleucine (µmol/L) | 58.28 (14.56) | 55.66 (13.31) | 59.85 (15.09) | 63.66 (15.47) | <0.001 |
| Citrate (µmol/L) | 2.09 (0.48) | 2.02 (0.45) | 2.12 (0.46) | 2.24 (0.52) | <0.001 |
| Questionnaire | |||||
| Age (years) | 54 (12) | 48 (10) | 57 (11) | 64 (10) | <0.001 |
| Males, n (%) | 2998 (49.5) | 1464 (44.8) | 871 (51.4) | 663 (60.6) | <0.001 |
| Alcohol consumers, n (%) | 4528 (74.8) | 2565 (78.6) | 1215 (71.7) | 748 (68.4) | <0.001 |
| Smokers, n (%) | <0.001 | ||||
| Never smokers | 1744 (28.8) | 1062 (32.5) | 461 (27.2) | 221 (20.2) | |
| Former smokers | 2618 (43.2) | 1241 (38.0) | 783 (46.2) | 594 (54.3) | |
| Light current smokers | 649 (10.7) | 370 (11.3) | 166 (9.8) | 113 (10.3) | |
| Heavy current smokers | 1043 (17.2) | 592 (18.1) | 285 (16.8) | 166 (15.2) | |
| Chronic diseases | |||||
| History of T2D, n (%) | 367 (6.1) | 0 (0.0) | 77 (4.5) | 290 (26.5) | <0.001 |
| History of CVD, n (%) | 180 (3.0) | 0 (0.0) | 19 (1.1) | 161 (14.7) | <0.001 |
| History of CKD, n (%) | 971 (16.0) | 0 (0.0) | 267 (15.8) | 704 (64.4) | <0.001 |
| History of hypertension, n (%) | 2037 (33.6) | 0 (0.0) | 1038 (61.2) | 999 (91.3) | <0.001 |
| History of CRD, n (%) | 553 (9.1) | 0 (0.0) | 261 (15.4) | 292 (26.7) | <0.001 |
| History of cancer, n (%) | 111 (1.8) | 0 (0.0) | 33 (1.9) | 78 (7.1) | <0.001 |
| Physical measurements | |||||
| BMI (kg/m2) | 26.7 (4.4) | 25.5 (3.7) | 27.5 (4.4) | 29.0 (4.9) | <0.001 |
| SBP (mmHg) | 126 (19) | 117 (11) | 133 (19) | 142 (21) | <0.001 |
| DBP (mmHg) | 73 (9) | 70 (7) | 76 (9) | 78 (10) | <0.001 |
| Lipid and renal markers | |||||
| Total cholesterol (mmol/L) | 5.43 (1.05) | 5.35 (1.02) | 5.58 (1.06) | 5.43 (1.10) | <0.001 |
| HDL-C (mmol/L) | 1.26 (0.31) | 1.30 (0.31) | 1.24 (0.30) | 1.17 (0.31) | <0.001 |
| Triglycerides (mmol/L) | 1.12 (0.81, 1.61) | 1.00 (0.74, 1.41) | 1.22 (0.90, 1.76) | 1.39 (1.02, 1.91) | <0.001 |
| Creatinine (mg/dl) | 0.83 (0.24) | 0.80 (0.13) | 0.81 (0.16) | 0.93 (0.45) | <0.001 |
| Cystatin C (mg/L) | 0.91 (0.21) | 0.85 (0.13) | 0.91 (0.16) | 1.08 (0.36) | <0.001 |
| Estimated GFR (mL/min/1.73 m2) | 84.0 (10.6) | 87.4 (5.6) | 83.5 (9.1) | 74.5 (16.7) | <0.001 |
| No Disease | One Disease | Multimorbidity | p-Value * | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events/Total | Model 1 HR (95% CI) | p-Value | Model 2 HR (95% CI) | p-Value | Events/Total | Model 1 HR (95% CI) | p-Value | Model 2 HR (95% CI) | p-Value | Events/Total | Model 1 HR (95% CI) | p-Value | Model 2 HR (95% CI) | p-Value | ||
| MVX | 186/3265 | 1.40 (1.20–1.63) | <0.001 | 1.32 (1.13–1.54) | <0.001 | 267/1695 | 1.28 (1.12–1.45) | <0.001 | 1.23 (1.08–1.40) | 0.002 | 458/1094 | 1.35 (1.22–1.49) | <0.001 | 1.29 (1.16–1.43) | <0.001 | 0.74 |
| IVX | 186/3265 | 1.29 (1.08–1.39) | 0.001 | 1.22 (1.05–1.42) | 0.010 | 267/1695 | 1.23 (1.08–1.39) | 0.002 | 1.17 (1.03–1.34) | 0.015 | 458/1094 | 1.31 (1.19–1.45) | <0.001 | 1.25 (1.13–1.38) | <0.001 | 0.74 |
| MMX | 186/3265 | 1.28 (1.12–1.48) | <0.001 | 1.29 (1.11–1.48) | 0.001 | 267/1695 | 1.15 (1.01–1.30) | 0.032 | 1.16 (1.02–1.31) | 0.024 | 458/1094 | 1.15 (1.05–1.26) | 0.004 | 1.14 (1.03–1.25) | 0.009 | 0.33 |
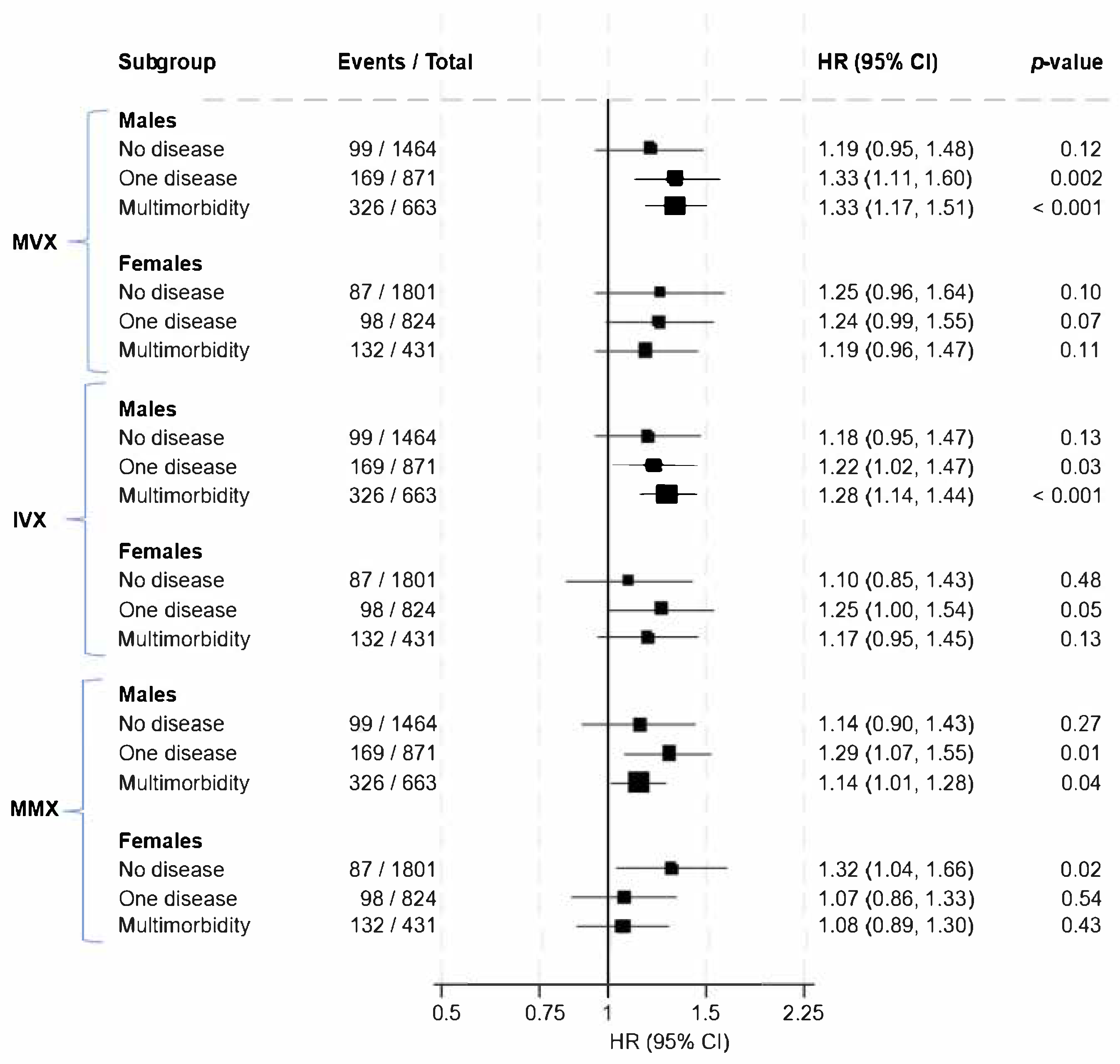
| No Disease | One Disease | Multimorbidity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males Events/Total | Females Events/Total | RHR (Males/Females) (95% CI) | Males Events/Total | Females Events/Total | RHR (Males/Females) (95% CI) | Males Events/Total | Females Events/Total | RHR (Males/Females) (95% CI) | |
| MVX | 99/1464 | 87/1801 | 0.95 (0.67–1.34) | 169/871 | 98/824 | 1.08 (0.81–1.44) | 326/663 | 132/431 | 1.12 (0.87–1.43) |
| IVX | 99/1464 | 87/1801 | 1.08 (0.76–1.51) | 169/871 | 98/824 | 0.98 (0.74–1.31) | 326/663 | 132/431 | 1.09 (0.86–1.39) |
| MMX | 99/1464 | 87/1801 | 0.86 (0.62–1.20) | 169/871 | 98/824 | 1.20 (0.91–1.60) | 326/663 | 132/431 | 1.05 (0.84–1.32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunutsor, S.K.; Rikhtehgaran, R.; Connelly, M.A.; Shalaurova, I.; Bakker, S.J.L.; Dullaart, R.P.F. Associations of Multimarkers of Metabolic Malnutrition and Inflammation with All-Cause Mortality by Multimorbidity Status. Nutrients 2025, 17, 1747. https://doi.org/10.3390/nu17111747
Kunutsor SK, Rikhtehgaran R, Connelly MA, Shalaurova I, Bakker SJL, Dullaart RPF. Associations of Multimarkers of Metabolic Malnutrition and Inflammation with All-Cause Mortality by Multimorbidity Status. Nutrients. 2025; 17(11):1747. https://doi.org/10.3390/nu17111747
Chicago/Turabian StyleKunutsor, Setor K., Reyhaneh Rikhtehgaran, Margery A. Connelly, Irina Shalaurova, Stephan J. L. Bakker, and Robin P. F. Dullaart. 2025. "Associations of Multimarkers of Metabolic Malnutrition and Inflammation with All-Cause Mortality by Multimorbidity Status" Nutrients 17, no. 11: 1747. https://doi.org/10.3390/nu17111747
APA StyleKunutsor, S. K., Rikhtehgaran, R., Connelly, M. A., Shalaurova, I., Bakker, S. J. L., & Dullaart, R. P. F. (2025). Associations of Multimarkers of Metabolic Malnutrition and Inflammation with All-Cause Mortality by Multimorbidity Status. Nutrients, 17(11), 1747. https://doi.org/10.3390/nu17111747










