Sex-Specific Associations of Gut Microbiota Composition with Sarcopenia Defined by the Asian Working Group for Sarcopenia 2019 Consensus in Older Outpatients: Prospective Cross-Sectional Study in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definition of SA and MS Indicators
2.3. Fecal DNA Preparation and Microbiota Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
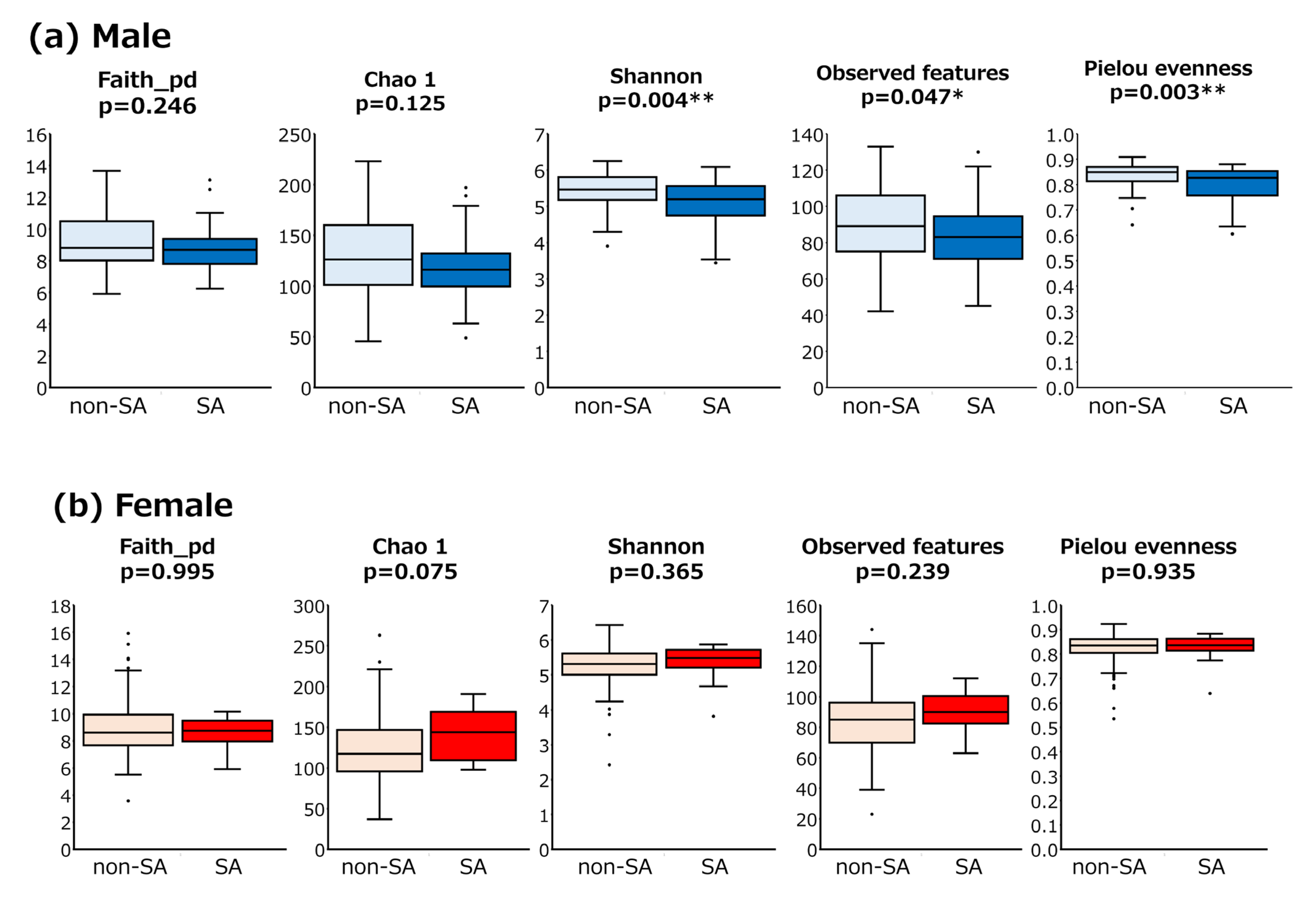
3.2. GM Analysis Between SA and Non-SA Individuals
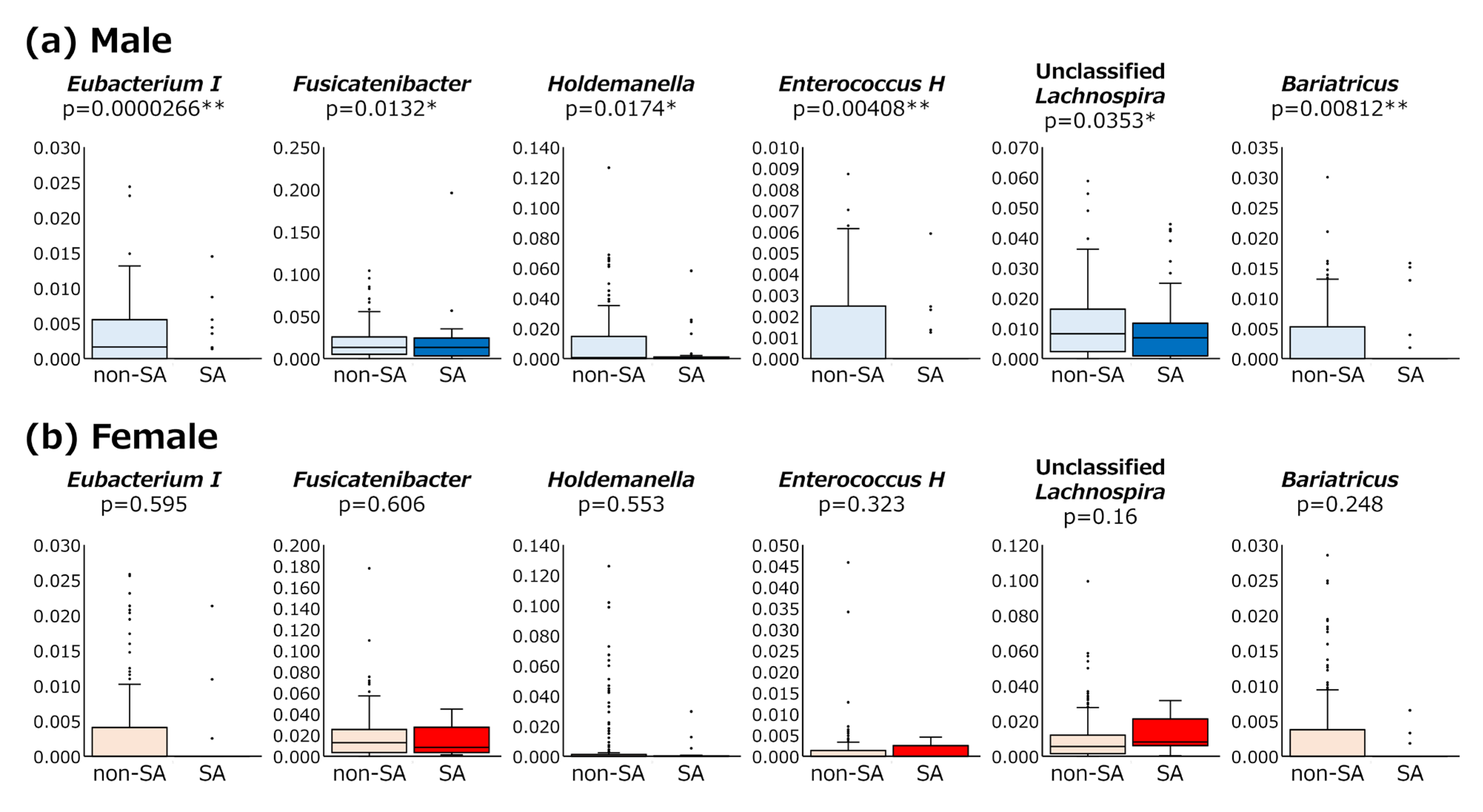
3.3. Abundance of Bacterial Genera Between SA and Non-SA Patients
3.4. Detection Rates of Bacterial Genera in SA and Non-SA Patients
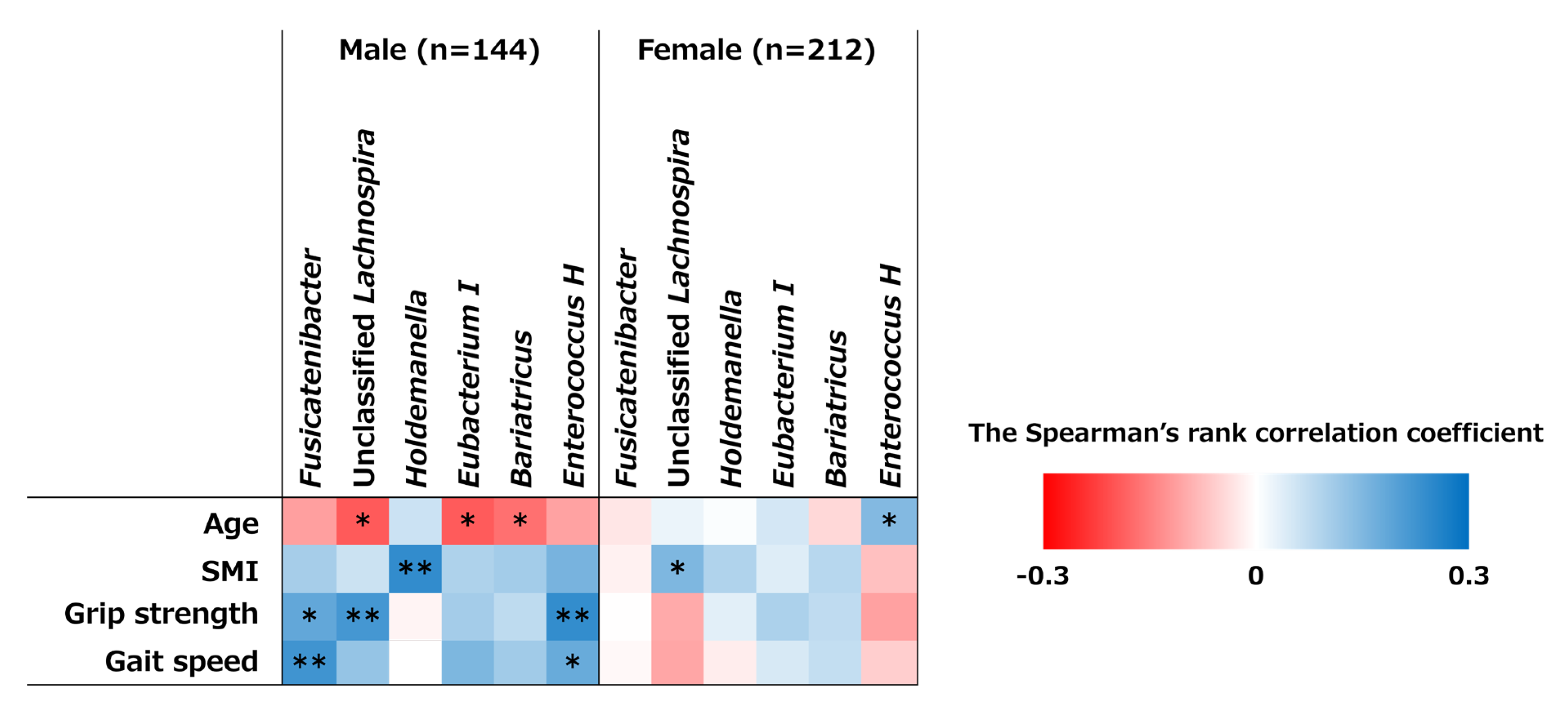
3.5. Correlations Between Bacterial Genera and MM and MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GM | gut microbiota |
| SCFA | short-chain fatty acid |
| AWGS | Asian Working Group for Sarcopenia |
| MM | muscle mass |
| MS | muscle strength |
| SMI | skeletal muscle mass index |
| SA | sarcopenia |
References
- Wang, D.X.M.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Cho, M.-R.; Lee, S.; Song, S.-K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J Korean Med Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sheng, R.W.; Cao, M.M.; Rui, Y.F. Exploring the Relationship Between Gut Microbiota and Sarcopenia Based on Gut-Muscle Axis. Food Sci. Nutr. 2024, 12, 8779–8792. [Google Scholar] [CrossRef]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef]
- Prokopidis, K.; Cervo, M.M.; Gandham, A.; Scott, D. Impact of Protein Intake in Older Adults with Sarcopenia and Obesity: A Gut Microbiota Perspective. Nutrients 2020, 12, 2285. [Google Scholar] [CrossRef]
- Daily, J.W.; Park, S. Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle. Cells 2022, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Chambers, E.; Ni Lochlainn, M.; Witard, O.C. Mechanisms Linking the Gut-Muscle Axis With Muscle Protein Metabolism and Anabolic Resistance: Implications for Older Adults at Risk of Sarcopenia. Front. Physiol. 2021, 12, 770455. [Google Scholar] [CrossRef]
- Ticinesi, A.; Mancabelli, L.; Tagliaferri, S.; Nouvenne, A.; Milani, C.; Del Rio, D.; Lauretani, F.; Maggio, M.G.; Ventura, M.; Meschi, T. The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing. Int. J. Mol. Sci. 2020, 21, 8946. [Google Scholar] [CrossRef]
- Barry, D.J.; Wu, S.S.X.; Cooke, M.B. The Relationship Between Gut Microbiota, Muscle Mass and Physical Function in Older Individuals: A Systematic Review. Nutrients 2024, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Papacosta, O.; Wannamethee, S.G. Sarcopenic obesity and risk of cardiovascular disease and mortality: A population-based cohort study of older men. J. Am. Geriatr. Soc. 2014, 62, 253–260. [Google Scholar] [CrossRef]
- Anbalagan, V.P.; Venkataraman, V.; Pradeepa, R.; Deepa, M.; Anjana, R.M.; Mohan, V. The prevalence of presarcopenia in Asian Indian individuals with and without type 2 diabetes. Diabetes Technol. Ther. 2013, 15, 768–775. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C. Molecular mechanisms and therapeutic strategies of gut microbiota modulation in Sarcopenia (Review). Oncol. Lett. 2024, 29, 104. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, G.; Jiang, S.; Ji, B.; Xie, W.; Li, H.; Sun, J.; Li, Y. Causal Relationship Between Gut Microbiota, Metabolites, and Sarcopenia: A Mendelian Randomization Study. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, 173. [Google Scholar] [CrossRef] [PubMed]
- Moriki, D.; Koumpagioti, D.; Francino, M.P.; Rufián-Henares, J.Á.; Kalogiannis, M.; Priftis, K.N.; Douros, K. How Different Are the Influences of Mediterranean and Japanese Diets on the Gut Microbiome? Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 1733–1745. [Google Scholar] [CrossRef]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.W.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef]
- Kato, K.; Ishida, S.; Tanaka, M.; Mitsuyama, E.; Xiao, J.Z.; Odamaki, T. Association between functional lactase variants and a high abundance of Bifidobacterium in the gut of healthy Japanese people. PLoS ONE 2018, 13, e0206189. [Google Scholar] [CrossRef]
- Hiraku, A.; Nakata, S.; Murata, M.; Xu, C.; Mutoh, N.; Arai, S.; Odamaki, T.; Iwabuchi, N.; Tanaka, M.; Tsuno, T.; et al. Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 1402–1420. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, E.J.; Kim, H.L.; Lee, Y.T.; Yoon, K.J.; Kim, H.N. Sex-specific associations between gut microbiota and skeletal muscle mass in a population-based study. J. Cachexia Sarcopenia Muscle 2022, 13, 2908–2919. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Sung, J.; Chang, Y.; Ryu, S.; Yoon, K.J.; Kim, H.L.; Kim, H.N. Metagenomic Analysis Identifies Sex-Related Gut Microbial Functions and Bacterial Taxa Associated With Skeletal Muscle Mass. J. Cachexia Sarcopenia Muscle 2024, 16, e13636. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Hird, S.M.; Chen, M.-H.; Xu, W.; Maas, K.; Cong, X. Sex differences in gut microbial development of preterm infant twins in early life: A longitudinal analysis. Front. Cell. Infect. Microbiol. 2021, 11, 671074. [Google Scholar] [CrossRef]
- d’Afflitto, M.; Upadhyaya, A.; Green, A.; Peiris, M. Association Between Sex Hormone Levels and Gut Microbiota Composition and Diversity-A Systematic Review. J. Clin. Gastroenterol. 2022, 56, 384–392. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human Gut Microbiota and Drug Metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Sandri, M.; Tessari, P. Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients 2023, 15, 4073. [Google Scholar] [CrossRef]
- Mai, X.; Yang, S.; Chen, Q.; Chen, K. Gut microbial composition is altered in sarcopenia: A systematic review and meta-analysis of clinical studies. PLoS ONE 2024, 19, e0308360. [Google Scholar] [CrossRef]
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci. Rep. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Lou, J.; Wang, Q.; Wan, X.; Cheng, J. Changes and correlation analysis of intestinal microflora composition, inflammatory index, and skeletal muscle mass in elderly patients with sarcopenia. Geriatr. Gerontol. Int. 2023, 24, 140–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Guo, Q.; Wang, W.; Zhang, L. High-throughput sequencing analysis of the characteristics of the gut microbiota in aged patients with sarcopenia. Exp. Gerontol. 2023, 182, 112287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, J.; Lin, Q.; Shi, L.; Zeng, Z.; Guan, L.; Ma, Y.; Zeng, Y.; Zhong, S.; Xu, L. Characteristics of the gut microbiome and metabolic profile in elderly patients with sarcopenia. Front. Pharmacol. 2023, 14, 1279448. [Google Scholar] [CrossRef]
- Nikkhah, A.; Ejtahed, H.S.; Ettehad Marvasti, F.; Taghavi, M.; Pakmehr, A.; Hajipour, F.; Larijani, B. The critical role of gut microbiota dysbiosis in skeletal muscle wasting: A systematic review. J. Appl. Microbiol. 2022, 134, lxac014. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Yang, Y.; Kanda, A.; Mawatari, A.; Tamada, Y.; Mikami, T.; Nakaji, S.; Ihara, K. Association between Gut Microbiota and Muscle Strength in Japanese General Population of the Iwaki Health Promotion Project. Microorganisms 2024, 12, 622. [Google Scholar] [CrossRef]
- Sugimura, Y.; Kanda, A.; Sawada, K.; Wai, K.M.; Tanabu, A.; Ozato, N.; Midorikawa, T.; Hisada, T.; Nakaji, S.; Ihara, K. Association between Gut Microbiota and Body Composition in Japanese General Population: A Focus on Gut Microbiota and Skeletal Muscle. Int. J. Environ. Res. Public Health 2022, 19, 7464. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Miyai, N.; Zhang, Y.; Sakamoto, Y.; Terada, K.; Utsumi, M.; Takeshita, T.; Arita, M. The gut microbiota genus Blautia is associated with skeletal muscle mass reduction in community-dwelling older Japanese adults: The Wakayama Study. Eur. Geriatr. Med. 2024, 16, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cheng, J.K.; Hu, Y.M. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res. Rev. 2022, 81, 101739. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Akazawa, N.; Nakamura, M.; Eda, N.; Murakami, H.; Nakagata, T.; Nanri, H.; Park, J.; Hosomi, K.; Mizuguchi, K.; Kunisawa, J.; et al. Gut microbiota alternation with training periodization and physical fitness in Japanese elite athletes. Front. Sports Act. Living 2023, 5, 1219345. [Google Scholar] [CrossRef]
- Takada, T.; Kurakawa, T.; Tsuji, H.; Nomoto, K. Fusicatenibacter saccharivorans gen. nov., sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2013, 63, 3691–3696. [Google Scholar] [CrossRef]
- Mascolo, N.; Rajendran, V.M.; Binder, H.J. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology 1991, 101, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rué, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wong, P.Y.; Wang, Q.; Wong, H.Y.; Huang, T.; Cui, C.; Zhang, N.; Cheung, W.H.; Wong, R.M.Y. Short-chain fatty acids enhance muscle mass and function through the activation of mTOR signalling pathways in sarcopenic mice. J. Cachexia Sarcopenia Muscle 2024, 15, 2387–2401. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.H.; Woldemariam, S.; Gisinger, C.; Dorner, T.E. Association of Gut Microbiome with Muscle Mass, Muscle Strength, and Muscle Performance in Older Adults: A Systematic Review. Int. J. Environ. Res. Public Health 2024, 21, 1246. [Google Scholar] [CrossRef]
- Simmering, R.; Pforte, H.; Jacobasch, G.; Blaut, M. The growth of the flavonoid-degrading intestinal bacterium, Eubacterium ramulus, is stimulated by dietary flavonoids in vivo. FEMS Microbiol. Ecol. 2002, 40, 243–248. [Google Scholar] [CrossRef]
- Sost, M.M.; Ahles, S.; Verhoeven, J.; Verbruggen, S.; Stevens, Y.; Venema, K. A Citrus Fruit Extract High in Polyphenols Beneficially Modulates the Gut Microbiota of Healthy Human Volunteers in a Validated In Vitro Model of the Colon. Nutrients 2021, 13, 3915. [Google Scholar] [CrossRef]
- Thomsen, M.; Tuukkanen, A.; Dickerhoff, J.; Palm, G.J.; Kratzat, H.; Svergun, D.I.; Weisz, K.; Bornscheuer, U.T.; Hinrichs, W. Structure and catalytic mechanism of the evolutionarily unique bacterial chalcone isomerase. Acta Crystallogr. Sect. D Struct. Biol. 2015, 71, 907–917. [Google Scholar] [CrossRef]
- Braune, A.; Gütschow, M.; Blaut, M. An NADH-Dependent Reductase from Eubacterium ramulus Catalyzes the Stereospecific Heteroring Cleavage of Flavanones and Flavanonols. Appl. Environ. Microbiol. 2019, 85, e01233–19. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Plagmann, L.S.; Yang, L.; Zielke, R.; Gombart, A.F.; Maier, C.S.; Sikora, A.E.; Blakemore, P.R.; Stevens, J.F. Reductive Metabolism of Xanthohumol and 8-Prenylnaringenin by the Intestinal Bacterium Eubacterium ramulus. Mol. Nutr. Food Res. 2019, 63, e1800923. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Tan, R.; Liu, Y. Effect of flavonoids on skeletal muscle mass, strength and physical performance in middle-aged and older adults with or without Sarcopenia: A meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 1013449. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, J.K. Flavonoids: Nutraceutical potential for counteracting muscle atrophy. Food Sci. Biotechnol. 2020, 29, 1619–1640. [Google Scholar] [CrossRef] [PubMed]
- Nazri, N.S.M.; Vanoh, D.; Soo, K.L. Natural Food for Sarcopenia: A Narrative Review. Malays. J. Med. Sci. 2022, 29, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Cailleaux, P.E.; Déchelotte, P.; Coëffier, M. Novel dietary strategies to manage sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Inoshita, H.; Asaoka, D.; Matsuno, K.; Yanagisawa, N.; Suzuki, Y.; Miyauchi, K. Cross-Sectional Study on the Association between Dietary Patterns and Sarcopenia in Elderly Patients with Chronic Kidney Disease Receiving Conservative Treatment. Nutrients 2023, 15, 4994. [Google Scholar] [CrossRef]
- Asaoka, D.; Sugano, K.; Matsuno, K.; Shibata, N.; Sugiyama, H.; Endo, N.; Iwase, Y.; Tajima, M.; Sakuma, N.; Inoue, M.; et al. Association between dietary variety status and sarcopenia as defined by the Asian Working Group for Sarcopenia 2019 consensus in older outpatients at a hospital specializing in geriatric medicine: A cross sectional study with baseline data of prospective cohort study (JUSTICE TOKYO study). Biomed. Rep. 2024, 21, 123. [Google Scholar]
- Guan, L.; Cao, Z.; Pan, Z.; Zhao, C.; Xue, M.; Yang, F.; Chen, J. Butyrate promotes C2C12 myoblast proliferation by activating ERK/MAPK pathway. Mol. Omics 2023, 19, 552–559. [Google Scholar] [CrossRef]
- Lee, M.C.; Tu, Y.T.; Lee, C.C.; Tsai, S.C.; Hsu, H.Y.; Tsai, T.Y.; Liu, T.H.; Young, S.L.; Lin, J.S.; Huang, C.C. Lactobacillus plantarum TWK10 Improves Muscle Mass and Functional Performance in Frail Older Adults: A Randomized, Double-Blind Clinical Trial. Microorganisms 2021, 9, 1466. [Google Scholar] [CrossRef]
- Baek, J.S.; Shin, Y.J.; Ma, X.; Park, H.S.; Hwang, Y.H.; Kim, D.H. Bifidobacterium bifidum and Lactobacillus paracasei alleviate sarcopenia and cognitive impairment in aged mice by regulating gut microbiota-mediated AKT, NF-κB, and FOXO3a signaling pathways. Immun. Ageing 2023, 20, 56. [Google Scholar] [CrossRef]
- Tzeng, P.L.; Lin, C.Y.; Lai, T.F.; Huang, W.C.; Pien, E.; Hsueh, M.C.; Lin, K.P.; Park, J.H.; Liao, Y. Daily lifestyle behaviors and risks of sarcopenia among older adults. Arch. Public Health 2020, 78, 113. [Google Scholar] [CrossRef]


| All Subjects Without Sarcopenia (n = 306) | All Subjects with Sarcopenia (n = 50) | p-Values | Male Subjects Without Sarcopenia (n = 109) | Male Subjects with Sarcopenia (n = 35) | p-Values | Female Subjects Without Sarcopenia (n = 197) | Female Subjects with Sarcopenia (n = 15) | p-Values | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 76.9 ± 6.1 | 79.5 ± 6.9 | 0.011 * | 76.1 ± 5.8 | 79.4 ± 6.7 | 0.004 ** | 77.0 ± 6.2 | 78.9 ± 6.4 | 0.530 |
| Hight (m) | 1.56 ± 0.10 | 1.58 ± 0.09 | 0.242 | 1.67 ± 0.06 | 1.62 ± 0.07 | 0.002 ** | 1.50 ± 0.06 | 1.51 ± 0.04 | 0.794 |
| Body weight (kg) | 57.0 ± 11.9 | 52.8 ± 9.3 | 0.057 | 67.3 ± 9.9 | 56.8 ± 7.4 | 0.000 ** | 51.2 ± 8.5 | 42.9 ± 4.8 | 0.003 ** |
| BMI | 23.3 ± 3.7 | 21.2 ± 2.7 | 0.000 ** | 24.3 ± 3.4 | 21.7 ± 2.6 | 0.001 ** | 22.8 ± 3.8 | 19.0 ± 2.4 | 0.009 ** |
| SMI (kg/m2) | 6.37 ± 0.97 | 5.80 ± 0.81 | 0.000 ** | 6.99 ± 0.90 | 6.15 ± 0.63 | 0.000 ** | 6.07 ± 0.84 | 5.31 ± 0.65 | 0.000 ** |
| Grip strength (kg) | 26.5 ± 9.0 | 24.9 ± 8.3 | 0.383 | 34.8 ± 8.2 | 27.9 ± 7.1 | 0.000 ** | 22.3 ± 5.7 | 19.0 ± 6.7 | 0.000 ** |
| Walking speed (m/s) | 1.28 ± 0.41 | 0.96 ± 0.31 | 0.000 ** | 1.41 ± 0.44 | 1.00 ± 0.28 | 0.000 ** | 1.23 ± 0.38 | 0.94 ± 0.25 | 0.005 ** |
| Detection Ratio | Male Non-Sarcopenia | Male Sarcopenia | Fisher’s Exact Test | Female Non-Sarcopenia | Female Sarcopenia | Fisher’s Exact Test |
|---|---|---|---|---|---|---|
| p__Firmicutes_A|c__Clostridia_258483|o__Lachnospirales|f__Lachnospiraceae|g__Eubacterium_I | 61% | 23% | 0.0002 ** | 37% | 27% | 0.581 |
| p__Firmicutes_A|c__Clostridia_258483|o__Lachnospirales|f__Lachnospiraceae|g__Fusicatenibacter | 100% | 97% | 0.243 | 97% | 100% | 1.000 |
| p__Firmicutes_D|c__Bacilli|o__Erysipelotrichales|f__Erysipelotrichaceae|g__Holdemanella | 71% | 46% | 0.009 ** | 46% | 33% | 0.427 |
| p__Firmicutes_D|c__Bacilli|o__Lactobacillales|f__Enterococcaceae|g__Enterococcus_H_360604 | 42% | 14% | 0.002 ** | 28% | 40% | 0.376 |
| p__Firmicutes_A|c__Clostridia_258483|o__Lachnospirales|f__Lachnospiraceae|__ | 98% | 91% | 0.093 | 91% | 100% | 0.622 |
| p__Firmicutes_A|c__Clostridia_258483|o__Lachnospirales|f__Lachnospiraceae|g__Bariatricus | 49% | 23% | 0.010 * | 32% | 20% | 0.401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asaoka, D.; Toda, K.; Yoshimoto, S.; Katsumata, N.; Odamaki, T.; Iwabuchi, N.; Tanaka, M.; Xiao, J.-Z.; Nishikawa, Y.; Nomura, O.; et al. Sex-Specific Associations of Gut Microbiota Composition with Sarcopenia Defined by the Asian Working Group for Sarcopenia 2019 Consensus in Older Outpatients: Prospective Cross-Sectional Study in Japan. Nutrients 2025, 17, 1746. https://doi.org/10.3390/nu17101746
Asaoka D, Toda K, Yoshimoto S, Katsumata N, Odamaki T, Iwabuchi N, Tanaka M, Xiao J-Z, Nishikawa Y, Nomura O, et al. Sex-Specific Associations of Gut Microbiota Composition with Sarcopenia Defined by the Asian Working Group for Sarcopenia 2019 Consensus in Older Outpatients: Prospective Cross-Sectional Study in Japan. Nutrients. 2025; 17(10):1746. https://doi.org/10.3390/nu17101746
Chicago/Turabian StyleAsaoka, Daisuke, Kazuya Toda, Shin Yoshimoto, Noriko Katsumata, Toshitaka Odamaki, Noriyuki Iwabuchi, Miyuki Tanaka, Jin-Zhong Xiao, Yuriko Nishikawa, Osamu Nomura, and et al. 2025. "Sex-Specific Associations of Gut Microbiota Composition with Sarcopenia Defined by the Asian Working Group for Sarcopenia 2019 Consensus in Older Outpatients: Prospective Cross-Sectional Study in Japan" Nutrients 17, no. 10: 1746. https://doi.org/10.3390/nu17101746
APA StyleAsaoka, D., Toda, K., Yoshimoto, S., Katsumata, N., Odamaki, T., Iwabuchi, N., Tanaka, M., Xiao, J.-Z., Nishikawa, Y., Nomura, O., Takeda, T., Nagahara, A., Koido, S., Ohkusa, T., & Sato, N. (2025). Sex-Specific Associations of Gut Microbiota Composition with Sarcopenia Defined by the Asian Working Group for Sarcopenia 2019 Consensus in Older Outpatients: Prospective Cross-Sectional Study in Japan. Nutrients, 17(10), 1746. https://doi.org/10.3390/nu17101746





