Obesity Is Associated with Fatty Liver and Fat Changes in the Kidneys in Humans as Assessed by MRI
Abstract
1. Introduction
2. Methods
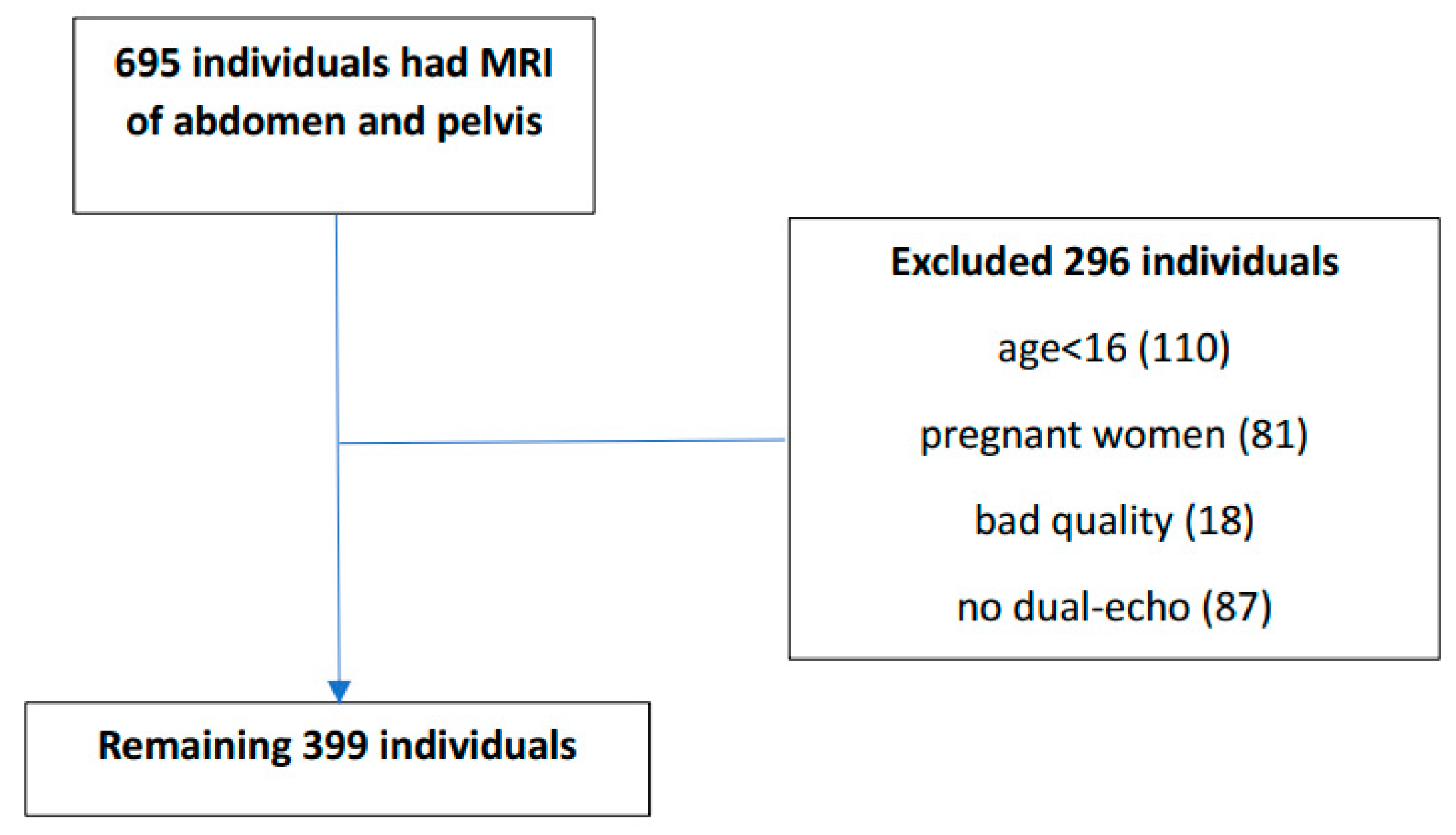
2.1. Study Population
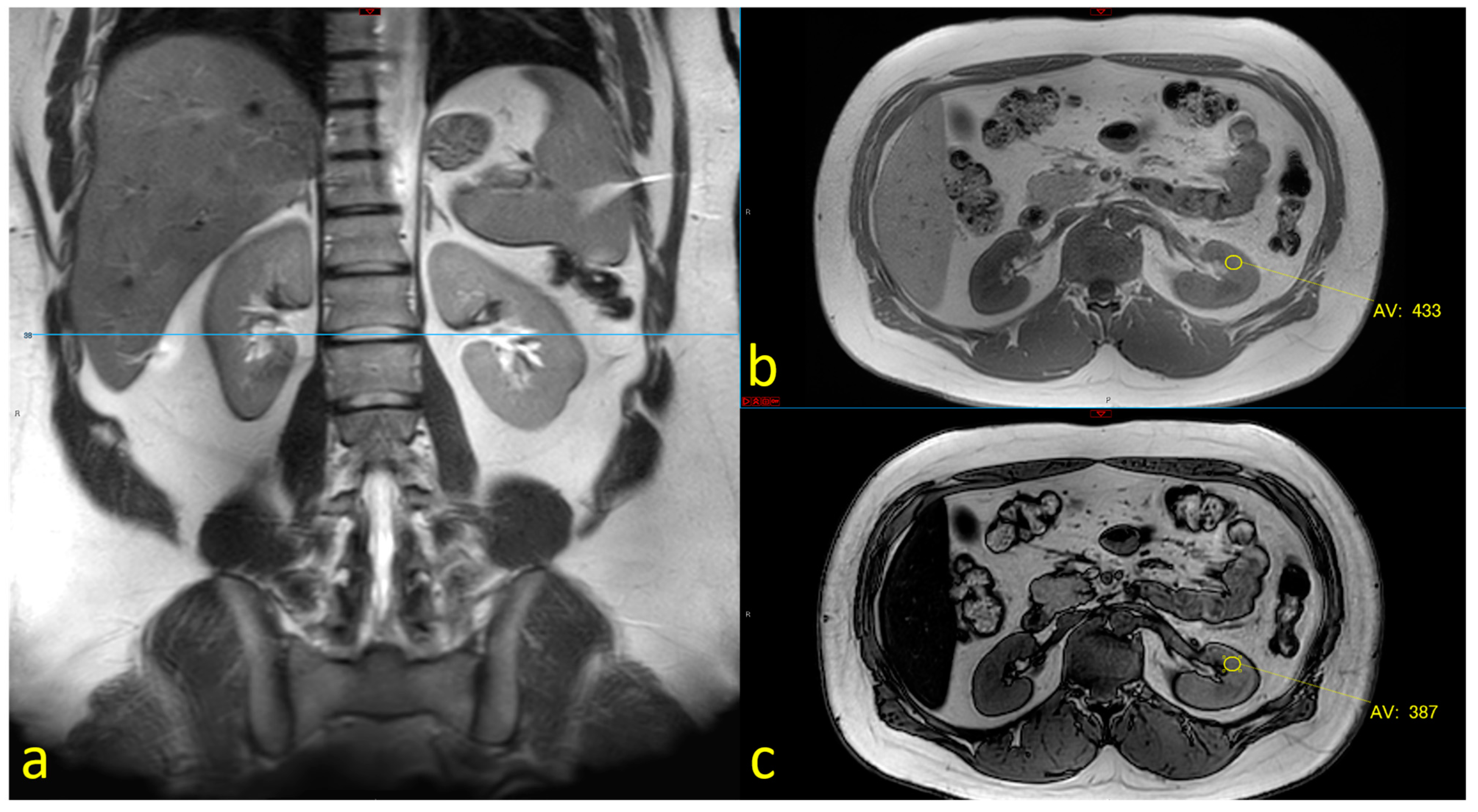
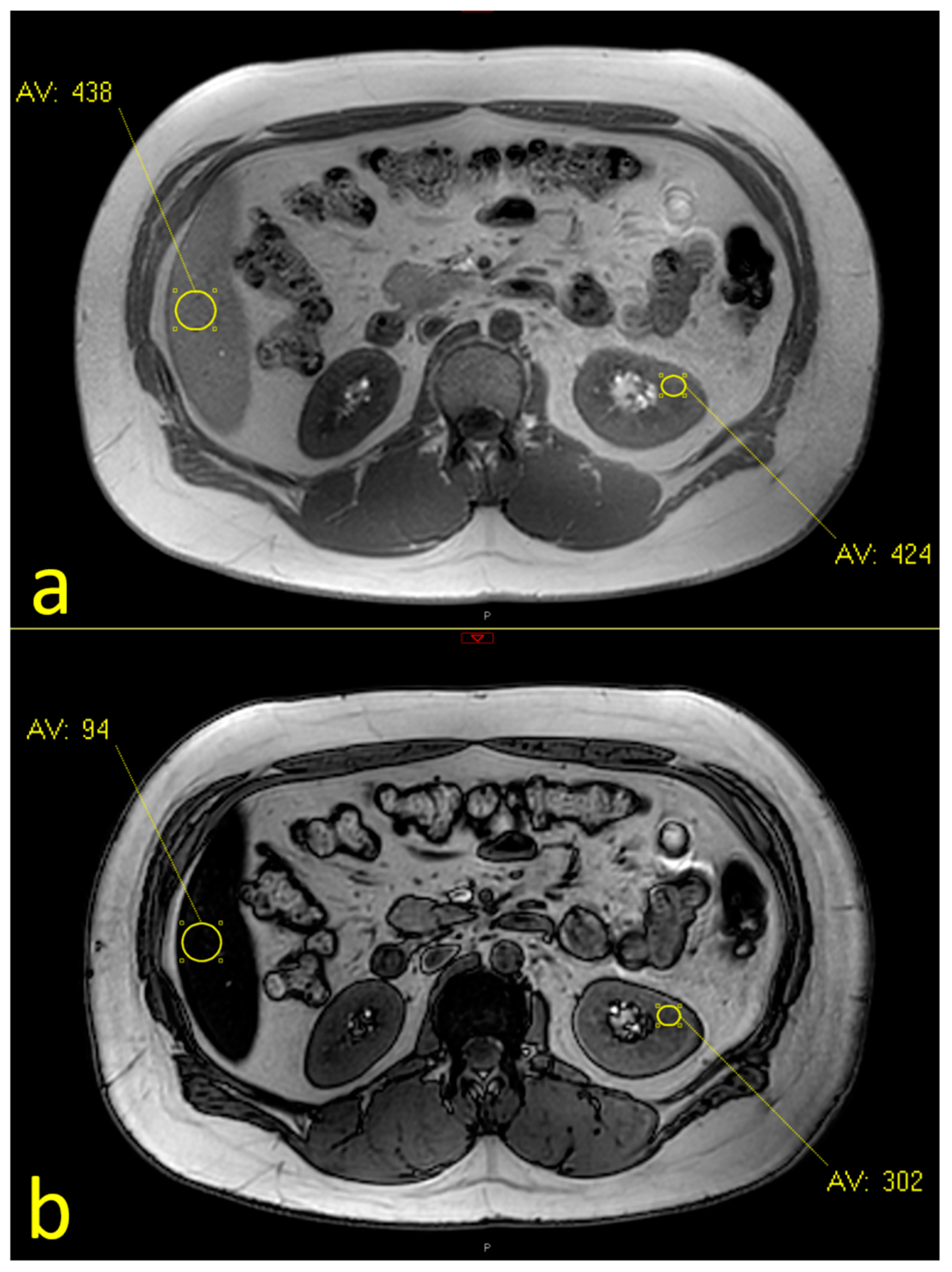
2.2. MRI Assessment
2.3. Statistical Analysis
3. Results
3.1. Subjects’ Characteristics
3.2. The rates of Fatty Liver and Fatty Kidney Disease
3.3. The Association between Body Mass Index and Fatty Liver and Fatty Kidneys
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collaborators GBDRF. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, A.; Bier, A.; Gilboa, M.; Peleg, E.; Barshack, I.; Grossman, E. Saccharin Increases Fasting Blood Glucose but Not Liver Insulin Resistance in Comparison to a High Fructose-Fed Rat Model. Nutrients 2018, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Khasbab, R.; Haberman, Y.; Braun, T.; Hadar, R.; Sosnovski, K.; Amir, A.; Leibowitz, A.; Grossman, E. Antibiotic Treatment Does Not Ameliorate the Metabolic Changes in Rats Presenting Dysbiosis After Consuming a High Fructose Diet. Nutrients 2020, 12, 203. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nakagawa, T.; Taniguchi, H.; Fujii, K.; Omatsu, T.; Nakajima, T.; Sarui, H.; Shimazaki, M.; et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2005, 143, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Bier, A.; Shapira, E.; Khasbab, R.; Sharabi, Y.; Grossman, E.; Leibowitz, A. High-Fructose Diet Increases Renal ChREBPbeta Expression, Leading to Intrarenal Fat Accumulation in a Rat Model with Metabolic Syndrome. Biology 2022, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, N.; Okabayashi, Y.; Shimizu, A.; Yokoo, T. The Renal Pathology of Obesity. Kidney Int. Rep. 2017, 2, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Dzyubak, B.; Li, J.; Chen, J.; Mara, K.C.; Therneau, T.M.; Venkatesh, S.K.; Ehman, R.L.; Allen, A.M.; Yin, M. Automated Analysis of Multiparametric Magnetic Resonance Imaging/Magnetic Resonance Elastography Exams for Prediction of Nonalcoholic Steatohepatitis. J. Magn. Reson. Imaging 2021, 54, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Starekova, J.; Hernando, D.; Pickhardt, P.J.; Reeder, S.B. Quantification of Liver Fat Content with CT and MRI: State of the Art. Radiology 2021, 301, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Waters, O.R.; Knuiman, M.W.; Elliott, R.R.; Olynyk, J.K. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: An eleven-year follow-up study. Am. J. Gastroenterol. 2009, 104, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Krievina, G.; Tretjakovs, P.; Skuja, I.; Silina, V.; Keisa, L.; Krievina, D.; Bahs, G. Ectopic Adipose Tissue Storage in the Left and the Right Renal Sinus is Asymmetric and Associated With Serum Kidney Injury Molecule-1 and Fibroblast Growth Factor-21 Levels Increase. EBioMedicine 2016, 13, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.C.; Hwang, S.J.; Porter, S.A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. Fatty kidney, hypertension, and chronic kidney disease: The Framingham Heart Study. Hypertension 2011, 58, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Rickards, E. Remarks on the Fatty Transformation of the Kidney. Br. Med. J. 1883, 2, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, H.L.; Morgan, T.M.; Rocco, M.; Stacey, B.; Brinkley, T.E.; Ding, J.; Nicklas, B.; Hamilton, C.; Hundley, W.G. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension 2010, 56, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Mende, C.; Einhorn, D. Fatty kidney disease: The importance of ectopic fat deposition and the potential value of imaging. J. Diabetes 2022, 14, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Mende, C.W.; Einhorn, D. Fatty Kidney Disease: A New Renal and Endocrine Clinical Entity? Describing the Role of the Kidney in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Endocr. Pract. 2019, 25, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Gjela, M.; Askeland, A.; Frokjaer, J.B.; Mellergaard, M.; Handberg, A. MRI-based quantification of renal fat in obese individuals using different image analysis approaches. Abdom. Radiol. 2022, 47, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dekkers, I.A.; Tao, Q.; Paiman, E.H.M.; Bizino, M.B.; Jazet, I.M.; Lamb, H.J. MR Assessed Changes of Renal Sinus Fat in Response to Glucose Regulation in West European and South Asian Patients With Type 2 Diabetes. J. Magn. Reson. Imaging 2024, 37, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Moritz, E.; Dadson, P.; Saukko, E.; Honka, M.J.; Koskensalo, K.; Seppala, K.; Pekkarinen, L.; Moriconi, D.; Helmio, M.; Salminen, P.; et al. Renal Sinus Fat Is Expanded in Patients with Obesity and/or Hypertension and Reduced by Bariatric Surgery Associated with Hypertension Remission. Metabolites 2022, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.B.; Vehbi, S.; Copur, S.; Gurses, B.; Siriopol, D.; Karakaya, B.A.D.; Hasbal, N.B.; Tekin, B.; Akyildiz, M.; van Raalte, D.H.; et al. Kidney and liver fat accumulation: From imaging to clinical consequences. J. Nephrol. 2024, 37, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Zelicha, H.; Schwarzfuchs, D.; Shelef, I.; Gepner, Y.; Tsaban, G.; Tene, L.; Yaskolka Meir, A.; Bilitzky, A.; Komy, O.; Cohen, N.; et al. Changes of renal sinus fat and renal parenchymal fat during an 18-month randomized weight loss trial. Clin. Nutr. 2018, 37, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Bassols, J.; Martinez-Calcerrada, J.M.; Prats-Puig, A.; Carreras-Badosa, G.; Xargay-Torrent, S.; Lizarraga-Mollinedo, E.; Feliu-Alsina, M.; Riera-Perez, E.; Osiniri, I.; de Zegher, F.; et al. Perirenal fat is related to carotid intima-media thickness in children. Int. J. Obes. 2018, 42, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.; Campobasso, N.; Nardecchia, A.; Triggiani, V.; Zupo, R.; Gesualdo, L.; Silvestris, F.; De Pergola, G. Relationship of para- and perirenal fat and epicardial fat with metabolic parameters in overweight and obese subjects. Eat Weight Disord. 2019, 24, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Bertoli, S.; Bedogni, G.; Vignati, L.; Pellizzari, M.; Battezzati, A. Association between Mediterranean Diet and Fatty Liver in Women with Overweight and Obesity. Nutrients 2022, 14, 3771. [Google Scholar] [CrossRef] [PubMed]
- Bobulescu, I.A.; Dubree, M.; Zhang, J.; McLeroy, P.; Moe, O.W. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am. J. Physiol. Renal Physiol. 2008, 294, F1315–F1322. [Google Scholar] [CrossRef] [PubMed]
- Deji, N.; Kume, S.; Araki, S.; Soumura, M.; Sugimoto, T.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Koya, D.; Haneda, M.; et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am. J. Physiol. Renal Physiol. 2009, 296, F118–F126. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, J.M.; Tallam, L.S.; Roberts, J.V.; Brandon, E.L.; Biglane, J.; da Silva, A.A.; Hall, J.E. Impact of obesity on renal structure and function in the presence and absence of hypertension: Evidence from melanocortin-4 receptor-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R803–R812. [Google Scholar] [CrossRef] [PubMed]
- Oron-Herman, M.; Kamari, Y.; Grossman, E.; Yeger, G.; Peleg, E.; Shabtay, Z.; Shamiss, A.; Sharabi, Y. Metabolic syndrome: Comparison of the two commonly used animal models. Am. J. Hypertens. 2008, 21, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Peltzer, K.; von Kruchten, R.; Lorbeer, R.; Rospleszcz, S.; Schulz, H.; Peters, A.; Bamberg, F.; Schlett, C.L.; Mujaj, B. Adipose tissue is associated with kidney function parameters. Sci. Rep. 2023, 13, 9151. [Google Scholar] [CrossRef] [PubMed]
- Aoko, O.; Maharaj, T.; Boland, F.; Cheriyan, D.; Ryan, J. Meta-analysis: Impact of intragastric balloon therapy on NAFLD-related parameters in patients with obesity. Aliment. Pharmacol. Ther. 2024, 59, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.C.M.; de Moura, D.T.H.; Kum, A.S.T.; de Oliveira, G.H.P.; Hirsch, B.S.; Ribeiro, I.B.; Gomes, I.L.C.; de Oliveira, C.P.M.; Mahmood, S.; Bernardo, W.M.; et al. Impact of Endoscopic Sleeve Gastroplasty in Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Obes. Surg. 2023, 33, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Yehnert, H.; Moustarah, F.; Schreiber, M.J.; Schauer, P.R.; Beddhu, S. Weight loss interventions in chronic kidney disease: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Chen, Y.; Still, C.; Wood, G.C.; Kirchner, H.L.; Lewis, M.; Kramer, H.; Hartle, J.E.; Carey, D.; Appel, L.J.; et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016, 90, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Wahed, A.S.; Wang, J.; Courcoulas, A.P.; Dakin, G.; Hinojosa, M.W.; Kimmel, P.L.; Mitchell, J.E.; Pomp, A.; Pories, W.J.; et al. Effect of Bariatric Surgery on CKD Risk. J. Am. Soc. Nephrol. 2018, 29, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 399) | Fatty Liver (n = 72) | Non-Fatty Liver (n = 327) | p | |
|---|---|---|---|---|
| Female sex, n, (%) | 209 (52.4) | 34 (47.2) | 175 (53.5) | 0.33 |
| Age, y, median (IQR) | 51 (35–67) | 56 (44–67) | 49 (33–67) | 0.141 |
| Weight, kg, median (IQR) * | 72 (60–85) | 88 (74–102) | 68.5 (58–79) | <0.0001 |
| Height, cm, median (IQR) * | 168 (162–175) | 167 (162–175) | 168 (161–175) | 0.800 |
| BMI, kg/ median (IQR) * | 24.6 (21.8–29.3) | 30.8 (27.4–34.1) | 24.1 (21.3–27.3) | <0.0001 |
| Systolic BP, mmHg, median # (IQR) | 121 (110–140) | 127 (115–142) | 120 (109–138) | 0.033 |
| Diastolic BP, mmHg, median # (IQR) | 73 (66–80) | 77 (72–81) | 73 (66–80) | 0.017 |
| Hypertension, n, (%) | 86 (21.6) | 22 (30.6) | 64 (19.6) | 0.040 |
| Diabetes Mellitus, n, (%) | 38 (9.5) | 12 (16.7) | 26 (8) | 0.023 |
| Dyslipidemia, n, (%) | 24 (6) | 9 (12.5) | 15 (4.6) | 0.011 |
| Total (n = 382) | Fatty Kidneys (n = 71) | Lack of Fatty Kidneys (n = 311) | p | |
|---|---|---|---|---|
| Female sex, n, (%) | 203 (53.1) | 31 (43.7) | 172 (55.3) | 0.076 |
| Age, y, median (IQR) | 50 (34–66) | 56 (35–70) | 49 (34–64) | 0.076 |
| Weight, kg, median (IQR) * | 71 (60–84) | 75 (65–88) | 70 (58–83) | 0.006 |
| Height, cm, median (IQR) * | 168 (160–175) | 169 (162–174) | 168 (160–175) | 0.616 |
| BMI, kg/ median (IQR) * | 24.6 (21.6–29.1) | 27.2 (23.1–30.0) | 24.4 (21.4–28.7) | 0.013 |
| Systolic BP, mmHg, median # (IQR) | 120 (110–140) | 126 (114–143) | 120 (109–137) | 0.032 |
| Diastolic BP, mmHg, median # (IQR) | 73 (66–80) | 76 (66–80) | 73 (66–80) | 0.464 |
| Hypertension, n, (%) | 79 (20.7) | 17 (23.9) | 62 (19.9) | 0.452 |
| Diabetes Mellitus, n, (%) | 35 (9.2) | 8 (11.3) | 27 (8.7) | 0.496 |
| Dyslipidemia, n, (%) | 20 (5.2) | 4 (5.6) | 16 (5.1) | 0.867 |
| Fatty Liver (n = 67) | Non-Fatty Liver (n = 315) | |||||
|---|---|---|---|---|---|---|
| Fatty Kidney (n = 23, 34.33%) | No Fatty Kidneys (n = 44, 65.67%) | p | Fatty Kidney (n = 48, 15.24%) | Non-Fatty Kidney (n = 267, 84.76%) | p | |
| Female sex, n, (%) | 12 (52.2) | 21 (47.7) | 0.730 | 19 (39.6) | 151 (56.6) | 0.030 |
| Age, y, median (IQR) | 56 (45–70) | 51 (40.5–61.75) | 0.326 | 54.5 (34–70) | 49 (33–65) | 0.201 |
| Weight, kg, median (IQR) * | 90 (81–101) | 85 (72–103) | 0.211 | 72 (61–81) | 68 (57–77) | 0.182 |
| Height, cm, median (IQR) * | 165 (159–178) | 168 (162–175) | 0.790 | 170 (165–173) | 168 (160–175) | 0.375 |
| BMI, kg/ median (IQR) * | 32.0 (29.7–35.6) | 29.8 (25.0–23.4) | 0.059 | 24.3 (21.6–27.6) | 24.0 (21.0–27.1) | 0.394 |
| Systolic BP, mmHg, median (IQR) # | 133 (114–156) | 120 (115–141) | 0.115 | 125 (111–140) | 120 (108–136) | 0.104 |
| Diastolic BP, mmHg, median (IQR) # | 78 (74–82) | 76 (70–80) | 0.440 | 73 (66–78) | 72 (65–80) | 0.864 |
| Hypertension, n, (%) | 8 (34.8) | 11 (25) | 0.399 | 9 (18.8) | 51 (19.1) | 0.955 |
| Diabetes Mellitus, n, (%) | 5 (21.7) | 5 (11.4) | 0.258 | 3 (6.3) | 22 (8.2) | 0.639 |
| Dyslipidemia, n, (%) | 2 (8.7) | 4 (9.1) | 0.957 | 2 (4.2) | 12 (4.5) | 0.919 |
| Parameter | Odds Ratio | CI 2.5% | CI 97.5% | p-Value |
|---|---|---|---|---|
| Age | 1.0 | 1.0 | 1.0 | 0.086 |
| Sex | 1.6 | 0.9 | 2.7 | 0.082 |
| Diabetes Mellitus | 1.0 | 0.4 | 2.5 | 0.987 |
| Hypertension | 0.9 | 0.4 | 1.8 | 0.688 |
| Dyslipidemia | 0.8 | 0.2 | 2.7 | 0.722 |
| Fatty Liver | 2.9 | 1.6 | 5.2 | 0.001 |
| Total (n = 322) | BMI ≤ 25 (n = 175, 54.3%) | 25 < BMI < 30 (n = 84, 26.1%) | BMI ≥ 30 (n = 63, 19.6%) | p Value | |
|---|---|---|---|---|---|
| Female sex, n, (%) | 166 (51.6) | 91 (52) | 40 (47.6) | 35 (55.6) | 0.625 |
| Age >50 y, n (%) | 166 (51.6) | 74 (42.3) | 49 (58.3) | 43 (68.3) | 0.0006 |
| Systolic BP > 130 mmHg, n (%) | 116 (36.0) | 44 (25.1) | 33 (39.2) | 39 (62.0) | <0.0001 |
| Diastolic BP > 80 mmHg, n (%) | 74 (23.0) | 37 (21.1) | 18 (21.4) | 19 (30.2) | 0.319 |
| Hypertension, n, (%) | 82 (25.5) | 27 (15.4) | 23 (27.4) | 32 (50.8) | <0.0001 |
| Diabetes Mellitus, n, (%) | 37 (11.5) | 13 (7.4) | 7 (8.3) | 17 (27) | <0.0001 |
| Dyslipidemia, n, (%) | 23 (7.1) | 8 (4.6) | 7 (8.3) | 8 (12.7) | 0.088 |
| Fatty liver, n, (%) | 57 (17.7) | 7 (4) | 18 (21.4) | 32 (50.8) | <0.0001 |
| Fatty kidney, n, (%) | 56 (17.4) | 22 (12.6) | 22 (26.2) | 12 (19) | 0.007 |
| Fatty liver and fatty kidney, n, (%) | 17 (5.3) | 0 (0) | 7 (8.3) | 10 (15.9) | <0.0001 |
| A | ||||
| Parameter | Odds Ratio | CI 2.5% | CI 97.5% | p-Value |
| Age | 1.0 | 1.0 | 1.0 | 0.086 |
| Sex | 1.6 | 0.9 | 2.7 | 0.082 |
| Diabetes mellitus | 1.0 | 0.4 | 2.5 | 0.987 |
| Hypertension | 0.9 | 0.4 | 1.8 | 0.688 |
| Dyslipidemia | 0.8 | 0.2 | 2.7 | 0.722 |
| Fatty Liver | 2.9 | 1.6 | 5.2 | 0.001 |
| B | ||||
| Parameter | Odds Ratio | CI 2.5% | CI 97.5% | p-Value |
| Age | 1.0 | 1.0 | 1.0 | 0.958 |
| Sex | 0.6 | 0.2 | 1.8 | 0.359 |
| Diabetes mellitus | 1.7 | 0.4 | 6.8 | 0.478 |
| Hypertension | 1.3 | 0.3 | 4.8 | 0.694 |
| Dyslipidemia | 0.5 | 0.1 | 4.9 | 0.545 |
| BMI ≥ 30 kg/m2 | 6.3 | 2.1 | 18.6 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raphael, H.; Klang, E.; Konen, E.; Inbar, Y.; Leibowitz, A.; Frenkel-Nir, Y.; Apter, S.; Grossman, E. Obesity Is Associated with Fatty Liver and Fat Changes in the Kidneys in Humans as Assessed by MRI. Nutrients 2024, 16, 1387. https://doi.org/10.3390/nu16091387
Raphael H, Klang E, Konen E, Inbar Y, Leibowitz A, Frenkel-Nir Y, Apter S, Grossman E. Obesity Is Associated with Fatty Liver and Fat Changes in the Kidneys in Humans as Assessed by MRI. Nutrients. 2024; 16(9):1387. https://doi.org/10.3390/nu16091387
Chicago/Turabian StyleRaphael, Hadar, Eyal Klang, Eli Konen, Yael Inbar, Avshalom Leibowitz, Yael Frenkel-Nir, Sara Apter, and Ehud Grossman. 2024. "Obesity Is Associated with Fatty Liver and Fat Changes in the Kidneys in Humans as Assessed by MRI" Nutrients 16, no. 9: 1387. https://doi.org/10.3390/nu16091387
APA StyleRaphael, H., Klang, E., Konen, E., Inbar, Y., Leibowitz, A., Frenkel-Nir, Y., Apter, S., & Grossman, E. (2024). Obesity Is Associated with Fatty Liver and Fat Changes in the Kidneys in Humans as Assessed by MRI. Nutrients, 16(9), 1387. https://doi.org/10.3390/nu16091387








