Abstract
Background: Obesity constitutes a public health problem worldwide and causes non-alcoholic fatty liver disease (MALFD), the leading cause of liver disease in developed countries, which progresses to liver cirrhosis and liver cancer. MAFLD is associated with obesity and can be evaluated by validated formulas to assess MAFLD risk using different parameters such as the body mass index (BMI) and waist circumference (WC). However, these parameters do not accurately measure body fat. As MAFLD is strongly associated with obesity, we hypothesize that measuring body and visceral fat by electrical bioimpedance is an efficient method to predict the risk of MAFLD. The objective of our work was to demonstrate that electrical bioimpedance is a more efficient method than the BMI or WC to predict an elevated risk of MAFLD. Methods: A cross-sectional, descriptive study involving 8590 Spanish workers in the Balearic Islands was carried out. The study’s sample of employees was drawn from those who underwent occupational medicine examinations between January 2019 and December 2020. Five MAFLD risk scales were determined for evaluating very high levels of body fat and visceral fat. The determination of body and visceral fat was performed using bioimpedanciometry. Student’s t-test was employed to ascertain the mean and standard deviation of quantitative data. The chi-square test was used to find prevalences for qualitative variables, while ROC curves were used to define the cut-off points for body and visceral fat. The calculations included the area under the curve (AUC), the cut-off points along with their Youden index, sensitivity, and specificity. Correlation and concordance between the various scales were determined using Pearson’s correlation index and Cohen’s kappa, respectively. Results: As both total body fat and visceral fat increase, the risk of MAFLD increases with a statistically significant result (p < 0.001), presenting a higher risk in men. The areas under the curve (AUC) of the five scales that assess overweight and obesity to determine the occurrence of high values of the different MAFLD risk scales were very high, most of them exceeding 0.9. These AUC values were higher for visceral and body fat than for the BMI or waist circumference. FLD-high presented the best results in men and women with the AUC at around 0.97, both for visceral fat and total body fat, with a high Youden index in all cases (women body fat = 0.830, visceral fat = 0.892; men body fat = 0.780, visceral fat = 0.881). Conclusions: In our study, all the overweight and obesity scales show a very good association with the scales assessing the risk of MAFLD. These values are higher for visceral and body fat than for waist circumference and the BMI. Both visceral fat and body fat are better associated than the BMI and waist circumference with MAFLD risk scales.
1. Introduction
Obesity is a metabolic disorder in which there is an accumulation of excessive fat in the body, which is harmful to health. Nowadays, it is considered a public health problem in both developed and developing countries and is recognized as a 21st century pandemic [1]. The impact of obesity on health is very high, both because of the pathology it presents in itself and because it is a risk factor for multiple diseases. Currently, it is a cause of greater mortality in developed countries than low weight in developing countries [2].
Among the pathologies that obesity promotes are heart disease and stroke [3], diabetes and insulin resistance [4], different types of cancer [3], musculoskeletal disorders [3], and different liver diseases such as non-alcoholic fatty liver disease (MAFLD), non-alcoholic steatohepatitis (MASH), liver cirrhosis, and hepatocellular carcinoma [5,6].
Fatty liver and obesity are closely related. This relationship is due to poor metabolic health that facilitates the development of what can be called fatty liver disease associated with metabolic dysfunction (MAFLD) [7]. MAFLD is currently defined as a chronic liver disease with >5% fat deposition in liver cells in the absence of excessive alcohol intake and other secondary causes [8]. This increase in fat in the liver is responsible for increased insulin resistance, which plays an essential role in the development of MAFLD. The adipocyte behaves like an endocrine organ and produces various cytokines, which include tumor necrosis factor (TNF-α), angiotensinogen, free fatty acids that are mainly responsible for lipotoxicity, and leptin. The latter increases in obese individuals—who produce resistance to it—exert a pro-inflammatory action and the development of MAFLD, and may well play an important role in the progression to liver fibrosis, which, in patients with MAFLD, constitutes the most important risk factor for developing liver cirrhosis and liver cancer [9].
About 20 years ago, MAFLD was strongly associated with obesity, type 2 diabetes mellitus (T2DM), metabolic syndrome (MS), and dyslipidemia [10]. Nowadays, a second type of MAFLD would be related to infectious diseases such as Hepatitis C and HIV or drugs [11]. However, the majority of MAFLD occurs in developed and developing countries due to an unhealthy lifestyle, with an intake of foods rich in carbohydrates and saturated fats (Fast Food), along with a lack of physical exercise, causing obesity [12].
Today, linked to obesity, MAFLD is the leading cause of chronic liver disease in developed countries, with a global prevalence of around 30%, which reaches 90% of patients with morbid obesity [13].
The gold standard for diagnosing any liver pathology is a biopsy; however, it is an aggressive, expensive, painful, and not risk-free test [14].
Liver ultrasound is a non-invasive method and well tolerated by the patient. However, the interpretation of hepatic steatosis is operator-dependent, requires training time for the technician [15], and the classification of steatosis is established subjectively at three levels: mild, moderate, and severe. Further, an experienced sonographer can only detect hepatic steatosis when the fat content in the liver is at least between 2.5 and 20% [16], so approximately 5% of patients with initial steatosis would not be diagnosed [17].
Transient elastography (LSM) is also useful for the diagnosis of MAFLD. However, although its usefulness has been demonstrated in cases of advanced fibrosis in MAFLD, with a sensitivity in these cases of 97% in patients with a BMI < 30 kg/m2, it is not useful in incipient or poorly developed cases [18]. It is an operator-dependent technique requiring training time; subcutaneous fat reduces the propagation of ultrasound, thereby affecting the measurements made in people with obesity; and it has the cost of the ultrasound machine [19].
As MAFLD is closely associated with obesity, different formulas have been validated to evaluate the risk of MAFLD. They use different parameters that evaluate obesity, such as the body mass index (BMI) and waist circumference (WC). However, although widely used, these parameters do not accurately measure body fat. The BMI is obtained by dividing weight (in kg) by height (in meters) squared. This formula has several limitations: firstly, it does not provide any information on how body fat is distributed; secondly, when establishing a relationship between height and weight, the BMI is not able to differentiate between muscle, bone, or adipose tissue, so individuals with large muscle mass will obtain a high BMI without having excess adipose tissue [20].
Visceral fat in adults is associated with insulin resistance [21], which plays a prominent role in the development of MAFLD. WC is a valid parameter for indirect measurement of visceral adipose tissue [22]; however, we consider that there are valid economic methods to assess both total body fat and visceral fat. These systems include bioelectrical impedance (BIA), which measures the electrical resistance of the different components of the body by applying a constant low intensity alternating current to them. It is a simple, fast, harmless, low-cost, and affordable technique in medical consultations. This technique is valid for studying body composition in healthy, normal weight individuals and in the study of overweight and moderate obesity. However, it does not seem to have the same usefulness in the study of morbid obesity [23].
It is important to highlight the fact that liver fibrosis can be prevented, reversed, or stabilized if the cause that triggers it is eliminated [24]; and if this is insufficient, immunosuppressive, anti-inflammatory, or antiviral drugs may be considered. New antifibrotic drugs, such as angiotensin inhibitors, are not yet available [25].
As previously mentioned, the most common cause of MAFLD today is unhealthy lifestyle habits, which facilitate the deposition of fat in the liver and are frequently associated with obesity. Modifying lifestyle habits to prevent MAFLD is the basis of preventive and restorative treatment for MAFLD [26]. Therefore, the detection of MAFLD in its early stages is of vital importance for establishing appropriate measures and preventing its evolution to liver fibrosis, liver cirrhosis, and hepatocellular carcinoma.
As MAFLD is strongly associated with obesity, we hypothesize that measuring body and visceral fat by electrical bioimpedance is an efficient method to predict the risk of MAFLD.
The objective of our work was to demonstrate that electrical bioimpedance is a more efficient method than the BMI or WC to predict an elevated risk of MAFLD.
2. Material and Methods
2.1. Participants
A cross-sectional, descriptive study including 8590 Spanish workers in the Balearic Islands was carried out. The study’s sample of employees was drawn from those who underwent occupational medicine exams between January 2019 and December 2020.
Inclusion criteria:
- –
- Individuals in the 18–69 age range;
- –
- Consent to take part in the research;
- –
- Giving permission for the data to be used for epidemiological research;
- –
- Being employed by one of the participating companies in the research and not being temporarily disabled at the time of the study;
- –
- A flowchart of the study participants is presented in Figure 1.
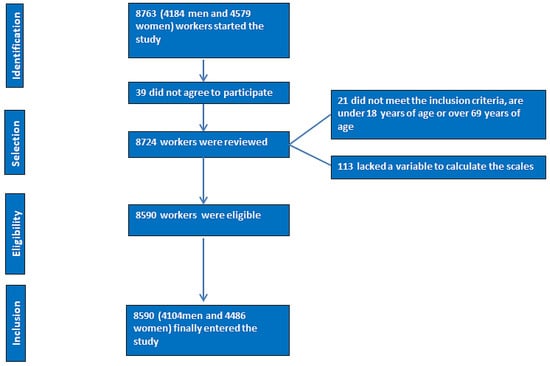 Figure 1. PRISMA flowchart of participants in the study.
Figure 1. PRISMA flowchart of participants in the study.
2.2. Determination of Variables
Following process standardization to prevent interobserver bias, occupational health professionals from the participating firms conducted all measurements, whether anthropometric (height, weight, and waist circumference), analytical, or clinical:
- –
- Variables such as age, sex, performing regular physical exercise, physical exercise days per week, and smoking were collected;
- –
- Anthropometric and clinical determinations: weight, height, waist and hip circumference, and both systolic and diastolic blood pressure;
- –
- Analytical determinations: fasting blood glucose, lipid profile, and hepatic enzymes.
2.2.1. Anthropometric Determinations
Measurements of height (in cm) and weight (in kg) were taken using a SECA 700 scale. The measurements were carried out following the ISAK’s international standards for anthropometric assessment [27].
With the subject standing, feet together, and abdomen relaxed, waist circumference was measured using a tape measure parallel to the floor at the midpoint between the last palpable rib and the iliac crest [28].
Body and visceral fat determination was performed by bioimpedanciometry using a Tanita DC 430MA model. High values of body and visceral fat are considered to be those shown by the bioimpedance scale (from 10 for visceral fat and variable according to age for body fat).
2.2.2. Clinical Determinations
Blood pressure was measured after 10 min of rest, with the subject seated and without crossed legs, using an OMRON-M3 model blood pressure monitor. Three measurements were made at one-minute intervals, and the average of the three was calculated.
2.2.3. Analytical Determinations
The blood sample was taken after a minimum of 12 h of fasting and was then processed in 48 to 72 h. The measurement of triglycerides, total cholesterol, and blood sugar was performed automatically by enzymatic procedures. The dextran sulfate-MgCl2 precipitation technique was employed for HDL-cholesterol.
By using the Friedewald formula, which is only reliable when triglycerides do not exceed 400, LDL-cholesterol can be calculated indirectly. The unit of measurement for all analytical parameters is mg/dL.
LDL = Total cholesterol total − HDL-c − triglycerides/5
2.2.4. Risk Scales
The non-alcoholic fatty liver disease and liver fibrosis risk scales listed below were applied:
- –
- FLI (fatty liver index) [29] FLI = (e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (ggt) + 0.053 × waist circumference − 15.745)/(1 + e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (ggt) + 0.053 × waist circumference − 15.745) × 100. High risk is defined as beginning at 60.
- –
- Hepatic steatosis index (HSI) [29] HSI = 8 × ALT/AST + BMI + 2 (if type 2 diabetes yes) + 2 (if female). Thirty-six is regarded as high risk.
- –
- Zhejian University index (ZJU) [29] ZJU index = BMI + FBG + TG + 3 × ALT/AST +2 (if female). Fasting blood glucose (FBG) was in mmol/L; WC was in cm, triglycerides (TG).
- –
- A high-risk situation is defined as 38.
- –
- Fatty Liver Disease Index (FLD) [30] FLD= BMI + TG + 3 × (ALT/AST) + 2 × Hyperglycemia (presence= 1; absence = 0)
- –
- High risk is defined as beginning at 37.
- –
- Lipid accumulation product (LAP) [31]. Men: (waist (cm) − 65) × (triglycerides (mMol)).
Women: (waist (cm) − 58) × (triglycerides (mMol)).
High risk is considered starting from 42.7.
Anyone who had smoked at least one cigarette in the previous month (or its equivalent in other forms of consumption) or who had given up smoking less than a year before was considered a smoker.
The Spanish Society of Epidemiology’s recommendation, based on the 2011 National Classification of Occupations, was used to determine socioeconomic class. Class I comprises managers, directors, and university professionals; Class II includes intermediate vocations and self-employed individuals; and Class III includes manual workers [32].
2.3. Statistical Analysis
Student’s t-test was employed to ascertain the mean and standard deviation of quantitative data. The chi-square test was used to find prevalences for qualitative variables. ROC curves were used to define the cut-off points for cardiac ages as moderate and high. The calculations included the area under the curve (AUC), the cut-off points along with their Youden index, sensitivity, and specificity. The correlation and concordance between the various scales were determined using Pearson’s correlation index and Cohen’s kappa, respectively. Statistical analysis was performed using SPSS 29.0, with p < 0.05 as the recognized threshold for statistical significance.
3. Results
The anthropometric and clinical details of the study participants are displayed in Table 1. The analyses comprised a total of 8590 workers (4104 men, 47.8%, and 4486 women, 52.2%). The average age of the sample was marginally higher than 41, with the bulk of participants in the 30- to 49-year-old age range. Labourers were primarily from social class I. In both genders, just over 15% smoked. Of the men and women, 25.9% and 35.1%, respectively, did not exercise regularly.

Table 1.
Characteristics of study participants.
Table 2 displays the average body and visceral fat values for both sexes based on the results of the various MAFLD risk scales.

Table 2.
Mean body fat and visceral fat values according to NAFLD risk scale values.
The mean body fat values were consistently higher in women and rose as the risk of MAFLD increased. Visceral fat and the MAFLD risk scales showed a similar link but with greater values in men in this instance.
Alongside the rise in the various scales measuring the risk of non-alcoholic fatty liver disease (MAFLD), there was a corresponding rise in the prevalence of extremely high body fat values. This prevalence was typically greater in women. Similar trends were revealed in the prevalence of high visceral fat levels, with men showing the highest prevalence in this instance. Table 3 contains all of the data.

Table 3.
Prevalence of very high body fat and visceral fat values according to MAFLD risk scales.
The areas under the curve (AUC) of the five scales that assess overweight and obesity to determine the occurrence of high values of the different MAFLD risk scales were very high, most of them exceeding 0.9. These AUC values were higher for visceral and body fat than for the BMI or waist circumference. In all cases, these AUCs were higher in women. See Figure 2 and Figure 3 and Table 4.
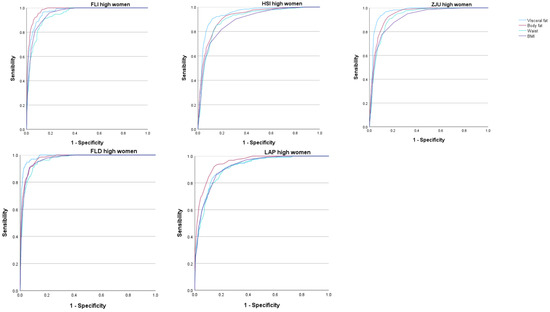
Figure 2.
ROC curves in women. FLI Fatty liver index, HSI Hepatic steatosis index, ZJU Zhejian University index, FLD Fatty liver disease index, LAP Lipid accumulation product.
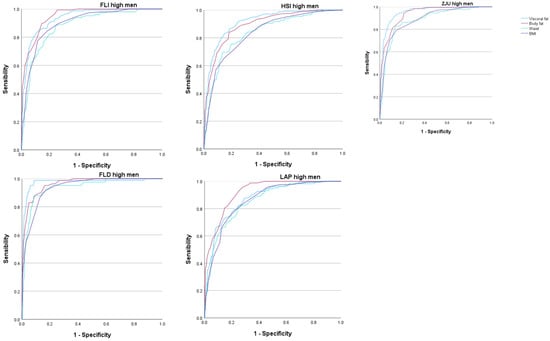
Figure 3.
ROC curves in men. FLI Fatty liver index, HSI Hepatic steatosis index, ZJU Zhejian University index, FLD Fatty liver disease index, LAP Lipid accumulation product.

Table 4.
Areas under the curve for body and visceral fat predict high values of MAFLD risk scales by sex.
Table 5 presents the cut-off points for all the overweight and obesity scales (with their sensitivity, specificity, and Youden index) to determine elevated values of the different MAFLD risk scales. As already seen for the AUC, the highest Youden index values were found for visceral and body fat, and these values were also higher in women.

Table 5.
Body and visceral fat cut-off points for predicting high values of MAFLD risk scales by sex.
Table 6a,b and Table 7a,b present the values of Pearson’s correlation coefficients and Cohen’s kappa indices for both the overweight and obesity scales and the MAFLD risk scales in both sexes. The degree of correlation in both sexes, although especially in women, was generally very high for both scales that assess overweightness and MAFLD.

Table 6.
a. Pearson’s correlation. b. Pearson’s correlation.

Table 7.
a. Kappa Cohen indices. b. Kappa Cohen indices.
The degree of concordance, determined with Cohen’s kappa index, shows much lower values for the overweight-obesity scales and higher values for the MAFLD scales, especially FLI with FLD and ZJU and HSI with ZJU.
4. Discussion
The ability of electrical bioimpedance to predict the high risk of MAFLD was evaluated in a sample of 8590 Spanish workers of both sexes: 4104 men and 4486 women. All of them belonged to the Autonomous Community of the Balearic Islands (Spain) and were between 18 and 69 years of age.
Regarding the characteristics of the population, it should be noted that they mostly belong to social class I, the majority of whom are between 30 and 49 years of age. The percentage of smokers is the same by sex, around 16%, and the group of women performs less physical exercise than the group of men. It is noteworthy that in our study population, the number of smokers is much lower than the data obtained in the European Health Survey in Spain in 2020, where the smoking population had a percentage of around 20% in women and 30% in men [33]. This could be influenced by the fact that all the participants in the sample come from the Balearic Islands, where the smoking habit is somewhat lower than the national average—approximately 17.2% according to the results of the INE (National Statistics Institute) of Spain in 2022 [34]—which is more in line with our results.
The objective of our study was to demonstrate that electrical bioimpedance is a more efficient method than the BMI or WC in the ability to predict a high risk of MAFLD. To do this, the amount of total body fat and visceral fat determined by electrical bioimpedance was compared against five validated formulas to calculate the risk of MAFLD, of which the BMI or WC are a part.
When we assessed the association between the average values of visceral fat and body fat with the five formulas (FLI, HSI, ZJU, FLD, LAP), we observed that as both total body fat and visceral fat increased, so did the risk of MAFLD, with a statistically significant result in all cases (p < 0.001 in all scales). These results coincide with a Spanish study carried out on 219,477 workers, where the relationship between several overweight and obesity scales (including the BMI and different estimators of body and visceral fat) and MAFLD risk scales was evaluated. Their results show that as overweight-obesity increases, the risk of MAFLD also increases in parallel in all the scales used [35]. Our results show that this increased risk is greater in the group of women than in the group of men. The association between increased body fat and MAFLD has already been defined in previous studies, in which an association of up to 80% is established between obesity and MAFLD [36].
When assessing the association between the risk of MAFLD and high body fat values, we found the same results. That is, there was a significant increase in the risk of MAFLD that was more pronounced in the group of women. However, when this association was assessed with high visceral fat values, the increased risk, in this case, was observed to be higher in the group of men. This is consistent with other studies that have found a greater association between visceral fat and the risk of MALFD in men [37]. In men, there is a greater predisposition to the accumulation of visceral fat, with more lipolytic capacity than subcutaneous fat and a greater supply of fatty acids to the liver, which may lead to MALFD [38].
In the analysis of the ROC curves, the AUC of the five scales used to assess overweight and obesity presented a very high value, with all of them showing a result between good and excellent. For women, for all five MAFLD risk scales used, the AUC was higher for both percent total body fat and visceral fat. Even when using the FLD high-risk scale, the AUC gave us a value greater than 0.97, which implies an excellent test result—as such a high result is obtained in very few tests. The same occurred when assessing the percentage of body fat with the FLI high-risk scale, in which the AUC also gave us a value greater than 0.97. Even so, it is worth noting that, for all the risk scales used, the AUC was greater than 0.90 for both the percentage of visceral fat and the percentage of body fat, which implies a very good test result.
In the case of men, the results were similar, although the AUC showed lower values than in women in practically all cases. Even so, we must highlight the highest AUC value when using the FLD high-risk scale for the percentage of total body fat, with a result greater than 0.97, which is excellent. On the other hand, the lowest AUC values were found for the LAP high-risk scales when assessing total body fat, with an AUC of 0.875, and in the HSI high-risk scale when assessing visceral fat, with an AUC of 0.895.
These values, being the lowest AUC obtained, offer a good test result that is very close to very good. Notably, for both risk scales, the AUC presented lower values when related to the BMI or WC.
In the five MALFD risk scales used, the AUC was higher when total body fat or visceral fat was used than when the BMI or WC was used.
The risk of MALFD for all scales was observed to increase more in men than in women as visceral fat increased. In the case of the ROC curves, the AUC for the percentage of visceral fat was higher in women. This tells us that although visceral fat is associated with a higher risk of MALFD in men, the Youden index is higher for women. That is to say, the performance of this test is greater or, in other words, has a greater sensitivity-specificity relationship. It can be seen how it oscillates from the lowest value with a Youden index of 0.708 for high LAP (sensitivity 85.4-specificity 85.4) to the highest value obtained with a high FLD, with a Youden index of 0.892 (sensitivity 94.7-specificity 94.5).
In the Pearson correlation coefficient, a linear correlation can be observed between body fat and visceral fat through the BMI and WC. This strength of correlation is very high, in all cases greater than 0.7 for both sexes, although it shows slightly higher figures in women.
Since MAFLD was not evaluated directly but rather indirectly through MAFLD risk formulas, the Pearson correlation coefficient between the different formulas was also performed to check the validity between them when determining said risk. In all the formulas used, a correlation between high and very high can be seen, with the highest value between ZJU and FLD of 0.996 in women and 0.991 in men, which implies a very high and almost total correlation. The lowest correlation was obtained between HSI and LAP, with a value of 0.678 in women and 0.595 in men. Even so, the results were greater than 0.5 in both cases, which expresses a high or strong correlation.
Likewise, in order to gauge the degree of agreement, Cohen’s Kappa index was evaluated. In the case of comparing the different scales to assess obesity, the greatest degree of agreement was found to have occurred between the BMI and total body fat, and between waist circumference and visceral fat, with good agreement, especially in the group of women. The good agreement between waist circumference and visceral fat confirms that waist circumference is a good indirect measure of the amount of visceral adipose tissue [39].
Regarding Cohen’s Kappa index between the different MAFLD risk formulas, a very good agreement was found between almost all of them except for what corresponds to the LAP. The latter presented values between insignificant and low in both men and women. This could be because the BMI is used in the other four formulas, whereas waist circumference is used in the LAP.
5. Strengths and Limitations
Among the strengths of the study, it is worth highlighting the large sample size, almost 9000 persons, and the fact that the determination of body and visceral fat was performed with objective and validated methods, such as bioimpedance measurement.
The main limitation of the study is that MAFLD was not determined by objective methods but by using risk scales, even though these are validated.
6. Conclusions
In our study, all the overweight and obesity scales show a very good association with the scales assessing the risk of MAFLD. Nonetheless, these values are higher for visceral and body fat than for waist circumference and the BMI.
Both visceral fat and body fat are better associated than the BMI and waist circumference with MAFLD risk scales.
The use of bioimpedanciometry in primary care and occupational medicine consultations can be very useful in predicting the risk of MAFLD.
Author Contributions
Conceptualization: Á.A.L.-G. and J.I.R.-M.; Data collection and analysis: M.G.S. and D.V. Data curation: M.G.S. Methodology: E.M.-A.R. and D.V. Validation: M.T.V.-H.; Formal analysis: Á.A.L.-G.; Investigation: M.G.S.; Draft: M.G.S., Á.A.L.-G., M.T.V.-H. and E.M.-A.R.; Revision: J.I.R.-M. and Á.A.L.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research team is committed to always adhering to the ethical guidelines for health sciences research that have been established on a national and international level (the Declaration of Helsinki), with particular consideration paid to participant anonymity and data confidentiality. The Ethics and Research Committee of the Balearic Islands (CEI-IB) was consulted in order to acquire approval (IB 4383/20), which was accomplished using indicator IB 4383/20. The study was voluntary, meaning that after being fully informed about its purpose, participants gave their written and verbal consent to take part in it. In order to do this, they were provided with an information sheet outlining the purpose of the study and an informed consent form. The codes used to identify the data collected for the study make it impossible for anybody other than the project coordinator to link the data to the participants. There will be no publication of the participants’ identities in any study report. No information that could be used to identify them will be shared by the investigators. The research team guarantees that all participants in this study may exercise their rights of access, rectification, cancellation, and opposition of the data collected. In any event, the team pledges to strictly adhere to Organic Law 3/2018, of December 5, on the protection of personal data and guarantee of digital rights.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Study data are stored in a database that complies with all security measures at the ADEMA-Escuela Universitaria. The Data Protection Delegate is Ángel Arturo López González.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Puljiz, Z.; Kumric, M.; Vrdoljak, J.; Martinovic, D.; Kurir, T.T.; Krnic, M.O.; Urlic, H.; Puljiz, Z.; Zucko, J.; Dumanic, P.; et al. Obesity, Gut Microbiota, and Metabolome: From Pathophysiology to Nutritional Interventions. Nutrients 2023, 15, 2236. [Google Scholar] [CrossRef] [PubMed]
- Hancková, M.; Betáková, T. Pandemics of the 21st Century: The Risk Factor for Obese People. Viruses 2021, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic obesity: Epidemiology, pathophysiology, cardiovascular disease, mortality, and management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Suárez, R.; Chapela, S.P.; Álvarez-Córdova, L.; Bautista-Valarezo, E.; Sarmiento-Andrade, Y.; Verde, L.; Frias-Toral, E.; Sarno, G. Epigenetics in Obesity and Diabetes Mellitus: New Insights. Nutrients 2023, 15, 811. [Google Scholar] [CrossRef] [PubMed]
- Koperska, A.; Wesołek, A.; Moszak, M.; Szulińska, M. Berberine in Non-Alcoholic Fatty Liver Disease—A Review. Nutrients 2022, 14, 3459. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Manent, J.I.; Gordito Soler, M.; Perelló Viola, N.; Montero Muñoz, N.; Altisench Jané, B. Anthropometric parameters related to high-risk values of different scales of nonalcoholic fatty liver disease and liver fibrosis in 146.318 spanish adults. Acad. J. Health Sci. 2022, 37, 73–82. [Google Scholar]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Stojsavljević, S.; Gomerčić Palčić, M.; Virović Jukić, L.; Smirčić Duvnjak, L.; Duvnjak, M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 18070–18091. [Google Scholar] [CrossRef]
- Cortez-Pinto, H.; Camilo, M.E.; Baptista, A.; De Oliveira, A.G.; De Moura, M.C. Non-alcoholic fatty liver: Another feature of the metabolic syndrome? Clin. Nutr. 1999, 18, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Nonguierma, E.; Lesco, E.; Olak, R.; Welch, H.; Alam, N.Z.; Bonyadi, J.; Hopkins, L. Improving Obesogenic Dietary Behaviors among Adolescents: A Systematic Review of Randomized Controlled Trials. Nutrients 2022, 14, 4592. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Chien, L.-W. Predictive Role of Neutrophil-Percentage-to-Albumin Ratio (NPAR) in Nonalcoholic Fatty Liver Disease and Advanced Liver Fibrosis in Nondiabetic US Adults: Evidence from NHANES 2017–2018. Nutrients 2023, 15, 1892. [Google Scholar] [CrossRef]
- Ballestri, S.; Nascimbeni, F.; Lugari, S.; Lonardo, A.; Francica, G. A critical appraisal of the use of ultrasound in hepatic steatosis. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 667–681. [Google Scholar] [CrossRef]
- Bril, F.; Ortiz-Lopez, C.; Lomonaco, R.; Orsak, B.; Freckleton, M.; Chintapalli, K.; Hardies, J.; Lai, S.; Solano, F.; Tio, F.; et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015, 35, 2139–2146. [Google Scholar] [CrossRef]
- Paige, J.S.; Bernstein, G.S.; Heba, E.; Costa, E.A.C.; Fereirra, M.; Wolfson, T.; Gamst, A.C.; Valasek, M.A.; Lin, G.Y.; Han, A.; et al. A Pilot Comparative Study of Quantitative Ultrasound, Conventional Ultrasound, and MRI for Predicting Histology-Determined Steatosis Grade in Adult Nonalcoholic Fatty Liver Disease. Am. J. Roentgenol. 2017, 208, W168–W177. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Vergniol, J.; Wong, G.L.-H.; Foucher, J.; Chan, H.L.-Y.; Le Bail, B.; Choi, P.C.-L.; Kowo, M.; Chan, A.W.-H.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2009, 51, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Bray, G.A. Beyond BMI. Nutrients 2023, 15, 2254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McLaughlin, T.; Lamendola, C.; Liu, A.; Abbasi, F. Preferential Fat Deposition in Subcutaneous Versus Visceral Depots Is Associated with Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2011, 96, E1756–E1760. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; González-Crespo, I.; Catalán, V.; Rodríguez, A.; Moncada, R.; Valentí, V.; Romero, S.; Ramírez, B.; Silva, C.; Gil, M.J.; et al. Clinical usefulness of abdominal bioimpedance (ViScan) in the determination of visceral fat and its application in the diagnosis and management of obesity and its comorbidities. Clin. Nutr. 2017, 37, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [PubMed]
- Lee, K.-C.; Wu, P.-S.; Lin, H.-C. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin. Mol. Hepatol. 2023, 29, 77–98. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; Ridder, H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry–ISAK: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Fang, H.; Berg, E.; Cheng, X.; Shen, W. How to best assess abdominal obesity. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 360–365. [Google Scholar] [CrossRef]
- Mohebbi, V.; Aramayo, A.; Morales, J. Determination of scales related to cardiovascular risk and fatty liver in 5.370 Spanish farmers. Acad. J. Health Sci. 2021, 36, 26–33. [Google Scholar]
- Fuyan, S.; Jing, L.; Wenjun, C.; Zhijun, T.; Weijing, M.; Suzhen, W.; Yongyong, X. Fatty liver disease index: A simple screening tool to facilitate diagnosis of nonalcoholic fatty liver disease in the Chinese population. Dig. Dis. Sci. 2013, 58, 3326–3334. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26, Erratum in: BMC Cardiovasc. Disord. 2006, 6, 5. [Google Scholar] [CrossRef]
- Domingo-Salvany, A.; Bacigalupe, A.; Carrasco, J.M.; Espelt, A.; Ferrando, J.; Borrell, C.; del Grupo de Determinantes Sociales de Sociedad Española de Epidemiología. Propuestas de clase social neoweberiana y neomarxista a partir de la Clasificación Nacional de Ocupaciones 2011 [Proposals for social class classification based on the Spanish National Classification of Occupations 2011 using neo-Weberian and neo-Marxist approaches]. Gac Sanit. 2013, 27, 263–272. (In Spanish) [Google Scholar]
- Determinantes de Salud (Consumo de Tabaco, Exposición Pasiva al Humo de Tabaco, Alcohol, Problemas Medioambientales en la Vivienda). Available online: https://www.ine.es/ss/Satellite?L=es_ES&c=INESeccion_C&cid=1259926698156&p=1254735110672&pagename=ProductosYServicios/PYSLayout (accessed on 11 May 2024).
- INEbase/Indicadores de Calidad de Vida. Available online: https://www.ine.es/jaxi/Datos.htm?path=/t00/ICV/dim3/l0/&file=33201.px (accessed on 11 May 2024).
- Martínez-Almoyna Rifá, E.; Tomás-Gil, P.; Coll Villalonga, J.L.L.; Ramírez-Manent, J.I.; Martí-Lliteras, P.; López-González, A.A. Relationship between nonalcoholic fatty liver disease and liver fibrosis risk scales with overweight and obesity scales in 219,477 spanish workers. Acad. J. Health Sci. 2023, 38, 92–100. [Google Scholar]
- Lazarus, J.V.; Mark, H.E.; Villota-Rivas, M.; Palayew, A.; Carrieri, P.; Colombo, M.; Ekstedt, M.; Esmat, G.; George, J.; Marchesini, G. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge? J. Hepatol. 2022, 76, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Jung, S.J.; Shim, J.-S.; Jeon, Y.W.; Seo, E.; Kim, H.C. Comparison of Computed Tomography-based Abdominal Adiposity Indexes as Predictors of Non-alcoholic Fatty Liver Disease Among Middle-aged Korean Men and Women. J. Prev. Med. Public Health 2020, 53, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S. Non-alcoholic Fatty Liver Disease as a Canonical Example of Metabolic Inflammatory-Based Liver Disease Showing a Sex-Specific Prevalence: Relevance of Estrogen Signaling. Front. Endocrinol. 2020, 11, 572490. [Google Scholar] [CrossRef]
- Pouliot, M.-C.; Després, J.-P.; Lemieux, S.; Moorjani, S.; Bouchard, C.; Tremblay, A.; Nadeau, A.; Lupien, P.J. Waist circumference and abdominal sagittal diameter: Best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am. J. Cardiol. 1994, 73, 460–468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).