Ecdysterone and Turkesterone—Compounds with Prominent Potential in Sport and Healthy Nutrition
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. 20-Hydroxyecdysterone
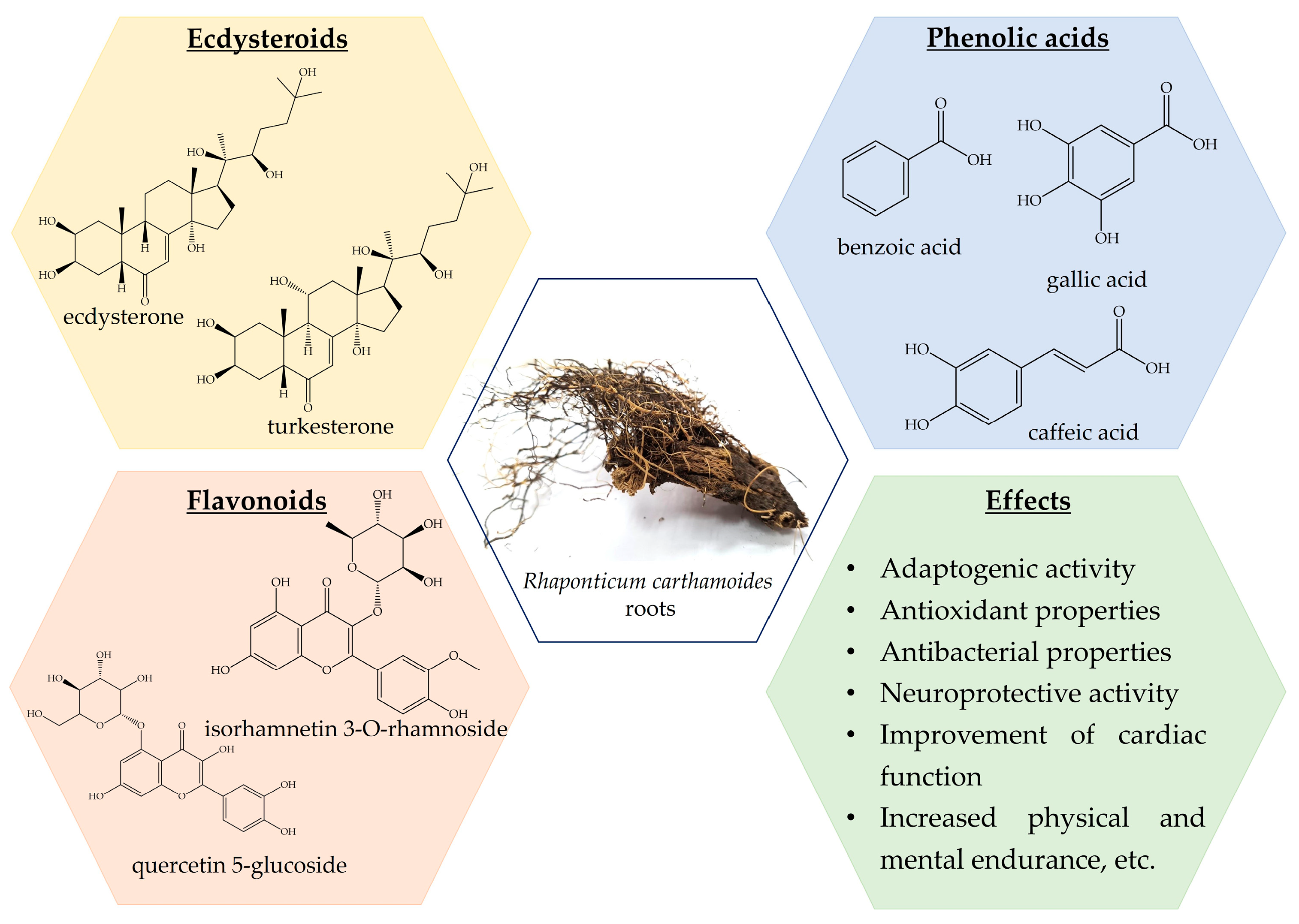
3.2. Rhaponticum carthamoides
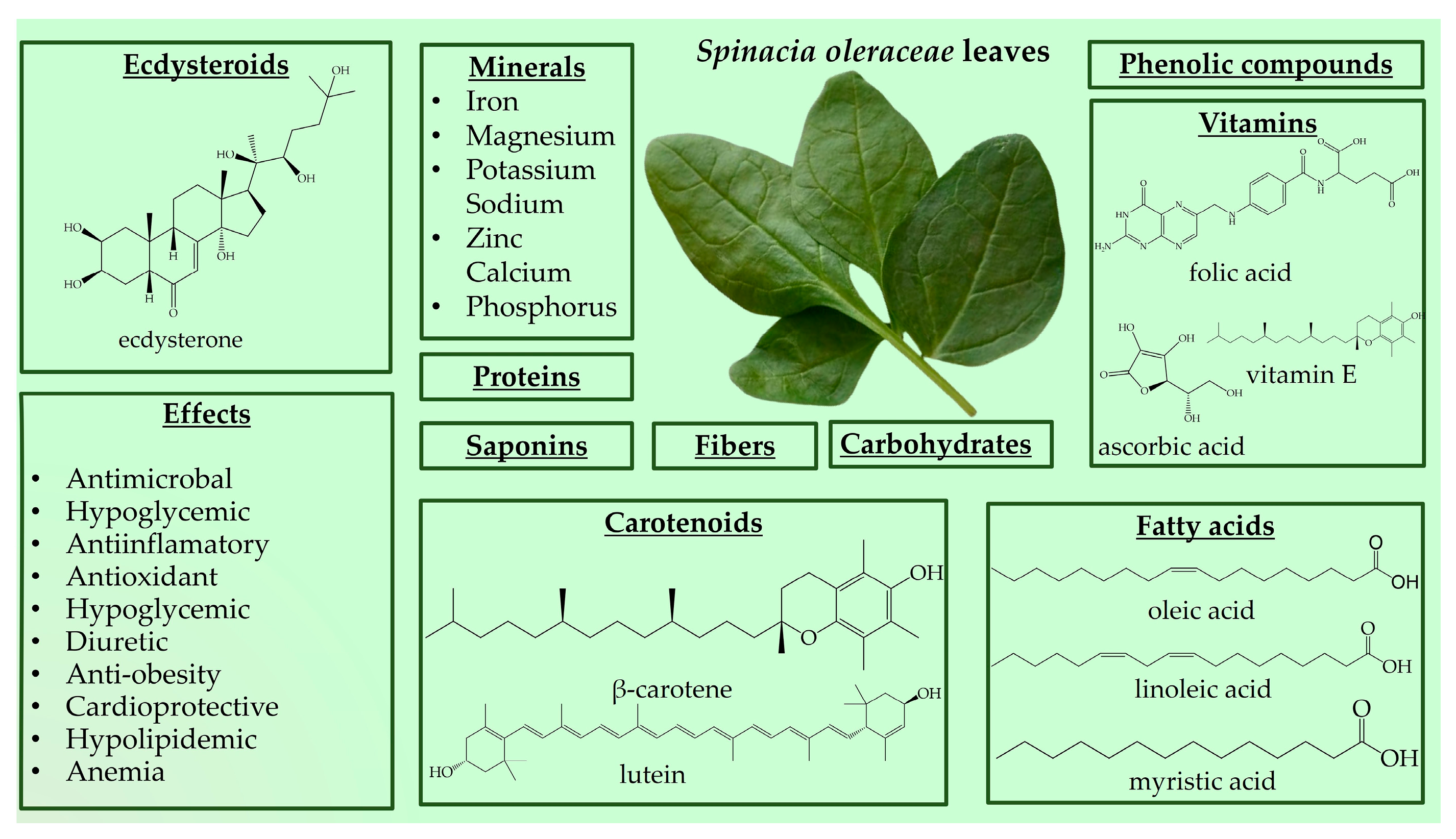
3.3. Spinacia oleracea
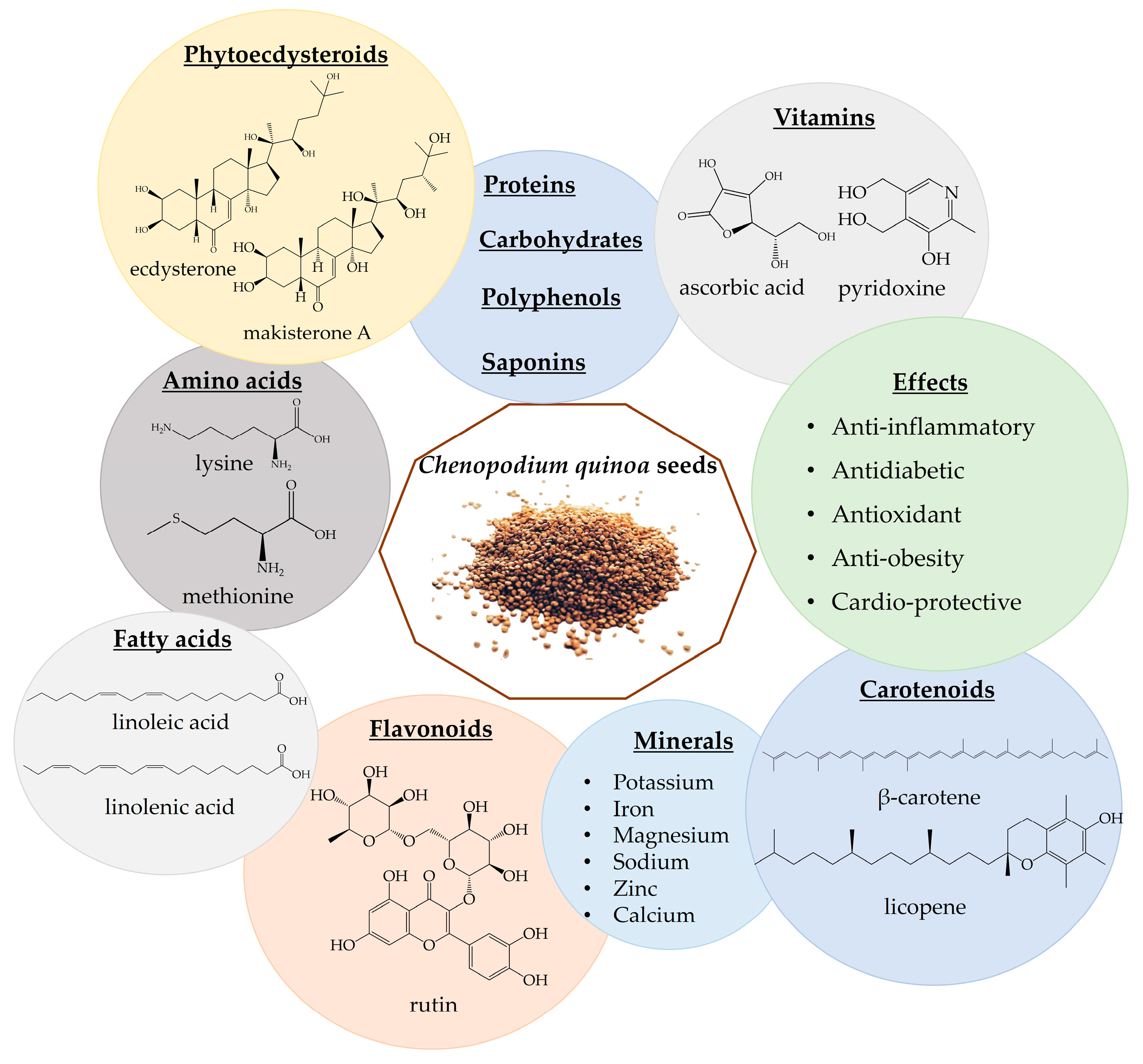
3.4. Chenopodium quinoa
3.5. Turkesterone and Ajuga turkestanica
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arif, Y.; Singh, P.; Bajguz, A.; Hayat, S. Phytoecdysteroids: Distribution, Structural Diversity, Biosynthesis, Activity, and Crosstalk with Phytohormones. Int. J. Mol. Sci. 2022, 23, 8664. [Google Scholar] [CrossRef]
- Lafont, R.; Balducci, C.; Dinan, L. Ecdysteroids. Encyclopedia 2021, 1, 1267–1302. [Google Scholar] [CrossRef]
- Huber, R.; Hoppe, W. Zur Chemie des Ecdysons, VII: Die Kristall-und Molekülstrukturanalyse des Insektenverpuppungshormons Ecdyson mit der automatisierten Faltmolekülmethode. Chem. Berichte 1965, 98, 2403–2424. [Google Scholar] [CrossRef]
- Butenandt, A.; Karlson, P. Über die Isolierung eines Metamorphose-Hormons der Insekten in kristallisierter Form. Z. Für Naturforschung 1954, 9, 389–391. [Google Scholar] [CrossRef]
- Ecdybase. The Ecdysone Handbook—A Free Online Ecdysteroids Database. Available online: https://ecdybase.org/ (accessed on 3 May 2023).
- Das, N.; Mishra, S.K.; Bishayee, A.; Ali, E.S.; Bishayee, A. The Phytochemical, Biological, and Medicinal Attributes of Phytoecdysteroids: An Updated Review. Acta Pharm. Sin. B 2021, 11, 1740–1766. [Google Scholar] [CrossRef]
- Nakanishi, K. The Ecdysones. Pure Appl. Chem. 1971, 25, 167–196. [Google Scholar] [CrossRef]
- Dinan, L. Phytoecdysteroids: Biological Aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Kubo, I.; Klocke, J.A.; Asano, S. Effects of Ingested Phytoecdysteroids on the Growth and Development of Two Lepidopterous Larvae. J. Insect Physiol. 1983, 29, 307–316. [Google Scholar] [CrossRef]
- Robbins, W.E.; Kaplanis, J.N.; Thompson, M.J.; Shortino, T.J.; Joyner, S.C. Ecdysones and Synthetic Analogs: Molting Hormone Activity and Inhibitive Effects on Insect Growth, Metamorphosis and Reproduction. Steroids 1970, 16, 105–125. [Google Scholar] [CrossRef]
- Dinan, L. Ecdysteroid Structure-Activity Relationships. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Bioactive Natural Products (Part J); Elsevier: Amsterdam, The Netherlands, 2003; Volume 29, pp. 3–71. [Google Scholar]
- Savchenko, R.G.; Veskina, N.A.; Odinokov, V.N.; Benkovskaya, G.V.; Parfenova, L.V. Ecdysteroids: Isolation, Chemical Transformations, and Biological Activity. Phytochem. Rev. 2022, 21, 1445–1486. [Google Scholar] [CrossRef]
- Kokoska, L.; Janovska, D. Chemistry and Pharmacology of Rhaponticum carthamoides: A Review. Phytochemistry 2009, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Mamarasulov, B.; Davranov, K.; Jabborova, D. Phytochemical, Pharmacological and Biological Properties of Ajuga turkestanica (Rgl.) Brig (Lamiaceae). Ann. Phytomed. Int. J. 2020, 9, 44–57. [Google Scholar] [CrossRef]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef]
- Bajguz, A.; Bąkała, I.; Talarek, M. Chapter 5—Ecdysteroids in Plants and Their Pharmacological Effects in Vertebrates and Humans. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 45, pp. 121–145. [Google Scholar]
- Odinokov, V.N.; Galyautdinov, I.V.; Nedopekin, D.V.; Khalilov, L.M.; Shashkov, A.S.; Kachala, V.V.; Dinan, L.; Lafont, R. Phytoecdysteroids from the Juice of Serratula coronata L. (Asteraceae). Insect Biochem. Mol. Biol. 2002, 32, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L.; Lafont, R. Chapter Two—Ecdysteroids as Defensive Chemicals. In Advances in Insect Physiology; Jurenka, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 63, pp. 107–154. [Google Scholar]
- Gorelick-Feldman, J.; MacLean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids Increase Protein Synthesis in Skeletal Muscle Cells. J. Agric. Food Chem. 2008, 56, 3532–3537. [Google Scholar] [CrossRef]
- Todorova, M.N.; Savova, M.S.; Mihaylova, L.V.; Georgiev, M.I. Icariin Improves Stress Resistance and Extends Lifespan in Caenorhabditis Elegans through Hsf-1 and Daf-2-Driven Hormesis. Int. J. Mol. Sci. 2023, 25, 352. [Google Scholar] [CrossRef] [PubMed]
- Savova, M.S.; Todorova, M.N.; Apostolov, A.G.; Yahubyan, G.T.; Georgiev, M.I. Betulinic Acid Counteracts the Lipid Accumulation in Caenorhabditis Elegans by Modulation of Nhr-49 Expression. Biomed. Pharmacother. 2022, 156, 113862. [Google Scholar] [CrossRef]
- Ivanova, S.; Delattre, C.; Karcheva-Bahchevanska, D.; Benbasat, N.; Nalbantova, V.; Ivanov, K. Plant-Based Diet as a Strategy for Weight Control. Foods 2021, 10, 3052. [Google Scholar] [CrossRef]
- Kraiem, S.; Al-Jaber, M.Y.; Al-Mohammed, H.; Al-Menhali, A.S.; Al-Thani, N.; Helaleh, M.; Samsam, W.; Touil, S.; Beotra, A.; Georgakopoulas, C.; et al. Analytical Strategy for the Detection of Ecdysterone and Its Metabolites in vivo in uPA(+/+)-SCID Mice with Humanized Liver, Human Urine Samples, and Estimation of Prevalence of Its Use in Anti-doping Samples. Drug Test. Anal. 2021, 13, 1341–1353. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Allison, D.B.; Coates, P.M. Dietary Supplements in Weight Reduction. J. Am. Diet. Assoc. 2005, 105, 80–86. [Google Scholar] [CrossRef]
- Staynova, R.; Yanachkova, V. Weight Management Strategies and Food Supplement Intake among Bulgarian Adults: Results of a National Survey. Pharmacia 2023, 70, 1119–1126. [Google Scholar] [CrossRef]
- Isenmann, E.; Ambrosio, G.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Zimmer, P.; Kazlauskas, R.; Goebel, C.; Botrè, F.; Diel, P.; et al. Ecdysteroids as Non-Conventional Anabolic Agent: Performance Enhancement by Ecdysterone Supplementation in Humans. Arch. Toxicol. 2019, 93, 1807–1816. [Google Scholar] [CrossRef]
- Ambrosio, G.; Wirth, D.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Botrè, F.; Parr, M.K. How Reliable Is Dietary Supplement Labelling? Experiences from the Analysis of Ecdysterone Supplements. J. Pharm. Biomed. Anal. 2020, 177, 112877. [Google Scholar] [CrossRef]
- Todorova, V.; Ivanov, K.; Karcheva-Bahchevanska, D.; Ivanova, S. Development and Validation of High-Performance Liquid Chromatography for Identification and Quantification of Phytoecdysteroids Ecdysterone and Turkesterone in Dietary Supplements. Processes 2023, 11, 1786. [Google Scholar] [CrossRef]
- Zubeldia, J.M.; Hernández-Santana, A.; Jiménez-del-Rio, M.; Pérez-López, V.; Pérez-Machín, R.; García-Castellano, J.M. In Vitro Characterization of the Efficacy and Safety Profile of a Proprietary Ajuga turkestanica Extract. Chin. Med. 2012, 2012, 26159. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, J.H.; Ham, H.J.; Kim, H.; Lee, J.; Baek, S.Y. Development of a Method for Simultaneous Screening of Four Natural-Derived Steroids and Their Analogues Used as Dietary Supplements via Liquid Chromatography-Quadrupole-Time of Flight Mass Spectrometry and Liquid Chromatography-Tandem Mass Spectrometry. Food Addit. Contam. Part A 2022, 39, 829–837. [Google Scholar] [CrossRef]
- Dinan, L.; Dioh, W.; Veillet, S.; Lafont, R. 20-Hydroxyecdysone, from Plant Extracts to Clinical Use: Therapeutic Potential for the Treatment of Neuromuscular, Cardio-Metabolic and Respiratory Diseases. Biomedicines 2021, 9, 492. [Google Scholar] [CrossRef]
- Pálinkás, Z.; Békési, D.; Utczás, M. Quantitation of Ecdysterone and Targeted Analysis of WADA-Prohibited Anabolic Androgen Steroids, Hormones, and Metabolic Modulators in Ecdysterone-Containing Dietary Supplements. Separations 2023, 10, 242. [Google Scholar] [CrossRef]
- The WADA 2020 Monitoring Program. Available online: https://www.wada-ama.org/sites/default/files/wada_2020_english_monitoring_program_.pdf (accessed on 3 May 2023).
- Hunyadi, A.; Herke, I.; Lengyel, K.; Báthori, M.; Kele, Z.; Simon, A.; Tóth, G.; Szendrei, K. Ecdysteroid-Containing Food Supplements from Cyanotis Arachnoidea on the European Market: Evidence for Spinach Product Counterfeiting. Sci. Rep. 2016, 6, 37322. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and Health Benefits of Quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Isenmann, E.; Yuliandra, T.; Touvleliou, K.; Broekmann, M.; de la Torre, X.; Botrè, F.; Diel, P.; Parr, M.K. Quinoa as Functional Food? Urinary Elimination of Ecdysterone after Consumption of Quinoa Alone and in Combination with spinach. bioRxiv 2023. [Google Scholar] [CrossRef]
- Schmidt, D.; Verruma-Bernardi, M.R.; Forti, V.A.; Borges, M.T.M.R. Quinoa and Amaranth as Functional Foods: A Review. Food Rev. Int. 2023, 39, 2277–2296. [Google Scholar] [CrossRef]
- Fehér, A.; Gazdecki, M.; Véha, M.; Szakály, M.; Szakály, Z. A Comprehensive Review of the Benefits of and the Barriers to the Switch to a Plant-Based Diet. Sustainability 2020, 12, 4136. [Google Scholar] [CrossRef]
- Sidorova, Y.S.; Petrov, N.A.; Shipelin, V.A.; Mazo, V.K. Spinach and quinoa—Prospective food sources of biologically active substances. Vopr. Pitan. 2020, 89, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Macáková, K.; Chlebek, J.; Hošt’álková, A.; Kulhánková, A.; Opletal, L. Ecdysterone and Its Activity on Some Degenerative Diseases. Nat. Prod. Commun. 2011, 6, 1934578X1100600527. [Google Scholar] [CrossRef]
- Chermnykh, N.S.; Shimanovskiĭ, N.L.; Shutko, G.V.; Syrov, V.N. The action of methandrostenolone and ecdysterone on the physical endurance of animals and on protein metabolism in the skeletal muscles. Farmakol. Toksikol. 1988, 51, 57–60. [Google Scholar]
- Kholodova, I.D.; Tugaĭ, V.A.; Zimina, V.P. Effect of vitamin D3 and 20-hydroxyecdysone on the content of ATP, creatine phosphate, carnosine and Ca2+ in skeletal muscles. Ukr. Biokhimicheskii Zhurnal (1978) 1997, 69, 3–9. [Google Scholar]
- Yoshida, T.; Otaka, T.; Uchiyama, M.; Ogawa, S. Effect of Ecdysterone on Hyperglycemia in Experimental Animals. Biochem. Pharmacol. 1971, 20, 3263–3268. [Google Scholar] [CrossRef]
- Lupien, P.J.; Hinse, C.; Chaudhary, K.D. Ecdysone as A Hypocholesterolemic Agent. Arch. Int. Physiol. Biochim. 1969, 77, 206–212. [Google Scholar] [CrossRef]
- Ogawa, S.; Nishimoto, N.; Matsuda, H. Pharmacology of Ecdysones in Vertebrates. In Invertebrate Endocrinology and Hormonal Heterophylly; Burdette, W.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1974; pp. 341–344. ISBN 978-3-642-65769-6. [Google Scholar]
- Seidlova-Wuttke, D.; Ehrhardt, C.; Wuttke, W. Metabolic Effects of 20-OH-Ecdysone in Ovariectomized Rats. J. Steroid Biochem. Mol. Biol. 2010, 119, 121–126. [Google Scholar] [CrossRef]
- Lafont, R.; Dinan, L. Practical Uses for Ecdysteroids in Mammals Including Humans: And Update. J. Insect Sci. 2003, 3, 7. [Google Scholar] [CrossRef]
- Syrov, V.N. Comparative Experimental Investigation of the Anabolic Activity of Phytoecdysteroids and Steranabols. Pharm. Chem. J. 2000, 34, 193–197. [Google Scholar] [CrossRef]
- Franco, R.R.; de Almeida Takata, L.; Chagas, K.; Justino, A.B.; Saraiva, A.L.; Goulart, L.R.; de Melo Rodrigues Ávila, V.; Otoni, W.C.; Espindola, F.S.; da Silva, C.R. A 20-Hydroxyecdysone-Enriched Fraction from Pfaffia glomerata (Spreng.) Pedersen Roots Alleviates Stress, Anxiety, and Depression in Mice. J. Ethnopharmacol. 2021, 267, 113599. [Google Scholar] [CrossRef]
- Kapur, P.; Wuttke, W.; Jarry, H.; Seidlova-Wuttke, D. Beneficial Effects of β-Ecdysone on the Joint, Epiphyseal Cartilage Tissue and Trabecular Bone in Ovariectomized Rats. Phytomedicine 2010, 17, 350–355. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, R.; Guo, H.; Dong, M. Phytoestrogen β-Ecdysterone Protects PC12 Cells Against MPP+-Induced Neurotoxicity In Vitro: Involvement of PI3K-Nrf2-Regulated Pathway. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 147, 28–38. [Google Scholar] [CrossRef]
- Mamadalieva, N.; Egamberdieva, D.; Tiezzi, A. In Vitro Biological Activities of the Components from Silene wallichiana. Med. Aromat. Plant Sci. Biotechnol. 2013, 7, 1–6. [Google Scholar]
- Takei, M.; Endo, K.; Nishimoto, N.; Shiobara, Y.; Inoue, S.; Matsuo, S. Effect of Ecdysterone on Histamine Release from Rat Peritoneal Mast Cells. J. Pharm. Sci. 1991, 80, 309–310. [Google Scholar] [CrossRef]
- Xu, T.; Niu, C.; Zhang, X.; Dong, M. β-Ecdysterone Protects SH-SY5Y Cells against β-Amyloid-Induced Apoptosis via c-Jun N-Terminal Kinase- and Akt-Associated Complementary Pathways. Lab. Invest. 2018, 98, 489–499. [Google Scholar] [CrossRef]
- Hung, T.-J.; Chen, W.-M.; Liu, S.-F.; Liao, T.-N.; Lee, T.-C.; Chuang, L.-Y.; Guh, J.-Y.; Hung, C.-Y.; Hung, Y.-J.; Chen, P.; et al. 20-Hydroxyecdysone Attenuates TGF-Β1-Induced Renal Cellular Fibrosis in Proximal Tubule Cells. J. Diabetes Complicat. 2012, 26, 463–469. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, B.; Ren, L.; Yang, J.; Zheng, Z.; Yao, F.; Ding, R.; Wang, J.; He, J.; Wang, W.; et al. 20-Hydroxyecdysone Inhibits Inflammation via SIRT6-Mediated NF-κB Signaling in Endothelial Cells. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2023, 1870, 119460. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Yue, Z.-S.; Li, G.-S.; Zeng, L.-R.; Xin, D.-W.; Hu, Z.-Q.; Xu, C.-D. Effect of Β-ecdysterone on Glucocorticoid-induced Apoptosis and Autophagy in Osteoblasts. Mol. Med. Rep. 2018, 17, 158–164. [Google Scholar] [CrossRef]
- Mamadalieva, N.; El-Readi, M.Z.; Ovidi, E.; Ashour, M.L.; Hamoud, R.; Sagdullaev, S.S.; Azimova, S.S.; Tiezzi, A.; Wink, M. Antiproliferative, Antimicrobial and Antioxidant Activities of the Chemical Constituents of Ajuga turkestanica. Phytopharmacology 2013, 4, 1–18. [Google Scholar]
- Chen, Q.; Xia, Y.; Qiu, Z. Effect of Ecdysterone on Glucose Metabolism in vitro. Life Sci. 2006, 78, 1108–1113. [Google Scholar] [CrossRef]
- Shuvalov, O.; Fedorova, O.; Tananykina, E.; Gnennaya, Y.; Daks, A.; Petukhov, A.; Barlev, N.A. An Arthropod Hormone, Ecdysterone, Inhibits the Growth of Breast Cancer Cells via Different Mechanisms. Front. Pharmacol. 2020, 11, 561537. [Google Scholar] [CrossRef]
- Smirnova, G.; Bezmaternykh, K.; Oktyabrsky, O.N. The Effect of 20-hydroxyecdysone on the Susceptibility of Escherichia coli to Different Antibiotics. J. Appl. Microbiol. 2016, 121, 1511–1518. [Google Scholar] [CrossRef]
- Martins, A.; Sipos, P.; Dér, K.; Csábi, J.; Miklos, W.; Berger, W.; Zalatnai, A.; Amaral, L.; Molnár, J.; Szabó-Révész, P.; et al. Ecdysteroids Sensitize MDR and Non-MDR Cancer Cell Lines to Doxorubicin, Paclitaxel, and Vincristine but Tend to Protect Them from Cisplatin. BioMed Res. Int. 2015, 2015, e895360. [Google Scholar] [CrossRef]
- Trenin, D.S.; Volodin, V.V. 20-Hydroxyecdysone as a Human Lymphocyte and Neutrophil Modulator: In Vitro Evaluation. Arch. Insect Biochem. Physiol. 1999, 41, 156–161. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; El-Readi, M.Z.; Janibekov, A.A.; Tahrani, A.; Wink, M. Phytoecdysteroids of Silene Guntensis and Their in Vitro Cytotoxic and Antioxidant Activity. Z. Für Naturforschung C 2011, 66, 215–224. [Google Scholar] [CrossRef]
- Romaniuk-Drapała, A.; Lisiak, N.; Totoń, E.; Matysiak, A.; Nawrot, J.; Nowak, G.; Kaczmarek, M.; Rybczyńska, M.; Rubiś, B. Proapoptotic and Proautophagic Activity of 20-Hydroxyecdysone in Breast Cancer Cells in vitro. Chem. Biol. Interact. 2021, 342, 109479. [Google Scholar] [CrossRef]
- Omanakuttan, A.; Bose, C.; Pandurangan, N.; Kumar, G.B.; Banerji, A.; Nair, B.G. Nitric Oxide and ERK Mediates Regulation of Cellular Processes by Ecdysterone. Exp. Cell Res. 2016, 346, 167–175. [Google Scholar] [CrossRef]
- Martucciello, S.; Paolella, G.; Muzashvili, T.; Skhirtladze, A.; Pizza, C.; Caputo, I.; Piacente, S. Steroids from Helleborus Caucasicus Reduce Cancer Cell Viability Inducing Apoptosis and GRP78 Down-Regulation. Chem. Biol. Interact. 2018, 279, 43–50. [Google Scholar] [CrossRef]
- Todorova, V.; Savova, M.S.; Ivanova, S.; Ivanov, K.; Georgiev, M.I. Anti-Adipogenic Activity of Rhaponticum carthamoides and Its Secondary Metabolites. Nutrients 2023, 15, 3061. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Xu, T.; Qin, S. β-Ecdysterone Suppresses Interleukin-1β-Induced Apoptosis and Inflammation in Rat Chondrocytes via Inhibition of NF-κB Signaling Pathway. Drug Dev. Res. 2014, 75, 195–201. [Google Scholar] [CrossRef]
- Catalan, R.E.; Aragones, M.D.; Godoy, J.E.; Martinez, A.M. Ecdysterone Induces Acetylcholinesterase in Mammalian Brain. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1984, 78, 193–195. [Google Scholar] [CrossRef]
- Dai, W.-W.; Wang, L.-B.; Jin, G.-Q.; Wu, H.-J.; Zhang, J.; Wang, C.-L.; Wei, Y.-J.; Lee, J.-H.; Lay, Y.-A.E.; Yao, W. Beta-Ecdysone Protects Mouse Osteoblasts from Glucocorticoid-Induced Apoptosis in vitro. Planta Med. 2017, 83, 888–894. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.; Zhou, J.; Wang, X.; Guo, L. Isolation and Purification of Three Ecdysteroids from the Stems of Diploclisia Glaucescens by High-Speed Countercurrent Chromatography and Their Anti-Inflammatory Activities in vitro. Molecules 2017, 22, 1310. [Google Scholar] [CrossRef]
- Yan, C.-P.; Wang, X.-K.; Jiang, K.; Yin, C.; Xiang, C.; Wang, Y.; Pu, C.; Chen, L.; Li, Y.-L. β-Ecdysterone Enhanced Bone Regeneration through the BMP-2/SMAD/RUNX2/Osterix Signaling Pathway. Front. Cell Dev. Biol. 2022, 10, 883228. [Google Scholar] [CrossRef]
- Catalán, R.E.; Martinez, A.M.; Aragones, M.D.; Miguel, B.G.; Robles, A.; Godoy, J.E. Alterations in Rat Lipid Metabolism Following Ecdysterone Treatment. Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 81, 771–775. [Google Scholar] [CrossRef]
- Gholipour, P.; Komaki, A.; Ramezani, M.; Parsa, H. Effects of the Combination of High-Intensity Interval Training and Ecdysterone on Learning and Memory Abilities, Antioxidant Enzyme Activities, and Neuronal Population in an Amyloid-Beta-Induced Rat Model of Alzheimer’s Disease. Physiol. Behav. 2022, 251, 113817. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.M.; Zwetsloot, K.A.; Arthur, S.T.; Sherman, C.A.; Huot, J.R.; Badmaev, V.; Grace, M.; Lila, M.A.; Nieman, D.C.; Shanely, R.A. Phytoecdysteroids Do Not Have Anabolic Effects in Skeletal Muscle in Sedentary Aging Mice. Int. J. Environ. Res. Public. Health 2021, 18, 370. [Google Scholar] [CrossRef]
- Kizelsztein, P.; Govorko, D.; Komarnytsky, S.; Evans, A.; Wang, Z.; Cefalu, W.T.; Raskin, I. 20-Hydroxyecdysone Decreases Weight and Hyperglycemia in a Diet-Induced Obesity Mice Model. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E433–E439. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, L.; Lay, Y.-A.E.; Chen, H.; Jin, G.; Zhang, H.; Kot, A.; Ritchie, R.O.; Lane, N.E.; Yao, W. Prevention of Glucocorticoid Induced Bone Changes with Beta-Ecdysone. Bone 2015, 74, 48–57. [Google Scholar] [CrossRef]
- Wu, J.; Gao, L.; Shang, L.; Wang, G.; Wei, N.; Chu, T.; Chen, S.; Zhang, Y.; Huang, J.; Wang, J.; et al. Ecdysterones from Rhaponticum carthamoides (Willd.) Iljin Reduce Hippocampal Excitotoxic Cell Loss and Upregulate mTOR Signaling in Rats. Fitoterapia 2017, 119, 158–167. [Google Scholar] [CrossRef]
- Catalan, R.E.; Martinez, A.M.; Aragones, M.D.; Miguel, B.G.; Robles, A.; Godoy, J.E. Effect of Ecdysterone Treatment on the Cyclic AMP-Protein Kinase System in Adipose Tissue. J. Steroid Biochem. 1982, 16, 573–576. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Q.; Liu, R.; Wang, Z.; Tang, N.; Liu, F.; Huang, G.; Jiang, X.; Gui, G.; Wang, L.; et al. Effects of 20-Hydroxyecdysone on Improving Memory Deficits in Streptozotocin-Induced Type 1 Diabetes Mellitus in Rat. Eur. J. Pharmacol. 2014, 740, 45–52. [Google Scholar] [CrossRef]
- You, W.-L.; Xu, Z.-L. β-Ecdysone Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. J. Gene Med. 2020, 22, e3207. [Google Scholar] [CrossRef]
- Catalan, R.E.; Aragonés, M.D.; Martínez, A.M. Effect of Ecdysterone on Cyclic AMP and Cyclic GMP in Mouse Plasma. Biochem. Biophys. Res. Commun. 1979, 87, 1018–1023. [Google Scholar] [CrossRef]
- Catalán, R.E.; Aragonés, M.D.; Martínez, A.M. Effect of Ecdysterone on the Cyclic AMP-Protein Kinase System in Mouse Liver. Biochem. Biophys. Res. Commun. 1979, 89, 44–49. [Google Scholar] [CrossRef]
- Parr, M.K.; Zhao, P.; Haupt, O.; Ngueu, S.T.; Hengevoss, J.; Fritzemeier, K.H.; Piechotta, M.; Schlörer, N.; Muhn, P.; Zheng, W.-Y.; et al. Estrogen Receptor Beta Is Involved in Skeletal Muscle Hypertrophy Induced by the Phytoecdysteroid Ecdysterone. Mol. Nutr. Food Res. 2014, 58, 1861–1872. [Google Scholar] [CrossRef]
- Marschall, M.J.M.; Ringseis, R.; Gessner, D.K.; Grundmann, S.M.; Most, E.; Wen, G.; Maheshwari, G.; Zorn, H.; Eder, K. Effect of Ecdysterone on the Hepatic Transcriptome and Lipid Metabolism in Lean and Obese Zucker Rats. Int. J. Mol. Sci. 2021, 22, 5241. [Google Scholar] [CrossRef]
- Lim, H.-S.; Yoon, K.; Lee, D.H.; Lee, Y.-S.; Chung, J.H.; Park, G. Effects of 20-Hydroxyecdysone on UVB-Induced Photoaging in Hairless Mice. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 164, 114899. [Google Scholar] [CrossRef]
- Gao, L.; Cai, G.; Shi, X. Beta-Ecdysterone Induces Osteogenic Differentiation in Mouse Mesenchymal Stem Cells and Relieves Osteoporosis. Biol. Pharm. Bull. 2008, 31, 2245–2249. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Wessels, J.T.; Wuttke, W.; Seidlová-Wuttke, D. The Effects of 20-Hydroxyecdysone and 17β-Estradiol on the Skin of Ovariectomized Rats. Menopause 2011, 18, 323–327. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, D.-L.; Liu, Z.-X.; Sun, X.-H.; Li, Y. Beneficial Effect of 20-hydroxyecdysone Exerted by Modulating Antioxidants and Inflammatory Cytokine Levels in Collagen-induced Arthritis: A Model for Rheumatoid Arthritis. Mol. Med. Rep. 2017, 16, 6162–6169. [Google Scholar] [CrossRef]
- Anthony, T.G.; Mirek, E.T.; Bargoud, A.R.; Phillipson-Weiner, L.; DeOliveira, C.M.; Wetstein, B.; Graf, B.L.; Kuhn, P.E.; Raskin, I. Evaluating the Effect of 20-Hydroxyecdysone (20HE) on Mechanistic Target of Rapamycin Complex 1 (mTORC1) Signaling in the Skeletal Muscle and Liver of Rats. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 1324–1328. [Google Scholar] [CrossRef]
- Konovalova, N.P.; Mitrokhin, Y.I.; Volkova, L.M.; Sidorenko, L.I.; Todorov, I.N. Ecdysterone Modulates Antitumor Activity of Cytostatics and Biosynthesis of Macromolecules in Tumor-Bearing Mice. Biol. Bull. Russ. Acad. Sci. 2002, 29, 530–536. [Google Scholar] [CrossRef]
- Fenner, R.; Zimmer, A.R.; Neves, G.; Kliemann, M.; Gosmann, G.; Rates, S.M.K. Hypnotic Effect of Ecdysterone Isolated from Pfaffia glomerata (Spreng.) Pedersen. Rev. Bras. Farmacogn. 2008, 18, 170–176. [Google Scholar] [CrossRef]
- Fedorov, V.N.; Punegova, N.V.; Zainullin, V.G.; Punegov, V.V.; Sychev, R.L. Extraction of Ecdysterone-80 from Serratula coronata L. and Evaluation of Its Pharmacological Actions. II. Cardioprotective Properties. Effects on Hormone-Transmitter Balance in Chronic Cardiac Failure. Pharm. Chem. J. 2009, 43, 36–40. [Google Scholar] [CrossRef]
- Ramazanov, N.S.; Bobayev, I.D.; Yusupova, U.Y.; Aliyeva, N.K.; Egamova, F.R.; Yuldasheva, N.K.; Syrov, V.N. Phytoecdysteroids-Containing Extract from Stachys Hissarica Plant and Its Wound-Healing Activity. Nat. Prod. Res. 2017, 31, 593–597. [Google Scholar] [CrossRef]
- Yang, L.; Pan, J. Therapeutic Effect of Ecdysterone Combine Paeonol Oral Cavity Direct Administered on Radiation-Induced Oral Mucositis in Rats. Int. J. Mol. Sci. 2019, 20, 3800. [Google Scholar] [CrossRef]
- Tóth, N.; Szabó, A.; Kacsala, P.; Héger, J.; Zádor, E. 20-Hydroxyecdysone Increases Fiber Size in a Muscle-Specific Fashion in Rat. Phytomed. Int. J. Phytother. Phytopharm. 2008, 15, 691–698. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Wuttke, W. In a Placebo-Controlled Study ß-Ecdysone (ECD) Prevented the Development of the Metabolic Syndrome. Planta Med. 2012, 78, CL37. [Google Scholar] [CrossRef]
- Dioh, W.; Tourette, C.; Del Signore, S.; Daudigny, L.; Dupont, P.; Balducci, C.; Dilda, P.J.; Lafont, R.; Veillet, S. A Phase 1 Study for Safety and Pharmacokinetics of BIO101 (20-Hydroxyecdysone) in Healthy Young and Older Adults. J. Cachexia Sarcopenia Muscle 2023, 14, 1259–1273. [Google Scholar] [CrossRef]
- Wilborn, C.D.; Taylor, L.W.; Campbell, B.I.; Kerksick, C.; Rasmussen, C.J.; Greenwood, M.; Kreider, R.B. Effects of Methoxyisoflavone, Ecdysterone, and Sulfo-Polysaccharide Supplementation on Training Adaptations in Resistance-Trained Males. J. Int. Soc. Sports Nutr. 2006, 3, 19–27. [Google Scholar] [CrossRef]
- Bathori, M.; Toth, N.; Hunyadi, A.; Marki, A.; Zador, E. Phytoecdysteroids and Anabolic-Androgenic Steroids—Structure and Effects on Humans. Curr. Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef]
- Łotocka, B.; Geszprych, A. Anatomy of the Vegetative Organs and Secretory Structures of Rhaponticum carthamoides (Asteraceae). Bot. J. Linn. Soc. 2004, 144, 207–233. [Google Scholar] [CrossRef]
- Timofeev, N.P. Leuzea carthamoides DC.: Application Prospects as Pharmpreparations and Biologically Active Components. In Functional Foods for Chronic Diseases; D&A Incorporated: Richardson, TX, USA, 2006. [Google Scholar]
- Brekhman, I.I.; Dardymov, I.V. New Substances of Plant Origin Which Increase Nonspecific Resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef]
- Opletal, L.; Sovová, M.; Dittrich, M.; Solich, P.; Dvorák, J.; Krátký, F.; Cerovský, J.; Hofbauer, J. Phytotherapeutic aspects of diseases of the circulatory system. 6. Leuzea carthamoides (Willd.) DC: The status of research and possible use of the taxon. Ceska Slov. Farm. 1997, 46, 247–255. [Google Scholar] [PubMed]
- Todorova, V.; Ivanov, K.; Ivanova, S. Comparison between the Biological Active Compounds in Plants with Adaptogenic Properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants 2022, 11, 64. [Google Scholar] [CrossRef]
- Sharaf, M.; Skiba, A.; Weglarz, Z.; El-Ansari, M.A. Two Flavonol 5-O-Glycosides from the Roots of Leuzea carthamoides. Fitoterapia 2001, 72, 940–942. [Google Scholar] [CrossRef]
- Skiba, A.; Weglarz, Z. Phenolic acids of Rhaponticum carthamoides. Acta Hortic. 2003, 597, 119–124. [Google Scholar] [CrossRef]
- Szendrei, K.; Varga, E.; Hajdú, Z.; Herke, I.; Lafont, R.; Girault, J.P. Ajugasterone C and 5-Deoxykaladasterone, an Ecdysteroid Artifact, from Leuzea Carthamoides. J. Nat. Prod. 1988, 51, 993–995. [Google Scholar] [CrossRef]
- Píš, J.; Buděšínskў, M.; Vokáč, K.; Laudová, V.; Harmatha, J. Ecdysteroids from the Roots of Leuzea carthamoides. Phytochemistry 1994, 37, 707–711. [Google Scholar] [CrossRef]
- Girault, J.-P.; Lafont, R.; Varga, E.; Hajdu, Z.; Herke, I.; Szendrei, K. Ecdysteroids from Leuzea Carthamoides. Phytochemistry 1988, 27, 737–741. [Google Scholar] [CrossRef]
- Bastaev, U.A.; Abubakirov, N.K. Phytoecdysteroids of Rhaponticum carthamoides. Chem. Nat. Compd. 1987, 23, 565–568. [Google Scholar] [CrossRef]
- Skała, E.; Rijo, P.; Garcia, C.; Sitarek, P.; Kalemba, D.; Toma, M.; Szemraj, J.; Pytel, D.; Wysokińska, H.; Śliwiński, T. The Essential Oils of Rhaponticum carthamoides Hairy Roots and Roots of Soil-Grown Plants: Chemical Composition and Antimicrobial, Anti-Inflammatory, and Antioxidant Activities. Oxid. Med. Cell. Longev. 2016, 2016, 8505384. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Ivanova, S.; Georgieva, Y.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Benbassat, N.; Savova, M.S.; Georgiev, M.I.; Ivanov, K. Chemical Composition and Histochemical Localization of Essential Oil from Wild and Cultivated Rhaponticum carthamoides Roots and Rhizomes. Plants 2022, 11, 2061. [Google Scholar] [CrossRef]
- Havlik, J.; Budesinsky, M.; Kloucek, P.; Kokoska, L.; Valterova, I.; Vasickova, S.; Zeleny, V. Norsesquiterpene Hydrocarbon, Chemical Composition and Antimicrobial Activity of Rhaponticum carthamoides Root Essential Oil. Phytochemistry 2009, 70, 414–418. [Google Scholar] [CrossRef]
- Geszprych, A.; Weglarz, Z. Composition of Essential Oil from Underground and Aboveground Organs of Rhaponticum carthamoides [Willd.] Iljin. Herba Pol. 2002, 48, 188–192. [Google Scholar]
- Krasnov, E.A.; Saratikov, A.S.; Yakunina, G.D. Inokosterone and Ecdysterone from Rhaponticum carthamoides. Chem. Nat. Compd. 1976, 12, 494–495. [Google Scholar] [CrossRef]
- Ramazanov, N.S.; Makshimov, E.S.; Saatov, Z.; Mamatkhanov, A.U.; Abdullaev, N.D. Phytoecdysteroids of Plants of the genus Rhaponticum I. Carthamosterone a from Rh. Carthamoides. Chem. Nat. Compd. 1997, 33, 301–302. [Google Scholar] [CrossRef]
- Martirosyan, D.M. Functional Foods for Chronic Diseases; D&A Inc.: Richardson, TX, USA, 2006; ISBN 978-0-9767535-2-0. [Google Scholar]
- Skała, E.; Kowalczyk, T.; Toma, M.; Szemraj, J.; Radek, M.; Pytel, D.; Wieczfinska, J.; Wysokińska, H.; Śliwiński, T.; Sitarek, P. Induction of Apoptosis in Human Glioma Cell Lines of Various Grades through the ROS-Mediated Mitochondrial Pathway and Caspase Activation by Rhaponticum carthamoides Transformed Root Extract. Mol. Cell. Biochem. 2018, 445, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Skała, E.; Sitarek, P.; Toma, M.; Szemraj, J.; Radek, M.; Nieborowska-Skorska, M.; Skorski, T.; Wysokińska, H.; Śliwiński, T. Inhibition of Human Glioma Cell Proliferation by Altered Bax/Bcl-2-P53 Expression and Apoptosis Induction by Rhaponticum carthamoides Extracts from Transformed and Normal Roots. J. Pharm. Pharmacol. 2016, 68, 1454–1464. [Google Scholar] [CrossRef]
- Zheng, Z.; Xian, Y.; Jin, Z.; Yao, F.; Liu, Y.; Deng, Y.; Wang, B.; Chen, D.; Yang, J.; Ren, L.; et al. Rhaponticum carthamoides Improved Energy Metabolism and Oxidative Stress through the SIRT6/Nrf2 Pathway to Ameliorate Myocardial Injury. Phytomedicine 2022, 105, 154197. [Google Scholar] [CrossRef]
- Jurkštienė, V.; Pavilonis, A.; Garšvienė, D.; Juozulynas, A.; Samsonienė, L.; Daukšienė, D.; Jankauskienė, K.; Šimonienė-Kazlauskienė, G.; Stankevičius, E. Investigation of the Antimicrobial Activity of Rhaponticum (Rhaponticum carthamoides D.C. Iljin) and Shrubby Cinquefoil (Potentilla fruticosa L.). Medicina 2011, 47, 24. [Google Scholar] [CrossRef]
- Peschel, W.; Kump, A.; Prieto, J.M. Effects of 20-Hydroxyecdysone, Leuzea carthamoides Extracts, Dexamethasone and Their Combinations on the NF-κB Activation in HeLa Cells. J. Pharm. Pharmacol. 2011, 63, 1483–1495. [Google Scholar] [CrossRef]
- Gaube, F.; Wölfl, S.; Pusch, L.; Werner, U.; Kroll, T.C.; Schrenk, D.; Hartmann, R.W.; Hamburger, M. Effects of Leuzea carthamoides on Human Breast Adenocarcinoma MCF-7 Cells Determined by Gene Expression Profiling and Functional Assays. Planta Med. 2008, 74, 1701–1708. [Google Scholar] [CrossRef]
- Skała, E.; Sitarek, P.; Różalski, M.; Krajewska, U.; Szemraj, J.; Wysokińska, H.; Śliwiński, T. Antioxidant and DNA Repair Stimulating Effect of Extracts from Transformed and Normal Roots of Rhaponticum carthamoides against Induced Oxidative Stress and DNA Damage in CHO Cells. Oxid. Med. Cell. Longev. 2016, 2016, 5753139. [Google Scholar] [CrossRef] [PubMed]
- Publication—Composition and Biological Activity of Rhaponticum carthamoides Extracts Obtained from Plants Collected in Poland and Russia—University of Gdańsk. Available online: https://repozytorium.bg.ug.edu.pl/info/article/UOGab0a3e66748d484882bc3dd7288a480a/ (accessed on 22 February 2024).
- Šelepcová, L.; Jalč, D.; Javorský, P.; Baran, M. Influence of Rhaponticum carthamoides Wild on the Growth of Ruminal Bacteria in Vitro and on Fermentation in an Artificial rumen (Rusitec). Arch. Für Tierernaehrung 1993, 43, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kokoska, L.; Janovska, D.; Rada, V.; Nepovim, A.; Vanek, T. In Vitro. Antibacterial Activity of Four Leuzea. Species. Pharm. Biol. 2005, 43, 8–11. [Google Scholar] [CrossRef]
- Kokoska, L.; Polesny, Z.; Rada, V.; Nepovim, A.; Vanek, T. Screening of Some Siberian Medicinal Plants for Antimicrobial Activity. J. Ethnopharmacol. 2002, 82, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Mácsai, L.; Datki, Z.L.; Csupor, D.; Horváth, A.; Zomborszki, Z.P. Biological Activities of Four Adaptogenic Plant Extracts and Their Active Substances on a Rotifer Model. Evid.-Based Complement. Altern. Med. 2018, 2018, 3690683. [Google Scholar] [CrossRef]
- Mosharrof, A.H. Effects of Extract from Rhapontcum carthamoides (Willd) Iljin (Leuzea) on Learning and Memory in Rats. Acta Physiol. Pharmacol. Bulg. 1987, 13, 37–42. [Google Scholar] [PubMed]
- Petkov, V.; Roussinov, K.; Todorov, S.; Lazarova, M.; Yonkov, D.; Draganova, S. Pharmacological Investigations on Rhaponticum carthamoides. Planta Med. 1984, 50, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Dushkin, M.; Khrapova, M.; Kovshik, G.; Chasovskikh, M.; Menshchikova, E.; Trufakin, V.; Shurlygina, A.; Vereschagin, E. Effects of Rhaponticum carthamoides versus Glycyrrhiza glabra and Punica granatum Extracts on Metabolic Syndrome Signs in Rats. BMC Complement. Altern. Med. 2014, 14, 33. [Google Scholar] [CrossRef]
- Roumanille, R.; Vernus, B.; Brioche, T.; Descossy, V.; Van Ba, C.T.; Campredon, S.; Philippe, A.G.; Delobel, P.; Bertrand-Gaday, C.; Chopard, A.; et al. Acute and Chronic Effects of Rhaponticum carthamoides and Rhodiola Rosea Extracts Supplementation Coupled to Resistance Exercise on Muscle Protein Synthesis and Mechanical Power in Rats. J. Int. Soc. Sports Nutr. 2020, 17, 58. [Google Scholar] [CrossRef]
- Baev, A.Y.; Charyshnikova, O.S.; Khasanov, F.A.; Nebesnaya, K.S.; Makhmudov, A.R.; Rahmedova, M.T.; Khushbaktova, Z.A.; Syrov, V.N.; Levitskaya, Y.V. Ecdysterone Prevents Negative Effect of Acute Immobilization Stress on Energy Metabolism of Rat Liver Mitochondria. J. Steroid Biochem. Mol. Biol. 2022, 219, 106066. [Google Scholar] [CrossRef]
- Shakhmurova, G.A.; Syrov, V.N.; Khushbaktova, Z.A. Immunomodulating and Antistress Activity of Ecdysterone and Turkesterone under Immobilization-Induced Stress Conditions in Mice. Pharm. Chem. J. 2010, 44, 7–9. [Google Scholar] [CrossRef]
- Neves, C.S.; Gomes, S.S.L.; Dos Santos, T.R.; De Almeida, M.M.; De Souza, Y.O.; Garcia, R.M.G.; Otoni, W.C.; Chedier, L.M.; Viccini, L.F.; De Campos, J.M.S. The Phytoecdysteroid β-Ecdysone Is Genotoxic in Rodent Bone Marrow Micronuclei and Allium cepa L. Assays. J. Ethnopharmacol. 2016, 177, 81–84. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Aliev, O.I.; Vasil’ev, A.S.; Andreeva, V.Y.; Krasnov, E.A.; Kalinkina, G.I. Effect of Rhaponticum carthamoides Extract on Structural and Metabolic Parameters of Erythrocytes in Rats with Cerebral Ischemia. Bull. Exp. Biol. Med. 2008, 146, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Selepcova, L.; Sommer, A.; Vargova, M. Effect of Feeding on a Diet Containing Varying Amounts of Rhaponticum carthamoides Hay Meal on Selected Morphological Parameters in Rats. EJE 2013, 92, 391–397. [Google Scholar]
- Ryan, E.D.; Gerstner, G.R.; Mota, J.A.; Trexler, E.T.; Giuliani, H.K.; Blue, M.N.M.; Hirsch, K.R.; Smith-Ryan, A.E. The Acute Effects of a Multi-Ingredient Herbal Supplement on Performance Fatigability: A Double-Blind, Randomized, and Placebo-Controlled Trial. J. Diet. Suppl. 2021, 18, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Nešković, M.; Ćulafić, L. Spinach (Spinacia oleracea L.). In Crops II; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1988; pp. 370–385. ISBN 978-3-642-73520-2. [Google Scholar]
- Murcia, M.A.; Jiménez-Monreal, A.M.; Gonzalez, J.; Martínez-Tomé, M. Chapter 11—Spinach. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 181–195. ISBN 978-0-12-812780-3. [Google Scholar]
- Pandey, S.C.; Kalloo, G. 23—Spinach: Spinacia oleracea L. In Genetic Improvement of Vegetable Crops; Kalloo, G., Bergh, B.O., Eds.; Pergamon: Amsterdam, The Netherlands, 1993; pp. 325–336. ISBN 978-0-08-040826-2. [Google Scholar]
- Huda-Faujan, N.; Zubairi, S.I.; Baker, A.A.A. Nutritional and Bioactive Constituents of Antioxidant and Antimicrobial Properties in Spinacia oleracea: A Review. Sains Malays. 2023, 52, 2571–2585. [Google Scholar] [CrossRef]
- Babu, N.R.; Divakar, J.; Krishna, U.L.; Vigneshwaran, C. Study of Antimicrobial, Antioxidant, Anti-Inflammatory Activities and Phytochemical Analysis of Cooked and Uncooked Different Spinach Leaves. J. Pharmacogn. Phytochem. 2018, 7, 1798–1803. [Google Scholar]
- Simko, I.; Hayes, R.J.; Mou, B.; McCreight, J.D. Lettuce and Spinach. In Yield Gains in Major U.S. Field Crops; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 53–85. ISBN 978-0-89118-620-5. [Google Scholar]
- Roberts, J.L.; Moreau, R. Functional Properties of Spinach (Spinacia oleracea L.) Phytochemicals and Bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Morelock, T.E.; Correll, J.C. Spinach. In Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Handbook of Plant Breeding; Springer: New York, NY, USA, 2008; pp. 189–218. ISBN 978-0-387-30443-4. [Google Scholar]
- Salehi, B.; Tumer, T.B.; Ozleyen, A.; Peron, G.; Dall’Acqua, S.; Rajkovic, J.; Naz, R.; Nosheen, A.; Mudau, F.N.; Labanca, F.; et al. Plants of the genus Spinacia: From Bioactive Molecules to Food and Phytopharmacological Applications. Trends Food Sci. Technol. 2019, 88, 260–273. [Google Scholar] [CrossRef]
- Ambo, A.I.; Patience, O.; Ayakeme, E.B. Evaluation of the proximate composition and metal content of spinach (Spinacia oleracea) from selected towns in nasarawa state, Nigeria. Sci. World J. 2023, 18, 26–30. [Google Scholar]
- Lomnitski, L.; Bergman, M.; Nyska, A.; Ben-Shaul, V.; Grossman, S. Composition, Efficacy, and Safety of Spinach Extracts. Nutr. Cancer 2003, 46, 222–231. [Google Scholar] [CrossRef]
- Olasupo, A.; Aborisade, A.; Olagoke, O. Phytochemical Analysis and Antibacterial Activities of Spinach Leaf. Am. J. Phytomed. Clin. Ther. 2018, 6, 8. [Google Scholar]
- Bakrim, A.; Maria, A.; Sayah, F.; Lafont, R.; Takvorian, N. Ecdysteroids in Spinach (Spinacia oleracea L.): Biosynthesis, Transport and Regulation of Levels. Plant Physiol. Biochem. 2008, 46, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, J.; Iraqi, R.H.; Bernstein, N. Ecdysteroid Content and Therapeutic Activity in Elicited Spinach Accessions. Plants 2020, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Grebenok, R.J.; Galbraith, D.W.; Bowers, W.S. Insect-Induced Synthesis of Phytoecdysteroids in Spinach, Spinacia oleracea. J. Chem. Ecol. 1999, 25, 1739–1757. [Google Scholar] [CrossRef]
- Bajkacz, S.; Rusin, K.; Wolny, A.; Adamek, J.; Erfurt, K.; Chrobok, A. Highly Efficient Extraction Procedures Based on Natural Deep Eutectic Solvents or Ionic Liquids for Determination of 20-Hydroxyecdysone in Spinach. Molecules 2020, 25, 4736. [Google Scholar] [CrossRef]
- Fang, X.; Szołtysik, R.; Tang, J.; Bajkacz, S. Efficient Extraction and Sensitive HPLC-MS/MS Quantification of Selected Ecdysteroids in Plants. J. Food Compos. Anal. 2022, 110, 104580. [Google Scholar] [CrossRef]
- Saeng-ngam, S.; Juntawong, N.; Vajarothai, S.; Visetson, S. Comparative study of moulting hormone content in different plant species. In Proceedings of the 42nd Kasetsart University Annual Conference, Kasetsart, Thailand, 3–6 February 2004; pp. 284–290. [Google Scholar]
- Grucza, K.; Wicka, M.; Drapała, A.; Kwiatkowska, D. Determination of Ecdysterone in Dietary Supplements and Spinach by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Separations 2022, 9, 8. [Google Scholar] [CrossRef]
- Bohlooli, S.; Barmaki, S.; Khoshkhahesh, F.; Nakhostin-Roohi, B. The Effect of Spinach Supplementation on Exercise-Induced Oxidative Stress. J. Sports Med. Phys. Fit. 2015, 55, 609–614. [Google Scholar]
- Yuliandra, T.; Touvleliou, K.; de la Torre, X.; Botrè, F.; Loke, S.; Isenmann, E.; Valder, S.; Diel, P.; Parr, M.K. Urinary Excretion of Ecdysterone and Its Metabolites Following Spinach Consumption. Mol. Nutr. Food Res. 2023, 67, 2200518. [Google Scholar] [CrossRef]
- Panda, V.; Shinde, P.; Dande, P. Consumption of Spinacia oleracea (Spinach) and Aerobic Exercise Controls Obesity in Rats by an Inhibitory Action on Pancreatic Lipase. Arch. Physiol. Biochem. 2020, 126, 187–195. [Google Scholar] [CrossRef]
- Panda, V.; Shinde, P. Appetite Suppressing Effect of Spinacia oleracea in Rats: Involvement of the Short Term Satiety Signal Cholecystokinin. Appetite 2017, 113, 224–230. [Google Scholar] [CrossRef]
- Bhatia, A.L.; Jain, M. Spinacia oleracea L. Protects against Gamma Radiations: A Study on Glutathione and Lipid Peroxidation in Mouse Liver. Phytomed. Int. J. Phytother. Phytopharm. 2004, 11, 607–615. [Google Scholar] [CrossRef]
- Rahati, S.; Kamalinezhad, M.; Ebrahimi, A.; Eshraghian, M.; Pishva, H. Accelerated Wound Healing Induced by Spinach Extract in Experimental Model Diabetic Rats with Streptozotocin. Sci. Rep. 2023, 13, 14933. [Google Scholar] [CrossRef]
- Heo, J.-C.; Park, C.-H.; Lee, H.-J.; Kim, S.-O.; Kim, T.-H.; Lee, S.-H. Amelioration of Asthmatic Inflammation by an Aqueous Extract of Spinacia oleracea Linn. Int. J. Mol. Med. 2010, 25, 409–414. [Google Scholar] [CrossRef]
- Ko, S.-H.; Park, J.-H.; Kim, S.-Y.; Lee, S.W.; Chun, S.-S.; Park, E. Antioxidant Effects of Spinach (Spinacia oleracea L.) Supplementation in Hyperlipidemic Rats. Prev. Nutr. Food Sci. 2014, 19, 19–26. [Google Scholar] [CrossRef]
- Breitbart, E.; Lomnitski, L.; Nyska, A.; Malik, Z.; Bergman, M.; Sofer, Y.; Haseman, J.K.; Grossman, S. Effects of Water-Soluble Antioxidant from Spinach, NAO, on Doxorubicin-Induced Heart Injury. Hum. Exp. Toxicol. 2001, 20, 337–345. [Google Scholar] [CrossRef]
- Pérez-Piñero, S.; Ávila-Gandía, V.; Rubio Arias, J.A.; Muñoz-Carrillo, J.C.; Losada-Zafrilla, P.; López-Román, F.J. A 12-Week Randomized Double-Blind Placebo-Controlled Clinical Trial, Evaluating the Effect of Supplementation with a Spinach Extract on Skeletal Muscle Fitness in Adults Older than 50 Years of Age. Nutrients 2021, 13, 4373. [Google Scholar] [CrossRef]
- Maruyama, C.; Kikuchi, N.; Masuya, Y.; Hirota, S.; Araki, R.; Maruyama, T. Effects of Green-Leafy Vegetable Intake on Postprandial Glycemic and Lipidemic Responses and α-Tocopherol Concentration in Normal Weight and Obese Men. J. Nutr. Sci. Vitaminol. 2013, 59, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nikrad, N.; Farhangi, M.A.; Fard Tabrizi, F.P.; Vaezi, M.; Mahmoudpour, A.; Mesgari-Abbasi, M. The Effect of Calorie-Restriction along with Thylakoid Membranes of Spinach on the Gut-Brain Axis Pathway and Oxidative Stress Biomarkers in Obese Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Ovarian Res. 2023, 16, 216. [Google Scholar] [CrossRef]
- Saeidi, A.; Saei, M.A.; Mohammadi, B.; Zarei, H.R.A.; Vafaei, M.; Mohammadi, A.S.; Barati, M.; Montazer, M.; Razi, O.; Kiyumi, M.H.A.; et al. Supplementation with Spinach-Derived Thylakoid Augments the Benefits of High Intensity Training on Adipokines, Insulin Resistance and Lipid Profiles in Males with Obesity. Front. Endocrinol. 2023, 14, 1141796. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Chu, J.; Beyl, R.; Edwall, D.; Erlanson-Albertsson, C.; Greenway, F.L. Acute Effects of a Spinach Extract Rich in Thylakoids on Satiety: A Randomized Controlled Crossover Trial. J. Am. Coll. Nutr. 2015, 34, 470–477. [Google Scholar] [CrossRef]
- Schirrmacher, G.; Skurk, T.; Hauner, H.; Grassmann, J. Effect of Spinacia oleraceae L. and Perilla frutescens L. on Antioxidants and Lipid Peroxidation in an Intervention Study in Healthy Individuals. Plant Foods Hum. Nutr. 2010, 65, 71–76. [Google Scholar] [CrossRef]
- Corte-Real, J.; Guignard, C.; Gantenbein, M.; Weber, B.; Burgard, K.; Hoffmann, L.; Richling, E.; Bohn, T. No Influence of Supplemental Dietary Calcium Intake on the Bioavailability of Spinach Carotenoids in Humans. Br. J. Nutr. 2017, 117, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Lefsrud, M.G.; Kopsell, D.E.; Wenzel, A.J.; Gerweck, C.; Curran-Celentano, J. Spinach Cultigen Variation for Tissue Carotenoid Concentrations Influences Human Serum Carotenoid Levels and Macular Pigment Optical Density Following a 12-Week Dietary Intervention. J. Agric. Food Chem. 2006, 54, 7998–8005. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Syed, Q.A.; Ishaq, A.; Al-Ghamdi, A.A.; Hatamleh, A.A. Botany, Nutritional Value, Phytochemical Composition and Biological Activities of Quinoa. Plants 2021, 10, 2258. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Cao, B.; Wei, X.; Shen, Z.; Su, N. Assessment and Comparison of Nutritional Qualities of Thirty Quinoa (Chenopodium quinoa Willd.) Seed Varieties. Food Chem. X 2023, 19, 100808. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Policy, Global Affairs, Board on Science; Technology for International Development and Ad Hoc Panel of the Advisory Committee on Technology Innovation. Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation; National Academies Press: Washington, DC, USA, 1989; ISBN 978-0-309-04264-2. [Google Scholar]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.-E. Nutritional Value and Use of the Andean Crops Quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Bielecka, J.; Markiewicz-Żukowska, R.; Puścion-Jakubik, A.; Grabia, M.; Nowakowski, P.; Soroczyńska, J.; Socha, K. Gluten-Free Cereals and Pseudocereals as a Potential Source of Exposure to Toxic Elements among Polish Residents. Nutrients 2022, 14, 2342. [Google Scholar] [CrossRef]
- Pathan, S.; Siddiqui, R.A. Nutritional Composition and Bioactive Components in Quinoa (Chenopodium quinoa Willd.) Greens: A Review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef]
- Kozioł, M.J. Chemical Composition and Nutritional Evaluation of Quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Abugoch James, L.E. Chapter 1 Quinoa (Chenopodium quinoa Willd.). In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2009; Volume 58, pp. 1–31. [Google Scholar]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C. Chemical and Nutritional Characterization of Chenopodium quinoa Willd (Quinoa) Grains: A Good Alternative to Nutritious Food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef]
- Kumpun, S.; Maria, A.; Crouzet, S.; Evrard-Todeschi, N.; Girault, J.-P.; Lafont, R. Ecdysteroids from Chenopodium quinoa Willd., an Ancient Andean Crop of High Nutritional Value. Food Chem. 2011, 125, 1226–1234. [Google Scholar] [CrossRef]
- Zhu, N.; Kikuzaki, H.; Vastano, B.C.; Nakatani, N.; Karwe, M.V.; Rosen, R.T.; Ho, C.-T. Ecdysteroids of Quinoa Seeds (Chenopodium quinoa Willd.). J. Agric. Food Chem. 2001, 49, 2576–2578. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Walia, S.; Kumar, R. Functional Composition, Physiological Effect and Agronomy of Future Food Quinoa (Chenopodium quinoa Willd.): A Review. J. Food Compos. Anal. 2023, 118, 105192. [Google Scholar] [CrossRef]
- Ren, G.; Teng, C.; Fan, X.; Guo, S.; Zhao, G.; Zhang, L.; Liang, Z.; Qin, P. Nutrient Composition, Functional Activity and Industrial Applications of Quinoa (Chenopodium quinoa Willd.). Food Chem. 2023, 410, 135290. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Hernández-Ledesma, B. Nutritional and Biological Value of Quinoa (Chenopodium quinoa Willd.). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Vidaurre-Ruiz, J.M. Bioactive Compounds in Quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). In Biology and Biotechnology of Quinoa: Super Grain for Food Security; Varma, A., Ed.; Springer: Singapore, 2021; pp. 243–264. ISBN 9789811638329. [Google Scholar]
- Graf, B.L.; Kamat, S.; Cheong, K.Y.; Komarnytsky, S.; Driscoll, M.; Di, R. Phytoecdysteroid-Enriched Quinoa Seed Leachate Enhances Healthspan and Mitochondrial Metabolism in Caenorhabditis elegans. J. Funct. Foods 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Angeli, V.; Miguel Silva, P.; Crispim Massuela, D.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, E.G.; Roura, R.; Rizzolo, D.a.D.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd), from Nutritional Value to Potential Health Benefits: An Integrative Review. J. Nutr. Food Sci. 2016, 6, 3. [Google Scholar]
- Zevallos, V.F.; Herencia, I.L.; Chang, F.; Donnelly, S.; Ellis, J.H.; Ciclitira, P.J. Gastrointestinal Effects of Eating Quinoa (Chenopodium quinoa Willd.) in Celiac Patients. Off. J. Am. Coll. Gastroenterol. ACG 2014, 109, 270–278. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition Facts and Functional Potential of Quinoa (Chenopodium quinoa Willd.), an Ancient Andean Grain: A Review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Meneguetti, Q.A.; Brenzan, M.A.; Batista, M.R.; Bazotte, R.B.; Silva, D.R.; Garcia Cortez, D.A. Biological Effects of Hydrolyzed Quinoa Extract from Seeds of Chenopodium quinoa Willd. J. Med. Food 2011, 14, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Zeyneb, H.; Pei, H.; Cao, X.; Wang, Y.; Win, Y.; Gong, L. In Vitro Study of the Effect of Quinoa and Quinoa Polysaccharides on Human Gut Microbiota. Food Sci. Nutr. 2021, 9, 5735–5745. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Świeca, M.; Sułkowski, M.; Dziki, D.; Baraniak, B.; Czyż, J. Antioxidant and Anticancer Activities of Chenopodium Quinoa Leaves Extracts—In Vitro Study. Food Chem. Toxicol. 2013, 57, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol Composition and in Vitro Antioxidant Activity of Amaranth, Quinoa Buckwheat and Wheat as Affected by Sprouting and Baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Ren, G.; Zhu, Y.; Shi, Z.; Li, J. Detection of Lunasin in Quinoa (Chenopodium quinoa Willd.) and the in vitro Evaluation of Its Antioxidant and Anti-Inflammatory Activities. J. Sci. Food Agric. 2017, 97, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhao, Q.; Zhao, B. Physicochemical Properties, Structural Characterization and Biological Activities of Polysaccharides from Quinoa (Chenopodium quinoa Willd.) Seeds. Int. J. Biol. Macromol. 2021, 193, 1635–1644. [Google Scholar] [CrossRef]
- Nsimba, R.Y.; Kikuzaki, H.; Konishi, Y. Ecdysteroids Act as Inhibitors of Calf Skin Collagenase and Oxidative Stress. J. Biochem. Mol. Toxicol. 2008, 22, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, M.; Bustos, M.; Mendez, D.; Fuentes, E.; Palomo, I.; Lutz, M. In Vitro Assay of Quinoa (Chenopodium quinoa Willd.) and Lupin (Lupinus spp.) Extracts on Human Platelet Aggregation. Plant Foods Hum. Nutr. 2020, 75, 215–222. [Google Scholar] [CrossRef]
- Foucault, A.-S.; Mathe, V.; Lafont, R.; Even, P.; Dioh, W.; Veillet, S.; Tome, D.; Huneau, J.-F.; Hermier, D.; Quignard-Boulange, A. Quinoa Extract Enriched in 20-Hydroxyecdysone Protects Mice From Diet-Induced Obesity and Modulates Adipokines Expression. Obesity 2012, 20, 270–277. [Google Scholar] [CrossRef]
- Sidorova, Y.S.; Shipelin, V.A.; Petrov, N.A.; Zorin, S.N.; Mazo, V.K. Anxiolytic and Antioxidant Effect of Phytoecdysteroids and Polyphenols from Chenopodium quinoa on an in vivo Restraint Stress Model. Molecules 2022, 27, 9003. [Google Scholar] [CrossRef]
- Graf, B.L.; Poulev, A.; Kuhn, P.; Grace, M.H.; Lila, M.A.; Raskin, I. Quinoa Seeds Leach Phytoecdysteroids and Other Compounds with Anti-Diabetic Properties. Food Chem. 2014, 163, 178–185. [Google Scholar] [CrossRef]
- Foucault, A.-S.; Even, P.; Lafont, R.; Dioh, W.; Veillet, S.; Tomé, D.; Huneau, J.-F.; Hermier, D.; Quignard-Boulangé, A. Quinoa Extract Enriched in 20-Hydroxyecdysone Affects Energy Homeostasis and Intestinal Fat Absorption in Mice Fed a High-Fat Diet. Physiol. Behav. 2014, 128, 226–231. [Google Scholar] [CrossRef]
- Omran, N.H.; El-Bahy, A.A.Z.; Hosny, H.T.A.; Handoussa, H. Quinoa and Chia Modulate AMPK/PPAR-ɣ Signaling in High-Fat Diet–Induced Obesity Rat Model. Rev. Bras. Farmacogn. 2023, 33, 583–594. [Google Scholar] [CrossRef]
- Ballegaard, A.-S.R.; Larsen, J.M.; Rasmussen, P.H.; Untersmayr, E.; Pilegaard, K.; Bøgh, K.L. Quinoa (Chenopodium quinoa Willd.) Seeds Increase Intestinal Protein Uptake. Mol. Nutr. Food Res. 2021, 65, e2100102. [Google Scholar] [CrossRef] [PubMed]
- Al-Qabba, M.M.; El-Mowafy, M.A.; Althwab, S.A.; Alfheeaid, H.A.; Aljutaily, T.; Barakat, H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium quinoa Sprouts against CCl4-Induced Oxidative Stress in Rats. Nutrients 2020, 12, 2904. [Google Scholar] [CrossRef]
- Sláma, K.; Abubakirov, N.K.; Gorovits, M.B.; Baltaev, U.A.; Saatov, Z. Hormonal Activity of Ecdysteroids from Certain Asiatic Plants. Insect Biochem. Mol. Biol. 1993, 23, 181–185. [Google Scholar] [CrossRef]
- Guibout, L.; Mamadalieva, N.; Balducci, C.; Girault, J.-P.; Lafont, R. The Minor Ecdysteroids from Ajuga Turkestanica. Phytochem. Anal. 2015, 26, 293–300. [Google Scholar] [CrossRef]
- Janeczko, A.; Oklestkova, J.; Tarkowská, D.; Drygaś, B. Naturally Occurring Ecdysteroids in Triticum aestivum L. and Evaluation of Fenarimol as a Potential Inhibitor of Their Biosynthesis in Plants. Int. J. Mol. Sci. 2021, 22, 2855. [Google Scholar] [CrossRef]
- Suksamrarn, A.; Kumpun, S.; Yingyongnarongkul, B.-E. Ecdysteroids of Vitex Scabra Stem Bark. J. Nat. Prod. 2002, 65, 1690–1692. [Google Scholar] [CrossRef]
- Mamatkhanov, A.U.; Yakubova, M.R.; Syrov, V.N. Isolation of Turkesterone from the Epigeal Part of Ajuga Turkestanica and Its Anabolic Activity. Chem. Nat. Compd. 1998, 34, 150–154. [Google Scholar] [CrossRef]
- Syrov, V.N.; Saatov, Z.; Sagdullaev, S.S.; Mamatkhanov, A.U. Study of the Structure—Anabolic Activity Relationship for Phytoecdysteroids Extracted from Some Plants of Central Asia. Pharm. Chem. J. 2001, 35, 667–671. [Google Scholar] [CrossRef]
- Abdukadirov, I.T.; Yakubova, M.R.; Nuriddinov, K.R.; Mamatkhanov, A.U.; Turakhozhaev, M.T. Ecdysterone and Turkesterone in Ajuga Turkestanica Determined by HPLC. Chem. Nat. Compd. 2005, 41, 475–476. [Google Scholar] [CrossRef]
- Abdukadirov, I.T.; Khodzhaeva, M.A.; Turakhozhaev, M.T.; Mamatkhanov, A.U. Carbohydrates from Ajuga Turkestanica. Chem. Nat. Compd. 2004, 40, 85–86. [Google Scholar] [CrossRef]
- Syrov, V.N.; Khushbaktova, Z.A.; Tolibaev, I.; Eletskaya, N.V.; Mamatkhanov, A.U. Effect of a Lipid Concentrate from the Aboveground Portion of Ajuga Turkestanica on the Metabolic Processes and Dynamics of Healing Skin Wounds Experimentally. Pharm. Chem. J. 1994, 28, 837–840. [Google Scholar] [CrossRef]
- Dinan, L.; Harmatha, J.; Volodin, V.; Lafont, R. Phytoecdysteroids: Diversity, Biosynthesis and Distribution. In Ecdysone: Structures and Functions; Smagghe, G., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 3–45. ISBN 978-1-4020-9112-4. [Google Scholar]
- Kuchkarova, L.S.; Rokhimova, S.O.; Syrov, V.N. Effect of Turkesterone on the Pancreas Histology and Function in Diabetic Rats. Int. J. Curr. Res. Rev. 2020, 12, 2–5. [Google Scholar] [CrossRef]
- Arthur, S.T.; Zwetsloot, K.A.; Lawrence, M.M.; Nieman, D.C.; Lila, M.A.; Grace, M.H.; Howden, R.; Cooley, I.D.; Tkach, J.F.; Keith, M.D.; et al. Ajuga Turkestanica Increases Notch and Wnt Signaling in Aged Skeletal Muscle. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2584–2592. [Google Scholar]
- Kutepova, T.A.; Syrov, V.N.; Khushbaktova, Z.A.; Saatov, Z. Hypoglycemic Activity of the Total Ecdysteroid Extract from Ajuga turkestanica. Pharm. Chem. J. 2001, 35, 608–609. [Google Scholar] [CrossRef]

| Study Objectives | Cell Lines | Main Results | Ref. |
|---|---|---|---|
| Neuroprotective effects of 20HE in Parkinson’s disease. | PC12 cells. | β-ecdysterone’s neuroprotective effects against MPPþ toxicity may be due to its antioxidant properties, which affect the PI3K-Nrf2 pathway. | [52] |
| Methanol, butanol, chloroform and water extracts and phytoecdysteroids (including ecdysterone) isolated from Silene wallichiana Klotsch. | Bacterial strains: Klebsiella oxytoca 6653, K. pneumoniae 40602, K. aerogenes NCTC8172, Citrobacter freundii 82073, Staphylococcus aureus MRSA16, Enterococcus faecalis NCTC775, Proteus rettgeri NCIMB9570, Pseudomonas aeruginosa NCTC6749, Escherichia coli NCTC9001, Enterobacter hormaechei T2, Acinetobacter sp. T132, Pantoea agglomerans T26, and Bacillus cereus T80. HeLa and HepG-2 cell lines. | At a MIC of 2.5 mg/mL, the methanolic extract inhibited the growth of Acinetobacter sp., E. faecalis, K. oxytoca, P. agglomerans, P. rettgeri, P. aeruginosa, and S. aureus, while E. coli and K. pneumoniae were inhibited at a MIC of 1.25 mg/mL. The chloroform extract significantly suppressed the growth of cancer cells. Aqueous and butanol extracts have high antioxidant activity. | [53] |
| 20-hydroxyecdysone effects on histamine release. | Rat peritoneal mast cells. | 20-hydroxyecdysone decreased the anti-IgE-induced histamine production by mast cells in a dose-dependent manner. | [54] |
| 20-hydroxyecdysone protects SH-SY5Y cells from β-induced apoptosis. | Human neuroblastoma cells (SH-SY5Y). | 20-hydroxyecdysone exerts neuroprotective effects through different and complementary pathways. It is a promising agent for the treatment of Alzheimer’s disease. | [55] |
| 20-hydroxyecdysone | Human proximal tubular epithelial cells (HK-2). | 20-hydroxyecdysone is a potential anti-fibrosis agent for renal proximal tubule cells. | [56] |
| Protective effects of 20-HE against endothelial dysfunction and its targets. | Human vascular endothelial cell line—HUVECs (CRL-1730 cells). | 20-hydroxyecdysone inhibits inflammation via SIRT6-mediated NF-κB signaling in endothelial cells. 20-hydroxyecdysone could be a promising therapeutic avenue for addressing cardiovascular ailments. | [57] |
| Effect of glucocorticoids in osteoblasts and the role of 20HE in the pathogenesis of glucocorticoid-induced osteoporosis. | Bone marrow mesenchymal stem cells, isolated from C57BL/6 mice. | The protective effect of 20HE on dexamethasone-induced osteoporosis was also shown. It can induce osteogenic differentiation and autophagy. | [58] |
| Ajuga turkestanica, isolated compounds (including ecdysterone and turkesterone) antioxidant, cytotoxic, and antimicrobial activities. | HeLa (cervical cancer), HepG-2 (hepatic cancer), and MCF-7 (breast cancer) human cell lines. Growth of Enterococcus VanB VRE ATCC 902291, E. VanB VRE ATCC 31299, S. aureus MRSA ATCC 1000/93, and S. aureus MRSA ATCC 10442, S. aureus MRSA ATCC 10442, S. pyogenes ATCC 12344, Candida albicans ATCC 90028, Candida glabrata ATCC MYA 2950, K. pneumonia ATCC 700603, P. aeruginosa ATCC 27853. | The isolated ecdysteroids and iridoid glucosides from A. turkestanica were found to lack antioxidant properties and did not exhibit significant cytotoxic or antimicrobial effects. Interestingly, the chloroform extract, containing more lipophilic compounds, demonstrated higher activity. | [59] |
| Investigation of the potential of ecdysterone to lower glucose levels in hepatocytes and whether it stimulates insulin secretion. | HepG2 and βTC3 cell lines. | Ecdysterone may exert the glucose-lowering effect in hepatocytes that is insulin-independent and shows no effect on insulin release. | [60] |
| Effect of ecdysterone on human breast cancer cell lines. | MCF7, MDA-MB-231, MDAMB-468, DF2, and WI-38—breast cancer cell lines. | Tumor suppressive effect of 20HE on a panel of breast cancer cell lines. It shows synergism with doxorubicin to induce cell death in breast cancer cell lines. | [61] |
| Examine the effect of 20HE on the susceptibility of Escherichia coli to antibiotics. | Escherihia coli BW25113 (wild type) and E. coli strains NM3031 and NM3041. | In E. coli, 20HE altered antibiotic bactericidal effect. | [62] |
| 20-hydroxyecdysone and its derivatives have the ability to sensitize multidrug-resistant (MDR) and non-MDR cancer cell lines to chemotherapeutic agents, and liposomal formulations. | MDR and non-MDR cancer cell. | 20-hydroxyecdysone-diacetonide can sensitize both MDR and non-MDR cancer cell lines to chemotherapeutic agents, and liposomal formulations. | [63] |
| Effect of 20HE and ecdysteroid fraction isolated from Serratula coronata L. on human blood cells and neutrophils. | Erythrocyte rosette formation. | 20-hydroxyecdysone exhibits potent immunomodulatory effects. It’s more effective than levamisole. | [64] |
| Silene guntensis B. Fedtsch and three isolated phytoecdysteroids incl. ecdysterone—antiproliferative and antioxidant effects. | Hepatocellular carcinoma (HepG-2), breast adenocarcinoma (MCF-7), and human cervix adenocarcinoma (HeLa) cell lines. | The chloroform extract showed potent cytotoxic effects, while the water and n-butanol extracts demonstrated notable antioxidant activities. | [65] |
| Investigation of 20HE, ajugasterone C, and polypodine B isolated from Serratula coronata effects on breast cancer. | Human breast cancer cells. | 20-hydroxyecdysone and ajugasterone C demonstrated notable inhibition of viability in the triple-negative cell line. 20-hydroxyecdysone induction of proapoptotic activity in both MDA-MB-231 and T-47D cells. | [66] |
| Interaction between the ecdysterone isolated from Sesuvium portulacastrum and nitric oxide synthase. | 3T3-L1 mouse fibroblast cells and RAW 264.7 mouse macrophage cells. | Ecdysterone boosts Nitric Oxide production and regulates intricate cellular mechanisms by activating ERK1/2 via the EGF pathway. | [67] |
| Cytotoxic effects of the butanolic extract from the underground parts of Helleborus caucasicus and the purified compounds: furostanol derivative, 20HE, and deglucohellebrin, on human cancer cell lines. | Calu-1—human epithelial lung cancer cells, MRC-5—human normal lung fibroblasts, and Caco-2—human colon cancer cells. | The extract of H. caucasicus and isolated compounds decreased cell viability in vitro on a lung cancer cell line and exerted a strong cytotoxic effect. Moreover, 20-HE and deglucohellebrin possess pro-apoptotic activities. | [68] |
| Antiadipogenic activity of R. carthamoides extract, ecdysterone, turkesterone, and ponasterone A. | SGBS human adipocytes. | Rhaponticum carthamoides extract, ecdysterone, and turkesterone reduced lipid accumulation in human adipocytes and R. carthamoides and ecdysterone stimulated lipolysis. | [69] |
| Effects of ecdysterone on interleukin-1β (IL-1β)-induced apoptosis and inflammation in rat chondrocytes. | Chondrocytes isolated from knee joints of 3 to 4-week-old male Sprague–Dawley rats. | Ecdysterone exhibited anti-apoptosis and anti-inflammatory effects in rat chondrocytes induced by IL-1β, which may be related to the NF-κB signaling pathway. | [70] |
| Effects of ecdysterone on brain acetylcholinesterase (AChE). | Rat cerebral cortex slices from Wistar albino rats. | Ecdysterone caused an increase in AChE in rat brain slices. No effect was observed in an in vitro assay using purified AChE. | [71] |
| Effects of β-ecdysone on osteoblast viability by assessing apoptosis following treatment with excess glucocorticoids. | BMSCs were isolated from the femurs and tibiae of mice—male BALB/C mice aged 8 weeks (20 ± 2 g body weight). | Beta-ecdysone prevented glucocorticoid-induced osteoblast apoptosis in vitro. | [72] |
| Anti-inflammatory activities of ecdysteroids from Diploclisia glaucescens (incl. ecdysterone). | Rat polymorphonuclear leukocytes (PMNs). | Tested compounds incl. ecdysterone showed significant anti-inflammatory activities. | [73] |
| Study Objectives | Study Design | Main Results | Ref. |
|---|---|---|---|
| Mechanism of action of phytoecdysteroids (20-HE, polypodine B, and ponesterone) and A. turkestanica and S. oleracea in mammalian tissue. | A mouse skeletal muscle cell line, C2C12 (ATCC CRL-1772). Male Sprague–Dawley rats, divided into four groups: vehicle, 50 mg/kg 20HE, 1000 mg/kg spinach extract, or 10 mg/kg methandrostenolone given orally. Duration: 28 days. | In vitro, phytoecdysteroids increased protein synthesis by up to 20% in C2C12 murine and human primary myotubes. Improved grip strength in rats, according to in vivo research. Similarly, plant extracts had comparable results. | [19] |
| Metabolic effects of 20HE. | Sprague–Dawley rats. Five groups 12 rats/group: control—soy-free, pelleted food, estradiol group—10 mg/kg food, 20HE—1 g/kg food, 3 g/kg food and 6 g/kg food. | Ecdysterone has beneficial effects on fat and muscle tissue and may have a non-estrogenic mechanism for the prevention of metabolic syndrome and sarcopenia. | [47] |
| Ecdysteroids (including ecdysterone and turkesterone) isolated from R. carthamoides, Silene brahuica Boiss, Silene praemixta M. Pop., and Ajuga turkestanika (Rgl.)Brig. | Male rats. Phytoecdysteroids were administered perorally—single daily dose—5 mg/kg for 10 days. Reference group received methylandrostenediol or nerobol at a dose of 10 mg/kg. | Ecdysteroids are potential agents capable of stimulating protein synthesis in the body without affecting the endocrine system. | [49] |
| 20-hydroxyecdysone-enriched fraction from Pfaffia glomerata roots. | 125 Male C57BL/6J mice. Naive group (not stressed or treated mice). Second: stress-inducted mice without treatment. Tirth: stressed mice and treated with 3, 10, 30 mg/kg of 20HE-enriched fraction. | A 30 mg/kg dose of 20HE-enriched fraction was able to reduce stress, anxiety, and depression, in addition to maintaining antioxidant defenses of the cortex striatum. | [50] |
| The effects of 20HE in ovariectomized rats on morphological changes in the joint, epiphyseal cartilage, and trabecular tissue. | Female Wistar rats, n = 6/group: control, ecdysterone (3 g/kg food), and estradiol (10 mg/kg) groups. | Ecdysterone induced a significant increase in the thickness of joint cartilage and increased the whole epiphyseal growth plate and its proliferative and hypertrophic zones. | [51] |
| β-ecdysterone effects on osteoblast differentiation and bone regeneration in vitro and in vivo. | MC3T3-E1 cells. Fifteen male Sprague–Dawley rats. Rats with bone abnormalities (n = 10) were divided into two groups: intraperitoneal injections of PBS (n = 5) or 72 mg/kg of 20HE (n = 5), every three days. | β-ecdysterone is identified as a regulator for bone regeneration, can stimulate the BMP-2/Smad/Runx2/Osterix pathway and MC3T3-E1 cell regeneration and initiate bone rebuilding. | [74] |
| The effect of ecdysterone on lipid metabolism. | Male Wistar rats. Ecdysterone was injected intraperitoneally in a dose of 0.5 mg/kg, injection volume was 0.2 mL. | Ecdysterone: reduces free fatty acids, diglycerides, triglyceride lipase activity, and specific activity of phosphatidylcholine, increases the specific activity of phosphatidylethanolamine and phosphatidylserine in the liver. | [75] |
| Evaluate the effect of high-intensity interval training exercise (HIT) and ecdysterone consumption synergistically after Alzheimer’s disease. | 72 adult male Wistar rats, 9 groups (n = 8 per group): control group, 10 mg/kg/day 20HE, performed HIIT or injection of Aβ peptide + 0.9% saline. | Due to the free radical scavenging and neuroprotective qualities, the combination of HIT exercise and ecdysterone therapy may be a viable therapeutic strategy for Alzheimer’s disease. | [76] |
| Extract from A. turkestanica or 20HE to sedentary aging mice would activate the key control point of protein synthesis. | Aging male C57BL/6 mice, 20 months old (n = 36). Vehicle group (n = 12), A. turkestanica extract (50 mg/kg/day; n = 12), or 20HE (50 mg/kg/day; n = 12). Duration: 28 days. | Treatment did not alter body, muscle, or organ mass; fiber cross-sectional area; or fiber type in the triceps brachii or plantaris muscles and muscle mass, nor did it activate protein synthesis. | [77] |
| 20-hydroxyecdysone effects on obesity and diabetes. | H4IIE rat hepatoma cells. Six-week-old male C57BL/6J mice (n = 10/group): low-fat diet or a high-fat diet (HFD). The HFD animals randomized into: a control group (n = 10), and a treatment group (n = 10)—10 mg/kg/body 20HE. Duration: 13 weeks. | Anti-obesity and anti-diabetic effects of 20-HE. | [78] |
| Treatment with 20HE may reduce glucocorticoid-induced osteoporosis. | Male Swiss–Webster mice. Two groups (n = 8–10/group): placebo and prednisolone (3.3 mg/kg/day). Then separated into control group and 20HE (0.5 mg/kg/day). Duration: 21 days. | 20-hydroxyecdysone showed increases in bone production and higher cortical bone mass. Concurrent treatment with 20HE and glucorticosteroids reduced bone formation rate, trabecular bone volume, and partially reversed cortical bone loss caused by glucocorticoid. | [79] |
| Examine ecdysterones derived from R. carthamoides neuroprotective mechanism of action. | In silico and 35 pathogen-free male Sprague–Dawley rats, aged 6–8 weeks. Seven groups from which 3 groups treated with ecdysterone (5, 10, and 20 mg/kg, respectively). | Neuroprotective effect and Removed glutamatergic excitotoxicity. | [80] |
| Ecdysterone effects on the cyclic AMP kinase system in mouse adipose tissue. | Male mice were injected intraperitoneally with 10 µg of ecdysterone or control mice received saline. | Decrease AMP-binding protein activity and cyclic AMP-dependent protein kinase. | [81] |
| Efficacy of 20-HE in ameliorating memory deficits within an animal model of type 1 diabetes mellitus. | Adult male Sprague–Dawley rats, divided into 2 control groups (n = 10) and animals (n = 70) in high-fat group were treated with a high-fat diet for 15 weeks. Other groups were received 20HE (1, 10, and 100 mg/kg/day) for 12 weeks. | 20-hydroxyecdysterone has a protective role in memory deficits in rats with diabetes mellitus. | [82] |
| Effect of 20HE on the osteogenic differentiation ability of bone marrow mesenchymal stem cells. | Human bone marrow mesenchymal stem cells and 40 Sprague–Dawley rats (5 groups): control group, model group, 20HE: 40 mg/kg, 20 mg/kg) and 10 mg/kg for 14 days. | β-ecdysterone may be beneficial for the recovery of osteonecrosis of bone marrow mesenchymal stem cells. | [83] |
| Effects of ecdysterone on cAMP and cGMP levels in mouse plasma. | Male mice, ecdysterone was given intraperitoneally at a dosage of 10 μg/animal. | The heterophilic effects of ecdysterone in mammals may be facilitated by modulation of the cyclic AMP system. | [84] |
| Effects of ecdysterone on cAMP and cGMP levels in mouse liver. | Male mice, ecdysterone was given intraperitoneally at a dosage of 10 μg/animal. | The cyclic AMP-protein kinase system is likely implicated in mediating the heterophilic effects of ecdysterone. | [85] |
| Investigation the underlying molecular mechanisms, in particular the role of estrogen receptor beta. | Male Wistar rats, groups (n = 6) treated for 21 days with 5 mg/kg 20HE or vehicle. | Ecdysterone led to an increase in muscle fiber size, accompanied by elevated serum insulin-like growth factor 1 (IGF-1) levels and decreased levels of corticosterone and 17β-estradiol (E2). Hypertrophy was induced by treatment with 20HE, dihydrotestosterone, IGF-1, and E2. | [86] |
| Ecdysterone lipid-lowering effects in obese Zucker rats. | 16 male, homozygous obese Zucker rats and 16 male, 25-week-old, heterozygous Zucker rats. Two groups (n = 8/group). The obese rats were allocated to two groups (n = 8/group): control and ecdysterone (0.5 g/kg/food). | Ecdysterone supplementation lacks lipid-lowering effects in liver and plasma of lean and obese Zucker rats. | [87] |
| Effects of 20HE on CYP11B1/2 levels and activity in UVB-induced photoaging and skin lesions in hairless mice. | PC12 cell line, HaCaT cell line, and male hairless mice (CrlOri:SKH1), 20HE or osilodrostat administered orally for 7 days. | 20-hydroxyecdysone prevents UVB-induced skin aging by inhibiting aldosterone synthase. It is a promising candidate for anti-aging therapy. | [88] |
| Effect of β-ecdysterone on osteogenic differentiation of bone marrow mesenchymal stem cells and its dependents on the estrogen receptor. | MSCs isolated from 8- to 10-week-old male Babl/c mice. Control, all-trans-retinoic acid, and ecdysterone groups. β-ecdysterone (1 mg/kg body weight) was administered intravenously daily for 3 weeks. | In vitro, β-ecdysterone stimulated osteogenic differentiation of mesenchymal stem cells in an estrogen receptor-dependent way, reducing osteoporotic processes in a mouse model. | [89] |
| 20-hydroxyecdysone for improving skin conditions in postmenopausal women, investigation in ovariectomized rats. | After ovariectomy rats. 20HE (18, 57, or 116 mg/animal/day) or 17A-estradiol-3-benzoate (60 Kg/kg body weight) for 12 weeks. | Ecdysterone prevented the ovariectomy-induced decrease in subcutaneous musculature, contrasting with the effects of 17β-estradiol. | [90] |
| Effect of 20HE on oxidative stress and inflammation in a collagen-induced rheumatoid arthritis rat model. | Forty healthy male Sprague–Dawley rats, (4 gropus, n = 10). Rats given saline for 28 days. Groups I and II: collagen-induced arthritis-induced rats and groups III and IV: rats treated with 10 or 20 mg/kg body weight 20HE for 28 days. | Administering 20HE (20 mg/kg) to collagen-induced rheumatoid arthritis rat models successfully reduces inflammation and oxidative stress, resulting in anti-rheumatoid arthritis effects. | [91] |
| Ability of 20HE to activate mTORC1 signaling in both skeletal muscle and liver tissues. | Male Sprague–Dawley rats. Dose-response study: Rats were separated into groups (n = 5–6) and administered doses of 0, 10, 50, or 200 mg/kg of 20HE via gavage. Time-course study: Rats were separated into groups and given either 200 mg/kg of 20HE or an excipient. Combination study: Rats were separated into groups and given either an excipient, 200 mg/kg of 20HE, or 200 mg/kg of 20HE plus 1.35 g/kg L-leucine by gavage. | 20-hydroxyecdysone does not quickly activate mTORC1 signaling in muscle or liver. | [92] |
| Ecdysterone as a modulator of cytostatic therapy. | Hybrid mice BDF1. On days 2, 4, 6, and 8, mice were given cisplatin (2 or 4 mg/kg) or cisplatin with ecdysterone (2 and 10 mg/kg). | Ecdysterone enhanced the chemotherapeutic impact of low doses of cytostatic. | [93] |
| Ethanol extract from Pfaffia glomerata and isolated ecdysterone hypnotic effect. | Adult male Wistar rats and adult male CF1 mice. Treatments: saline i.p. (n = 18); first—saline + polysorbate 80 1% intra peritoneal (n = 13); aquas 500 mg/kg i.p. (n = 9); butanol 500 mg/kg i.p. (n = 9); oranic 1 -500 mg/kg i.p. (n = 10); diazepam 1 mg/kg i.p. (n = 16). | The lipophilic fraction derived from P. glomerata demonstrates a hypnotic effect, with ecdysterone identified as one of the compounds responsible for this central nervous system activity. | [94] |
| “Ecdysterone-80”—an extract of phytoecdysteroids from Serratula coronata L., dominated by 20HE (81–86%). | 168 male rats. Ecdysterone-80 at a dosage of 20 mg/kg for 60 days beginning in the second month of the trial. | Ecdysteroids confer a cardioprotective effect. | [95] |
| Stachys hissarica and isolated ecdysteroids (ecdysterone etc.) effect on skin wounds healing. | Rats were orally administered the extract at a repeated dose of 10 mg/kg. | The ecdysteroid-containing preparation derived from S. hissarica exhibited notable effectiveness in promoting wound healing activity. | [96] |
| Ecdysterone derived from Achyranthes bidentate and Paeonol derived from Cortex Moutan. | Male Sprague–Dawley rats. 10% ecdysterone and 5% paeonol. Rats in each group were given vehicle or compound treatments three times a day. Duration: 12 days. | Orally given ecdysterone-paeonol reduced radiation-induced oral mucositis in rats. | [97] |
| 20-hydroxyecdysone isolated from Silene viridiflora influence on size of the different muscle fiber types | Twenty male Wistar rats (5 groups, n = 4/group): 0.9% NaCl, a daily subcutaneous injection of 5 mg/kg body weight of 20HE, snake venom (notexin) injections. From the 5th day post-injection: a daily subcutaneous injection of 5 mg/kg body mass weight of 20HE for 7 days or a daily subcutaneous injection of 0.5 mg/kg body mass weight of 20HE for 7 days. | 20-hydroxyecdysone also augmented the myonuclear count within the fibres of both normal and regenerating muscles. | [98] |
| 20-hydroxyecdysone from the dietary supplement “Peak Ecdysone”. | Fourty-six men (mean age 25.6 ± 3.7 years). Twenty men took 200 mg of 20HE, ten took 800 mg, and twelve received a placebo. Control group—12 volunteers consumed 200 mg of 20HE without training. Duration: 10 weeks. | 20-hydroxyecdysone increases body weight and muscle mass. Increased power and strength in performance. No adverse impacts on creatinine, gamma-glutamyl transferase, glutamate-oxaloacetate transaminase, or glutamate-pyruvate transaminase. The steroid profile was not affected. | [26] |
| Ecdysterone, a spinach component, has the potential to prevent metabolic syndrome. | Overweight women and men (18 receiving placebo, 21 receiving verum) consumed 2 × 50 mg of 20HE, along with 2 × 450 mg of spinach powder, daily. | It has been determined that this product can be utilized to treat and prevent the development of metabolic syndrome. | [99] |
| 20-hydroxyecdysone—randomised double-blind Phase 1 study for safety, tolerance pharmacokinetics and pharmacodynamics in healthy young and older adults (age ≥ 65 years). | Part 1: Single ascending dose (SAD): Three cohorts: A, B, and C. Cohorts A and B each had eight young individuals ranging in age from 18 to 55. Cohort C consists of eight senior participants (65 years or older). In the fasting state, subjects were given escalating dosages of BIO101 or a placebo. Part 2: Multiple ascending doses (MAD): Dose levels based on Part 1 findings. Three cohorts of ten older people (aged 65 to 85). Subjects were randomized to receive BIO101 or a placebo once or twice daily. | BIO101 (20HE) is well tolerated at doses of 350 mg/day, 350 mg twice a day, and 450 mg twice a day. Showed good safety profile in young and older adults, without serious adverse effects. | [100] |
| Methoxyisoflavone, 20HE and sulfopolysaccharides Intake results on training adaptation. | 45 resistance-trained males: group 1—placebo, group 2—800 mg/day of methoxyisoflavone, group 4—200 mg of 20HE, and group 5—1000 mg/day of sulfopolysaccharide. Duration 8 weeks. | No changes were observed in training adaptation and in anabolic/catabolic effect. | [101] |
| Study Objectives | Cell Lines | Main Results | Ref. |
|---|---|---|---|
| Anti-adipogenic activity of R. carthamoides extract, ecdysterone, turkesterone and ponasterone A. | SGBS human adipocytes. | Rhaponticum carthamoides extract, ecdysterone and turkesterone reduced lipid accumulation in human adipocytes and R. carthamoides and ecdysterone stimulated lipolysis. | [69] |
| Influence of R. carthamoides transformed roots extract on the growth of grade II and III human glioma cells. | Human primary glioma cell lines (grade II and III astrocytoma). | The extract from R. carthamoides transformed roots shows anticancer properties by impeding the proliferation of glioma cells and prompting apoptotic cell demise. | [121] |
| The cytotoxic effect and apoptotic potential of extracts derived from R. carthamoides transformed roots and roots of soil-grown plants evaluated in human glioma primary cells. | Human glioma cancer cells and normal human astrocytes. | Rhaponticum carthamoides root extracts reduce cell proliferation and promote apoptosis in human glioma cells. | [122] |
| Efficacy of R. carthamoides against myocardial injury. | A myocardial ischaemia in male SD rats and H9c2 cell lines. | Rhaponticum carthamoides possess cardioprotective effect. | [123] |
| Antimicrobial activity of R. carthamoides and Potentilla fruticosa L. extracts. | Escherihia coli, K. pneumoniae, P. aeruginosa, Proteus mirabilis, Enterococcus faecalis, S. aureus, Bacillus subtilis, B. cereu, and C. albicans | Rhaponticum carthamoides and Potentilla fruticosa extracts, exhibit antimicrobial properties against E. coli, K. pneumoniae, P. aeruginosa, E. faecalis, B. subtilis, B. cereus, S. aureus, and C. albicans. | [124] |
| 20-hydroxyecdysone, R. carthamoides extracts, and selected steroidal/non-steroidal anti-inflammatory drugs influence on NF-κB-inhibiting activity. | Human cervical cancer HeLa-IL-6 cells. | The extracts showed a significant modulation of the NF-κB inhibitory effect of dexamethasone. | [125] |
| Rhaponticum carthamoides root extract and 20HE, effects on human breast adenocarcinoma. | Human breast adenocarcinoma MCF-7 cells. | 20-hydroxyecdysone does not affect cell proliferation, ERα protein, or 5α-reductase activity. The extract inhibited cell proliferation and affected AhR-agonistic activity. | [126] |
| Extracts from R. carthamoides transform roots and soil-grown plant roots’ effect on oxidative stress. | Chinese hamster ovary (CHO) cells. | Rhaponicum carthamoides possess antioxidant properties protecting CHO cells from oxidative stress. | [127] |
| Compare the composition and antioxidant activity of R. carthamoides leaf extracts taken from plants growing in Poland and Russia. | HL-60 cells (human leukaemia). | The extracts from the Polish material had better antioxidative and cytotoxic activity than those from the Russian. | [128] |
| The effect of R. carthamoides on the proliferation of a diverse population of rumen bacteria and their influence on the fermentation process of a feed mixture in an artificial rumen (Rusitec). | Rumen bacteria and artificial rumen (RUSITEC) | No significant influence of R. carthamoides on rumen microflora metabolism, nor any effect of stimulative chemicals found in the plant’s above-ground parts. | [129] |
| The antibacterial activity of crude ethanol extracts from the aerial parts and roots of four leuzea species incl. Leuzea carthamoides DC. | Bacillus subtilis, B. cereus, Bacteroides fragilis, E. faecalis, E. coli, P. aeruginosa, S. aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, and Streptococcus pyogenes. | Extracts from aerial parts of all examined species showed notable antibacterial activity, particularly against B. cereus, S. epidermidis, and B. fragilis. L. carthamoides was the most efficient. | [130] |
| Antimicrobial activity of crude ethanolic extracts derived from 16 Siberian medicinal plants (incl. R. carthamoides) | Escherihia coli, B. cereus, P. aeruginosa, and Salmonella enteritidis, C. albicans | Rhaponticumcarthamoides affect mostly Bergenia crassifolia and Staphylococcus aureus at MIC 15.63 and 62.50, respectively. | [131] |
| The effects of four adaptogenic plants (inkl. R. carthamoides) extracts on the life span. | Philodina acuticornis bdelloid rotifers. | Ajugasterone C from R. carthamoides exhibited lower toxicity than 20HE. | [132] |
| Study Objectives | Study Design | Main Results | Ref. |
|---|---|---|---|
| 20-hydroxyecdysone isolated from Achyranthes faurei influence on glucose levels. | Male Donryu rats and male ddy mice, alloxane-induced. Ecdysterone injected 0.1, 0.5, 1 and 10 mg/kg. | Ecdysterone did not affect blood glucose levels in non-diabetic animals but reduced blood glucose levels in alloxan-diabetic mice. | [44] |
| Rhaponticum carthamoides extract effects on rats’ learning and memory processes. | Rats were orally administered R. carthamoides extract at doses of 0.25, 0.5, and 2.5 g/kg body weight for 10 days preceding the training sessions. | The extract increased learning and memory indices to varied degrees, depending on the amount used. | [133] |
| The central neurotropic activity of an aqueous-alcoholic extract from R. carthamoides cultivated in Bulgaria. | Male Wistar rats. The extract in doses of 200, 500, and 1000 mg/kg was injected subcutaneously 1 h prior to or immediately after training. Effect of extract on blood pressure conducted on male cats weighing 3.0 to 4.0 kg, administered intraduodenally in 1 mL/kg body weight. | The extract of R. carthamoides showed significant central stimulating effects, including enhanced ambulation increased central nervous system excitability, and a minor antinarcotic action and rearing behavior, improved learning, memory ability, and physical endurance in rats. | [134] |
| Rhaponticum carthamoides extract effects compared to those of Glycyrrhiza glabra and Punica granatum extracts. | Male Wistar Albino Glaxo rats incorporated with 300 mg/kg/day extracts. | Rhaponticum carthamoides extract powder supplementation significantly enhanced glucose and lipid metabolism compared to Glycyrrhiza glabra and Punica granatum extracts. | [135] |
| Investigate the impact of R. carthamoides, R. rosea, and their combined administration on resistance exercise and mechanical power. | Eleven-week-old Wistar Han rats (n = 56), 7 groups (n = 8). Rhodiola group—43.5 mg/kg body weight; R. carthamoides group—43.5 mg/kg body weight; R. carthamoides and Rhodiola groups 1–4 87.1, 43.5, 21.8. 8.7 43.5 mg/kg body weight, respectively. | R. carthamoides extract promotes muscle protein synthesis. The combination with R. rosea improved muscle protein synthesis and power performance. | [136] |
| Ecdysterone from the roots of R. carthamoides, influence on the mitochondrial level. | White male mongrel rats. Ecdysterone was administered orally to experimental group for 10 days before the start of the experiment at a daily dose of 5 mg/kg. | Ecdysterone may directly influence mitochondrial bioenergetics. | [137] |
| Ecdysterone isolated from Rhaponticum integrifolium, turkesterone isolated from A. turkestanica. | Male mongrel white mice subjected to immobilization on their back for 6 h. Ecdysterone, turkesterone, and T-activin were administered intraperitoneally before immobilization. | Ecdysterone and turkesterone prevented and facilitated the consequences of stress and restored the immunological activity. | [138] |
| Genotoxic evaluation of β-ecdysone. | Twelve-week-old male Wistar rats. 0.0823, 0.4115, and 0.8230 mg/kg treatments. | The results suggest cytogenotoxic activity of 2HE. | [139] |
| Effect of R. carthamoides extract on the lipid content of the erythrocyte membrane in rats with cerebral ischemia. | Male Wistar rats (n = 18). R. carthamoides extract administration (150 mg/kg). | Rhaponticum carthamoides extract increased the total lipid and phospholipid contents. | [140] |
| The impact of different levels of R. carthamoides hay meal in rat diets. | 30 female and 30 male SPF Wistar rats, 10 groups (n = 6). Hay meal with R. carthamoides 5–50%. | The use of a diet containing 20% R. carthamoides has resulted in significant variability in teste weight. | [141] |
| Effect of an herbal supplement comprising a 70:30 combination of extracts R. carthamoides and R. rosea on performance fatigue. | Thirty healthy active men (age 22.3 ± 4.1 years): 350 mg dose of the DS, a 175 mg dose of the DS with 175 mg of maltodextrin, or a placebo—350 mg (maltodextrin). | No significant influence on performance fatigability during high-intensity, repetitive muscular activities. | [142] |
| Study Objectives | Study Design | Main Results | Ref. |
|---|---|---|---|
| Mechanism of action of PEs (20-HE, polypodine B, and ponesterone) and A. turkestanica and S. oleracea in mammalian tissue, focusing on protein synthesis and physical performance. | A skeletal muscle cell line (mouse), C2C12 (ATCC CRL-1772)—threatened with 20HE, turkesterone, ponesterone, polypodine B, methandrostenolone, A. turkestanica and S. oleracea extract. Male Sprague–Dawley rats, divided in four groups: vehicle, 50 mg/kg 20HE, 1000 mg/kg spinach extract, or 10 mg/kg methandrostenolone given orally. Duration: 28 days. | In vitro, PEs increased protein synthesis in C2C12 murine and human primary myotubes. Ecdysteroids improved grip strength in rats, according to in vivo research. Similarly, plant extracts containing ecdysteroids had comparable results. | [19] |
| Anti-hyperlipidaemic and anti-obesity effects of S. oleracea extract. | Sprague–Dawley female rats (7 groups, n = 6): control—normal diet, high fed diet control (HFD), orlistat (10 mg/kg), antioxidant-rich extract of S. oleracea (200 mg/kg, 400 mg/kg, p. o), group 4—HFD and aerobic exercise (AE) for 20 min daily, group VII (NAOEAE)—NAOE (400 mg/kg, p. o) 20 min before receiving HFD and AE for 20 min daily. Duration: 21 days. | S. oleracea extract demonstrated significantly reduced hyperlipidaemia. The combined therapy and aerobic exercise, underscored the importance of incorporating both exercise and antioxidant-rich dietary sources to managed lipid levels and obesity. | [164] |
| A flavonoid-rich extract from S. oleracea leaves effect on appetite in rats. | Sprague–Dawley female rats, 4 groups (n = 6) Control—received drinking water (1 mL/kg, per oral, reference standard—received fluoxetine (6 mg/kg, intraperitoneal), S. oleracea extract 200 mg/kg, or 400 mg/kg per oral. Duration: 14 days. | Spinach leaves extract shows promising appetite suppression effect. | [165] |
| Protective effect of spinach against radiation-induced oxidative stress. | Male Swiss albino mice, four groups (n = 30). Normal group, fed with spinacia extract (1100-mg/kg body weight/day or 1100-mg/kg body weight/day), and control group. Duration: 15 days. | Spinacia oleracea showed strong protective effects against radiation-induced oxidative stress in mouse livers, indicating its potential as a low-cost source of antioxidants. | [166] |
| Effect of Spinacia oleracea extracts on ulcer regeneration, particularly in diabetic conditions. | Seventy-two male Sprague–Dawley rats, six groups (n = 12). Group 1—diabetic rats—300 mg/kg saline. Group 2—non-diabetic rats—300 mg/kg normal saline. Group 3—diabetic rats—300 mg/kg S. oleracea aquatic extract (SOAE). Group 4—diabetic rats—300 mg/kg SOAE. Group 5—intact rats—300 mg/kg SOAE. Then, they were exposed to diabetes and received 300 mg/kg SOAE. Group 6—intact rats—300 mg/kg S. oleracea alcoholic extract. Then, they were exposed to diabetes and received 300 mg/kg S. oleracea alcoholic extract. Duration: 1 month. | Spinacia oleracea extracts may be effective in the regeneration of diabetic ulcers. | [167] |
| Anti-asthmatic activity of S. oleracea an aqueous extract. | B16 cells and SH-SY5Y neuroblastoma cells and ovalbumin-induced asthmatic mouse model: Mice weighing 25 ± 2.5 g, male, 7-week, the aqueous S. oleaceae extract. | In the ovalbumin-induced asthmatic mouse model, the aqueous spinach extract efficiently alleviates asthmatic symptoms. | [168] |
| Spinach—antioxidant and hyperlipidaemic effect. | HepG2 cells and male Sprague–Dawley rats (n = 24). Three groups of eight animals each. Normal diet, a high fat-cholesterol diet (HFCD), or a HFCD supplemented with 5% freeze-dried spinach powder. | Spinach led to improvements in lipid profiles, decreased oxidative stress markers in the liver and blood, and enhanced antioxidant enzyme activity. | [169] |
| Examined the preventive efficacy of spinach natural antioxidants. | B16 melanoma cell line and female Balb/c mice. Saline—treated controls; doxorubicin (DOX) injected intraperitoneally -20 mg/kg, group 2—spinach natural antioxidant (NAO) administration i.p. for 7 days before and for 6 days after DOX administration (130 mg/kg); group 3—NAO administration i.p. for 6 days after DOX administration (60 mg/kg); and NAO administered i.p. for 13 days (cumulative dose, 130 mg/kg). | Water-soluble antioxidants from spinach can reduce doxorubicin-induced cardiotoxicity without adverse effects. | [170] |
| Study Objectives | Study Design | Main Results | Ref. |
|---|---|---|---|
| The influence of chronic daily spinach supplementation on oxidative stress and muscle damage. | Twenty well-trained men, randomised in two groups: spinach (n = 10) and placebo (n = 10). The participants took spinach supplementation (1 g/kg body weight) or placebo daily for 14 days before running (21.1 km). | Persistent daily oral supplementation with spinach reduces indicators of oxidative stress and muscle injury after a half-marathon in well-trained healthy young men. | [162] |
| Effects of daily supplementation with a S. oleracea extract with a moderate-intensity exercise programmed on skeletal muscle. | Over 50-year-old adults (Caucasian men and postmenopausal women aged between 50 and 75 years). Daily intake of a spinach extract for 12 weeks and a 12-week physical exercise resistance training program on skeletal muscle. | Training combined with daily intake of S. oleracea extract may increase muscle-related factors and muscle quality. | [171] |
| Benefits of spinach, in a therapeutic diet for obese and insulin-resistant patients. | Fourteen normal-weight and ten obese men, 20 to 35 years, consumed three test meals of bread, as a control, bread and butter, and bread and butter with boiled spinach. | Green leafy vegetable intake with a fat-rich meal may effectively supply postprandial α-tocopherol in obese subjects. | [172] |
| Supplementation with thylakoid membranes from spinach, combined with a hypocaloric diet, effect on lipopolysaccharide (LPS) levels, neurotrophic factors, and oxidative stress in polycystic ovary syndrome (PCOS). | Forty-eight obese women diagnosed with PCOS, aged 20–45 years: thylakoid (n = 21) and placebo groups (n = 23). Supplementation with a thylakoid-rich spinach extract (5 g/day) or a placebo (5 g cornstarch. Duration: 12 weeks. | Supplementation with thylakoid from spinach, combined with a hypocaloric diet, resulted in reduced LPS levels, increased neurotrophic factor levels, and improved glycaemic and sex hormone profiles in PCOS patients. | [173] |
| High-intensity functional training (HIFT) combined with spinach-derived thylakoid supplementation effect on selected adipokines and insulin resistance in males with obesity. | Sixty-eight participants, mean age: 27.6 ± 8.4 years, four groups (n = 17): control group, supplement group, training group, and training with supplement group. The two training groups started the 12 weeks of exercise training program. The eligible participants received 5 g/day of thylakoid-rich spinach extract or matching placebo as 5 g/day of raw corn starch. Duration: 12 weeks. | Potential benefits of combining HIFT with spinach-derived thylakoid supplementation in improving adipokine profiles and insulin resistance in males with obesity over a 12-week period. | [174] |
| The impact of taking a single dosage of concentrated extract of thylakoids from spinach on satiety, food intake, lipids, and glucose. | Sixty overweight and obese individuals aged 18–65 years. They consumed the spinach extract or placebo. Blood was drawn for assessments of lipids and glucose before a breakfast meal, followed 4 h later by a 5 g dose of the extract and a standard lunch. Two hours after lunch a second blood draw was conducted. | Consuming the spinach extract significantly reduced hunger. Postprandial plasma glucose concentrations were increased following. There were no differences in plasma lipids and energy intake at dinner. | [175] |
| Carotenoid-rich leafy vegetables—spinach and perilla effect on carotenoid concentration and oxidative stress in human blood plasma. | Twelve volunteers, aged 19–44 years. Examination after a 10-day consumption of perilla or spinach, after the washout phase (10 days), and after the following 10-day consumption. Duration: 10 days. | Significant increases in lutein content were observed in human blood plasma after consumption of both spinach and perilla preparations. Both perilla and spinach preparations influence lipid peroxidation. | [176] |
| Calcium possibility to affect bioavailability of carotenoids from a vegetable matrix—spinach. | Twenty-five non-obese men aged 20–46 years. Each participant received 270 g of spinach-based meals (8.61 mg carotenoids/100 g fresh weight), 0, 500, or 1000 mg of Ca. | Calcium did not affect the bioavailability of carotenoids from a spinach-based meal. | [177] |
| To characterize tissue lutein and β-carotene concentrations in various spinach cultigens, and to determine serum carotenoid and macular pigment optical density (MPOD) responses in human subjects consuming spinach cultigens. | Thirty individuals (21–60 years). Three groups (n = 10/group): control group, high-lutein spinach group, and low-lutein spinach group. | Consumption of low-lutein spinach led to a 22% increase in average serum lutein concentrations, while consumption of high-lutein spinach resulted in a 33% increase, and consuming high-lutein spinach demonstrated significant increases in MPOD. | [178] |
| Study Objectives | Cell Lines | Main Results | Ref. |
|---|---|---|---|
| Quinoa and its polysaccharides effects on the gut microbiota. | In vitro, including three different stages: simulated oral, gastric, and small intestine digestion. | Quinoa and its polysaccharides improved microbiota composition and short-chain fatty acid synthesis. They are potential prebiotics. | [201] |
| The effect of C. quinoa leaves phenolic compounds on cancer cells. | Rat prostate cancer AT-2 and MAT-LyLu, HTB-140 and normal mouse 3T3 fibroblasts. | The phenolic compounds may exert a chemopreventive and anticarcinogenic effect on oxidative stress. | [202] |
| The polyphenol composition and antioxidant properties of methanolic extracts from amaranth, quinoa, buckwheat, and wheat. | Antioxidant capacity is determined by using the radical DPPH scavenging capacity assay. | Total phenol content and antioxidant activity were shown to rise after sprouting, but levels decreased after breadmaking. | [203] |
| The presence and bioactivity of lunasin in quinoa. | RAW 264.7 macrophage cells | The lunasin isolated and purified from quinoa inhibited the production of nitric oxide, tumor necrosis factor-α, and interleukin-6. | [204] |
| Antioxidant, anti-diabetic, and immunoregulatory activities of the isolated polysaccharides from quinoa seeds. | Antioxidant activities (DPPH radical scavenging activity and ABTS radical scavenging activity. Antidiabetic activities (alpha-glucosidase inhibitory activity and alpha-amylase inhibitory activity), RAW264.7 cells for immunostimulatory activities. | Dose-dependent antioxidant and antidiabetic activities of the polysaccharides isolated from quinoa seeds, along with immunoregulatory activity. | [205] |
| The inhibition of collagenase activity of quinoa extracted ecdysteroids. | DPPH free-radical-scavenging. Collagenase inhibition assay—method of Sawabe. | The potential of ecdysteroids from quinoa in modulating collagenase activity. | [206] |
| In vitro antiplatelet activity of quinoa and lupin bean extracts. | Six healthy young male volunteers (ages 20–30) participated in the study. 10 mL venous blood samples were obtained for platelets. | Quinoa extracts did not exert an anti-aggregatory effect, whereas lupin extracts showed a significant effect. | [207] |
| Study Objectives | Study Design | Main Results | Ref. |
|---|---|---|---|
| Effects of hydrolyzed quinoa. | Wistar rats (n = 64): control groups, treated group with hydrolyzed quinoa 2.000 mg/kg and treated and exercised group for 30 days. | Hydrolyzed quinoa decreased body weight, blood triacylglycerol level, and fat deposition. | [200] |
| The ability of quinoa extract enriched in 20-HE to prevent obesity in mice. | Male C57BL/6J mice (6-week-old), 4 groups (n = 12/group). The high-fat group was supplemented or not with either quinoa extract or 20HE (6 mg/day/kg body weight). | Quinoa possesses anti-obesity activity; similar effect was observed with ecdysterone. | [208] |
| Anxiolytic and antioxidant effects of PEs (incl. 20-HE) and polyphenols from C. quinoa. | Sixty male Wistar rats. Three groups (n = 12): CTRL, IMM, and IMM-FFI. The CTRL and IMM groups were treated with a standard half-synthetic diet for 36 days. The third group, IMM-FFI, was treated with a standard diet with the addition of FFIs in the dose of 0.055 ± 0.003%. | The nutritional support from C. quinoa effectively normalized malondialdehyde and catecholamine levels under induced oxidative/nitrosative stress, while maintaining high superoxide dismutase activity, improving behavioral reactions and cognitive functions. | [209] |
| Hypoglycemic activity of quinoa leachate (QL). | Five-week-old male C57Bl/6J mice. Mice were randomly divided into an oral ingestion of 0.25 mL/50 g body weight of vehicle (70% Labrasol®) or QL (250 and 500 mg/kg) (n = 7). Metformin (300 mg/kg) was administered as a positive control. | The optimized quinoa leachate, including 0.86% 20-HE, 1.00% total Pes, 2.59% flavonoid glycosides, 11.9% oil, and 20.4% protein, notably reduced fasting blood glucose levels in obese, hyperglycaemic mice. | [210] |
| The impact of chronic intake of quinoa extract and 20HE on the regulation of energy homeostasis in mice. | Male C57BL/6J mice (6-week-old). Fed with a high-fat (HF) diet, supplemented or not with quinoa extract (0.28%) or 20HE (6 mg/day/kg body weight) for 3 weeks. | Both quinoa extract and 20HE promoted a higher rate of glucose oxidation. Quinoa extract possesses an anti-obesity effect. | [211] |
| Investigate the potential weight management effects of hydroalcoholic extracts from quinoa seeds and chia (Salvia hispanica L., Lamiaceae), and assess the hepatoprotective, anti-inflammatory, and antioxidant activities. | Albino Wistar rats, five groups (n = 6/group): normal food diet (NFD)—normal food diet and saline, high-fat diet (HFD)—high-fat diet and saline, (HFD and green tea)—HFD and green tea extract (250 mg/kg/day), (HFD and quinoa)—HFD and quinoa (250 mg/kg/day), (HFD and chia)—HFD and chia (250 mg/kg/day) for 6 weeks. | Chia and quinoa seed extracts showed hepatoprotective, anti-inflammatory, and antioxidant activity in obese rats given a high-fat diet. These extracts also influenced important metabolic indicators such as leptin, adiponectin, serum lipids, and glycaemic levels. | [212] |
| Effect of quinoa on intestinal permeability and inflammation. | BN rats and Caco-2/TC7 cells. BN rats with an age of 7–12 weeks, five groups (n = 24). Daily administered for one week: control group, quinoa flour (300 mg) with or without control, or capsaicin (12 mg). | Quinoa has the ability to enhance intestinal permeability and promote compartment-specific protein uptake, potentially through mechanisms distinct from those of cholera toxin and capsaicin. | [213] |
| Polyphenol composition of red and yellow quinoa seeds, effect of antioxidant bioactivity of both raw and germinated quinoa seeds in vitro and effect against carbon tetrachloride (CCl4)-induced oxidative stress in rats. | Markers of oxidative stress were evaluated in Wistar rats, which were categorized into five groups, each comprising six rats. Group I received an intraperitoneal injection of fresh olive oil (1.0 mL/kg/twice per week) along with 0.5 mL of distilled water orally per day. Group II received an intraperitoneal injection of a fresh mixture of CCl4 and olive oil (1.0 mL/kg/twice per week). Group III was administered an intramuscular injection of vitamin E and selenium (at a dosage of 30 mg/kg/twice per week) in combination with CCl4. Group IV was given an oral dose of 30 mg/kg gallic acid-yellow quinoa extract for 4 weeks concurrently with CCl4. Lastly, group V received 30 mg/kg gallic acid-red quinoa extract for 4 weeks along with CCl4. | Both red quinoa sprouts and yellow quinoa sprouts contain naturally synthesized polyphenols with superior antioxidant activity. The ethanolic extracts of quinoa sprouts demonstrated promising effects in counteracting oxidative stress. | [214] |
| Study Objectives | Study Design | Main Results | Ref. |
|---|---|---|---|
| Antiadipogenic activity of R. carthamoides extract, ecdysterone, turkesterone, and ponasterone A. | SGBS human adipocytes. | Rhaponticum carthamoides extract, ecdysterone, and turkesterone reduced lipid accumulation in human adipocytes and R. carthamoides- and ecdysterone-stimulated lipolysis. | [69] |
| Ecdysterone isolated from Rhaponticum integrifolium, turkesterone isolated from A. turkestanica. | Male mongrel white mice subjected to immobilization on their back for 6 h. Ecdysterone, turkesterone, and T-activin were administered intraperitoneally. | Ecdysterone and turkesterone prevented and facilitated the consequences of stress and restored immunological activity. | [138] |
| Turkesterone isolated from the roots of A. turkestanica and its anabolic effect. | Sexually immature male rats weighing 60–80 g. Turkesterone was administered orally at a dose of 5 mg/kg. For comparison, nerobol (methandrostenolone) was used in a dose of 10 mg/kg. | Turkesterone exhibited pronounced anabolic, leading to significant increases in body weight, comparable in anabolic effect to nerobol. It did not exhibit androgenic effects. | [219] |
| The impact of turkesterone on the endocrine and exocrine function in alloxan-induced diabetic rats. | White outbred rats, divided into 4 groups: saline group and single dose of alloxan group (170 mg/kg, intraperitoneal) and/or turkesterone groups (10 mg/kg/24 h) and glibenclamide (5 mg/kg/24 h) for 10 days. | Turkesterone improved the exocrine and endocrine function in alloxan-induced diabetic rats. | [225] |
| Study Objectives | Study Design | Main Results | Ref. |
|---|---|---|---|
| Mechanism of action of Pes (20HE, polypodine B, and ponesterone) and A. turkestanica and S. oleracea in mammalian tissue, focusing on protein synthesis and physical performance. | A mouse skeletal muscle cell line, ‘C2C1’ (ATCC CRL-1772)—threatened with 20HE, turkesterone, ponesterone, polypodine B, methandrostenolone, A.turkestanica, and S. oleracea extract. Male Sprague–Dawley rats, divided into four groups: vehicle, 50 mg/kg 20HE, 1000 mg/kg spinach extract, or 10 mg/kg methandrostenolone given orally. Duration: 28 days. | In vitro, phytoecdysteroids increased protein synthesis by up to 20% in C2C12 murine and human primary myotubes. Ecdysteroids improved grip strength in rats, according to in vivo research. Plant extracts containing ecdysteroids had comparable results. | [19] |
| Ajuga turkestanica, isolated compounds (including ecdysterone and turkesterone) antioxidant, cytotoxic, and antimicrobial activities. | HeLa, HepG-2, and MCF-7 human cell lines. Growth of Enterococcus VanB VRE ATCC 902291, E. VanB VRE ATCC 31299, Staphylococcus aureus MRSA ATCC 1000/93, and S. aureus MRSA ATCC 10442, S. aureus MRSA ATCC 10442, S. pyogenes ATCC 12344, C. albicans ATCC 90028, Candida glabrata ATCC MYA 2950, K. pneumonia ATCC 700603, and P. aeruginosa ATCC 27853. | The isolated ecdysteroids and iridoid glucosides from A. turkestanica were found to lack antioxidant properties and did not exhibit significant cytotoxic or antimicrobial effects. Interestingly, the chloroform extract, containing more lipophilic compounds, demonstrated higher activity. | [59] |
| Extract from A. turkestanica or 20HE to sedentary aging mice would activate the key control point of protein synthesis. | Aging male C57BL/6 mice, 20 months old (n = 36). Mice were randomly assigned to one of three treatment groups: vehicle (n = 12), A. turkestanica extract (50 mg/kg/day, n = 12), or 20HE (50 mg/kg/day, n = 12). Duration: 28 days. | Treatment did not alter body, muscle, or organ mass; fiber cross-sectional area; or fiber type in the triceps brachii or plantaris muscles. Phytoecdysteroid treatment does not alter muscle mass or fibre type. | [77] |
| Phytoecdysteroid enriched extract from A. turkestanica affects Notch and Wnt signaling in aged skeletal muscle. | Male C57BL/6 mice (20 months old) were randomly assigned to Control and A. turkestanica extract (50 mg/kg/day) groups. Duration: 28 days. | Ajuga turkestanica extract supplementation in aged mice increases Notch and Wnt signaling in triceps brachii muscle. | [226] |
| Total ecdysteroid extract from A. turkestanica (including 22-acetylcyasterone, cyasterone, ecdysterone, and turkesterone) and to assess the hypoglycemic activity of this extract on the model of alloxan-induced hyperglycemia and diabetes. | White mongrel male rats. The hyperglycemia model was induced by alloxan (150 mg/kg, s.c.). The PE preparation was introduced once per day in a dose of 5 mg/kg over a period of seven days. | Phytoecdysteroids extracted from A. turkestanica may be considered a promising hypoglycemic preparation. | [227] |
| Screen of A. turkestanica extract, rich in ecdysteroids for efficacy and safety. | C2C12 mouse myotube cell line. | The extract showed no androgenic activity within the dose range used. The potential for an A. turkestanica to support muscle mass without androgenic side effects. | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, V.; Ivanova, S.; Chakarov, D.; Kraev, K.; Ivanov, K. Ecdysterone and Turkesterone—Compounds with Prominent Potential in Sport and Healthy Nutrition. Nutrients 2024, 16, 1382. https://doi.org/10.3390/nu16091382
Todorova V, Ivanova S, Chakarov D, Kraev K, Ivanov K. Ecdysterone and Turkesterone—Compounds with Prominent Potential in Sport and Healthy Nutrition. Nutrients. 2024; 16(9):1382. https://doi.org/10.3390/nu16091382
Chicago/Turabian StyleTodorova, Velislava, Stanislava Ivanova, Dzhevdet Chakarov, Krasimir Kraev, and Kalin Ivanov. 2024. "Ecdysterone and Turkesterone—Compounds with Prominent Potential in Sport and Healthy Nutrition" Nutrients 16, no. 9: 1382. https://doi.org/10.3390/nu16091382
APA StyleTodorova, V., Ivanova, S., Chakarov, D., Kraev, K., & Ivanov, K. (2024). Ecdysterone and Turkesterone—Compounds with Prominent Potential in Sport and Healthy Nutrition. Nutrients, 16(9), 1382. https://doi.org/10.3390/nu16091382








