Evaluation of the Diagnostic Utility of Selected Serum Adipokines and Cytokines in Subjects with MASLD—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
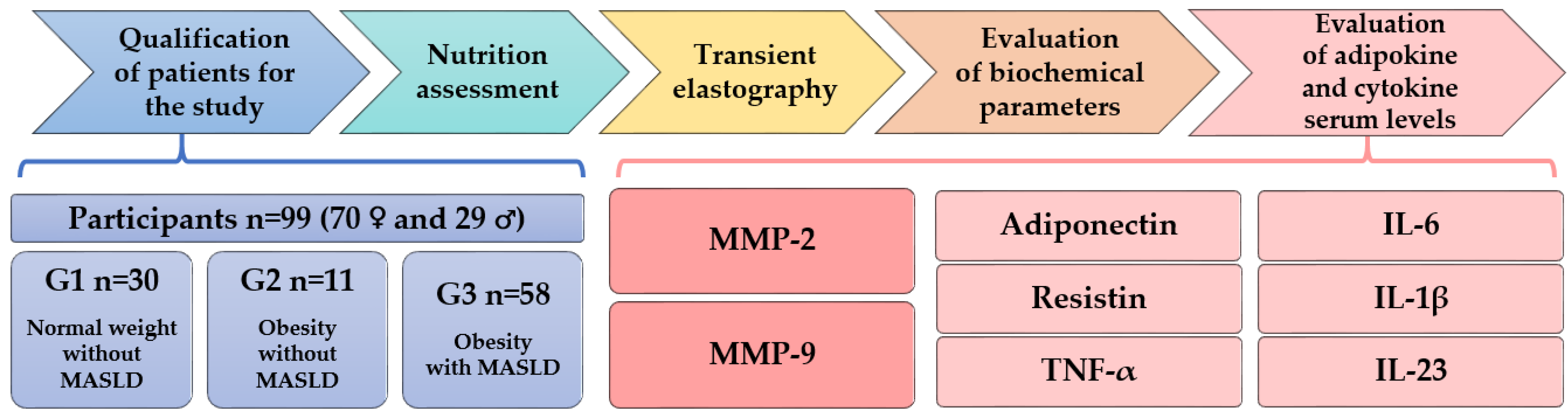
2.1. Criteria for Qualifying Patients for the Study
- 🗸
- BMI ≥25 kg/m² or waist circumference >94 cm (men) and >80 cm (women);
- 🗸
- Fasting serum glucose ≥5.6 mmol/L [100 mg/dL] or 2 h post-load glucose levels ≥7.8 mmol/L [≥140 mg/dL];
- 🗸
- Blood pressure ≥130/85 mmHg or specific antihypertensive drug treatment;
- 🗸
- Plasma triglycerides ≥1.70 mmol/L (150 mg/dL) or lipid lowering treatment;
- 🗸
- Plasma HDL-cholesterol ≤1.0 mmol/L (40 mg/dL) (men) and ≤1.3 mmol/L (50 mg/dL) (women) OR lipid lowering treatment.
- ▪
- Group G1 comprised individuals with normal weight without MASLD (n = 30).
- ▪
- Group G2 included those with obesity without MASLD (n = 11).
- ▪
- Group G3 encompassed those with obesity and MASLD (with or without hepatic fibrosis) (n = 58).
2.2. Nutrition Assessment
2.3. Transient Elastography Measurement of Liver Stiffness and Steatosis
2.4. Evaluation of Biochemical Parameters and Adipokine and Cytokine Serum Levels
2.4.1. Collection of Blood Samples
2.4.2. Methods for Evaluating Selected Biochemical Parameters
2.4.3. Methods for Determining Serum Levels of Adipokines and Cytokines
2.5. Statistical Analysis of the Results
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; Tan, D.J.H.; Tang, A.S.P.; Tay, P.; Xiao, J.; et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 20–30. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Cembrowska, P.; Stefańska, A.; Odrowąż-Sypniewska, G. Obesity phenotypes: Normal-weight individuals with metabolic disorders versus metabolically healthy obese. Med. Res. J. 2017, 1, 95–99. [Google Scholar] [CrossRef]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef]
- Wang, H.; Mehal, W.; Nagy, L.E.; Rotman, Y. Immunological mechanisms and therapeutic targets of fatty liver diseases. Cell. Mol. Immunol. 2021, 18, 73–91. [Google Scholar] [CrossRef]
- Maltron International Ltd. BioScan 920 User Guide; Maltron International Ltd.: Essex, UK, 2014. [Google Scholar]
- Janczewska, E.; Pisula, A.; Simon, K. Recommendations for elastography-based imaging of liver. Prz. Epidemiol. 2015, 69, 317–321. [Google Scholar]
- Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chan, H.L.; Le Bail, B.; Choi, P.C.; Kowo, M.; Chan, A.W.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Garnov, N.; Böhm, S.; Tenckhoff, H.; Wittekind, C.; Wiese, M.; Schiefke, I.; Linder, N.; Schaudinn, A.; et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS ONE 2014, 9, e91987. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281. [Google Scholar] [CrossRef] [PubMed]
- Zyśk, B.; Ostrowska, L.; Smarkusz-Zarzecka, J.; Witczak-Sawczuk, K.; Gornowicz, A.; Bielawska, A. Pro-Inflammatory Adipokine and Cytokine Profiles in the Saliva of Obese Patients with Non-Alcoholic Fatty Liver Disease (NAFLD)-A Pilot Study. Int. J. Mol. Sci. 2023, 24, 2891. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.N., Jr.; Harrison, S.A. Noninvasive imaging methods to determine severity of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2016, 64, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Fracanzani, A.L.; Valenti, L.; Bugianesi, E.; Andreoletti, M.; Colli, A.; Vanni, E.; Bertelli, C.; Fatta, E.; Bignamini, D.; Marchesini, G.; et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology 2008, 48, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Skelly, M.M.; James, P.D.; Ryder, S.D. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J. Hepatol. 2001, 35, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Mofrad, P.; Contos, M.J.; Haque, M.; Sargeant, C.; Fisher, R.A.; Luketic, V.A.; Sterling, R.K.; Shiffman, M.L.; Stravitz, R.T.; Sanyal, A.J. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003, 37, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Moghbeli, M.; Khedmatgozar, H.; Yadegari, M.; Avan, A.; Ferns, G.A.; Ghayour Mobarhan, M. Cytokines and the immune response in obesity-related disorders. Adv. Clin. Chem. 2021, 101, 135–168. [Google Scholar] [CrossRef]
- Borges, M.D.; Franca, E.L.; Fujimori, M.; Silva, S.M.C.; de Marchi, P.G.F.; Deluque, A.L.; Honorio-Franca, A.C.; de Abreu, L.C. Relationship between Proinflammatory Cytokines/Chemokines and Adipokines in Serum of Young Adults with Obesity. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 260–267. [Google Scholar] [CrossRef]
- Jhuma, K.A.; Giasuddin, A.S.; Hossain, M.S. Status of Serum Pro-inflammatory Cytokines (IL-1, IL-6, TNF-α) and Anti-inflammatory Cytokines (IL-4, IL-10, IL-13) in Newly Diagnosed Bangladeshi Patients with Type 2 Diabetes Mellitus. Mymensingh Med. J. 2023, 32, 1149–1155. [Google Scholar] [PubMed]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines with Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, M.; Verhelst, X.; Geerts, A.; Lapauw, B.; Calders, P. Association of recently described adipokines with liver histology in biopsy-proven non-alcoholic fatty liver disease: A systematic review. Obes. Rev. 2016, 17, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liao, L.; Liang, Z.; Yu, S.; Guo, Z. Correlation Analysis of IL-17, IL-21, IL-23 with Non-Alcoholic Liver Fibrosis and Cirrhosis. J. Inflamm. Res. 2024, 17, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Naim, A.; Pan, Q.; Baig, M.S. Matrix Metalloproteinases (MMPs) in Liver Diseases. J. Clin. Exp. Hepatol. 2017, 7, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Vessby, J.; Ekstedt, M.; Shang, Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J. Hepatol. 2024, 80, e76–e77. [Google Scholar] [CrossRef] [PubMed]
- Baltieri, L.; Chaim, E.A.; Chaim, F.D.M.; Utrini, M.P.; Gestic, M.A.; Cazzo, E. Correlation between nonalcoholic fatty liver disease features and levels of adipokines and inflammatory cytokines among morbidly obese individuals. Arq. Gastroenterol. 2018, 55, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Hadinia, A.; Doustimotlagh, A.H.; Goodarzi, H.R.; Arya, A.; Jafarinia, M. Circulating Levels of Pro-inflammatory Cytokines in Patients with Nonalcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Iran. J. Immunol. 2019, 16, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Fontes-Cal, T.C.M.; Mattos, R.T.; Medeiros, N.I.; Pinto, B.F.; Belchior-Bezerra, M.; Roque-Souza, B.; Dutra, W.O.; Ferrari, T.C.A.; Vidigal, P.V.T.; Faria, L.C.; et al. Crosstalk Between Plasma Cytokines, Inflammation, and Liver Damage as a New Strategy to Monitoring NAFLD Progression. Front. Immunol. 2021, 12, 708959. [Google Scholar] [CrossRef]
- Hegazy, M.; Abo-Elfadl, S.; Mostafa, A.; Ibrahim, M.; Rashed, L.; Salman, A. Serum Resistin Level and Its Receptor Gene Expression in Liver Biopsy as Predictors for the Severity of Nonalcoholic Fatty Liver Disease. Euroasian J. Hepatogastroenterol. 2014, 4, 59–62. [Google Scholar] [CrossRef]
- Ariya, M.; Koohpayeh, F.; Ghaemi, A.; Osati, S.; Davoodi, S.H.; Razzaz, J.M.; Javedan, G.; Ehrampoush, E.; Homayounfar, R. Assessment of the association between body composition and risk of non-alcoholic fatty liver. PLoS ONE 2021, 16, e0249223. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Yang, H.; Qi, X.; Bai, R.; Zhang, S.; Gong, J.; Mei, Y.; Hu, P. Gender differences in the ideal cutoffs of visceral fat area for predicting MAFLD in China. Lipids Health Dis. 2022, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, K.W.; Lee, J. Sex-specific Cutoff Values of Visceral Fat Area for Lean vs. Overweight/Obese Nonalcoholic Fatty Liver Disease in Asians. J. Clin. Transl. Hepatol. 2022, 10, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Makri, E.; Goulas, A.; Polyzos, S.A. Epidemiology, Pathogenesis, Diagnosis and Emerging Treatment of Nonalcoholic Fatty Liver Disease. Arch. Med. Res. 2021, 52, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Motamed, N.; Miresmail, S.J.; Rabiee, B.; Keyvani, H.; Farahani, B.; Maadi, M.; Zamani, F. Optimal cutoff points for HOMA-IR and QUICKI in the diagnosis of metabolic syndrome and non-alcoholic fatty liver disease: A population based study. J. Diabetes Complicat. 2016, 30, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Isokuortti, E.; Zhou, Y.; Peltonen, M.; Bugianesi, E.; Clement, K.; Bonnefont-Rousselot, D.; Lacorte, J.M.; Gastaldelli, A.; Schuppan, D.; Schattenberg, J.M.; et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: A population-based and inter-laboratory study. Diabetologia 2017, 60, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, M.H.; Baranova, A.; Collantes, R.; Ranard, B.; Stepanova, M.; Bennett, C.; Fang, Y.; Elariny, H.; Goodman, Z.; Chandhoke, V.; et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2008, 27, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Schlauch, K.; Elariny, H.; Jarrar, M.; Bennett, C.; Nugent, C.; Gowder, S.J.; Younoszai, Z.; Collantes, R.; Chandhoke, V.; et al. Gene expression patterns in hepatic tissue and visceral adipose tissue of patients with non-alcoholic fatty liver disease. Obes. Surg. 2007, 17, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Eren, F. Serum biomarkers of fibrosis and extracellular matrix remodeling in patients with nonalcoholic fatty liver disease: Association with liver histology. Eur. J. Gastroenterol. Hepatol. 2019, 31, 43–46. [Google Scholar] [CrossRef]
- Ando, W.; Yokomori, H.; Tsutsui, N.; Yamanouchi, E.; Suzuki, Y.; Oda, M.; Inagaki, Y.; Otori, K.; Okazaki, I. Serum matrixmetalloproteinase-1 level represents disease activity as opposed to fibrosis in patients with histologically proven nonalcoholic steatohepatitis. Clin. Mol. Hepatol. 2018, 24, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Munsterman, I.D.; Kendall, T.J.; Khelil, N.; Popa, M.; Lomme, R.; Drenth, J.P.H.; Tjwa, E.T.T.L. Extracellular matrix components indicate remodelling activity in different fibrosis stages of human non-alcoholic fatty liver disease. Histopathology 2018, 73, 612–621. [Google Scholar] [CrossRef] [PubMed]




| G1 (n = 30) Normal Weight without MASLD | G2 (n = 11) Obesity without MASLD | G3 (n = 58) Obesity with MASLD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | p | |||
| K-W All Groups | Post Hoc | |||||||||
| G1–G2 | G2–G3 | G1–G3 | ||||||||
| Age (years) | 35.50 | 29.00–43.00 | 39.00 | 30.00–46.00 | 46.00 | 40.00–52.00 | <0.001 * | 1.000 | 0.185 | <0.001 * |
| BMI (kg/m2) | 23.10 | 22.20–24.60 | 31.60 | 31.00–33.00 | 34.45 | 32.60–36.60 | <0.001 * | 0.003 * | 0.113 | <0.001 * |
| Total adipose tissue (%) | 27.69 | 22.19–30.39 | 38.34 | 33.52–45.15 | 42.52 | 33.83–46.91 | <0.001 * | 0.001 * | 1.000 | <0.001 * |
| VAT (cm2) | 87.50 | 65.00–132.00 | 220.00 | 148.00–261.00 | 291.00 | 199.00–350.00 | <0.001 * | 0.014 * | 0.328 | <0.001 * |
| SAT (cm2) | 66.50 | 57.00–92.00 | 112.00 | 92.00–145.00 | 135.50 | 110.00–151.00 | <0.001 * | 0.004 * | 0.666 | <0.001 * |
| VAT/SAT ratio | 1.37 | 0.94–1.62 | 1.89 | 1.42–2.51 | 2.18 | 1.56–2.86 | <0.001 * | 0.161 | 0.865 | <0.001 * |
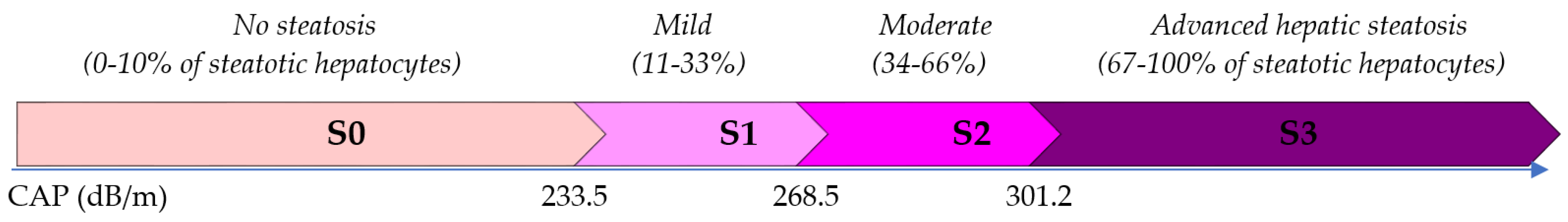
| CAP (dB/m) | 193.50 | 178.00–214.00 | 215.00 | 197.00–229.00 | 292.50 | 276.00–315.00 | <0.001 * | 1.000 | <0.001 * | <0.001 * |
| E (kPa) | 3.85 | 3.30–5.00 | 4.10 | 2.80–4.50 | 5.50 | 4.30–6.30 | <0.001 * | 1.000 | 0.042 * | <0.001 * |
| n | % | n | % | n | % | |||||
| Women | 26 | 86.67 | 8 | 72.73 | 36 | 62.07 | ||||
| Men | 4 | 13.33 | 3 | 27.27 | 22 | 37.93 | ||||
| G1 (n = 30) | G2 (n = 11) | G3 (n = 58) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | p | |||
| K-W All Groups | Post Hoc | |||||||||
| G1–G2 | G2–G3 | G1–G3 | ||||||||
| Adiponectin (ng/mL) | 6728.05 | 4453.80–11,906.50 | 7448.80 | 4717.90–8764.00 | 6283.30 | 4255.00–9762.30 | 0.585 | -- | -- | -- |
| Resistin (ng/mL) | 9.85 | 8.02–12.61 | 14.08 | 9.47–17.98 | 11.37 | 8.82–15.04 | 0.086 | -- | -- | -- |
| TNF-α (pg/mL) | 3.67 | 2.30–4.84 | 3.17 | 0.52–4.95 | 3.86 | 2.38–5.79 | 0.491 | -- | -- | -- |
| IL-6 (pg/mL) | 0.70 | 0.27–1.24 | 1.53 | 1.24–2.08 | 2.00 | 1.51–2.87 | <0.001 * | 0.025 * | 0.953 | <0.001 * |
| IL-1β (pg/mL) | 0.54 | 0.14–1.22 | 0.52 | 0.09–0.63 | 0.34 | 0.01–0.90 | 0.212 | -- | -- | -- |
| IL-23 (pg/mL) | 2.40 | 0.91–4.29 | 3.33 | 0.00–6.59 | 0.71 | 0.00–2.47 | 0.029 * | 1.000 | 0.308 | 0.052 |
| MMP-2 (ng/mL) | 399.83 | 308.68–530.28 | 375.64 | 291.78–628.68 | 339.40 | 270.76–436.70 | 0.087 | -- | -- | -- |
| MMP-9 (ng/mL) | 859.10 | 624.10–1083.80 | 1037.00 | 628.40–1630.80 | 811.55 | 656.10–1091.90 | 0.560 | -- | -- | -- |
| G1 (n = 30) | G2 (n = 11) | G3 (n = 58) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | p | |||
| K-W All Groups | Post Hoc | |||||||||
| G1–G2 | G2–G3 | G1–G3 | ||||||||
| CRP (mg/L) | 1.00 | 1.00–1.30 | 1.10 | 1.00–3.70 | 2.25 | 1.40–4.10 | <0.001 * | 0.774 | 0.132 | <0.001 * |
| Fasting glucose (mg/dL) | 91.00 | 85.00–94.00 | 95.00 | 89.00–104.00 | 96.00 | 89.00–106.00 | 0.032 * | 0.822 | 1.000 | 0.026 * |
| Glucose after 1 h (mg/dL) | 124.50 | 85.00–145.00 | 116.00 | 93.00–138.00 | 147.00 | 123.00–182.00 | 0.001 * | 1.000 | 0.065 | 0.001 * |
| Glucose after 2 h (mg/dL) | 97.50 | 84.00–110.00 | 98.00 | 80.00–107.00 | 106.50 | 98.00–127.00 | 0.005 * | 1.000 | 0.050 | 0.019 * |
| Fasting insulin (µU/mL) | 6.25 | 4.70–8.10 | 9.40 | 7.40–12.40 | 12.35 | 8.60–15.50 | <0.001 * | 0.039 * | 0.620 | <0.001 * |
| HOMA-IR | 1.41 | 1.03–1.89 | 2.28 | 1.67–3.03 | 2.84 | 1.93–4.22 | <0.001 * | 0.045 * | 0.645 | <0.001 * |
| QUICKI | 0.36 | 0.35–0.38 | 0.34 | 0.32–0.35 | 0.33 | 0.31–0.35 | <0.001 * | 0.043 * | 0.840 | <0.001 * |
| AST (U/L) | 17.50 | 16.00–19.00 | 20.00 | 18.00–26.00 | 20.00 | 16.00–27.00 | 0.073 | -- | -- | -- |
| ALT (U/L) | 15.00 | 13.00–19.00 | 23.00 | 18.00–30.00 | 25.00 | 20.00–34.00 | <0.001 * | 0.034 * | 1.000 | <0.001 * |
| GGTP (U/L) | 14.00 | 11.00–17.00 | 21.00 | 15.00–28.00 | 27.50 | 20.00–43.00 | <0.001 * | 0.058 | 0.425 | <0.001 * |
| ALP (IU/L) | 53.50 | 45.00–63.00 | 52.00 | 48.00–59.00 | 62.00 | 50.00–72.00 | 0.034 * | 1.000 | 0.150 | 0.102 |
| Cholesterol-T (mg/dL) | 190.50 | 179.00–231.00 | 216.00 | 182.00–232.00 | 199.50 | 181.00–231.00 | 0.562 | -- | -- | -- |
| HDL-C (mg/dL) | 61.50 | 51.00–76.00 | 55.00 | 43.00–72.00 | 48.50 | 42.00–56.00 | 0.001 * | 0.493 | 0.712 | <0.001 * |
| LDL-C (mg/dL) | 100.50 | 82.00–115.00 | 135.00 | 97.00–163.00 | 123.00 | 107.00–154.00 | 0.001 * | 0.050 | 1.000 | 0.001 * |
| TG (mg/dL) | 69.00 | 52.00–85.00 | 101.00 | 81.00–123.00 | 119.50 | 82.00–163.00 | <0.001 * | 0.070 | 1.000 | <0.001 * |
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | |
|---|---|---|---|---|---|---|---|---|
| Total adipose tissue (%) | β | 3.779 | 4.072 | |||||
| SE | 0.666 | 0.556 | ||||||
| p | <0.001 * | <0.001 * | ||||||
| VAT (cm2) | β | 0.296 * | 0.259 | 0.317 | ||||
| SE | 0.056 | 0.056 | 0.057 | |||||
| p | <0.001 | <0.001 * | <0.001 * | |||||
| VAT/SAT Ratio | β | 23.494 | 20.387 | |||||
| SE | 5.287 | 5.182 | ||||||
| p | <0.001 * | <0.001 * | ||||||
| HOMA-IR | β | 6.371 | 5.563 | 7.172 | 5.854 | 4.531 | 5.804 | 6.200 |
| SE | 2.380 | 2.501 | 2.548 | 2.323 | 2.509 | 2.649 | 2.507 | |
| p | 0.010 * | 0.030 * | 0.007 * | 0.014 * | 0.075 | 0.032 * | 0.016 * | |
| Adiponectin (ng/mL) | β | 0.001 | 0.001 | 0.001 | ||||
| SE | 0.001 | 0.001 | 0.001 | |||||
| p | 0.078 | 0.128 | 0.184 | |||||
| Resistin (ng/mL) | β | −0.631 | −2.049 | −2.111 | ||||
| SE | 1.103 | 1.135 | 1.194 | |||||
| p | 0.569 | 0.076 | 0.082 | |||||
| TNF-α (pg/mL) | β | 0.158 | 0.482 | 0.616 | ||||
| SE | 1.788 | 1.832 | 1.920 | |||||
| p | 0.930 | 0.793 | 0.749 | |||||
| Il-6 (pg/mL) | β | 3.370 | 12.374 | 16.354 | 11.692 | 15.245 | ||
| SE | 4.138 | 3.693 | 3.829 | 3.441 | 3.464 | |||
| p | 0.419 | 0.001 * | <0.001 * | 0.001 * | <0.001 * | |||
| IL-1β (pg/mL) | β | 17.783 | 17.861 | 16.165 | 15.763 | 13.296 | ||
| SE | 6.404 | 6.583 | 6.871 | 5.931 | 6.564 | |||
| p | 0.007 * | 0.009 * | 0.022 * | 0.010 * | 0.047 * | |||
| IL-23 (pg/mL) | β | −1.630 | −0.905 | −0.896 | ||||
| SE | 1.022 | 1.058 | 1.111 | |||||
| p | 0.116 | 0.396 | 0.423 | |||||
| MMP-2 (ng/mL) | β | −0.059 | −0.074 | −0.093 | ||||
| SE | 0.041 | 0.042 | 0.044 | |||||
| p | 0.153 | 0.083 | 0.039 * | |||||
| MMP-9 (ng/mL) | β | −0.006 | −0.016 | −0.013 | ||||
| SE | 0.014 | 0.015 | 0.015 | |||||
| p | 0.678 | 0.287 | 0.400 | |||||
| Adj. R2 | 0.554 | 0.529 | 0.483 | 0.536 | 0.482 | 0.427 | 0.442 | |
| Variable | Model 8 | Model 9 | Model 10 | Model 11 | Model 12 | |
|---|---|---|---|---|---|---|
| Total adipose tissue (%) | β | 3.160 | 3.496 | |||
| SE | 1.068 | 0.886 | ||||
| p | 0.008 * | 0.001 * | ||||
| VAT (cm2) | β | 0.303 | 0.317 | |||
| SE | 0.114 | 0.077 | ||||
| p | 0.016 * | <0.001 * | ||||
| VAT/SAT Ratio | β | 7.995 | ||||
| SE | 10.498 | |||||
| p | 0.456 | |||||
| HOMA-IR | β | −1.855 | 1.955 | 6.925 | −2.022 | 0.289 |
| SE | 6.771 | 6.581 | 7.343 | 4.064 | 3.847 | |
| p | 0.787 | 0.770 | 0.358 | 0.623 | 0.941 | |
| Adiponectin (ng/mL) | β | 0.001 | 0.001 | 0.003 | ||
| SE | 0.003 | 0.003 | 0.004 | |||
| p | 0.801 | 0.711 | 0.514 | |||
| Resistin (ng/mL) | β | 0.069 | −1.089 | 0.367 | ||
| SE | 1.686 | 1.866 | 2.078 | |||
| p | 0.968 | 0.567 | 0.862 | |||
| TNF-α (pg/mL) | β | 2.371 | −1.796 | 1.316 | ||
| SE | 3.171 | 3.680 | 4.176 | |||
| p | 0.464 | 0.631 | 0.756 | |||
| Il-6 (pg/mL) | β | 16.647 | 21.978 | 10.523 | 19.275 | 22.752 |
| SE | 10.741 | 11.932 | 12.858 | 7.521 | 7.500 | |
| p | 0.139 | 0.082 | 0.424 | 0.017 * | 0.006 * | |
| IL-1β (pg/mL) | β | 15.213 | 13.921 | 21.758 | ||
| SE | 20.498 | 21.392 | 25.280 | |||
| p | 0.468 | 0.523 | 0.401 | |||
| IL-23 (pg/mL) | β | 0.969 | 0.577 | 1.165 | ||
| SE | 0.913 | 0.976 | 1.102 | |||
| p | 0.302 | 0.562 | 0.304 | |||
| MMP-2 (ng/mL) | β | 0.021 | −0.028 | −0.071 | ||
| SE | 0.093 | 0.093 | 0.110 | |||
| p | 0.828 | 0.765 | 0.527 | |||
| MMP-9 (ng/mL) | β | −0.006 | 0.011 | 0.006 | ||
| SE | 0.021 | 0.023 | 0.028 | |||
| p | 0.795 | 0.651 | 0.825 | |||
| Adj. R2 | 0.337 | 0.291 | 0.045 | 0.413 | 0.432 | |
| Women (n = 70) | Men (n = 29) | |
|---|---|---|
| Parameter | Total adipose tissue (%) | |
| AUC (95% Cl) | 0.94 (0.88–1.00) | 0.77 (0.49–1.00) |
| p-Value AUC | <0.001 * | 0.063 |
| Cutoff point | 40.21 | 26.85 |
| Sensitivity | 92% | 100% |
| Specificity | 91% | 71% |
| Women (n = 70) | Men (n = 29) | |
| Parameter | VAT (cm2) | |
| AUC (95% Cl) | 0.87 (0.78–0.95) | 0.80 (0.59–1.00) |
| p-Value AUC | <0.001 * | 0.005 * |
| Cutoff point | 156 | 155 |
| Sensitivity | 89% | 100% |
| Specificity | 77% | 43% |
| Women (n = 70) | Men (n = 29) | |
| Parameter | VAT/SAT Ratio | |
| AUC (95% Cl) | 0.75 (0.63–0.86) | 0.58 (0.29–0.86) |
| p-Value AUC | <0.001 * | 0.591 |
| Cutoff point | 2.05 | 2.04 |
| Sensitivity | 56% | 82% |
| Specificity | 85% | 57% |
| Women (n = 70) | Men (n = 29) | |
|---|---|---|
| Parameter | HOMA-IR | HOMA-IR |
| AUC (95% Cl) | 0.79 (0.69–0.90) | 0.76 (0.58–0.94) |
| p-Value AUC | <0.001 * | 0.005 * |
| Cutoff point | 1.81 | 1.91 |
| Sensitivity | 86% | 82% |
| Specificity | 65% | 71% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyśk, B.; Ostrowska, L.; Smarkusz-Zarzecka, J.; Orywal, K.; Mroczko, B.; Cwalina, U. Evaluation of the Diagnostic Utility of Selected Serum Adipokines and Cytokines in Subjects with MASLD—A Pilot Study. Nutrients 2024, 16, 1381. https://doi.org/10.3390/nu16091381
Zyśk B, Ostrowska L, Smarkusz-Zarzecka J, Orywal K, Mroczko B, Cwalina U. Evaluation of the Diagnostic Utility of Selected Serum Adipokines and Cytokines in Subjects with MASLD—A Pilot Study. Nutrients. 2024; 16(9):1381. https://doi.org/10.3390/nu16091381
Chicago/Turabian StyleZyśk, Beata, Lucyna Ostrowska, Joanna Smarkusz-Zarzecka, Karolina Orywal, Barbara Mroczko, and Urszula Cwalina. 2024. "Evaluation of the Diagnostic Utility of Selected Serum Adipokines and Cytokines in Subjects with MASLD—A Pilot Study" Nutrients 16, no. 9: 1381. https://doi.org/10.3390/nu16091381
APA StyleZyśk, B., Ostrowska, L., Smarkusz-Zarzecka, J., Orywal, K., Mroczko, B., & Cwalina, U. (2024). Evaluation of the Diagnostic Utility of Selected Serum Adipokines and Cytokines in Subjects with MASLD—A Pilot Study. Nutrients, 16(9), 1381. https://doi.org/10.3390/nu16091381










