Protein Intake and Physical Activity Levels as Determinants of Sarcopenia Risk in Community-Dwelling Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Risk of Sarcopenia Categorisation
2.4. Body Composition
2.5. Strength and Physical Function
2.6. Questionnaires
2.6.1. Dietary Intake
2.6.2. Physical Activity
2.6.3. General Health and Health Related Quality of Life
2.7. Sarcopenia Assessment
2.8. Statistical Analysis
Sample Size Calculation
3. Results
3.1. Baseline Characteristics
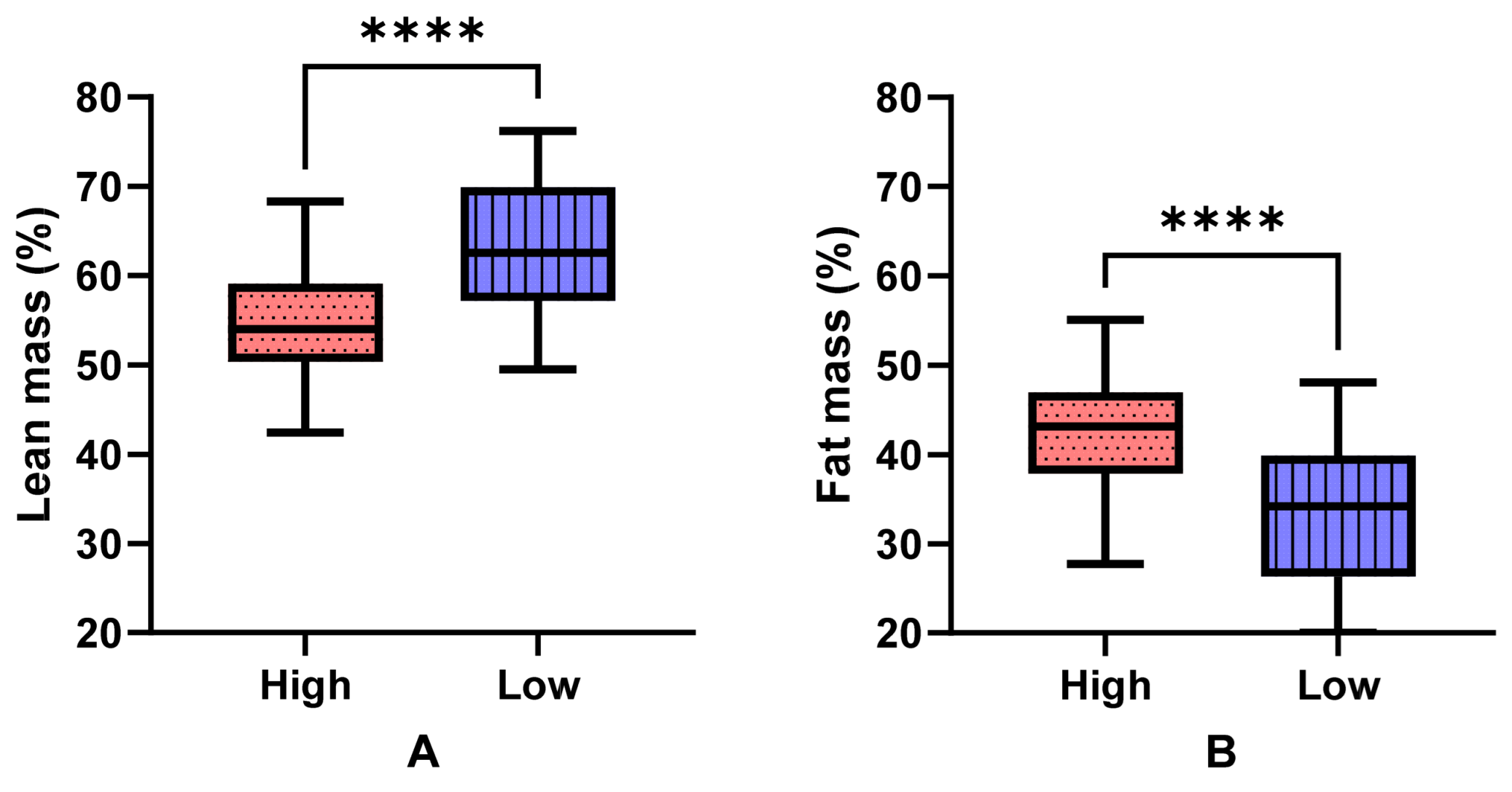
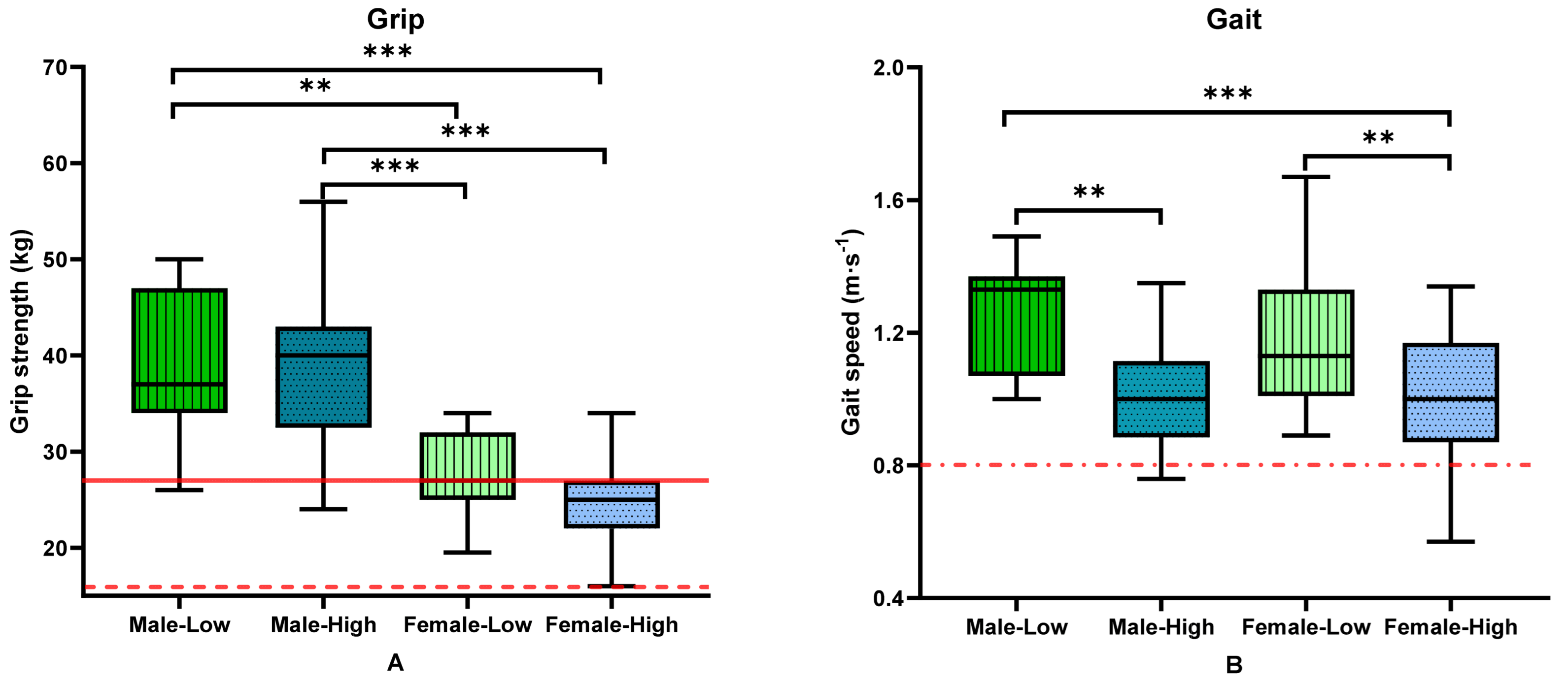
3.2. Body Composition, Strength and Physical Function by Group and Sex
3.3. Sarcopenia Diagnosis according to the EWGSOP Definition of Sarcopenia
3.4. Dietary Intake
3.5. Physical Activity
3.6. Quality of Life
3.7. Relationship between Determinants and Clinical Sarcopenia Outcomes
4. Discussion
Limitations
5. Conclusions
6. Clinical Significance
- A screening tool assessing lifestyle-based criteria, which does not require access to specialist equipment and training, may assist in identifying older adults at risk of sarcopenia.
- Older adults who are active and eat well appear to be at lower risk of developing sarcopenia.
- Older adults who are sedentary and consume low-protein diets may be at higher risk of developing sarcopenia, with higher risk in females than males.
- Assessing and improving the activity levels and protein intake, of older adults prior to sarcopenia diagnosis may prevent functional decline linked with sarcopenia
- Individuals with early functional decline might not meet current sarcopenia definitions, but may already have impaired body composition, strength and physical function. Community-dwelling older adults should engage with allied health professionals such as dietitians, physiotherapists and exercise physiologists early for nutrition and physical activity-based sarcopenia prevention strategies.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Ageing 2019: Highlights; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Bachettini, N.P.; Bielemann, R.M.; Barbosa-Silva, T.G.; Menezes, A.M.B.; Tomasi, E.; Gonzalez, M.C. Sarcopenia as a mortality predictor in community-dwelling older adults: A comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur. J. Clin. Nutr. 2020, 74, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Lera, L.; Angel, B.; Marquez, C.; Saguez, R.; Albala, C. Besides Sarcopenia, Pre-Sarcopenia Also Predicts All-Cause Mortality in Older Chileans. Clin. Interv. Aging 2021, 16, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.M.; Ingles, M.; Salvador-Pascual, A.; Cominetti, M.R.; Gomez-Cabrera, M.C.; Viña, J. Sarcopenia, frailty and their prevention by exercise. Free Radic. Biol. Med. 2019, 132, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Leach, S.; Pasco, J.A. Associations Between Muscle Quality and Cognitive Function in Older Men: Cross-Sectional Data From the Geelong Osteoporosis Study. J. Clin. Densitom. 2021, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Beasley, J.M.; Shikany, J.M.; Thomson, C.A. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr. Clin. Pr. 2013, 28, 684–690. [Google Scholar] [CrossRef]
- Reijnierse, E.M.; de van der Schueren, M.A.E.; Trappenburg, M.C.; Doves, M.; Meskers, C.G.M.; Maier, A.B. Lack of knowledge and availability of diagnostic equipment could hinder the diagnosis of sarcopenia and its management. PLoS ONE 2017, 12, e0185837. [Google Scholar] [CrossRef]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of Resistance Training on Functional Strength and Muscle Mass in 70-Year-Old Individuals With Pre-sarcopenia: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef]
- Pereira, F.B.; Leite, A.F.; de Paula, A.P. Relationship between pre-sarcopenia, sarcopenia and bone mineral density in elderly men. Arch. Endocrinol. Metab. 2015, 59, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Orprayoon, N.; Wainipitapong, P.; Champaiboon, J.; Wattanachanya, L.; Jaisamrarn, U.; Chaikittisilpa, S. Prevalence of pre-sarcopenia among postmenopausal women younger than 65 years. Menopause 2021, 28, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: A randomized controlled trial. Arch. Osteoporos. 2018, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Maruya, K.; Asakawa, Y.; Ishibashi, H.; Fujita, H.; Arai, T.; Yamaguchi, H. Effect of a simple and adherent home exercise program on the physical function of community dwelling adults sixty years of age and older with pre-sarcopenia or sarcopenia. J. Phys. Ther. Sci. 2016, 28, 3183–3188. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, Y.; Makizako, H.; Nakai, Y.; Taniguchi, Y.; Tomioka, K.; Sato, N.; Wada, A.; Doi, T.; Kiyama, R.; Takenaka, T. Associations of alpha-actinin-3 genotype with thigh muscle volume and physical performance in older adults with sarcopenia or pre-sarcopenia. Exp. Gerontol. 2021, 154, 111525. [Google Scholar] [CrossRef] [PubMed]
- Krzymińska-Siemaszko, R.; Tobis, S.; Lewandowicz, M.; Wieczorowska-Tobis, K. Comparison of four sarcopenia screening questionnaires in community-dwelling older adults from Poland using six sets of international diagnostic criteria of sarcopenia. PLoS ONE 2020, 15, e0231847. [Google Scholar] [CrossRef] [PubMed]
- Kera, T.; Kawai, H.; Hirano, H.; Kojima, M.; Watanabe, Y.; Motokawa, K.; Fujiwara, Y.; Osuka, Y.; Kojima, N.; Kim, H.; et al. Limitations of SARC-F in the diagnosis of sarcopenia in community-dwelling older adults. Arch. Gerontol. Geriatr. 2020, 87, 103959. [Google Scholar] [CrossRef] [PubMed]
- Ackermans, L.L.G.C.; Rabou, J.; Basrai, M.; Schweinlin, A.; Bischoff, S.C.; Cussenot, O.; Cancel-Tassin, G.; Renken, R.J.; Gómez, E.; Sánchez-González, P.; et al. Screening, diagnosis and monitoring of sarcopenia: When to use which tool? Clin. Nutr. ESPEN 2022, 48, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Phu, S.; Boersma, D.; Duque, G. Exercise and Sarcopenia. J. Clin. Densitom. 2015, 18, 488–492. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. Physical Activity and Sedentary Behaviour Guidelines—Adults (18–64 Years); Department of Health: Canberra, Australia, 2014.
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Physical Activity across the Life Stages; PHE 225; Australian Institute of Health and Welfare: Canberra, Australia, 2018; p. 72.
- Schutz, Y.; Kyle, U.U.G.; Pichard, C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int. J. Obes. 2002, 26, 953–960. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- GE Healthcare. enCORE™ Operator’s Manual Documentation Version: Rev F11/2006; GE Healthcare: Madison, WI, USA, 2006. [Google Scholar]
- Baghurst, P.; Magarey, A. A Modelling System to Inform the Revision of the Australian Guide to Healthy Eating; Dietitians Association of Australia for Department of Health and Ageing and National Health and Medical Research Council: Canberra, Australia, 2011.
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Goldberg, G.; Black, A.; Jebb, S.; Cole, T.; Murgatroyd, P.; Coward, W.; Prentice, A. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar] [PubMed]
- Dipietro, L.; Caspersen, C.J.; Ostfeld, A.M.; Nadel, E.R. A survey for assessing physical activity among older adults. Med. Sci. Sports Exerc. 1993, 25, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Taft, C.; Karlsson, J.; Sullivan, M. Do SF-36 summary component scores accurately summarize subscale scores? Qual. Life Res. 2001, 10, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P. SF36: Stata Module to Calculate Summary Statistics for the SF-36 Health Survey Instrument. 1999. Available online: https://ideas.repec.org/c/boc/bocode/s377601.html (accessed on 1 November 2021).
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S, discussion 1229S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; O’Connell, S.L.; Mundell, N.L.; Grimes, C.A.; Dunstan, D.W.; Nowson, C.A. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: A cluster randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, N.; Hengeveld, L.M.; Visser, M.; Presse, N.; Canhão, H.; Simonsick, E.M.; Kritchevsky, S.B.; Newman, A.B.; Gaudreau, P.; Jagger, C. Low protein intake, physical activity, and physical function in European and North American community-dwelling older adults: A pooled analysis of four longitudinal aging cohorts. Am. J. Clin. Nutr. 2021, 114, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, S.; Sirola, J.; Rikkonen, T.; Erkkilä, A.T.; Kröger, H.; Qazi, S.L.; Isanejad, M. Interaction of recommended levels of physical activity and protein intake is associated with greater physical function and lower fat mass in older women: Kuopio Osteoporosis Risk Factor- (OSTPRE) and Fracture-Prevention Study. Br. J. Nutr. 2020, 123, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, J.; Ellison, R.C.; Singer, M.R.; Bradlee, M.L.; Kalesan, B.; Holick, M.F.; Moore, L.L. Dietary Protein and Preservation of Physical Functioning Among Middle-Aged and Older Adults in the Framingham Offspring Study. Am. J. Epidemiol. 2018, 187, 1411–1419. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Gallagher, D. Body composition changes with aging: The cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition 2010, 26, 152–155. [Google Scholar] [CrossRef]
- Buch, A.; Carmeli, E.; Boker, L.K.; Marcus, Y.; Shefer, G.; Kis, O.; Berner, Y.; Stern, N. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp. Gerontol. 2016, 76, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919, quiz 920. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.P.; Chen, W.L.; Peng, T.C.; Wu, L.W.; Liaw, F.Y.; Kao, T.W. Examining the association between muscle mass, muscle function, and fat indexes in an elderly population. Nutrition 2021, 83, 111071. [Google Scholar] [CrossRef] [PubMed]
- Stavrinou, P.; Aphamis, G.; Andreou, E.; Pantzaris, M.; Giannaki, C. Association of body composition with functional capacity and cognitive function in older adults living in nursing homes. Curr. Aging Sci. 2021, 15, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hiol, A.N.; von Hurst, P.R.; Conlon, C.A.; Mugridge, O.; Beck, K.L. Body composition associations with muscle strength in older adults living in Auckland, New Zealand. PLoS ONE 2021, 16, e0250439. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Koster, A.; Visser, M. Adiposity, Muscle Mass, and Muscle Strength in Relation to Functional Decline in Older Persons. Epidemiol. Rev. 2013, 35, 51–65. [Google Scholar] [CrossRef]
- Choe, H.J.; Cho, B.L.; Park, Y.S.; Roh, E.; Kim, H.J.; Lee, S.G.; Kim, B.J.; Kim, M.; Won, C.W.; Park, K.S.; et al. Gender differences in risk factors for the 2 year development of sarcopenia in community-dwelling older adults. J. Cachexia Sarcopenia Muscle 2022, 13, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Gheller, B.J.; Riddle, E.S.; Lem, M.R.; Thalacker-Mercer, A.E. Understanding Age-Related Changes in Skeletal Muscle Metabolism: Differences Between Females and Males. Annu. Rev. Nutr. 2016, 36, 129–156. [Google Scholar] [CrossRef]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef]
- Maltais, M.L.; Desroches, J.; Dionne, I.J. Changes in muscle mass and strength after menopause. J. Musculoskelet. Neuronal Interact. 2009, 9, 186–197. [Google Scholar]
- Basualto-Alarcón, C.; Jorquera, G.; Altamirano, F.; Jaimovich, E.; Estrada, M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med. Sci. Sports Exerc. 2013, 45, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J. Steroid Biochem. Mol. Biol. 2019, 191, 105375. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Woo, J.; Scott, D.; Hoogendijk, E.O. Sarcopenia measurement in research and clinical practice. Eur. J. Intern. Med. 2021, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, T.P.; Hull, S.G.; McNutt, S.; Mittl, B.; Islam, N.; Guenther, P.M.; Thompson, F.E.; Potischman, N.A.; Subar, A.F. Challenges in converting an interviewer-administered food probe database to self-administration in the National Cancer Institute Automated Self-administered 24-Hour Recall (ASA24). J. Food Compos. Anal. 2009, 22, S48–S51. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.; Rollo, M.; Hutchesson, M.J.; Burrows, T.L. Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin. Nutr. 2014, 33, 906–914. [Google Scholar] [CrossRef]
- Ugarte Ll, J.; Vargas, R.F. Timed up and go values in older people with and without a history of falls. Rev. Med. Chil. 2021, 149, 1302–1310. [Google Scholar] [CrossRef]
- Bohannon, R.W. Reference Values for the Timed Up and Go Test: A Descriptive Meta-Analysis. J. Geriatr. Phys. Ther. 2006, 29, 64–68. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Stähelin, H.B.; Monsch, A.U.; Iversen, M.D.; Weyh, A.; von Dechend, M.; Akos, R.; Conzelmann, M.; Dick, W.; Theiler, R. Identifying a cut-off point for normal mobility: A comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 2003, 32, 315–320. [Google Scholar] [CrossRef]
- van Staveren, W.A.; de Groot, L.C.; Blauw, Y.H.; van der Wielen, R.P. Assessing diets of elderly people: Problems and approaches. Am. J. Clin. Nutr. 1994, 59, 221S–223S. [Google Scholar] [CrossRef]
- Washburn, R.A. Assessment of physical activity in older adults. Res. Q. Exerc. Sport 2000, 71, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Schrader, E. Dietary assessment methods for older persons: What is the best approach? Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 534–540. [Google Scholar] [CrossRef] [PubMed]
| Group | Usual Protein Intake | Usual Physical Activity Levels |
|---|---|---|
| High risk of developing sarcopenia | ≤1.0 g∙kg−1∙day−1 | <150 moderate intensity and/or <80 vigorous intensity min∙week−1 ≤1 resistance exercise session∙week−1 |
| Low risk of developing sarcopenia | >1.0 g∙kg−1∙day−1 | ≥150 moderate intensity and/or ≥80 intensity min∙week−1 >1 resistance exercise session∙week−1 |
| High Risk (n = 92) | Low Risk (n = 31) | p-Values | |
|---|---|---|---|
| Age | 72.3 (68.7–75.9) | 70.3 (67.1–74.2) | 0.062 |
| Sex, female, n (%) | 71 (77%) | 20 (65%) | 0.165 |
| Ex-smokers, n (%) | 44 (48%) | 13 (42%) | 0.569 |
| Pack years | 6.4 (1.3–19) | 2 (0.45–16.5) | 0.270 |
| Health Conditions | |||
| Hypertension, n (%) | 55 (60%) | 10 (32%) | 0.008 |
| Arthritis, n (%) | 55 (60%) | 18 (58%) | 0.866 |
| Joint replacement, n (%) Knee, n (%) Hip, n (%) | 11 (12%) 4 (4%) | 2 (6%) 1 (3%) | 0.514 1.000 |
| Spine BMD T-scores, n (%) Normal (≥−1.0) Osteopenia (−1.0 to −2.5) Osteoporosis (≤2.5) | 48 (52%) 39 (42%) 5 (5%) | 16 (52%) 12 (39%) 3 (10%) | 0.679 |
| Femur BMD T-scores, n (%) Normal (≥−1.0) Osteopenia (−1.0 to −2.5) Osteoporosis (≤2.5) | 67 (73%) 20 (22%) 5 (5%) | 17 (55%) 12 (39%) 2 (7%) | 0.138 |
| Diabetes, n (%) | 9 (10%) | 1 (3%) | 0.449 |
| Prediabetes, n (%) | 3 (3%) | 1 (3%) | 1.000 |
| Anxiety, n (%) | 10 (11%) | 5 (16%) | 0.526 |
| Depression, n (%) | 7 (8%) | 1 (3%) | 0.678 |
| Medications and supplements | |||
| Reflux, n (%) | 22 (24%) | 7 (23%) | 0.880 |
| Cholesterol-lowering, n (%) | 44 (48%) | 8 (26%) | 0.032 |
| Any supplement, n (%) | 58 (63%) | 21 (68%) | 0.637 |
| Total | p-Values | Male | Female | p-Values | ||||
|---|---|---|---|---|---|---|---|---|
| High Risk (n = 92) | Low Risk (n = 31) | Low Risk (n = 11) | High Risk (n = 21) | Low Risk (n = 20) | High Risk (n = 71) | |||
| Body Composition | ||||||||
| BMI (kg/m2) | 29.6 (26.8–34.3) | 25.0 (23.2–27.4) | <0.001 | 25.04 (22.62–26.83) | 30.05 (27.15–33.33) | 25.07 (23.775–27.865) | 29.17 (26.56–34.28) | <0.001 ACF |
| FFM (kg) | 44.52 (41.52–51.32) | 41.85 (40.22–58.59) | 0.450 | 59.55 (55.27–61.03) | 58.83 (55.41–65.23) | 40.48(39.54–41.77) | 42.56(40.80–46.33) | <0.001 BCDE |
| FFMI (kg/m2) | 16.89 (15.89–18.84) | 16.13 (15.89–18.18) | 0.221 | 18.44 (1.07) | 19.76 (1.92) | 15.93 (1.19) | 16.73 (1.57) | <0.001 BCDE |
| ASMM (kg) | 18.66 (17.02–22.11) | 17.26 (16.44–23.52) | 0.461 | 25.63 (23.04–26.64) | 25.13 (23.75–27.68) | 16.60 (15.48–17.21) | 17.76 (16.48–19.31) | <0.001 BCDE |
| ASMMI (kg/m2) | 7.10 (6.51–8.07) | 6.71 (6.28–7.81) | 0.269 | 7.89 (0.58) | 8.46 (1.00) | 6.51 (0.69) | 6.96 (0.79) | <0.001 BCDE |
| Fat mass (kg) | 33.86 (27.28–41.31) | 23.75 (18.67–27.40) | <0.001 | 19.94 (5.66) | 32.80 (9.53) | 25.29 (7.05) | 35.57 (9.24) | <0.001 ACF |
| Fat mass index (kg/m2) | 12.43 (10.45–16.04) | 8.27 (6.76–10.74) | <0.001 | 6.28 (1.65) | 10.84 (3.11) | 10.02 (2.91) | 13.71 (3.53) | <0.001 ABCEF |
| VAT mass (kg) | 1.34 (0.96–1.81) | 0.60 (0.28–1.20) | <0.001 | 1.05 (0.62–1.51) | 2.13 (1.53–2.88) | 0.48 (0.21–0.85) | 1.17 (0.87–1.51) | <0.001 ADEF |
| Total body bone mineral content (kg) | 2.26 (2.05–2.69) | 2.09 (1.90–2.96) | 0.333 | 3.21 (2.95–3.27) | 3.17 (2.97–3.30) | 1.93 (1.81–2.08) | 2.16 (2.03–2.40) | <0.001 BCDE |
| Strength and Physical Function | ||||||||
| Five chair stand test (s) | 11.00 (9.49–14.00) | 8.68 (8.00–10.4) | <0.001 | 9 (8.57–10.00) | 9.86 (9.06–13.08) | 8.49 (7.38–10.46) | 11.06 (10–14.00) | <0.001 CF |
| Thirty second sit-to-stand (stands) | 12.67 (2.90) | 17.47 (3.75) | <0.001 | 16 (15–19) | 13 (13–16) | 17 (16–19) | 12 (10–14) | <0.001 CDF |
| Grip strength, total (kg) | 26.0 (23.0–30.0) | 29.0 (26.0–34.0) | 0.012 | 37 (34–47) | 40 (34.00–42.00) | 27.00 (25.00–32.00) | 25.00 (22.00–27.00) | <0.001 BCDE |
| Shoulder adduction strength (kg) | 11.5 (7.5–15.0) | 16.75 (13.0–22.5) | 0.001 | 23.00 (16.50–33.50) | 21.50 (13.50–24.00) | 13.50 (10.0–18.0) | 10.0 (7.0–13.0) | <0.001 BCE |
| Shoulder abduction strength (kg) | 6.8 (3.5–10.8) | 10.0 (8.0–15.0) | 0.002 | 14.00 (11.50–16.00) | 13.00 (8.5–19.00) | 9.00 (6.50–10.00) | 5.50 (3.0–8.5) | <0.001 CE |
| SPPB score (total) <10/12 n (%) ≥10/12 n (%) | 11 (10–12) 17 (18.5%) 75 (81.5%) | 12 (12–12) 0 (0%) 30 (100%) | 0.001 0.012 | 12 (12–12) | 12 (9–12) | 12 (12–12) | 11 (10–12) | 0.003 F |
| Gait speed (m∙s−1) | 1.00 (0.87–1.15) | 1.15 (1.03–1.33) | <0.001 | 1.33 (1.07–1.37) | 1.00 (0.89–1.08) | 1.13 (1.01–1.33) | 1.00 (0.87–1.17) | <0.001 ACF |
| TUG (s) | 7.00 (6.70–7.91) | 5.80 (5.00–6.18) | <0.001 | 5.53 (5.00–6.83) | 7.00 (6.49–7.49) | 5.87 (5.01–6.18) | 7.00 (6.75–7.97) | <0.001 ACDF |
| EWGSOP Sarcopenia Categorisation | ||||||||
| No sarcopenia, n (%) Probable sarcopenia, n (%) Sarcopenia, n (%) Severe sarcopenia, n (%) | 77 (84%) 14 (15%) 0 (0%) 1 (1%) | 29 (94%) 1 (3%) 1 (3%) 0 (0%) | 0.087 | 10 (91%) 0 (0%) 1 (9%) 0 (0%) | 15 (71%) 5 (24%) 0 (0%) 1 (5%) | 19 (95%) 1 (5%) 0 (0%) 0 (0%) | 62 (87%) 9 (13%) 0 (0%) 0 (0%) | 0.048 |
| Functional criteria only, n (%) | 17 (19%) | 0 (0%) | 0.012 | 0 (0%) | 3 (14%) | 0 (0%) | 14 (20%) | 0.071 |
| High Risk (n = 92) | Low Risk (n = 31) | p-Values | Energy-Adjusted p-Values | |
|---|---|---|---|---|
| Macronutrients | ||||
| Energy (kJ∙day−1) | 5915 (5272–7213) | 7913 (6566–8707) | <0.001 | |
| Energy (kJ∙kg−1∙day−1) | 76.9 (63.1–76.9) | 107.6 (94.5–107.6) | <0.001 | |
| Protein (g∙day−1) | 64.7 (12.8) | 92.0 (15.8) | <0.001 | <0.001 |
| Protein (g∙kg−1∙day−1) | 0.9 (0.7–0.9) | 1.2 (1.1–1.5) | <0.001 | <0.001 |
| Carbohydrate (g∙day−1) | 148.4 (126.6–184.3) | 158.6 (131.9–246.2) | 0.057 | 0.007 |
| Fibre (g∙day−1) | 19.3 (15.3–25.8) | 26.8 (22.8–36.0) | <0.001 | 0.077 |
| Fat (g∙day−1) | 57.3 (18.2) | 76.9 (20.5) | <0.001 | 0.675 |
| SFA (g∙day−1) | 22.0 (8.3) | 24.9 (7.5) | 0.086 | 0.018 |
| MUFA (g∙day−1) | 20.4 (15.9–26.5) | 29.0 (24.1–36.6) | <0.001 | 0.054 |
| PUFA (g∙day−1) | 8.0 (6.4–9.9) | 11.5 (8.8–18.5) | <0.001 | 0.126 |
| Under-reporters | ||||
| n, (%) | 27 (30%) | 0 (0%) | 0.001 | |
| High Risk (n = 92) | Low Risk (n = 31) | p-Values | |
|---|---|---|---|
| Activity Dimension Indices | |||
| Vigorous Activity index (units∙month−1) | 5.0 (0.0–10.0) | 30.0 (20.0–40.0) | <0.001 |
| Leisure walking index (units∙month−1) | 16.0 (8.0–24.0) | 16.0 (8.0–16.0) | 0.995 |
| Moving index (units∙month−1) | 9.0 (6.0–9.0) | 9.0 (9.0–12.0) | 0.001 |
| Standing index (units∙month−1) | 4.0 (2.0–4.0) | 4.0 (2.0–4.0) | 0.860 |
| Sitting index (units∙month−1) | 2.0 (2.0–3.0) | 2.0 (1.0–2.0) | 0.002 |
| Total activity dimension indices | 36.0 (23.5–47.5) | 52.0 (47.0–69.0) | <0.001 |
| Activities | |||
| Brisk walking (hours∙week−1) | 0.0 (0.0–1.8) | 1.7 (1.0–3.0) | <0.001 |
| Stretch/yoga/tai chi (hours∙week−1) | 0.0 (0.0–1.0) | 0.8 (0.0–1.5) | 0.032 |
| Aerobics (hours∙week−1) | 0.0 (0.0–0.0) | 1.0 (0.0–1.8) | <0.001 |
| Cycling (hours∙week−1) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.901 |
| Lap swimming (hours∙week−1) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.045 |
| Strength exercise (hours∙week−1) | 0.0 (0.0–0.0) | 1.5 (1.0–2.0) | <0.001 |
| Leisurely walking (hours∙week−1) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.213 |
| FFMI | ASMMI | FMI | Five Chair Stand | 30STS | Grip | Shoulder Adduction | Shoulder Abduction | Gait Speed | TUG | Protein Intake | Energy Intake | Exercise Time | Strength Time | Aerobics Time | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FFMI | 1 | ||||||||||||||
| ASMMI | 0.946 | 1 | Key | ||||||||||||
| FMI | 0.163 | 0.205 | 1 | p > 0.05 | |||||||||||
| Five chair stand | −0.093 | −0.091 | 0.230 * | 1 | p ≤ 0.05 | ||||||||||
| 30STS | 0.092 | 0.081 | −0.424 | −0.761 | 1 | p ≤ 0.01 | |||||||||
| Grip strength | 0.368 | 0.435 | −0.380 | −0.352 | 0.352 | 1 | p ≤ 0.001 | ||||||||
| Shoulder adduction | 0.437 | 0.463 | −0.190 | −0.326 | 0.333 | 0.577 | 1 | ||||||||
| Shoulder abduction | 0.336 | 0.377 | −0.231 | −0.149 | 0.154 | 0.519 | 0.658 | 1 | |||||||
| Gait speed | −0.118 | −0.042 | −0.428 | −0.401 | 0.45 | 0.266 * | 0.391 | 0.294 | 1 | ||||||
| TUG | 0.060 | 0.006 | 0.330 | 0.492 | −0.474 | −0.442 | −0.322 | −0.294 | −0.503 | 1 | |||||
| Protein intake | −0.194 | −0.220 | −0.577 | −0.169 | 0.299 | 0.173 | 0.074 | 0.079 | 0.266 | −0.298 | 1 | ||||
| Energy intake | 0.231 | 0.230 | −0.302 | −0.237 | 0.1641 | 0.365 | 0.357 | 0.326 | 0.193 | −0.294 | 0.758 | 1 | |||
| Exercise time | −0.123 | −0.043 | −0.229 | −0.202 | 0.20 | 0.128 | 0.122 * | 0.22 | 0.321 | −0.167 | 0.258 | 0.262 | 1 | ||
| Strength time | −0.011 | −0.016 | −0.393 | −0.342 | 0.302 | 0.251 | 0.211 | 0.273 | 0.304 | −0.364 | 0.471 | 0.394 | 0.444 | 1 | |
| Aerobics time | −0.010 | −0.055 | −0.292 | −0.082 | 0.131 | 0.182 | 0.109 | 0.277 | 0.154 | −0.227 | 0.314 | 0.284 | 0.219 | 0.281 | 1 |
| Gait Speed (m∙s−1) | TUG (s) | Grip Strength (kg) | Shoulder Adduction Strength (kg) | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Model 1 n = 121 | R2 = 0.1327 | <0.001 | R2 = 0.1876 | <0.001 | R2 = 0.0106 | 0.496 | R2 = 0.0510 | 0.1330 |
| Protein (g∙kg−1∙day−1) | 0.19 (0.05, 0.33) | 0.007 | −0.19 (−0.35, −0.02) | 0.025 | 0.03 (−0.16, 0.22) | 0.782 | −0.96 (−6.65, 4.72) | 0.738 |
| Total exercise time (h∙week−1) | 0.01 (0.00, 0.02) | 0.194 | −0.01 (−0.02, 0.00) | 0.034 | 0.01 (−0.01, 0.02) | 0.438 | 0.51 (−0.02, 1.05) | 0.061 |
| Model 2 n = 121 | R2 = 0.1548 | <0.001 | R2 = 0.2654 | <0.001 | R2 = 0.5561 | <0.001 | R2 = 0.3918 | <0.001 |
| Protein (g∙kg−1∙day−1) | 0.18 (0.04, 0.31) | 0.012 | −0.15 (−0.31, 0.00) | 0.049 | −0.02 (−0.15, 0.11) | 0.739 | −1.56 (−5.79, 2.67) | 0.465 |
| Total exercise time (h∙week−1) | 0.01 (0.00, 0.01) | 0.241 | −0.01 (−0.02, 0.00) | 0.066 | 0.00 (−0.01, 0.02) | 0.661 | 0.45 (0.06, 0.85) | 0.025 |
| Age | −0.01 (−0.01, 0.00) | 0.136 | 0.01 (0.00, 0.02) | 0.009 | −0.01 (−0.02, 0.00) | 0.002 | −0.08 (−0.32, 0.16) | 0.504 |
| Sex (male) | 0.03 (−0.04, 0.11) | 0.398 | −0.07 (−0.14, 0.01) | 0.092 | 0.45 (0.36, 0.55) | <0.001 | 10.11 (7.06, 13.16) | <0.001 |
| Model 3 n = 121 | R2 = 0.1558 | <0.001 | R2 = 0.2723 | <0.001 | R2 = 0.5884 | <0.001 | R2 = 0.4498 | <0.001 |
| Protein (g∙kg−1∙day−1) | 0.17 (0.01, 0.33) | 0.038 | −0.11 (−0.29, 0.07) | 0.234 | 0.07 (−0.05, 0.20) | 0.256 | 2.02 (−2.46, 6.51) | 0.373 |
| Total exercise time (h∙week−1) | 0.01 (0.00, 0.02) | 0.229 | −0.01 (−0.02, 0.00) | 0.045 | 0.00 (−0.01, 0.01) | 0.861 | 0.38 (0.01, 0.74) | 0.043 |
| Age | −0.01 (−0.01, 0.00) | 0.131 | 0.01 (0.00, 0.02) | 0.010 | −0.01 (−0.02, 0.00) | 0.006 | −0.01 (−0.24, 0.22) | 0.932 |
| Sex (male) | 0.05 (−0.11, 0.21) | 0.529 | −0.15 (−0.33, 0.03) | 0.099 | 0.27 (0.12, 0.43) | 0.001 | 3.30 (−2.00, 8.59) | 0.220 |
| Lean muscle mass (kg) | 0.00 (−0.01, 0.01) | 0.807 | 0.01 (0.00, 0.01) | 0.234 | 0.01 (0.00, 0.02) | 0.004 | 0.43 (0.15, 0.71) | 0.003 |
| Spine BMD | Femur BMD | Lean Muscle (%) | Fat Mass (%) | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | |
| Model 1 n = 121 | R2 = 0.0740 | 0.005 | R2 = 0.0366 | 0.082 | R2 = 0.2789 | <0.001 | R2 = 0.2752 | <0.001 |
| Protein (g∙kg−1∙day−1) | −0.17 (−0.28, −0.06) | 0.004 | −0.09 (−0.17, −0.01) | 0.036 | 0.08 (0.02, 0.13) | 0.005 | −0.08 (−0.14, −0.02) | 0.006 |
| Total exercise time (h∙week−1) | 0.00 (−0.01, 0.01) | 0.758 | 0.00 (−0.01, 0.01) | 0.506 | 0.01 (0.00, 0.01) | 0.003 | −0.01 (−0.01, 0.00) | 0.004 |
| Model 2 n = 121 | R2 = 0.3599 | <0.001 | R2 = 0.2004 | <0.001 | R2 = 0.6532 | <0.001 | R2 = 0.6542 | <0.001 |
| Protein (g∙kg−1∙day−1) | −0.17 (−0.26, 0.09) | <0.001 | −0.10 (−0.18, −0.02) | 0.017 | 0.08 (0.04, 0.11) | <0.001 | −0.08 (−0.12, −0.04) | <0.001 |
| Total exercise time (h∙week−1) | 0.00 (−0.01, 0.01) | 0.779 | 0.00 (−0.01, 0.01) | 0.500 | 0.00 (0.00, 0.10) | <0.001 | −0.01 (−0.01, 0.00) | <0.001 |
| Age | 0.00 (0.00, 0.01) | 0.759 | −0.00 (−0.01, 0.00) | 0.575 | 0.00 (0.00, 0.00) | 0.143 | 0.00 (0.00, 0.00) | 0.147 |
| Sex (male) | 0.22 (0.17, 0.28) | <0.001 | 0.11 (0.07, 0.16) | <0.001 | 0.10 (0.08, 0.12) | <0.001 | −0.11 (−0.13, −0.09) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoodley, I.L.; Berthon, B.S.; Scott, H.A.; Williams, E.J.; Baines, P.J.; Knox, H.; Wood, S.; Paradzayi, B.; Cameron-Smith, D.; Wood, L.G. Protein Intake and Physical Activity Levels as Determinants of Sarcopenia Risk in Community-Dwelling Older Adults. Nutrients 2024, 16, 1380. https://doi.org/10.3390/nu16091380
Stoodley IL, Berthon BS, Scott HA, Williams EJ, Baines PJ, Knox H, Wood S, Paradzayi B, Cameron-Smith D, Wood LG. Protein Intake and Physical Activity Levels as Determinants of Sarcopenia Risk in Community-Dwelling Older Adults. Nutrients. 2024; 16(9):1380. https://doi.org/10.3390/nu16091380
Chicago/Turabian StyleStoodley, Isobel L., Bronwyn S. Berthon, Hayley A. Scott, Evan J. Williams, Penelope J. Baines, Hannah Knox, Sophie Wood, Beauty Paradzayi, David Cameron-Smith, and Lisa G. Wood. 2024. "Protein Intake and Physical Activity Levels as Determinants of Sarcopenia Risk in Community-Dwelling Older Adults" Nutrients 16, no. 9: 1380. https://doi.org/10.3390/nu16091380
APA StyleStoodley, I. L., Berthon, B. S., Scott, H. A., Williams, E. J., Baines, P. J., Knox, H., Wood, S., Paradzayi, B., Cameron-Smith, D., & Wood, L. G. (2024). Protein Intake and Physical Activity Levels as Determinants of Sarcopenia Risk in Community-Dwelling Older Adults. Nutrients, 16(9), 1380. https://doi.org/10.3390/nu16091380







