Abstract
Black trumpet (Craterellus cornucopioides) is a mushroom present in many countries but underestimated. The aim of this publication is to present the latest state of knowledge about the chemical composition and bioactivity of C. cornucopioides and the possibility of its application in food. According to researchers, black trumpet is very rich in nutritional compounds, including unsaturated fatty acids (mainly oleic and linoleic acids), β-glucans, minerals, and vitamins as well as polyphenols and tannins. It also contains compounds influencing the sensory properties, like free amino acids and nucleotides as well as sugars and polyols, mainly mannitol. Many of the described components show high nutritional and bioactive properties. Therefore, C. cornucopioides shows antioxidant activity and immunostimulating, anti-inflammatory, and anticancer effects as well as antibacterial, antifungal, antiviral, and antihyperglycemic effects. This makes black trumpet, also called horn of plenty, a mushroom with great potential for use both in medicine and directly in food. So far, black trumpet is not widely used in food, especially processed food. There are only a few studies on the use of dried black trumpet in sausages, but there is great potential for its use in food.
1. Introduction
Black trumpet (Craterellus cornucopioides) is an edible wild mushroom that is not very popular in Europe as a food ingredient. Its taste is usually known only to mushroom enthusiasts who are looking for new culinary experiences. Although its chemical composition is also relatively poorly known due to its bioactivity and the possibility of using it in medicine, this mushroom can be an element that changes the sensory value of dishes, and it can supplement the diet with health-promoting ingredients. In recent years, several works have appeared on the composition and functional properties of black trumpet. However, there are significant differences in the results presented by different authors. In turn, in the review of Bumbu et al. [1], the authors mainly presented the bioactive ingredients and biological properties of black trumpet without taking into account the basic composition or potential applications. Therefore, the aim of this publication is to present the latest state of knowledge about the chemical composition and bioactivity of C. cornucopioides, including differences between the results of different studies. Moreover, as it is a novelty, the possibilities of using this mushroom and the substances it contains in medicine and the production of functional food will be taken into account.
2. Characteristics and Occurrence
Black trumpet [Craterellus cornucopioides (L.) Pers], also called horn of plenty, black chanterelle, or trumpet of the dead, is a mushroom belonging to the phylum Basidiomycota, order Cantharellales. Its appearance is unusual for mushrooms: the mature fruiting body is very dark (dark brown to black) and has the shape of a slightly wrinkled, narrow funnel with a hollow center. The upper part of the fruiting body is rolled outwards (Figure 1). Fruiting bodies usually grow in scattered groups or clusters [2,3,4].

Figure 1.
Fruiting bodies of the black trumpet (Craterellus cornucopioides). Photo: Pierino Bigoni.
This mushroom occurs in deciduous and mixed forests. It belongs to the group of ectomycorrhizal organisms and enters into trophic systems with both deciduous trees (usually oaks and beeches) and conifers [2,3,4,5]. Its presence has been found in North America, Europe, and Asia [3,6] as well as South America and Australia [7]. Although it is a mushroom imported from Turkey [8], it still occurs only naturally (the technology for its cultivation has not been developed yet) [9].
3. Chemical Composition
3.1. Proximate Composition
The caloric value of the fruiting bodies of Craterellus cornucopioides was estimated at 248.0–413.2 kcal [10,11,12,13]. This is due, among other aspects, to the high water content (62.5–93.2% [11,12,13,14]) and low fat content (4.9–6.9% dw [10,11,12,13,14,15]). Only the study by Ouali et al. [16] showed a very high fat content (61.7% dw); however, they had used a different method of lipid content determination (gravimetrical from chloroform–methanol extracts instead of the Soxhlet method) than other researchers. Quali et al. [16] studied nine species of mushrooms and the lipid content in two of them was extremely high (>60%). In the case of all other studies, the crude lipid content was much lower than in other species.
The protein content in these mushrooms varied greatly and ranged from 11.8% to 69.4% dw. Differences in protein content may be caused, among other aspects, by the correction factor used for the calculation of protein content from nitrogen determined by the Kjeldahl method: either 4.38 [12,13,16] or 6.25 [11,15]. According to research by Turfan et al. [17], C. cornucopioides contains the highest amount of total soluble protein (126.6 mg/g) among the 15 compared taxa (species and strains) of mushrooms. Large differences were also observed in the content of carbohydrates—their total content ranged from 6.2% to 45.6% dw. Differences in protein content and lipid content affected the calculated amounts of carbohydrates. The lowest value (6.2%) was demonstrated by Quali et al. [16], who found the highest lipid content. Among saccharides, sugars (glucose, fructose, sucrose) and sugar alcohols (mannitol) have to be mentioned as well as the very important nondigestible polysaccharide β-glucans, which are classified as dietary fiber [10,12,17].
The ash content ranged from 10.1% to 17.4% dw [10,11,12,14,15,16,18] (Figure 2). Differences in the amount of minerals in mushrooms occur frequently and depend strongly on the composition of the soil (the differences can be huge). Dimopolou et al. [15] showed that the fruiting bodies of C. cornucopioides contained 4.7% dw fibers and 0.1% dw salt. The share of crude fiber in the fruiting bodies examined by Odoh et al. [14] was as much as 16.1% dw.
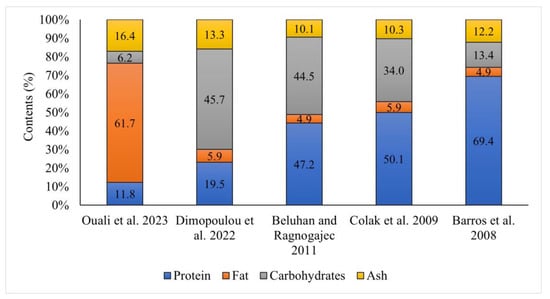
Figure 2.
Basic composition of fruiting bodies of Craterellus cornucopioides (dry matter) [10,11,12,15,16].
3.2. β-Glucans
According to the analyses of Özcan and Ertan [19], β-glucans constituted 11.3% of the mass of black trumpet fruiting bodies, which was the lowest value among the five compared species (the most of these ingredients, 14.6%, was contained in the fruiting bodies of Agaricus bisporus). Similarly, Mirończuk-Chodakowska [20] showed that black trumpet fruiting bodies contained 15 g of β-glucan per 100 g dw, which was one of the lowest values among the compared mushroom species (the highest amount was found in Tricholomopsis rutilans—40.9 g/100 g dw). The total content of 1,3-1,6-β-D-glucan in C. cornucopioides was 4.5 g/100 g dw, which was also one of the lowest values compared to other tested species. The fruiting bodies of Auricularia auricula-judae contained the most of these substances—16.8 g/100 g dw [19]. According to the research of Guo et al. [21,22], black trumpet also contains triple helix polysaccharides. Radović et al. [13] found that thermal treatment (cooking of dried fruiting bodies of C. cornucopioides) caused a more than 10-fold decrease in the content of both α-glucans and β-glucans. The content of total glucans changed from 16.0 g/100 g dw to 1.5 g/100 g dw, and in the case of β-glucans, from 15.7 g/100 g dw to 1.4 g/100 g dw.
3.3. Fatty Acids
Barros et al. [10] and Radović et al. [13] showed that among the fatty acids occurring in the fruiting bodies of Craterellus cornucopioides, unsaturated acids (UFA) predominated (accounting 75.9–83.6% of all fatty acids), the majority of which were monounsaturated fatty acids (MUFAs, accounting for almost 60% to 61.4%). Similar conclusions were reached by Dimopoulou et al. [15], who found the advantage of unsaturated fatty acids (69.6% of all fatty acids) over saturated acids, and by Ouali et al. [16] (UFA accounted 54.9% of all fatty acids). However, in the study by Ouali et al. [16], unlike in studies by other authors, polyunsaturated fatty acids (PUFAs) dominated among UFAs, accounting for 33.0%.
According to the research of Barros et al. [10], the most abundant fatty acids were oleic and linoleic acids (51.9% and 23.7%), followed by stearic and palmitic acids (7.9 and 6.7%). In the mushrooms studied by Radović et al. [13], oleic acid also dominated, accounting for 60.8% of all fatty acids, but stearic acids (12.4%), linoleic acid (10.8%), and palmitic acids (10.0%) also had a large share. Ouali et al. [16] found that among fatty acids, linoleic and oleic acids had the largest share (21.0% and 20.3%, respectively), followed by palmitic acid (13.9%), dihomo-γ-linolenic (10.9%), and margaric acid (8.9%).
3.4. Mineral Composition
Research on the mineral composition showed large differences depending on the origin of the research material (mushrooms). The fruiting bodies of Craterellus cornucopioides analyzed by Vetter [18] were the richest in phosphorus and potassium among those compared in Table 1, the most copper and zinc were contained in mushrooms collected in Turkey [17], and those from Tunisia showed the highest content of magnesium and iron [16] (Table 1). According to research by Yildiz et al. [23] and Ouali et al. [16], these mushrooms may also be a good source of calcium.

Table 1.
Mineral composition of fruiting bodies of Craterellus cornucopioides according to different authors [14,15,16,17,18,23].
3.5. Vitamins
3.5.1. Ascorbic Acid
Barros et al. [10] and Liu et al. [24] showed a similar content of vitamin C in the fruiting bodies of Craterellus cornucopioides (0.87 mg/g and 0.81 mg/g dw, respectively), while Cağlarirmak [25] reported 1.89 mg/100 g wet weight (ww). According to Barros et al. [10], these mushrooms contained the most ascorbic acid among the compared mushrooms, although a similar content was found in the fruiting bodies of Cantharellus cibarius (0.86 mg/g dw). Vamanu and Nita [26] found that extracts from C. cornucopioides contained less ascorbic acid than extracts obtained from the M2191 strain of Pleurotus ostreatus mushrooms and from Marasmius oreades mushrooms (0.87 mg/100 g of extract, 2.71 mg/100 g of extract, and 2.62 mg/100 g of extract, respectively).
3.5.2. Tocopherols
The content of total tocopherols in C. cornucopioides is low compared to other species of tested mushrooms. Barros et al. [10] showed only 1.87 µg/g dw in black trumpet fruiting bodies, while in Agaricus bisporus it was 2.41 µg/g dw, in A. silvicola—3.23 µg/g dw, and in Boletus edulis as much as 10.65 µg/g dw. A similarly low content, 1.94 µg/g, was found by Liu et al. [24].
According to Barros et al. [10], the highest share of tocopherols in the black trumpet was β-tocopherol (1.55 µg/g dw), while α-tocopherol and γ-tocopherol contents were much smaller (0.24 µg/g dw and 0.08 µg/g dw, respectively). Similarly, in the study by Liu et al. [24], α-tocopherol dominated quantitatively (1.15 µg/g dw), while γ-tocopherol and δ-tocopherol had a small share in the total tocopherol content (0.62 µg/g dw and 0.17 µg/g dw, respectively). Fruiting bodies of C. cornucopioides studied by Radović et al. [13] contained 0.479 mg/g dw of α-tocopherol. Barros et al. [10] found a greater amount of α-tocopherol in Agaricus silvicola (1.30 µg/g dw), A. bisporus (0.75 µg/g dw), and A. silvaticus (0.49 µg/g dw). For β-tocopherol, the values were Boletus edulis (8.90 µg/g dw), A. silvicola (1.93 µg/g dw), and A. bisporus (1.66 µg/g dw), and for γ-tocopherol, the values were B. edulis (1.42 µg/g dw), Marasimus oreades (1.30 µg/g dw), and Calocybe gambosa (0.14 µg/g dw).
Extraction using a fluidized bed allowed researchers to obtain extracts containing 117.4 mg of α-tocopherol per 100 g of extract (the most among the compared seven taxa of fungi) and 6.42 mg/100 g of extract γ-tocopherol (the least among the tested species, although its occurrence was not found in four taxa) [26]. Research conducted by Radović et al. [13] showed the presence of vitamin E only in cyclohexane extract in an amount of 2816 mg/100 g of dry extract, while water and methanol extracts did not contain it.
3.5.3. Other Vitamins
Liu et al. [24] showed the presence of ergosterol in C. cornucopioides, a precursor of vitamin D, in the amount of 3.27 mg/g dw; however, in the study by Gil-Ramirez et al. [27], it was much less, only 0.79 mg/g dw. Villares et al. [28] also reported low content of ergosterol in black trumpet, 0.44 mg/g, so five among eight studied mushrooms contained much more ergosterol. According to Despatliev et al. [29], ergosterol accounted for 72.8% of all sterols present in C. cornucopioides, while in Cantharellus cibarius it was only 42.4%. Generally, when mushrooms are exposed to UV radiation, ergosterol is transformed into a pro-vitamin and then to ergocalciferol, vitamin D2 [30]. However, the levels of ergocalciferol in mushrooms are usually low and both compounds are reduced by cooking processes [31,32]. Cyclohexane extract prepared from the fruiting bodies of C. cornucopioides also contained vitamin D3 (cholecalciferol) in the amount of 89.3 mg/100 g of dry extract (which corresponded to 1.52 mg/100 g dw of mushroom) and vitamin A (16.1 mg/100 g dry extract); however, the first result was not supported by any publication [13]. Radović et al. [13] found that methanol and water extracts also contained vitamins B1, B2, B3, and B6. There was more vitamin B1 in water extract than in methanol extract (54.7 and 19.4 mg/100 g of dry extract, respectively). The remaining vitamins were present in greater amounts in methanol extracts than in water extracts. The greatest difference was found in the case of vitamins B3 (367.0 and 249.0 mg/100 g dry extract, respectively) and B6 (17.0 and 8.3 mg/100 g dry extract). Cağlarirmak [25] found the presence of vitamins B1, B2, B3, B6, and B9 in the fruiting bodies of this species in amounts 0.11, 0.06, 3.34, 0.86, and 17.83 mg/100 g ww, respectively. Watanabe et al. [33] showed that black trumpet fruiting bodies from four regions in Europe contain from 1.79 to 2.65 µg/100 g dw of vitamin B12. Compared to five other mushroom species, these were the highest values.
3.5.4. β-Carotene
Barros et al. [10] showed that the fruiting bodies of C. cornucopioides were very rich in β-carotene (containing 12.77 µg/g). The higher amount occurred only in Cantharellus cibarius (13.56 µg/g), and the lowest in Agaricus bisporus (1.95 µg/g). Vamanu and Nita [26] found that the ethanol extract prepared from C. cornucopioides contained 0.14 mg/100 g of β-carotene. A higher concentration was found only in the extract prepared from the Tuber melanosporum (0.18 mg/100 g of extract) and the lowest in the extract from Agaricus bisporus (0.002 mg/100 g of extract). Kol et al. [34] showed that methanol extract contained 6.34 µg/mg of β-carotene and water extract 3.89 µg/mg.
3.6. Lycopene
According to the research of Barros et al. [10], the fruiting bodies of C. cornucopioides were the richest in lycopene among the eight compared species and contained 5.13 µg/g of extract. This value was 0.07 µg/g higher than the content found in the fruiting bodies of Cantharellus cibarius, which are considered a rich source of this ingredient. For comparison, the lowest lycopene content was found in Agaricus bisporus (0.91 µg/g of extract) [10]. Vamanu and Nita [26] also showed that ethanol extracts of C. cornucopioides contained lycopene, but it was less than in one of the tested strains of Pleurotus ostreatus and less than in Tuber melanosporum (0.07 mg/100 g of extract in C. cornucopioides, 0.12 mg/100 g of extract in P. ostreatus, and 0.1 mg/100 g of extract in T. melanosporum). Kol et al. [34] showed that depending on the solvent, the lycopene content in the extracts of C. cornucopioides varied, and it was 2.49 µg/mg for water extract and 5.55 µg/mg for methanol extract.
3.7. Phenolic Compounds, Flavonoids, and Tannins
Most studies found that the fruiting bodies of C. cornucopioides contained small amounts of total phenolic compounds, from 2.13 mg/g dw [10] to about 5 mg/g dw [16]; however, Turfan et al. [17] found as much as 37.5 mg/g dw. In research conducted by Barros et al. [10], among the eight compared species, the largest amount of these substances was found in the fruiting bodies of Agaricus silvaticus (8.94 mg/g dw), and smaller amounts than that recorded in C. cornucopioides were found only in Calocybe gambosa (1.7 mg/g dw) and Cantherellus cibarius (0.88 mg/g dw). Among the nine species compared by Ouali et al. [16], the highest total phenolic compounds were found in Hericium erinaceus (above 11 mg/g dw), and among the 15 taxa studied by Turfan et al. [17] in Boletus edulis (157.4 mg/g dw). A very low content of total phenolic compounds, amounting to 1.6 mg GA/g dw in the fruiting bodies of C. cornucopioides, was also demonstrated by Dospatiev et al. [35]. Vamanu and Nita [26] found that the extract obtained from the fruiting bodies of C. cornucopioides contained total polyphenols at the level of 88.4 mg GA/100 g of extract, which was one of the average values among the seven compared mushroom species. The largest amount was contained in the fruiting bodies of Tuber melanosporum (122.4 mg of gallic acid/100 g of extract). According to research by Radović et al. [13], the total phenolic content in the water extract was almost twice as high compared to the methanol extract (17.0 and 8.9 mg GA/g extract, respectively).
Both Barros et al. [10], Turfan et al. [17], and Ouali et al. [16] showed that the fruiting bodies of C. cornucopioides contained small amounts of flavonoids (1.71 mg/g, 8.8 mg/g, and about 5 mg/g dw, respectively). The largest amount of these substances Barros et al. [10] found in Agaricus silvaticus (3.4 mg/g), Turfan et al. [17] in Ganoderma lucidum (30.7 mg/g), and Ouali et al. [16] in Ramaria flavescens (above 17 mg/g dw). Similarly, Vamanu and Nita [26] found that the extract prepared from C. cornucopioides contained only 151.5 µg of quercetin/100 g of extracted flavonoids, while the largest amount among the seven fungal taxa tested was contained in the extract from Tuber melanosporum (414.0 µg of quercitin/100 g of extract).
Similar observations regarding the low content of total phenolic compounds and total flavonoids in black trumpet fruiting bodies were made by Palacios et al. [36]. Their studies showed that ferulic, gallic, p-hydroxybenzoic, homogentisic and protocatechuic acids, myricetin, and pyrogallol were present in methanol extracts.
Fruiting bodies of C. cornucopioides contained approximately 20 mg/g dry weight of tannin compounds, which was the average value among nine fungal taxa studied by Ouali et al. [16], while Lactarius deliciosus contained the most of these substances (above 25 mg/g).
3.8. Ingredients Influencing the Sensory Properties of Black Trumpet
Although raw fruiting bodies of C. cornucopioides have a mild taste and pleasant smell [3], they should be eaten after heat treatment. Properly prepared, they are tasty and delicate in taste and also have a pleasant smell [4]. They are considered the most aromatic among other edible mushrooms from the order Cantherellales [37]. Both fresh and soaked, they are flexible after drying [4].
The taste of mushrooms is related to the content and composition of non-volatile components, including the combination of free amino acids and nucleotides characteristic of a given species [12,17,38,39,40,41] and soluble sugars and polyols. Turfan et al. [17] and Beluhan and Ragnogajec [12] believe that the relatively high content of sugars and polyols, which affects the sweetish taste of mushrooms, is a feature particularly desired by consumers (Figure 3).
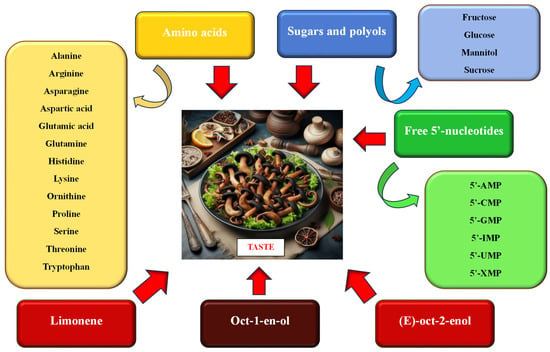
Figure 3.
Chemical ingredients affecting the taste of food products containing black trumpet fruiting bodies (data sources: [10,12,13,16,17,40,42,43,44]; the figure was prepared by the authors, and the picture was generated using artificial intelligence in Bing AI).
3.8.1. Sugars and Polyols
Fruit bodies of Craterellus cornucopioides contain from 10.8 to 15.2 g/100 g dw total soluble carbohydrates [10,12,16] (Figure 4). However, according to a comparison conducted by Beluhan and Ragnogajec [12], these mushrooms are the poorest in these ingredients among the 10 edible species compared.
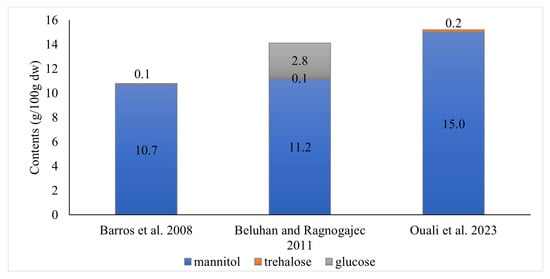
Figure 4.
Composition of soluble sugars and polyols in fruiting bodies of Craterellus cornucopioides according to different authors [10,12,16].
Turfan et al. [17] showed the presence of glucose (51.6 mg/g; it constituted 15.0% of all soluble sugars and polyols), fructose (9.2 mg/g; 2.7%), and sucrose (1.8 mg/g; 0.5%) in the fruiting bodies of C. cornucopioides. On the other hand, the presence of maltose and melezitose [10] and mannose [12] has not been found in them so far, although the presence of these substances has been demonstrated in other species of mushrooms.
Compared to other mushroom species, the fruiting bodies of C. cornucopioides were among the richest in terms of mannitol content (a higher amount was found only in Agaricus campestris), but the poorest in trehalose and glucose [12].
3.8.2. Amino Acids and Free Nucleotides
Amino acids found in food products differ in their solubility in various solvents and the taste sensation they produce. Some of them (proline, hydroxyproline, alanine, and glycine) easily dissolve in water, and depending on the pH, they give the products a sweet (all four amino acids) or bitter (proline and hydroxyproline) taste. The threshold values for the perception of taste sensation for most of them are very low and are 3.0, 0.5, 0.6, and 1.3 mg/cm3, respectively. The solubility in water of other amino acids giving sweet (e.g., serine), sour (e.g., aspartic acid and glutamic acid) or bitter tastes (phenylalanine, isoleucine, leucine, methionine, tryptophan, and valine) is weaker [42,45,46]. Monosodium glutamate, sodium aspartate, glutamic acid, and 5′-nucleotides, responsible for the umami taste, play a special role in food [12,38,42,47]. To a lesser extent, aspartic acid, glutamine, serine, and methionine are also responsible for the perception of this taste [42].
Turfan et al. [17] found that the fruiting bodies of C. cornucopioides contained one of the highest values of the total amount of free amino acids among the 15 compared fungal taxa, i.e., 6.6 mg/g (the highest value was found in Marasmius oreades—7.6 mg/g). According to research by Dospatliev et al. [40], dry fruit bodies contained an average of 30.4 mg amino acids /kg dw, the largest shares of which were glutamine (11.9 mg/kg dw), arginine (4.1 mg/kg dw), ornithine (3.2 mg/kg dw), glutamic acid (2.8 mg/kg dw), and serine (1.6 mg/kg dw). The remaining amino acids had a smaller contribution (Figure 5). The dominant group of amino acids found in these mushrooms is mostly responsible for the sweet, sour, and umami tastes (glutamine, serine, ornithine, and glutamic acid) [42,43], and only arginine is associated with a bitter taste. Research by Radović et al. [13] showed that the largest share in the fruiting bodies of C. cornucopioides were amino acids, which, according to the classification of Beluhan and Ragnogajec [12], are responsible for the bitter taste. They constituted 45.1% of all amino acids found in these mushrooms (3.1 mg/g dw). These mushrooms contained the most arginine (32.1% of all amino acids; 2.2 mg/g dw) and glutamic acid (20.1%, 1.4 mg/g dw), responsible for the bitter and monosodium glutamate-like tastes, respectively.
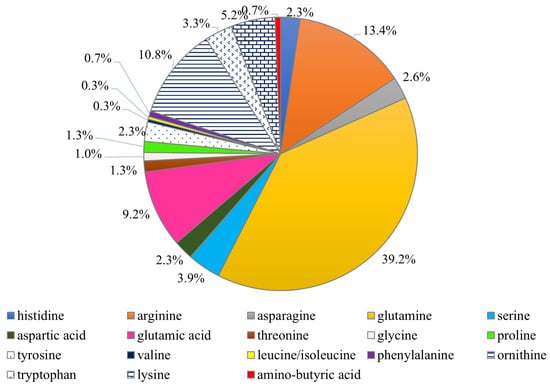
Figure 5.
The percentage of free amino acids present in the fruiting bodies of Craterellus cornucopioides (data from: Dospatliev et al. [40]).
Beluhan and Ragnogajec [12] found that in the fruiting bodies of C. cornucopioides collected in the forests of Croatia, the amino acid determining the umami taste (glutamic acid) was quantitatively dominant. It constituted 70.0% of all amino acids present in these mushrooms. Amino acids responsible for bitter and sweet tastes (12.0% and 10.8%, respectively) and tasteless amino acids (7.2%) had a much smaller share. Amino acids with a significant share were lysine (accounting for 7.0% of all amino acids), threonine (6.8%), histidine (5.4%), and alanine (3.5%). The remaining amino acids were present in small amounts. On the other hand, according to the research of Beluhan and Ragnogajec [12], the fruiting bodies of C. cornucopioides contained the highest amount of free 5′-nucleotides among other tested fungi (35.4 mg/g dw), which included 5′-adenosine monophosphate (5′-AMP), 5′-inosine monophosphate (5′-IMP), 5′-xanthosine monophosphate (5′-XMP), 5′-cytosine monophosphate (5′-CMP), 5′-guanosine monophosphate (5′-GMP), and 5′-uridine monophosphate (5′-UMP). These ingredients determine the taste of the product. A special role is played by 5′-GMP, which enhances the flavor and at the same time gives the products a meaty taste [31]. However, the sum of the 5′-GMP, 5′-IMP, and 5′-XMP content is considered to be the factor that demonstrates the intensity of the product taste [48]. According to Beluhan and Ragnogajec [12], this coefficient for C. cornucopioides was the highest among other compared fungi and amounted to 13.9 mg/g dw, while for the remaining species it ranged from 0.4 to 5.3 mg/g dw. However, the EUC index, which is an indicator of the umami taste as a result of the synergism of monosodium glutamate-like (MSG-like) components and free 5′-nucleotides, in the case of C. cornucopioides, was one of the lowest among the compared species and amounted to only 120.9 g MSG/100 g dw (in the case of Boletus edulis, this index reached a value of as much as 1186.5 g MSG/100 g dw). In the study by Radović et al. [13], the largest share of 5′-UMP was demonstrated (1.6 mg/g dw, which accounted for 38.8% of all 5′-nucleotides), and the other three nucleotides had a smaller share (5′-AMP: 0.9 mg/g dw, 21.8%; 5′-GMP: 0.8 mg/g dw, 20.8%; 5′-CMP: 0.7 mg/g dw, 18.5%).
Fons et al. [44] found that the volatile components responsible for the sensory properties of C. cornucopioides fruit bodies were mainly oct-1-en-3-ol, limonene, and (E)-Oct-2-enol constituting, respectively, 17.1%, 14.8%, and 12.1% of the volatile compounds present in these mushrooms.
3.9. Other Ingredients
Liu et al. [49], examining the chemical composition of C. cornucopioides culture broth, isolated three substances in the form of colorless oils: 4-oxohex-5-enyl acetate (C8H12O3Na), 4-oxohex-1,6-diyl diacetate (C10H17O5), and 6-hydroxy-4-oxohexyl acetate (C8H12O3Na), belonging to the group of keto esters. Additionally, eight components belonging to the sesquiterpenoid group were isolated from black trumpet cultures: gymnomitr-3-en-10β,15-diol, illudalenol, illudin F, illudin M, illudin T, and craterellins A-C [50].
4. Bioactivity of Black Trumpet Fruiting Bodies
4.1. Antioxidant Activity
The antioxidant activity of black trumpet has been studied by a few authors, but the results differed depending on the extraction method, the testing method, and the expression of the results (Table 2).

Table 2.
Antioxidant activities of black trumpet (Craterellus cornucopioides) extracts and extracted black trumpet polysaccharides (EC50—median effective concentration, causing 50% of antioxidant activity; IC50—concentration causing 50% inhibition of absorbance; AAE—ascorbic acid equivalents/g; TE—Trolox equivalent; Eq—equivalents).
According to Queirós et al. [51], Craterellus cornucopioides was characterized by average antioxidant activity, which was the third highest among five species examined. The concentration of dry extract necessary to inhibit β-carotene bleaching was low, 1.70 mg/mL, so the fruiting bodies of black trumpet compounds were effective to prevent lipid oxidation. In case of DPPH scavenging activity and reducing power, the effective concentrations causing 50% of antioxidant activity (EC50) were lower than 7.5 mg/mL, so they were considered by the authors as low (high antioxidant activity). However, according to Vasdekis et al. [52], C. cornucopioides’ ability to scavenge DPPH free radicals, expressed as EC50 of dry extract, was higher than 40 mg/mL and did not differ compared to other fungi from the Cantharellaceae family (Cantharellus cibarius and Cantharellus cinereus) and was similar to another 14 fungal species among the 29 studied. The highest antioxidative properties were found in Amanita citrina (4.0 ± 0.0 mg/mL), Ganoderma lucidum (4.0 ± 0.0 mg/mL), and Agaricus urinascens (4.9 ± 0.1 mg/mL). There was clear dependency between antioxidant activity and total polyphenol content; C. cornucopioides was in the group of four species with the lowest polyphenol content, although not in terms of flavonoids [52].
Mešić et al. [53], examining the relationship between the composition and morphological features of 16 species of wild mushrooms (in 23 samples), showed that C. cornucopioides were characterized by the third highest DPPH scavenging ability (31 µmol Trolox equivalent TE/g dw) after Psathyrella piluliformis (40 µmol TE/g dw) and Lycoperdon perlatum (35 µmol TE/g dw). It showed the fifth highest ferric reducing power (31 µmol Fe3+/g dw), and P. piluliformis also showed the highest FRAP values of 70 and 52 µmol TE/g dw (in two independent tested samples).
In turn, Costea et al. [54] investigated the antioxidant activity of C. cornucopioides, depending on the type of extracts. They found that water dry extract was characterized by the highest ability to scavenge DPPH and ABTS free radicals as well as ferric reducing power, expressed as ascorbic acid equivalents per 1 g of dry extract (AAE/g), while methanolic dry extract showed the highest chelating activity, expressed as Na2-EDTA equivalents per one gram of dry extract. Ethanolic extracts showed the lowest antioxidant properties. Comparison of the effect of dry extract concentration (0.2–1.8 g/mL) on 50% inhibition of absorbance (IC50) confirmed that water dry extracts caused higher inhibition of DPPH and ABTS free radicals than other extracts, and ethanol extracts showed the lowest inhibition and the lowest absorbance. In turn, methanol extracts showed the highest inhibition in chelating activity. This was due to the content of polyphenols in these extracts; in the water extract, they were more than twice as high as in the ethanol and methanol extracts [54].
Kosanić et al. [56] showed that acetone extract of black trumpet was characterized by antioxidant activity expressed as DPPH radical scavenging activity, and the IC50 was 19.7 ± 1.1 µg/mL, while ascorbic acid as positive control was 6.41 ± 0.2 µg/mL. Superoxide anion scavenging IC50, 221.8 ± 3.1 µg/mL, was two times greater than ascorbic acid. However, ferric reducing power was very low.
Generally, it can be said that the black trumpet is characterized by relatively low DPPH scavenging activity, but its antioxidant activity against ABTS cation radicals is high.
Ferric reducing power was rather low [54], especially in acetone extract [56]; however, copper reducing power was three times lower than that of α-tocopherol [55]. Extracts were efficient as ferrous chelating agents [54] and agents preventing lipid oxidation determined as inhibition of β-carotene bleaching [51] and linoleic acid oxidation [36,55].
The antioxidant activity of mushrooms is related to the presence of polyphenols, flavonoids, tocopherols, etc., as well as polysaccharides. Yang et al. [57] showed that methanolic extract of polysaccharides extracted from black trumpet fruiting bodies showed high antioxidant activity against DPPH and ABTS radicals (0.10 and 0.15 mg/mL, respectively).
4.2. Immunostimulating and Anti-Inflammatory Effects
Research conducted in Tanzania has shown that a preparation consisting of the powdered fruiting bodies of several species of mushrooms, including C. cornucopioides, effectively strengthens immunity in HIV-infected patients [58].
According to the research of Guo et al. [21,22,59], polysaccharides occurring in the fruiting bodies of this mushroom species, especially polysaccharides characterized by a triple helix, may be responsible for the extremely beneficial immunostimulating effect. They have the ability to stimulate the functioning of the immune system in mice by stimulating the activity of RAW264.7 macrophages. Ding et al. [60] came to similar conclusions as a result of examining the bioactivity of the CC-M polysaccharide isolated from C. cornucopioides. It increased the activity of T and B lymphocytes and RAW264.7 macrocytes and stimulated the signaling pathways of MAPK, PI3K-Akt, NF-κB, and NOD-like receptor signaling pathway receptors as a result of stimulating the secretion of CD163, IL-1β, IL-6, IL-10, and TNF-α. Also, Xu et al. [61] found that CCPP-1 polysaccharides obtained from C. cornucopioides can affect the NF-kB signaling pathway and alleviate inflammation by reducing the amount of pro-inflammatory cytokines IL-1β, IL-18, and TNF-α and the mediator iNOS and by reducing the amount of ROS and NO in cells. Moreover, CCPP-1 polysaccharides had the ability to restore GPx and CAT levels to appropriate levels and inhibit the formation of MDA and reactive oxygen species (ROS) inside cells. This action helps protect erythrocytes against changes caused by oxidative stress (hemolysis) [57].
Zhang et al. [62] found that the polysaccharide CCP2 with a catenarian pyranose structure, consisting of galactose, glucose, mannose, and xylose, isolated from C. cornucopioides, also exhibited immunomodulatory effects in in vitro and in vivo studies. It activated the increased production of cytokines IL-2, IL6, and IL-8, TLR4 protein, and protein kinases NF-κB p 65, TRAF6, and TRIF. This stimulated the activity of macrophages and immune processes in cells.
The effectiveness of C. cornucopioides extract in preventing or reducing inflammation by reducing the expression of NO and IL-6 was also previously demonstrated by O’Callaghan et al. [63]. Moro et al. [64] showed that the methanol extract from this mushroom only inhibits the production of NO and the expression of iNOS, without causing changes in the levels of IL-1b and IL-6 (Figure 6).
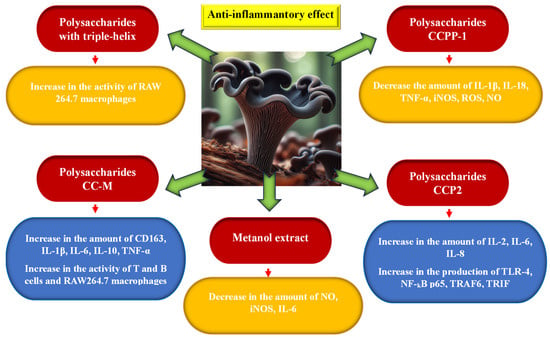
Figure 6.
Anti-inflammatory activity of Craterellus cornucopioides fruiting bodies (data sources: [21,22,57,59,60,61,62,63,64]; the figure was prepared by the authors, and the picture was generated using artificial intelligence in Bing AI).
4.3. Antibacterial, Antifungal, and Antiviral Effects
It was shown that methanol and acetone extracts obtained from C. cornucopioides inhibited the development of Staphylococcus aureus. Moreover, they were characterized by antibacterial activity against Klebsiella pneumoniae, but this effect was observed only at the highest tested concentration (200 mg/mL) [19]. Kosanić and colleagues [56] found that acetone extracts affect not only Staphyllococcus aureus but also inhibit the activity of Bacillus cereus, B. subtilis, Escherichia coli, and Proteus mirabilis. Kol et al. [34] demonstrated strong antibacterial effectiveness of methanol and water extracts against Agrobacterium tumefaciens, Bacillus licheniformis, B. subtilis, Enterococcus faecalis, Escherichia coli, and Staphylococcus aureus (ATCC 2921). Acetone extracts also limited the activity of the fungi Aspergillus niger, Candida albicans, Mucor mucedo, Penicillium italicum, and Trichoderma viride [56].
It has also been shown that the CCP polysaccharide isolated from the fruiting bodies of C. cornucopioides influences the species and quantitative composition of microorganisms constituting the intestinal flora and their activity, promoting species that have a beneficial effect on digestive processes. The authors of the study related the research results to the possibility of using CCP in the prevention of digestive system diseases [65].
The literature also contains information about the antiviral effect of aqueous extracts of this fungus against Vaccinia virus [66].
4.4. Anticancer Effect
In order to examine the anticancer activity of C. cornucopioides, the following were used: extracts obtained from the fruiting bodies of the fungus using various solvents (water, methanol, acetone, cyclohexane, or dichloromethane), selected substances isolated from liquid extracts, and powdered dried mushrooms (a mixture of several species) (Figure 7).
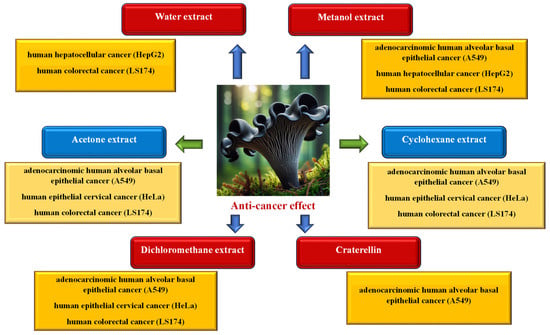
Figure 7.
Anticancer activity of Craterellus cornucopioides fruiting bodies (data sources: [13,34,50,52,56]; the figure was prepared by the authors, and the picture was generated using artificial intelligence in Bing AI).
Kol et al. [34] showed that both aqueous and methanol extracts of C. cornucopioides had the ability to inhibit the growth of HepG2 tumor cells (human hepatocellular cancer), but this effect was more pronounced in the case of methanol extracts (IC50 for the methanol extract was 3.14 mg/mL and for the water extract 18.41 mg/mL). It was observed that the anticancer effectiveness of both types of extracts increased with the increase in the applied concentration. Also, Vasdekis et al. [52] found that the methanol extract showed very strong anticancer activity against the A549 cancer cell line (adenocarcinomic human alveolar basal epithelial cells; IC50 < 1 mg/mL); moreover, this activity was significantly higher compared to other tested fungal species.
The acetone extract, however, showed a much greater effect on the HeLa cell line (human epithelial cervical cancer cells; IC50 65.5 μg/mL) than on A549 (IC50 108.2 μg/mL) and LS174 (human colorectal cancer cells; IC50 131.7 μg/mL) [56]. Testing the effect of four types of extracts (aqueous, methanol, cyclohexane, and dichloromethane) on the A549, HeLa, and LS174 cancer cell lines showed that cyclohexane and dichloromethane extracts have anticancer activity against all tested cell lines. Cyclohexane extract had a strong effect on HeLa (IC50 78.3 µg/mL) and a moderate effect on LS174 (IC50 139.1 µg/mL) and on A549 (IC50 141.9 µg/mL). Dichloromethane extract had a similar moderate effect on HeLa and LS174 (IC50 135.6 and 135.7 µg/mL, respectively) and a weaker effect on A549 (IC50 153.2 µg/mL). Aqueous and methanolic extracts showed only low cytotoxic activity towards LS174 (IC50 191.5 and 186.6 µg/mL, respectively) [13].
The anticancer activity of eight sesquiterpenoids isolated from C. cornucopioides fruiting bodies against five cancer cell lines was also tested, but only one of them—craterellin C (C15H22O4)—showed moderate toxicity towards A549 cells (IC50 21.0 μM) [50].
The health-promoting effects of mushrooms, including their anticancer effects, should be associated with the polysaccharides they contain, including beta-glucans [67,68]. However, according to comparative studies by Mirończuk-Chodakowska et al. [20], black trumpet fruiting bodies are not the richest in these substances. Moreover, according to the literature reports, GACOCA powder, obtained after grinding several species of dried mushrooms (Cantharellus cibarius, C. cornucopioides, C. isabellinus, Fomes fomentarius, Ganoderma applanatum, G. lucidum, G. pfefferi, Phellinus igniarus, Schizophyllum commune, and Trametes versicolor), has been successfully used in Tanzania as a therapeutic agent in the treatment of Kaposi’s sarcoma (skin cancer affecting patients with HIV/AIDS) [58].
4.5. Antihyperglycemic Effect
The ethanol extract from C. cornucopioides shows high α-glucosidase inhibitory activity (EC50 value 8.28 lg/mL) [24,69].
4.6. Other Activity
The methanol–water extract obtained from C. cornucopioides showed very weak inhibition of fat absorption as a pancreatic lipase inhibitor. Although the activity of the extract determined using the enzymatic kit was 95.6%, it increased significantly using the in vitro digestion model and amounted to as much as 181.0% [70].
5. Possibilities of Using Black Trumpet Fruiting Bodies in Food Production
Black trumpet fruiting bodies have a scent reminiscent of apricots, which is why they are appreciated by consumers [9]. On the one hand, their appearance is considered to be unappetizing due to its blackening during cooking [71], but according to Yamada [9], the natural shape of a black funnel is an additional stimulator of sensory experiences when consuming dishes with this mushroom.
Fruiting bodies of C. cornucopioides are most often used in dried form (as raw material for preparing various dishes) or dried and ground into mushroom flour (for preparing soups) [8]. The latter method of being used as a seasoning for soups and sauces was also mentioned in the Lexicon of Mushrooms [71]. In Denmark, C. cornucopioides is sold in two forms: fresh and dried [5].
The antioxidant and antibacterial effects of the black trumpet fruiting bodies, demonstrated in many studies, led to the study of the effect of the fruiting bodies of this mushroom together with two other species (Boletus edulis and Cantharellus cibarius) as an addition to frankfurters. All these mushroom species significantly influenced the texture of the sausages. An improvement (increase) in the hardness and chewiness of the final product was achieved due to the higher protein content, which created a denser matrix compared to frankfurters without the addition of mushrooms. However, in this study, samples with the addition of C. cornucopioides obtained lower scores than the other two species of mushrooms. What was significant in the tests was the very low color rating of the product with the addition of black trumpet. After adding this mushroom, an immediate deterioration in color was observed, which disqualified the final product after 2 months of storage. Frankfurters with the addition of the other two species received much better ratings here [72].
Due to its dark color, black trumpet can be a substituted for mun mushrooms (Auricularia auricula-judae), used as one of the main ingredients in Asian (mainly Chinese) dishes. Moreover, this mushroom can be used in the production of, e.g., pasta, and it can be a valuable ingredient in snacks and crackers. It can be also a valuable ingredient in vegan “blood sausage”.
6. Conclusions
Black trumpet, also called horn of plenty, contains a high amount of water; however, the composition of dry matter varies depending on the world region and study. It was found that C. cornucopioides contains, among other components, unsaturated fatty acids (mainly oleic and linoleic acids), β-glucans, minerals, and vitamins as well as polyphenols and tannins. It also contains compounds influencing the sensory properties, like free amino acids and nucleotides as well as sugars and polyols, mainly mannitol. The presence of bioactive compounds causes C. cornucopioides to show antioxidant activity and immunostimulating, anti-inflammatory, and anticancer properties. It also exhibits antibacterial, antifungal, antiviral, and antihyperglycemic effects. Although black trumpet has been applied in traditional medicine and it is consumed in freshly prepared dishes, it has not yet been used in ready-to-eat processed products. So far, a few works have presented its use only in sausages. However, the use of fresh or dried mushrooms in food can provide important health benefits and could therefore be used in new, interesting food products.
Author Contributions
Conceptualization, I.A. and K.F.; writing—original draft preparation, I.A. and K.F.; writing—review and editing, I.A. and K.F.; visualization, I.A. and K.F.; supervision, I.A.; funding acquisition, K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Many thanks are due to Pierino Bigoni and Renato Aldo Ferri for collecting and providing information about the culinary use of C. cornucopioides in Italy. Many thanks to Pierino Bigoni for sharing photos with the right to publish.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bumbu, M.-G.; Niculae, M.; Ielciu, I.; Hanganu, D.; Oniga, I.; Benedec, D.; Nechita, M.-A.; Nechita, V.-I.; Marcus, I. Comprehensive review of functional and nutraceutical properties of Craterellus cornucopioides (L.) Pers. Nutrients 2024, 16, 831. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, M.; Danell, E.; Spatafora, J.W. Molecular systematics of Craterellus: Cladistic analysis of nuclear LSU rDNA sequence data. Mycol. Res. 2000, 104, 388–394. [Google Scholar] [CrossRef]
- Pilz, D.; Norvell, L.; Danell, E.; Molina, R. Ecology and Management of Commercially Harvested Chanterelle Mushrooms; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Jefferson Way: Corvallis, OR, USA, 2002; p. 35.
- Škubla, P. Wielki Atlas Grzybów; Elipsa: Poznań, Poland, 2007; ISBN 978-83-245-9550-1. [Google Scholar]
- Gry, J.; Andersson, C. Mushrooms Traded as Food. Vol II Sec. 2. Nordic Risk Assessments and Background on Edible Mushrooms, Suitable for Commercial Marketing and Background Lists for Industry, Trade and Food Inspection. Risk Assessments of Mushrooms on the Four Guidance Lists; Norden Nordic Council of Ministers: Copenhagen, Denmark, 2014; pp. 169–170. [Google Scholar]
- Anusiya, G.; Gowthama Prabu, U.; Yamini, N.V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A review of the therapeutic and biological effects of edible and wild mushrooms. Bioengineered 2021, 12, 11239–11268. [Google Scholar] [CrossRef] [PubMed]
- ColFungi. Useful Fungi of Colombia. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://colfungi.org/taxon/urn:lsid:indexfungorum.org:names:153130 (accessed on 13 February 2024).
- Bulam, S.; Üstün, N.S.; Pekşen, A. The Most Popular Edible Wild Mushrooms in Vezirköprü District of Samsun Province. Turk. J. Agric.-Food Sci. Technol. 2018, 6, 189–194. [Google Scholar] [CrossRef]
- Yamada, A. Cultivation studies of edible ectomycorrhizal mushrooms: Successful establishment of ectomycorrhizal associations in vitro and efficient production of fruiting bodies. Mycoscience 2022, 63, 235–246. [Google Scholar] [CrossRef]
- Barros, L.; Cruz, T.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol. 2008, 46, 2742–2747. [Google Scholar] [CrossRef]
- Colak, A.; Faiz, Ö.; Sesli, E. Nutritional Composition of Some Wild Edible Mushrooms. Turk. J. Biochem. 2009, 34, 25–31. [Google Scholar]
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Radović, J.; Leković, A.; Tačić, A.; Dodevska, M.; Stanojković, T.; Marinković, T.; Jelić, Č.; Kundaković-Vasović, T. Black Trumpet, Craterellus cornucopioides (L.) Pers.: Culinary mushroom with Angiotensin Converting Enzyme Inhibitory and Cytotoxic Activity. Polish J. Food Nutr. Sci. 2022, 72, 171–181. [Google Scholar] [CrossRef]
- Odoh, R.; Ugwuja, D.I.; Udegbunam, I.S. Proximate composition and mineral profiles of selected edible mushroom consumed in northern part of Nigeria. Acad. J. Sci. Res. 2017, 5, 349–364. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Ouali, Z.; Chaar, H.; Venturella, G.; Cirlincione, F.; Gargano, M.L.; Jaouani, A. Chemical composition and nutritional value of nine wild edible mushrooms from Northwestern Tunisia. Ital. J. Mycol. 2023, 52, 32–49. [Google Scholar] [CrossRef]
- Turfan, N.; Pekşen, A.; Kibar, B.; Ünal, S. Determination of nutritional and bioactive properties in some selected wild growing and cultivated mushrooms from Turkey. Acta Sci. Pol. Hortorum Cultus 2018, 17, 57–72. [Google Scholar] [CrossRef]
- Vetter, J. Chemische Zusammensetzung von acht ebaren Pilzarten. Z. Lebensm. Unters. Forsch. 1993, 196, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Özcan, Ö.; Ertan, F. Beta-glucan Content, Antioxidant and Antimicrobial Activities of Some Edible Mushroom Species. Food Sci. Technol. 2018, 6, 47–55. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E.; Terlikowska, K.M. Quantitative evaluation of 1,3-1,6-β-D-glucan contents in wild-growing species of edible polish mushrooms. Rocz. Panstw. Zakl. Hig. 2017, 68, 281–290. [Google Scholar] [PubMed]
- Guo, M.-Z.; Meng, M.; Duan, S.-Q.; Feng, C.-C.; Wang, C.-L. Structure characterization, physicochemical property and immunomodulatory activity on RAW264.7 cells of a novel triple-helix polysaccharide from Craterellus cornucopioides. Int. J. Biol. Macromol. 2019, 126, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.-Z.; Meng, M.; Feng, C.-C.; Wang, X.; Wang, C.-L. A novel polysaccharide obtained from Craterellus cornucopioides enhances immunomodulatory activity in immunosuppressive mice models via regulation of the TLR4-NF-κB pathway. Food Funct. 2019, 10, 4792–4801. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Gürgen, A.; Ϛevik, U. Accumulation of metals in some wild and cultivated mushroom species. Sigma J. Eng. Nat. Sci. 2019, 37, 1375–1384. [Google Scholar]
- Liu, Y.T.; Sun, J.; Luo, Z.Y.; Rao, S.Q.; Su, Y.J.; Xu, R.R.; Yang, Y.J. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Cağlarirmak, N. Edible mushrooms: An alternative food item. In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7), Arcachon, France, 4–7 October 2011; pp. 548–554. [Google Scholar]
- Vamanu, E.; Nita, S. Biological activity of fluidized bed ethanol extracts from several edible mushrooms. Food Sci. Biotechnol. 2014, 23, 1483–1490. [Google Scholar] [CrossRef]
- Gil-Ramirez, A.; Clavijo, C.; Palanisamy, M.; Soler-Rivas, C.; Ruiz-Rodriguez, A.; Marin, F.R.; Reglero, G.; Perez, M. Edible mushrooms as potential sources of new hypocholesterolemic compounds. In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7), Arcachon, France, 4–7 October 2011; pp. 110–119. [Google Scholar]
- Villares, A.; Mateo-Vivaracho, L.; García-Lafuente, A.; Guillamón, E. Storage temperature and UV-irradiation influence on the ergosterol content in edible mushrooms. Food Chem. 2014, 147, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Dospatliev, L.; Petkova, Z.; Antova, G.; Angelova-Romova, M.; Ivanova, M.; Mustafa, S. Proximate composition of wild edible mushrooms from the Batak Mountain, Bulgaria. J. Microbiol. Biotech. Food Sci. 2023, 12, e4718. [Google Scholar] [CrossRef]
- Judprasong, K.; Chheng, S.; Chimkerd, C.; Jittinandana, S.; Tangsuphoom, N.; Sridonpai, P. Effect of Ultraviolet Irradiation on Vitamin D in Commonly Consumed Mushrooms in Thailand. Foods 2023, 12, 3632. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Sridonpai, P.; Suthipibul, P.; Boonyingsathit, K.; Chimkerd, C.; Jittinandana, S.; Judprasong, K. Vitamin D Content in Commonly Consumed Mushrooms in Thailand and Its True Retention after Household Cooking. Foods 2023, 12, 2141. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Schwarz, J.; Takenaka, S.; Miyamoto, E.; Ohishi, N.; Nelle, E.; Hochstrasser, R.; Yabuta, Y. Characterization of vitamin B12 compound in the wild edible mushrooms blact trumpet (Craterellus cornucopioides) and golden chanterelle (Cantharellus cibarius). J. Nutr. Sci. Vitaminol. 2012, 58, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Kol, S.; Bostanci, A.; Kocabas, A.; Uzun, Y.; Sadi, G. Cell growth inhibitory potential of Craterellus cornucopioides (L.) Pers. together with antioxidant and antimicrobial properties. Ant. J. Bot. 2018, 2, 60–64. [Google Scholar] [CrossRef]
- Dospatliev, L.K.; Petkova, Z.Y.; Bojilov, D.G.; Ivanova, M.T.; Antova, G.A.; Angelova-Romova, M.Y. A comparative study on the methods of antioxidant activity in wild edible mushrooms from the Batak Mountain, Bulgaria. Bulg. Chem. Commun. 2019, 51, 245–250. [Google Scholar]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, A.E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Arora, D. All That the Rain Promises and More: A Hip Pocket Guide to Western Mushrooms; Ten Speed Press: Berkeley, CA, USA, 2022; Volume 263, p. 1991. [Google Scholar]
- Litchfield, J.H. Morel mushroom mycelium as a food-flavouring material. Biotechnol. Bioeng. 1967, 9, 289–304. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lin, H.-C.; Mau, J.-L. Non-volatile taste components of several commercial mushrooms. Food Chem. 2001, 72, 465–471. [Google Scholar] [CrossRef]
- Dospatliev, L.; Lozanov, V.; Ivanova, M.; Papazov, P.; Sugareva, P. Amino acids in edible wild mushroom from the Batak Mountain, Bulgaria. Bulg. Chem. Commun. 2019, 51, 92–96. [Google Scholar]
- Valchev, N. Nutritional and amino acid content of stem and cap of Agaricus bisporus, Bulgaria. Bulg. J. Agric. Sci. 2020, 26 (Suppl. S1), 192–201. [Google Scholar]
- Kirimura, J.; Shimizu, A.; Kimizuka, A.; Nimomiya, T.; Katsuya, N. The contribution of peptides and amino acids to the taste of foodstuffs. J. Agric. Food Chem. 1969, 17, 689–695. [Google Scholar] [CrossRef]
- Kuramitsu, R.; Takahashi, M.; Tahara, K.; Nakamura, K.; Okai, H. Tastes Produced by Peptides Containing Ionic Groups and by Related Compounds. Biosci. Biotechnol. Biochem. 1996, 60, 1637–1642. [Google Scholar] [CrossRef]
- Fons, F.; Rapior, S.; Eyssartier, G.; Bessiere, J.-M. Les substances volatiles dans les genres Cantharellus, Craterellus et Hydnum. Cryptogam. Mycol. 2003, 24, 367–376. [Google Scholar]
- Belitz, H.-D.; Grosch, W. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Kalać, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Edible mushrooms: Functional foods or functional ingredients? A Focus on Pleurotus spp. AIMS Agric. Food 2023, 8, 391–439. [Google Scholar] [CrossRef]
- Chen, H.K. Studies on the Characteristics of Taste-Active Components in Mushroom Concentrate and Its Powderization. Master’s Thesis, National ChungHsing University, Taichung, Taiwan, 1986. [Google Scholar]
- Liu, R.; Zhou, Z.-Y.; Liu, J.-K. Three New Keto Esters from Cultures of the Basidiomycete Craterellus cornucopioides. Chin. J. Nat. Med. 2010, 8, 88–90. [Google Scholar] [CrossRef]
- Guo, H.; Diao, Q.-P.; Hou, D.-Y.; Li, Z.-H.; Zhou, Z.-Y.; Feng, T.; Liu, J.-K. Sesquiterpenoids from cultures of the edible mushroom Craterellus cornucopioides. Phytochem. Lett. 2017, 21, 114–117. [Google Scholar] [CrossRef]
- Queirós, B.; Barreira, J.C.M.; Sarmento, A.C.; Ferreira, I.C.F.R. In search of synergistic effects in antioxidant capacity of combined edible mushrooms. Int. J. Food Sci. Nutr. 2009, 60, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Vasdekis, E.P.; Karkabounas, A.; Giannakopoulos, J.; Savvas, D.; Lekka, M.E. Screening of mushrooms bioactivity: Piceatannol was identified as a bioactive ingredient in the order Cantharellales. Eur. Food Res. Technol. 2018, 244, 861–871. [Google Scholar] [CrossRef]
- Mešić, A.; Šamec, D.; Jadan, M.; Bahun, V.; Tkalčec, Z. Integrated morphological with molecular identification and bioactive compounds of 23 Croatian wild mushrooms samples. Food Biosci. 2020, 37, 100720. [Google Scholar] [CrossRef]
- Costea, T.; Hudiţă, A.; Olaru, O.T.; Gălăţeanu, B.; Gîrd, C.E.; Mocanu, M.M. Chemical composition, antioxidant activity and cytotoxic effects of romanian Craterellus cornucopioides (L.) pers. mushroom. Farmacia 2020, 68, 340–347. [Google Scholar] [CrossRef]
- Novaković, S.M. The Impact of the Addition of Porcini (Boletus edulis), Chanterelle (Cantharellus cibarius) and Horn of Plenty (Craterellus cornucopioides) on the Overall Quality of Cooked Sausages in the Type of Frankfurters. Ph.D. Thesis, University of Belgrade, Beograd, Serbia, 2020. [Google Scholar]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Radović-Jakovljević, M.; Cirić, A.; Grujičić, D.; Milošević-Djordjević, O. Craterellus cornucopioides edible mushroom as source of biologically active compounds. Nat. Product. Commun. 2019, 14, 1934578–19843610. [Google Scholar] [CrossRef]
- Yang, W.-W.; Li-Ming Wang, L.-M.; Gong, L.-L.; Lu, Y.-M.; Pan, W.-J.; Wang, Y.; Zhang, W.-N.; Chen, Y. Structural characterization and antioxidant activities of a novel polysaccharide fraction from the fruiting bodies of Craterellus cornucopioides. Int. J. Biol. Macromol. 2018, 117, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Sumba, J.D. GACOCA Formulation of East African Wild Mushrooms Show Promise in Combating Kaposi’s Sarcoma and HIV/AIDS. Int. J. Med. Mushrooms 2005, 7, 473–474. [Google Scholar] [CrossRef]
- Guo, M.; Meng, M.; Zhao, J.; Wang, X.; Wang, C. Immunomodulatory effects of the polysaccharide from Craterellus cornucopioides via activating the TLR4-NFκB signaling pathway in peritoneal macrophages of BALB/c mice. Int. J. Biol. Macromol. 2020, 160, 871–879. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, M.; Hou, Y. Comparative studies on the structure, biological activity and molecular mechanisms of polysaccharides from Craterellus cornucopioides (CC-M) and Dictyophora indusiata (Vent.ex Pers) Fisch (DI-Z). Food Sci. Technol. 2022, 42, e40421. [Google Scholar] [CrossRef]
- Xu, J.-J.; Gong, L.-L.; Li, Y.-Y.; Zhou, Z.B.; Yang, W.-W.; Wan, C.-X.; Zhang, W.-N. Anti-inflammatory effect of a polysaccharide fraction from Craterellus cornucopioides in LPS-stimulated macrophages. J. Food Biochem. 2021, 45, e13842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shu, Y.; Li, Y.; Guo, M. Extraction and immunomodulatory activity of the polysaccharide obtained from Craterellus cornucopioides. Front. Nutr. 2022, 9, 1017431. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, Y.C.; O’Brien, N.M.; Kenny, O.; Harrington, T.; Brunton, N.; Smyth, T.J. Anti-inflammatory effects of wild Irish mushroom extracts in RAW264.7 mouse macrophage cells. J. Med. Food 2015, 18, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villar, A.; Martinez, J.A.; Garcia-Lafuente, A.G. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, X.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, Y.; Wu, H.; Chen, H.; Wu, W. Effects of in vitro digestion and fecal fermentation on the stability and metabolic behavior of polysaccharides from Craterellus cornucopioides. Food Funct. 2020, 11, 6899–6910. [Google Scholar] [CrossRef] [PubMed]
- Sevindik, M.; Bal, C.; Eraslan, E.C.; Uysal, I.; Mohammed, F.S. Medicinal mushrooms: A comprehensive study on their antiviral potential. Prospect. Pharm. Sci. 2023, 21, 42–56. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Xie, J.; Chen, S. Structure, function and mechanism of edible fungus polysaccharides in human beings chronic diseases. Food Sci. Technol. 2023, 43, e111022. [Google Scholar] [CrossRef]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef]
- Perez-Moreno, J.; Martinez-Reyes, M. Edible Ectomycorrhizal Mushrooms: Biofactories for Sustainable Development. In Biosystems Engineering: Biofactories for Food Production in the Century XXI; Guevara-Gonzalez, R., Torres-Pacheco, I., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 151–233. [Google Scholar] [CrossRef]
- Palanisamy, M.; Gil-Ramirez, A.; Ruiz-Rodriguez, A.; Marin, F.R.; Regelo, G.; Soler-Rivas, C. Testing edible mushrooms to inhibit the pancreatic lipase activity by an in vitro digestion model. Int. J. Food Sci. Technol. 2012, 47, 1004–1010. [Google Scholar] [CrossRef]
- Jezierska, M. Lexicon of Mushrooms; Firma Księgarska Jacek i Krzysztof Olesiejuk Inwestycje: Warszawa, Poland, 2006; ISBN 3-89836-604-9. [Google Scholar]
- Novakovic, S. The potential of the application of Boletus edulis, Cantharellus cibarius and Craterellus cornucopioides in frankfurters: A review. In IOP Conference Series: Earth and Environmental Science 21, Proceedings of the 61st International Meat Industry Conference, Jaipur, Rajasthan, India, 5–6 March 2021; IOP Publishing: Bristol, UK, 2021; Volume 854, p. 012068. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).