Impact of a Whole-Food, High-Soluble Fiber Diet on the Gut–Muscle Axis in Aged Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Overview
2.2. Diets
2.3. Diet Composition Analysis
2.4. Stool DNA Extraction, High-Throughput Sequencing, and Taxonomy Assignment
2.5. Plasma SCFA and Clinical Chemistry Analyses
2.6. Body Composition Assessment and Measurement of Skeletal Muscle Mass
2.7. Physical Function Measures
2.8. Statistical Analysis
2.8.1. α-, β-Diversity
2.8.2. Within- and between-Group Differences for Food and Energy Intake, Taxonomy, SCFAs and Clinical Chemistry Analytes, Body Weight and Composition, and Muscle-Related Measures
3. Results
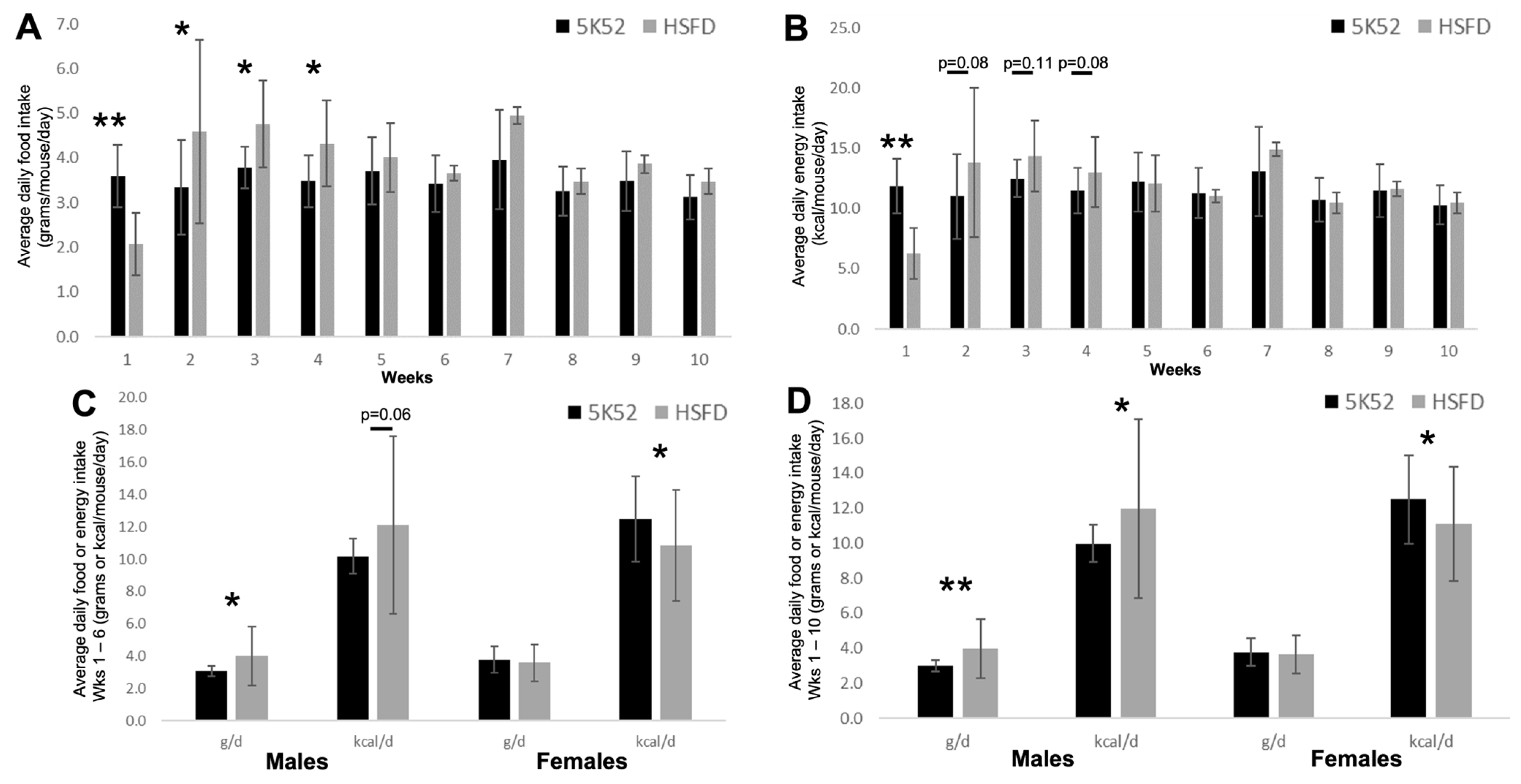
3.1. Daily Food and Energy Intake
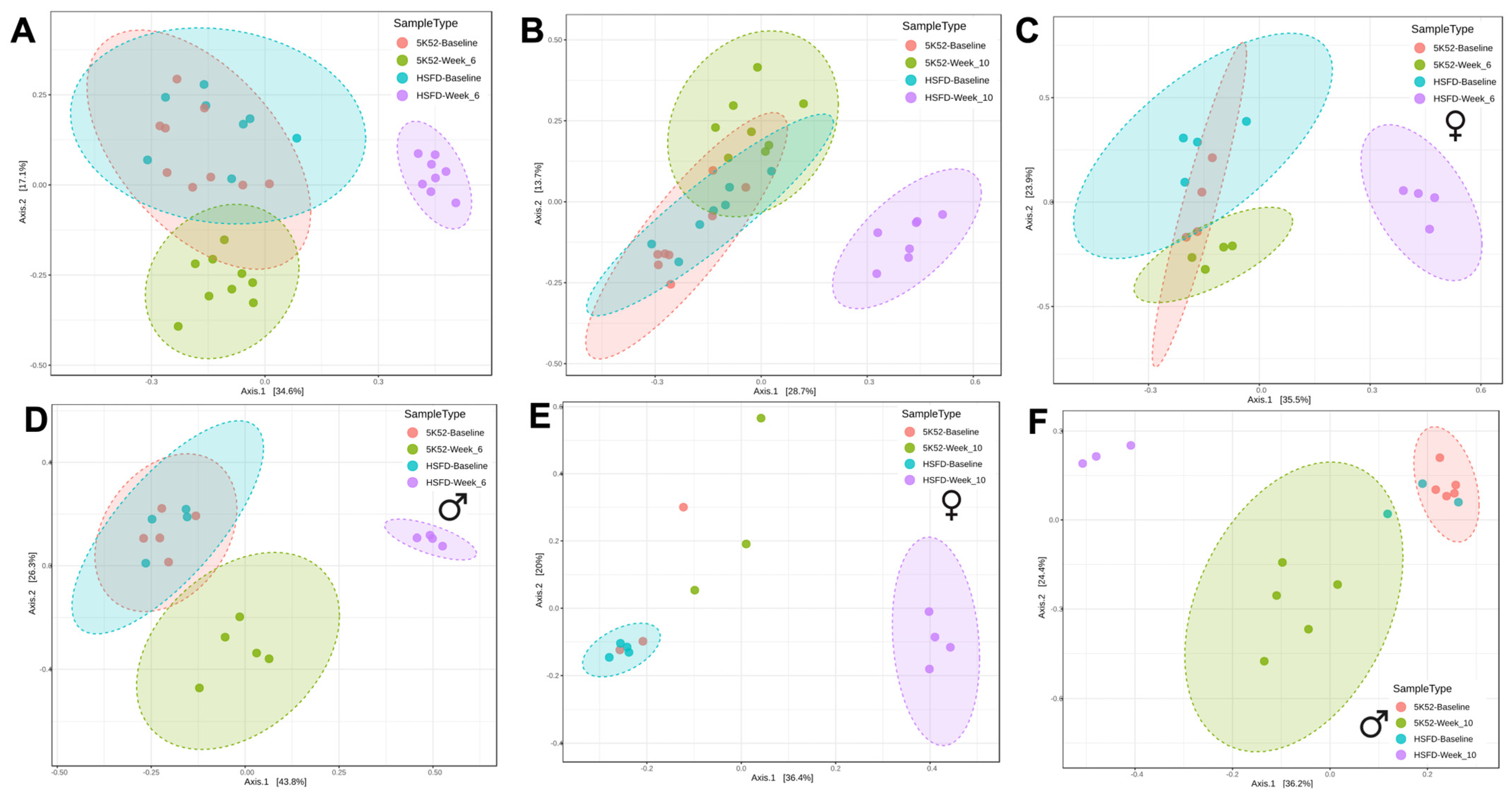
3.2. Altered Bacterial Community Structure in HSFD-Fed Mice
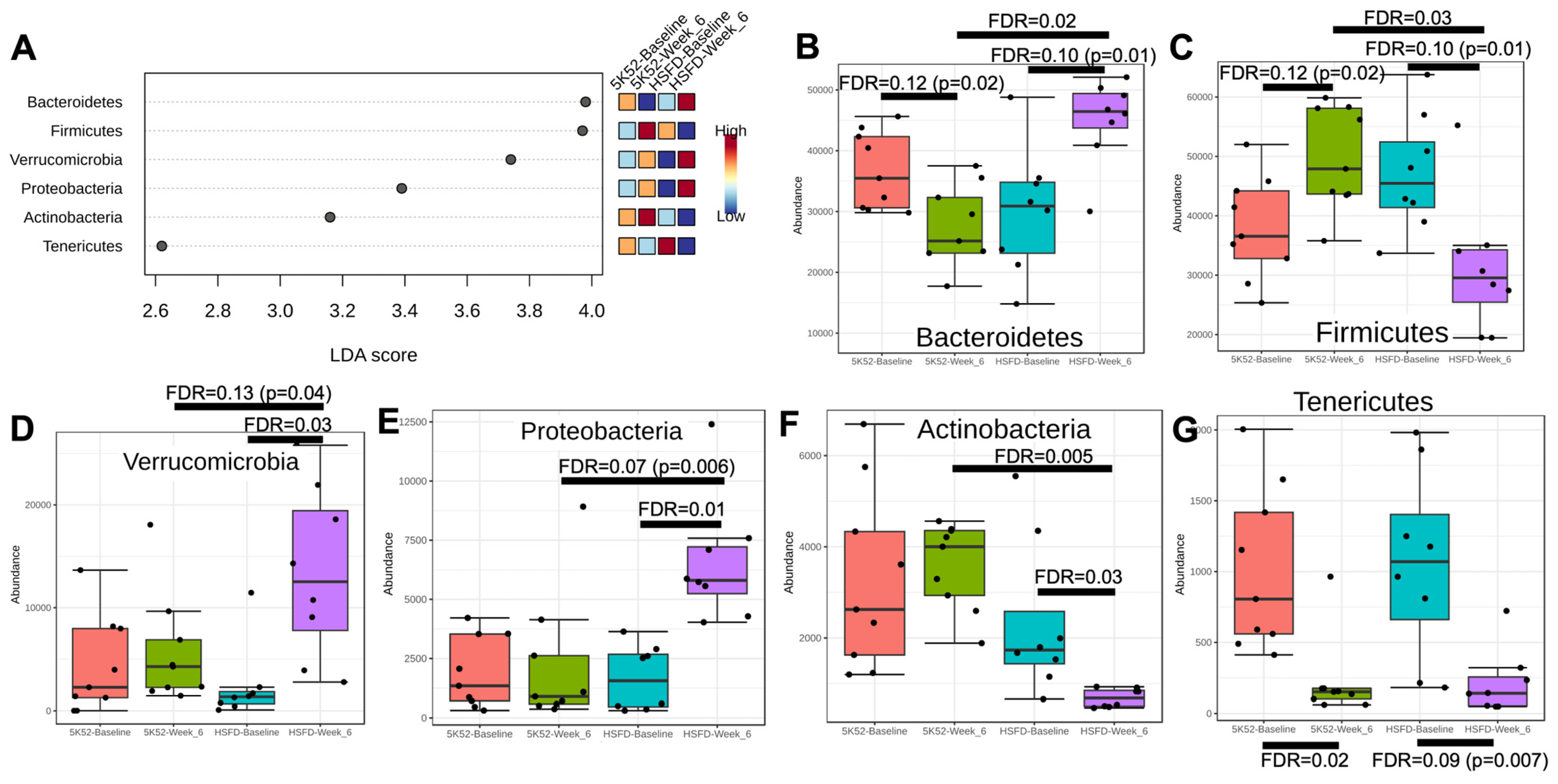
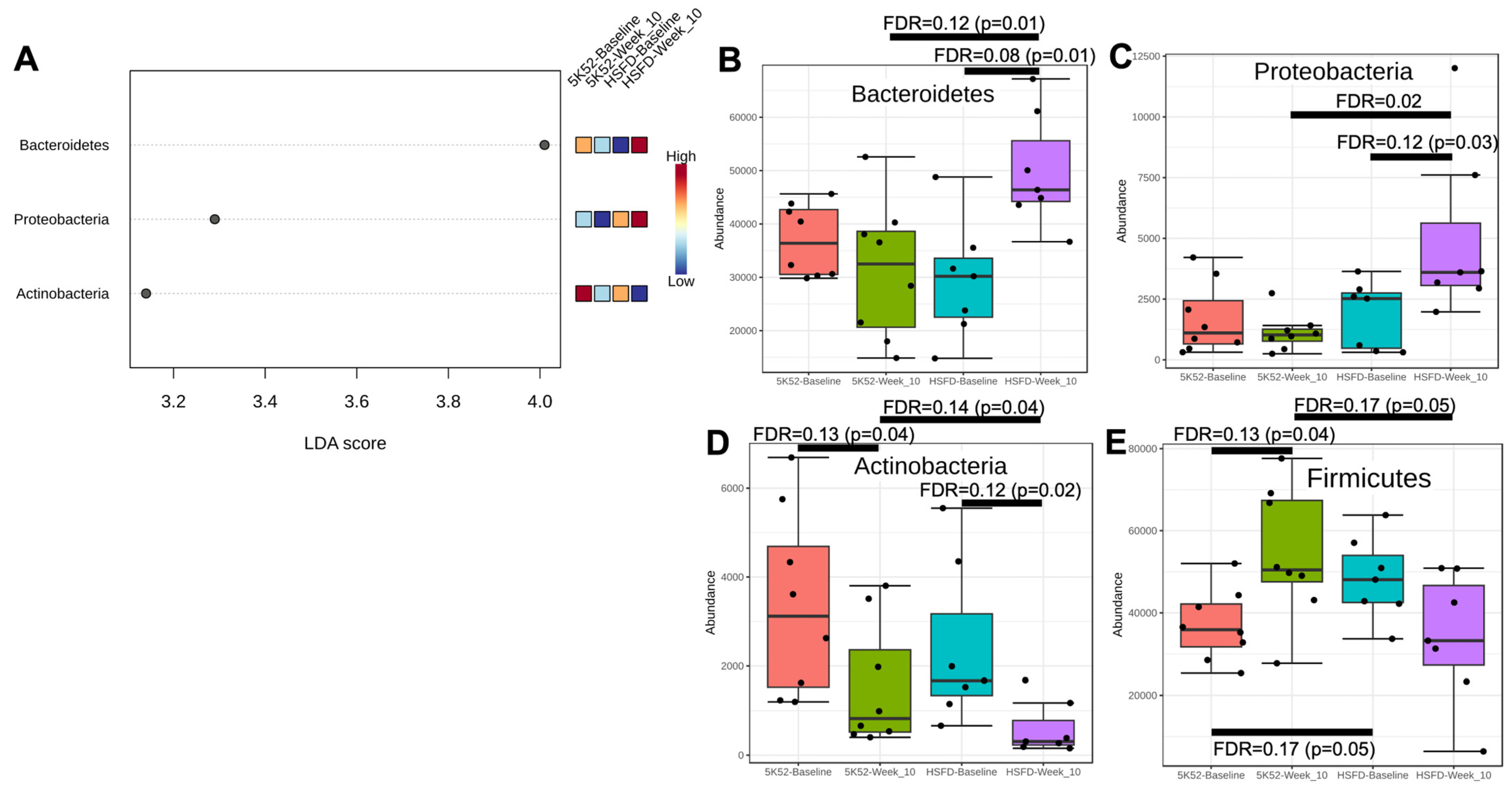
3.2.1. The Firmicutes/Bacteroidetes Ratio Is Reduced in HSFD-Fed Mice
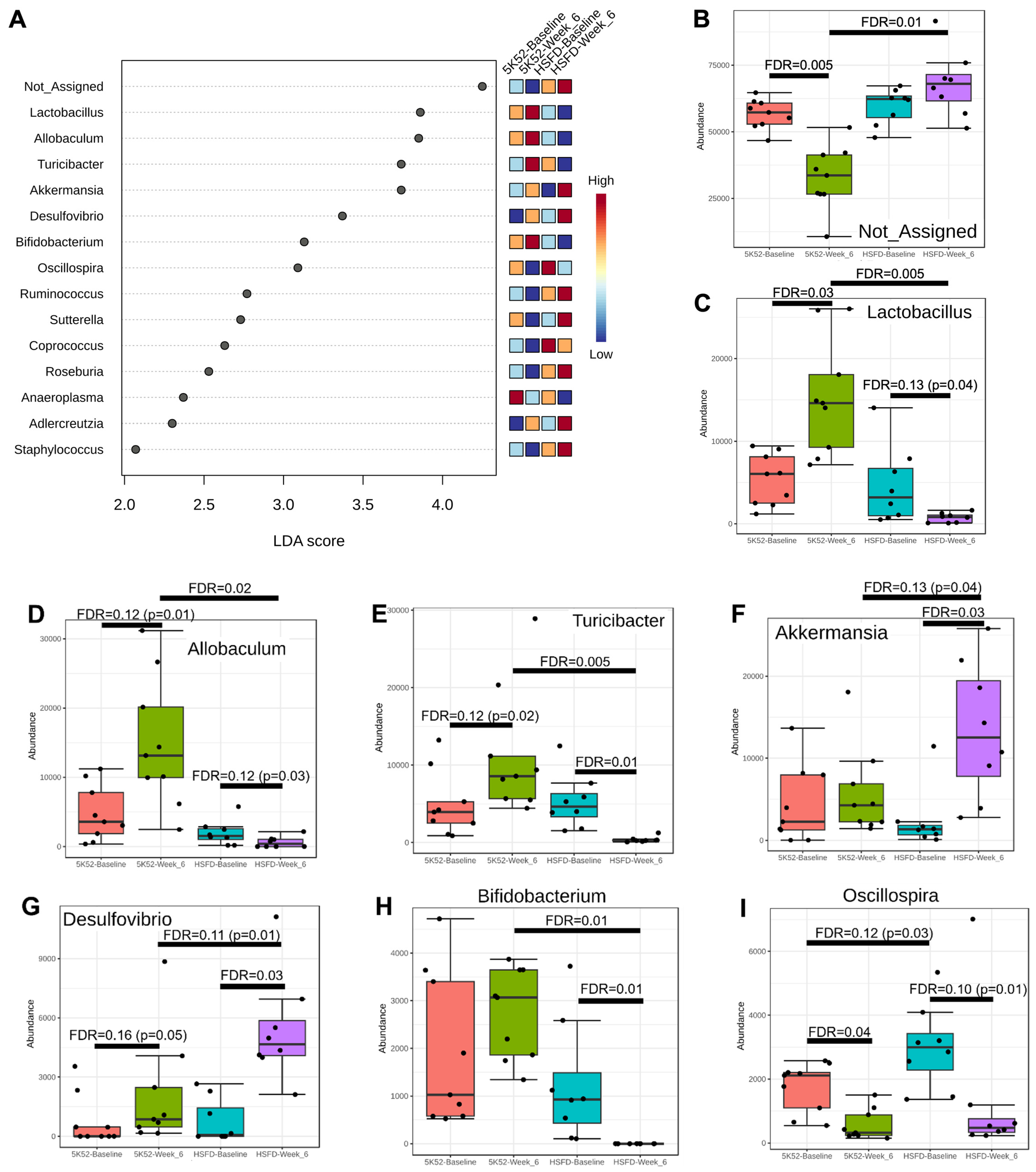
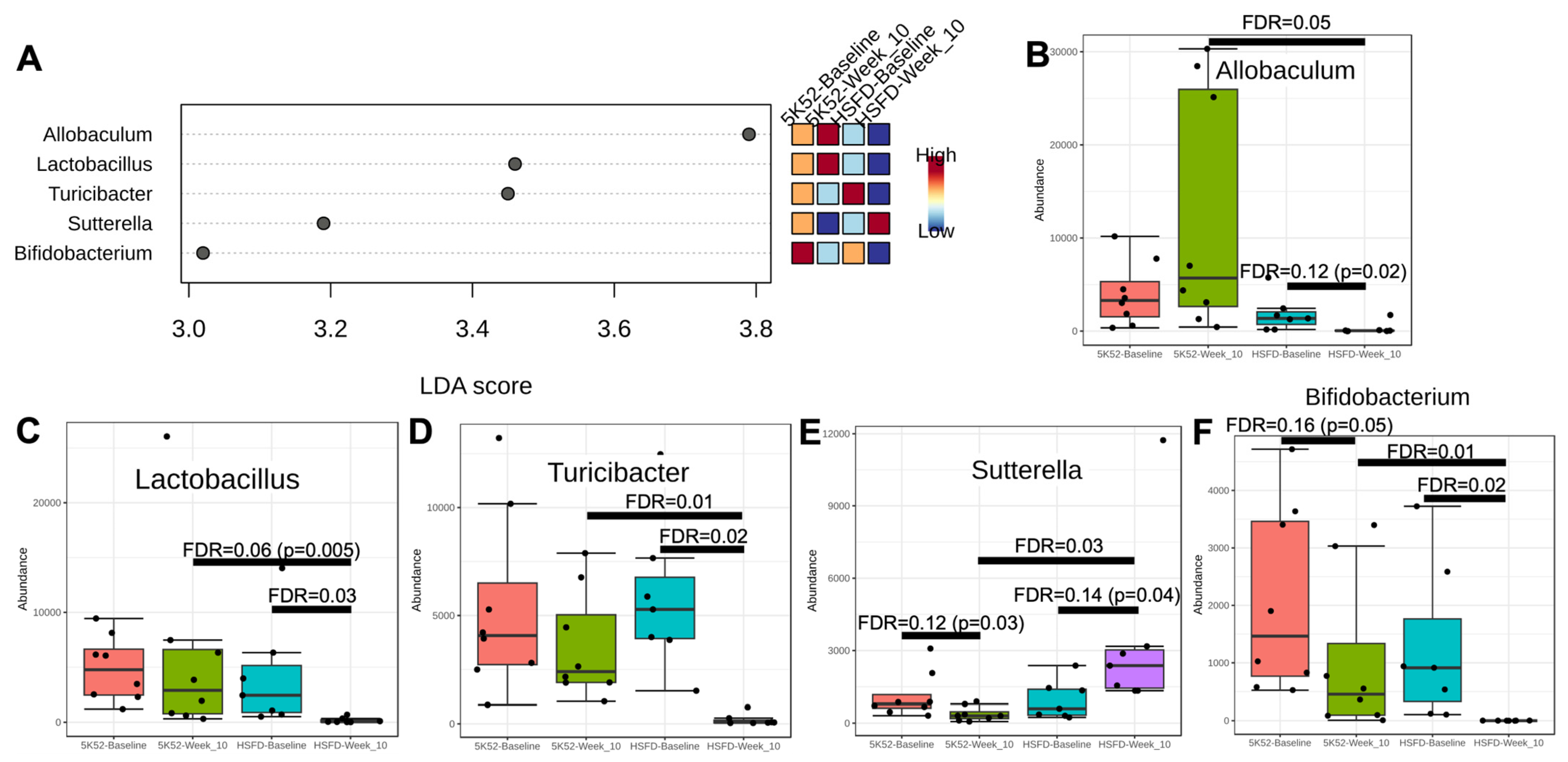
3.2.2. Reduced Allobaculum Lactobacillus, Turicibacter, and Bifidobacteria at Both Time Points within and between Groups for HSFD
3.2.3. Sex Differences for Phyla and Genus within and between Groups
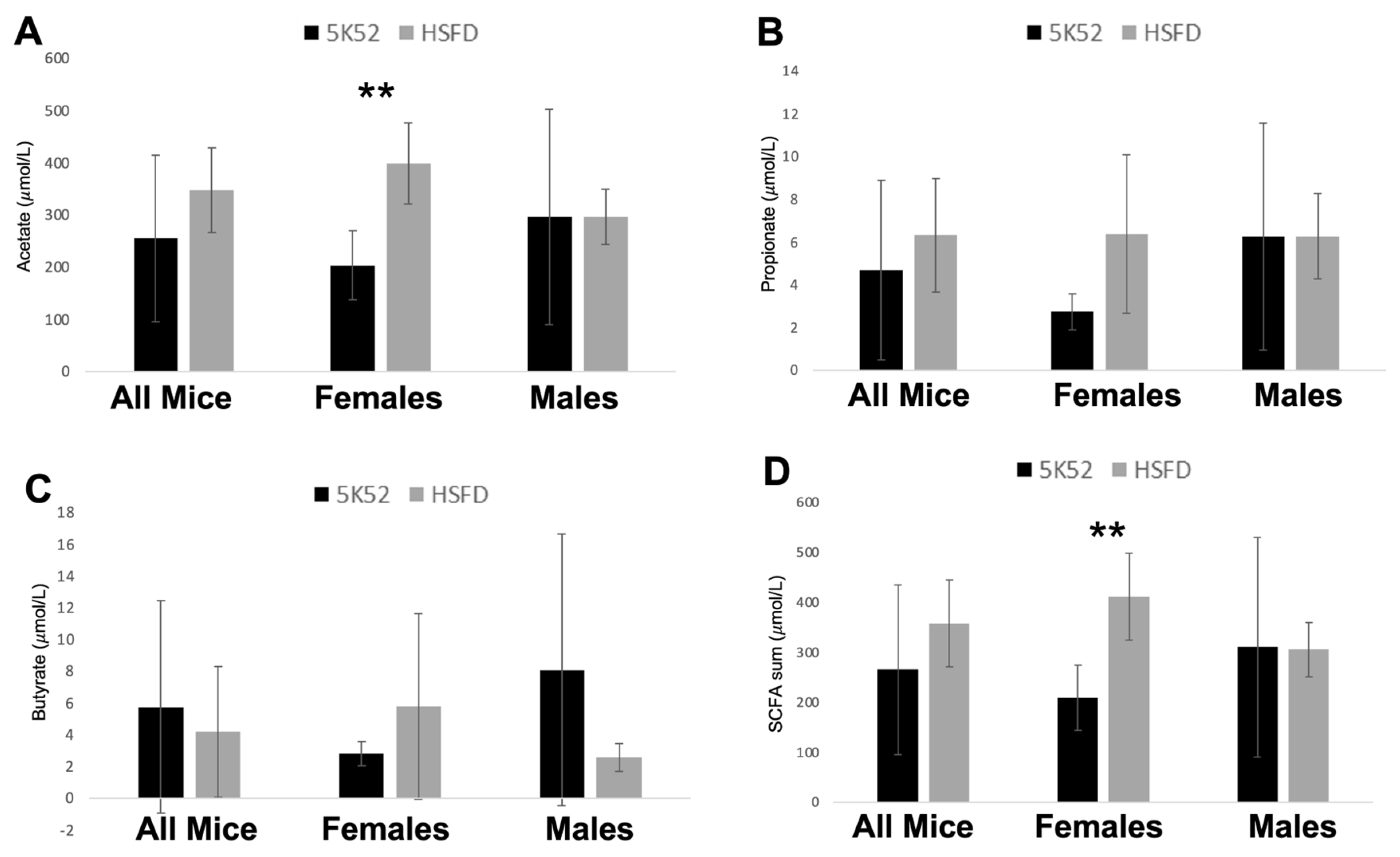
3.3. Plasma SCFAs Are Increased in Female but Not Male HSFD-Fed Mice
3.4. Reduced Body Weight and Improved Body Composition in HSFD-Fed Mice
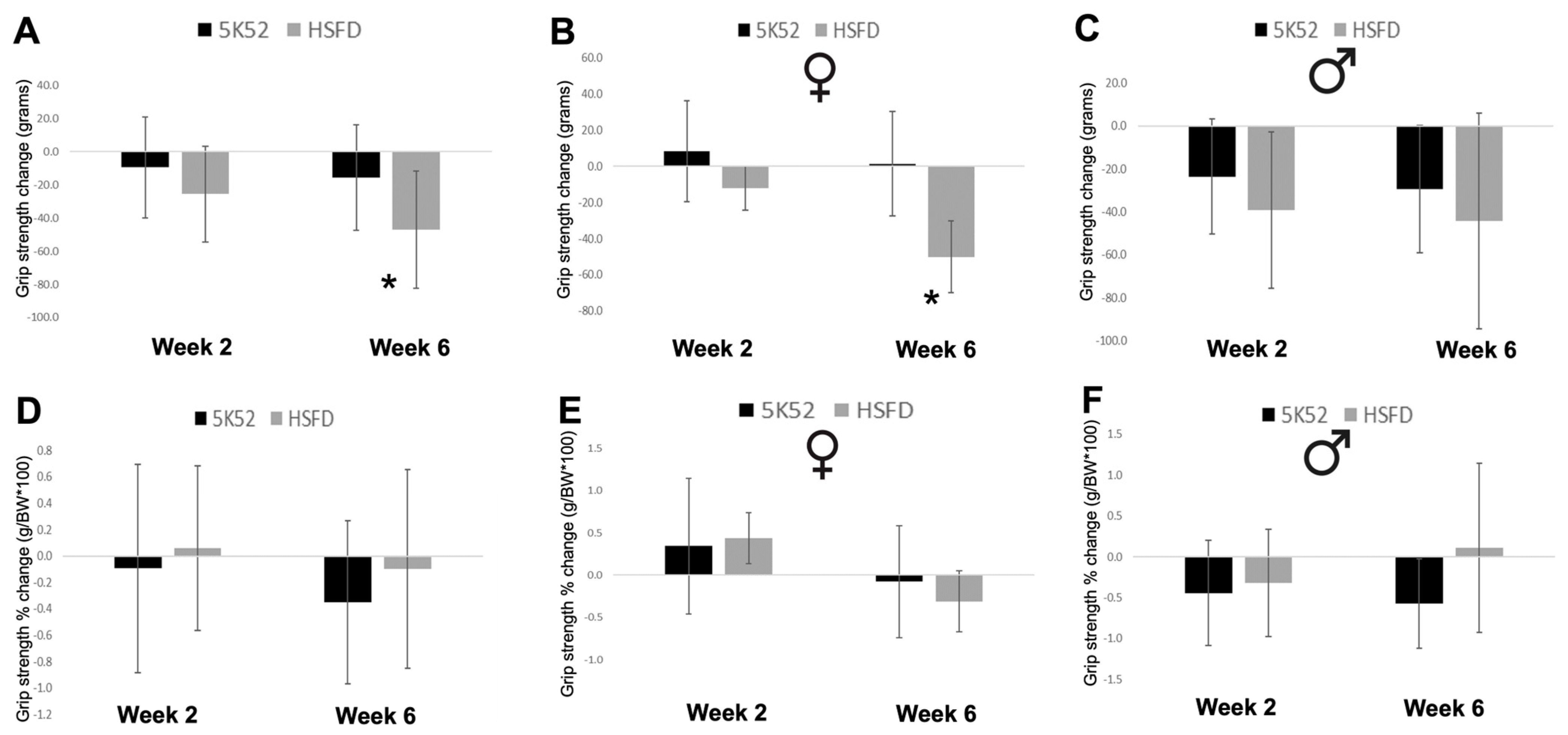
3.5. Grip Strength Is Reduced in HSFD-Fed Mice, but Not When Divided by Body Weight
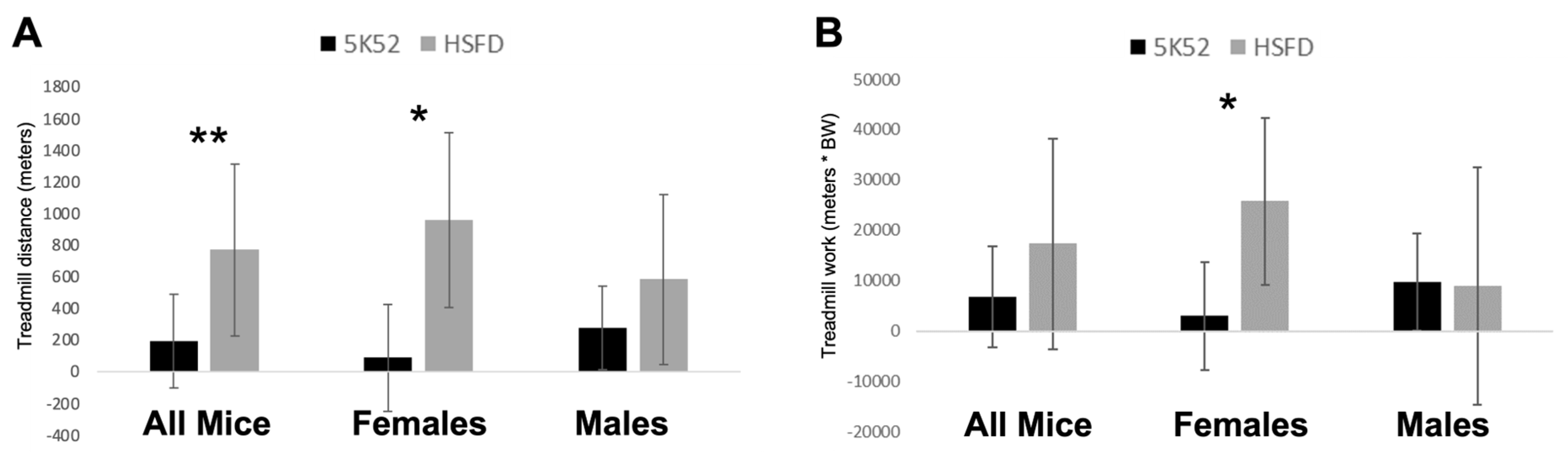
3.6. Improved Treadmill Endurance Capacity and Work, but Only in HSFD-Fed Female Mice
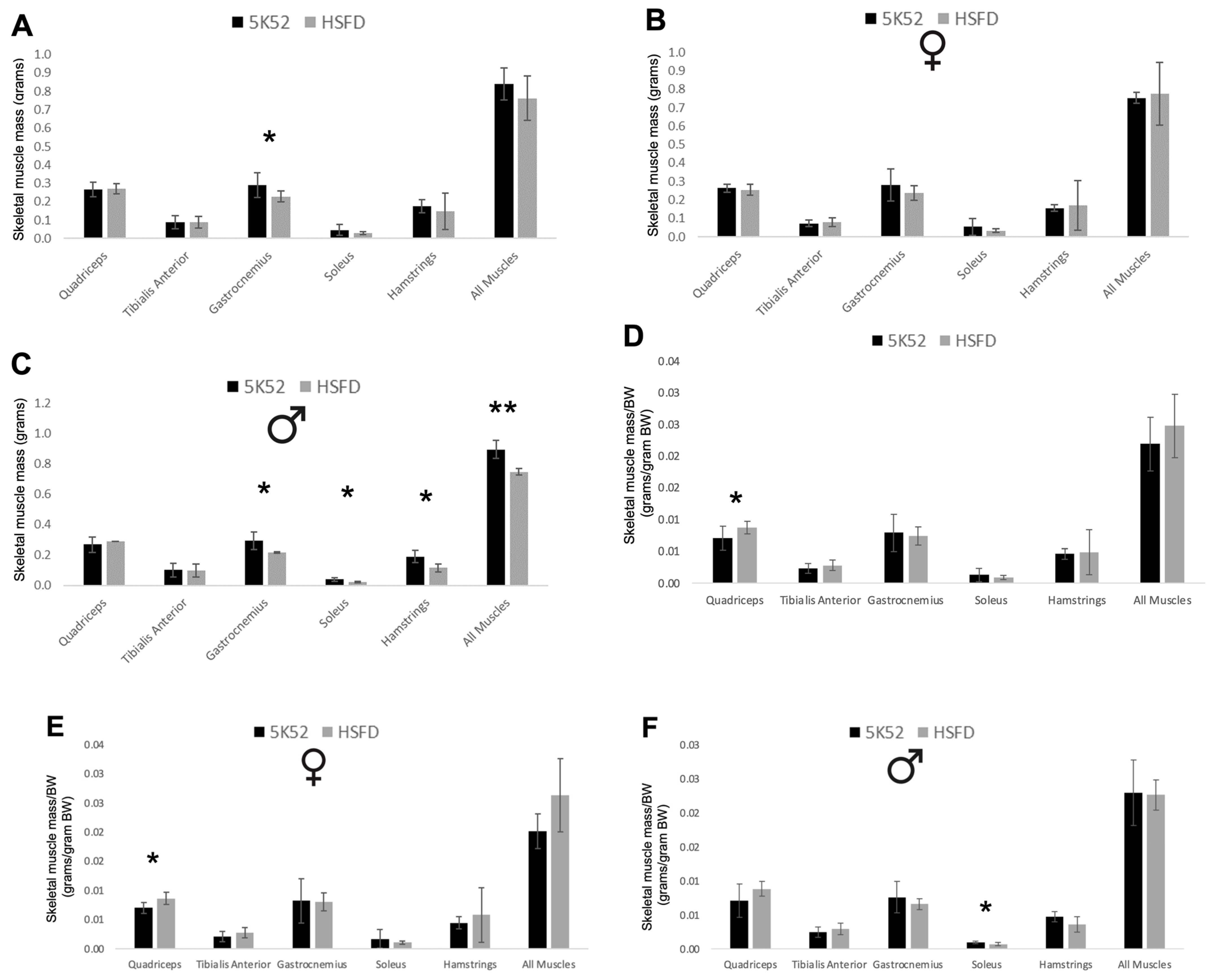
3.7. Muscle Mass Is Decreased in HSFD-Fed Mice, but Is Increased When Accounting for Body Weight
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cesari, M.; Leeuwenburgh, C.; Lauretani, F.; Onder, G.; Bandinelli, S.; Maraldi, C.; Guralnik, J.M.; Pahor, M.; Ferrucci, L. Frailty syndrome and skeletal muscle: Results from the Invecchiare in Chianti study. Am. J. Clin. Nutr. 2006, 83, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Frank-Wilson, A.W.; Farthing, J.P.; Chilibeck, P.D.; Arnold, C.M.; Davison, K.S.; Olszynski, W.P.; Kontulainen, S.A. Lower leg muscle density is independently associated with fall status in community-dwelling older adults. Osteoporos. Int. 2016, 27, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Cauley, J.A.; Tylavsky, F.; Bauer, D.; Cummings, S.; Harris, T.B.; Health, A.B.C.S. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: The health, aging, and body composition study. J. Bone Miner. Res. 2010, 25, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Rolland, Y.; Abellan Van Kan, G.; Bandinelli, S.; Vellas, B.; Ferrucci, L. Sarcopenia-related parameters and incident disability in older persons: Results from the “invecchiare in Chianti” study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Fox, K.M.; Gandra, S.R.; Delmonico, M.J.; Chiou, C.F.; Anthony, M.S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S.R.; et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J. Am. Geriatr. Soc. 2009, 57, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, I.; Kuipers, A.L.; Cauley, J.A.; Prasad, T.; Lee, C.G.; Ensrud, K.E.; Cawthon, P.M.; Hoffman, A.R.; Dam, T.T.; Gordon, C.L.; et al. Greater Skeletal Muscle Fat Infiltration Is Associated with Higher All-Cause and Cardiovascular Mortality in Older Men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef] [PubMed]
- de Sire, R.; Rizzatti, G.; Ingravalle, F.; Pizzoferrato, M.; Petito, V.; Lopetuso, L.; Graziani, C.; de Sire, A.; Mentella, M.C.; Mele, M.C.; et al. Skeletal muscle-gut axis: Emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva Gastroenterol. Dietol. 2018, 64, 351–362. [Google Scholar] [CrossRef]
- Ni Lochlainn, M.; Bowyer, R.C.E.; Steves, C.J. Dietary Protein and Muscle in Aging People: The Potential Role of the Gut Microbiome. Nutrients 2018, 10, 929. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mule, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Huang, Y.T.; Huang, C.C.; Chuang, H.L. Effect of intestinal microbiota on exercise performance in mice. J. Strength Cond. Res. 2015, 29, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Manickam, R.; Oh, H.Y.P.; Tan, C.K.; Paramalingam, E.; Wahli, W. Metronidazole Causes Skeletal Muscle Atrophy and Modulates Muscle Chronometabolism. Int. J. Mol. Sci. 2018, 19, 2418. [Google Scholar] [CrossRef] [PubMed]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Rue, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E158–E171. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animal 2019, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.L.; Zhang, W.; Iwaniuk, M.; Grilli, E.; Stahl, C.H. Dietary tributyrin, an HDAC inhibitor, promotes muscle growth through enhanced terminal differentiation of satellite cells. Physiol. Rep. 2018, 6, e13706. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- Bourquin, L.D.; Titgemeyer, E.C.; Fahey, G.C., Jr. Vegetable fiber fermentation by human fecal bacteria: Cell wall polysaccharide disappearance and short-chain fatty acid production during in vitro fermentation and water-holding capacity of unfermented residues. J. Nutr. 1993, 123, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Popovich, D.G.; Vidgen, E.; Mehling, C.C.; Vuksan, V.; Ransom, T.P.; Rao, A.V.; Rosenberg-Zand, R.; Tariq, N.; et al. Effect of a very-high-fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metab. Clin. Exp. 2001, 50, 494–503. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hwang, S.W.; Kim, S.; Lee, Y.S.; Kim, T.Y.; Lee, S.H.; Kim, S.J.; Yoo, H.J.; Kim, E.N.; Kweon, M.-N. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020, 11, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.M.; Kane, A.V.; Chang, J.; Tai, A.; Vickers, R.J.; Snydman, D.R. Enhanced preservation of the human intestinal microbiota by ridinilazole, a novel Clostridium difficile-targeting antibacterial, compared to vancomycin. PLoS ONE 2018, 13, e0199810. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- NIH (National Institute of Health). Guidelines for Survival Blood Collection in Mice and Rats. Available online: https://oacu.oir.nih.gov/system/files/media/file/2022-12/b2-Survival_Blood_Collection_Mice_Rats.pdf (accessed on 24 April 2024).
- Dei Cas, M.; Paroni, R.; Saccardo, A.; Casagni, E.; Arnoldi, S.; Gambaro, V.; Saresella, M.; Mario, C.; La Rosa, F.; Marventano, I.; et al. A straightforward LC-MS/MS analysis to study serum profile of short and medium chain fatty acids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1154, 121982. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Roschlau, P.; Bernt, E.; Gruber, W. [Enzymatic determination of total cholesterol in serum (author’s transl)]. Z Klin. Chem. Klin. Biochem. 1974, 12, 403–407. [Google Scholar]
- Trinder, P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969, 22, 158–161. [Google Scholar] [CrossRef]
- Bucolo, G.; David, H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Babson, A.L. The DPC Cirrus IMMULITE automated immunoassay system. J. Clin. Immunol. 1991, 14, 83–88. [Google Scholar]
- Fielding, R.A.; Reeves, A.R.; Jasuja, R.; Liu, C.; Barrett, B.B.; Lustgarten, M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019, 127, 110722. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Jang, Y.C.; Liu, Y.; Muller, F.L.; Qi, W.; Steinhelper, M.; Brooks, S.V.; Larkin, L.; Shimizu, T.; Shirasawa, T.; et al. Conditional knockout of Mn-SOD targeted to type IIB skeletal muscle fibers increases oxidative stress and is sufficient to alter aerobic exercise capacity. Am. J. Physiol. Cell Physiol. 2009, 297, C1520–C1532. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Paone, P.; Suriano, F.; Jian, C.; Korpela, K.; Delzenne, N.M.; Van Hul, M.; Salonen, A.; Cani, P.D. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes 2022, 14, 2152307. [Google Scholar] [CrossRef] [PubMed]
- Muthyala, S.D.V.; Shankar, S.; Klemashevich, C.; Blazier, J.C.; Hillhouse, A.; Wu, C.S. Differential effects of the soluble fiber inulin in reducing adiposity and altering gut microbiome in aging mice. J. Nutr. Biochem. 2022, 105, 108999. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Van Hee, V.F.; Piront, N.; De Backer, F.; Toussaint, O.; Cani, P.D.; Delzenne, N.M. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr. Diabetes 2012, 2, e28. [Google Scholar] [CrossRef]
- Barnard, N.D.; Scialli, A.R.; Turner-McGrievy, G.; Lanou, A.J.; Glass, J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am. J. Med. 2005, 118, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The Thermic Effect of Food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21, E607–E615. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Mitchell, S.J.; MacArthur, M.R.; Kane, A.E.; Sinclair, D.A.; Venable, E.M.; Chadaideh, K.S.; Carmody, R.N.; Grodstein, F.; Mitchell, J.R.; et al. Gut Microbiota Predicts Healthy Late-Life Aging in Male Mice. Nutrients 2021, 13, 3290. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Lyu, W.; Ren, Y.; Li, X.; Zhao, S.; Yang, H.; Xiao, Y. Allobaculum Involves in the Modulation of Intestinal ANGPTLT4 Expression in Mice Treated by High-Fat Diet. Front. Nutr. 2021, 8, 690138. [Google Scholar] [CrossRef] [PubMed]
- Suriano, F.; Bindels, L.B.; Verspreet, J.; Courtin, C.M.; Verbeke, K.; Cani, P.D.; Neyrinck, A.M.; Delzenne, N.M. Fat binding capacity and modulation of the gut microbiota both determine the effect of wheat bran fractions on adiposity. Sci. Rep. 2017, 7, 5621. [Google Scholar] [CrossRef]
- Kok, D.E.G.; Rusli, F.; van der Lugt, B.; Lute, C.; Laghi, L.; Salvioli, S.; Picone, G.; Franceschi, C.; Smidt, H.; Vervoort, J.; et al. Lifelong calorie restriction affects indicators of colonic health in aging C57Bl/6J mice. J. Nutr. Biochem. 2018, 56, 152–164. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improved Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Chen, M.J.; Huang, H.W.; Wu, W.K.; Lee, Y.W.; Kuo, H.C.; Huang, C.C. Probiotic Lactiplantibacillus plantarum Tana Isolated from an International Weightlifter Enhances Exercise Performance and Promotes Antifatigue Effects in Mice. Nutrients 2022, 14, 3308. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Kuo, Y.W.; Lin, W.Y.; Tsai, S.Y.; Chen, W.L.; Lin, C.L.; Huang, C.C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci. Rep. 2021, 11, 19469. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, B.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Intervention of five strains of Lactobacillus on obesity in mice induced by high-fat diet. J. Funct. Foods 2020, 72, 104078. [Google Scholar] [CrossRef]
- Bo, T.B.; Wen, J.; Zhao, Y.C.; Tian, S.J.; Zhang, X.Y.; Wang, D.H. Bifidobacterium pseudolongum reduces triglycerides by modulating gut microbiota in mice fed high-fat food. J. Steroid. Biochem. Mol. Biol. 2020, 198, 105602. [Google Scholar] [CrossRef] [PubMed]
- Jangid, A.; Fukuda, S.; Suzuki, Y.; Taylor, T.D.; Ohno, H.; Prakash, T. Shotgun metagenomic sequencing revealed the prebiotic potential of a grain-based diet in mice. Sci. Rep. 2022, 12, 6748. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Baranauskas, M.N.; Hinrichs, R.J.; Liu, Z.; Carter, S.J. Effect of dietary nitrate on human muscle power: A systematic review and individual participant data meta-analysis. J. Int. Soc. Sports Nutr. 2021, 18, 66. [Google Scholar] [CrossRef]
- Jones, A.M.; Ferguson, S.K.; Bailey, S.J.; Vanhatalo, A.; Poole, D.C. Fiber Type-Specific Effects of Dietary Nitrate. Exerc. Sport Sci. Rev. 2016, 44, 53–60. [Google Scholar] [CrossRef]
- Kadach, S.; Park, J.W.; Stoyanov, Z.; Black, M.I.; Vanhatalo, A.; Burnley, M.; Walter, P.J.; Cai, H.; Schechter, A.N.; Piknova, B.; et al. 15N-labeled dietary nitrate supplementation increases human skeletal muscle nitrate concentration and improves muscle torque production. Acta Physiol. 2023, 237, e13924. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Fulford, J.; Bailey, S.J.; Blackwell, J.R.; Winyard, P.G.; Jones, A.M. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol 2011, 589, 5517–5528. [Google Scholar] [CrossRef] [PubMed]
- Zoughaib, W.S.; Brault, J.J.; Coggan, A.R. Beeting atrophy: Dietary nitrate to protect the powerhouse of the cell? J. Physiol. 2023. early view. [Google Scholar] [CrossRef]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Cerda, B.; Periago, P.; Espin, J.C.; Tomas-Barberan, F.A. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef] [PubMed]
- Luan, P.; D’Amico, D.; Andreux, P.A.; Laurila, P.P.; Wohlwend, M.; Li, H.; Imamura de Lima, T.; Place, N.; Rinsch, C.; Zanou, N.; et al. Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci. Transl. Med. 2021, 13, eabb0319. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fielding, R.A.; Lustgarten, M.S. Impact of a Whole-Food, High-Soluble Fiber Diet on the Gut–Muscle Axis in Aged Mice. Nutrients 2024, 16, 1323. https://doi.org/10.3390/nu16091323
Fielding RA, Lustgarten MS. Impact of a Whole-Food, High-Soluble Fiber Diet on the Gut–Muscle Axis in Aged Mice. Nutrients. 2024; 16(9):1323. https://doi.org/10.3390/nu16091323
Chicago/Turabian StyleFielding, Roger A., and Michael S. Lustgarten. 2024. "Impact of a Whole-Food, High-Soluble Fiber Diet on the Gut–Muscle Axis in Aged Mice" Nutrients 16, no. 9: 1323. https://doi.org/10.3390/nu16091323
APA StyleFielding, R. A., & Lustgarten, M. S. (2024). Impact of a Whole-Food, High-Soluble Fiber Diet on the Gut–Muscle Axis in Aged Mice. Nutrients, 16(9), 1323. https://doi.org/10.3390/nu16091323





