Variability in Definitions and Criteria of Extrauterine Growth Restriction and Its Association with Neurodevelopmental Outcomes in Preterm Infants: A Narrative Review
Abstract
1. Introduction
1.1. Growth in the Preterm Infant
1.2. Growth Assessment in the NICU and Extrauterine Growth Restriction
2. Materials and Methods
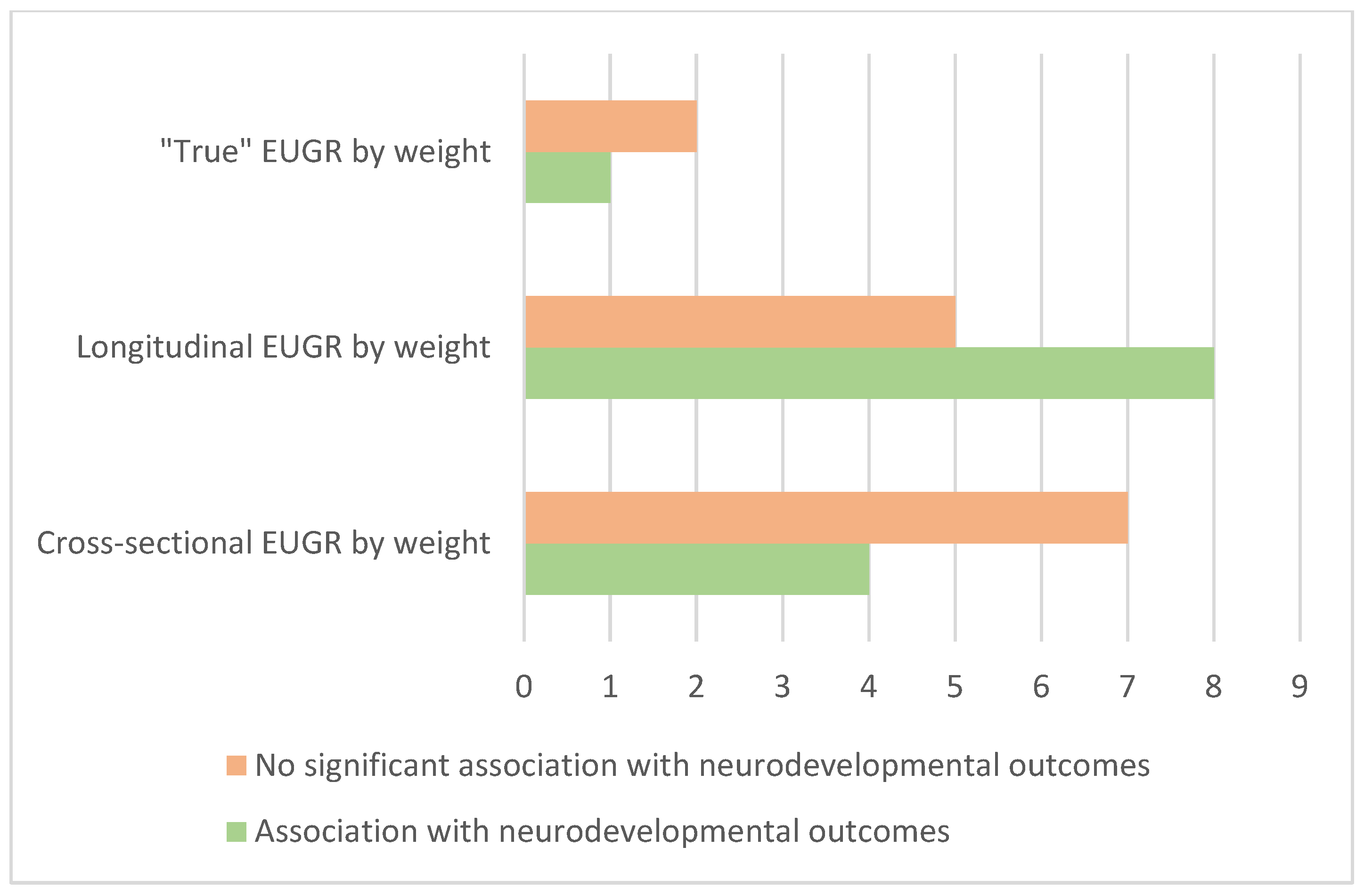
3. EUGR and Neurodevelopmental Outcomes
3.1. Cross-Sectional EUGR and Neurodevelopmental Outcomes
3.2. Longitudinal EUGR and Neurodevelopmental Outcomes
3.3. “True” EUGR and Neurodevelopmental Outcomes
3.4. EUGR by Length and Head Circumference and Neurodevelopmental Outcomes
4. Conclusions
Funding
Conflicts of Interest
References
- Barros, F.C.; Papageorghiou, A.T.; Victora, C.G.; Noble, J.A.; Pang, R.; Iams, J.; Cheikh Ismail, L.; Goldenberg, R.L.; Lambert, A.; Kramer, M.S.; et al. The Distribution of Clinical Phenotypes of Preterm Birth Syndrome: Implications for Prevention. JAMA Pediatr. 2015, 169, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Giuliani, F.; Barros, F.; Roggero, P.; Coronado Zarco, I.A.; Rego, M.A.S.; Ochieng, R.; Gianni, M.L.; Rao, S.; Lambert, A.; et al. Monitoring the Postnatal Growth of Preterm Infants: A Paradigm Change. Pediatrics 2018, 141, e20172467. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics, Committee on Nutrition. Nutritional Needs of Low-Birth-Weight Infants. Pediatrics 1977, 60, 519–530. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Younes, N.; Lemons, J.A.; Fanaroff, A.A.; Donovan, E.F.; Wright, L.L.; Katsikiotis, V.; Tyson, J.E.; Oh, W.; Shankaran, S.; et al. Longitudinal Growth of Hospitalized Very Low Birth Weight Infants. Pediatrics 1999, 104, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kiserud, T.; Piaggio, G.; Carroli, G.; Widmer, M.; Carvalho, J.; Neerup Jensen, L.; Giordano, D.; Cecatti, J.G.; Abdel Aleem, H.; Talegawkar, S.A.; et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS Med. 2017, 14, e1002220. [Google Scholar]
- Rochow, N.; Raja, P.; Liu, K.; Fenton, T.; Landau-Crangle, E.; Göttler, S.; Jahn, A.; Lee, S.; Seigel, S.; Campbell, D.; et al. Physiological Adjustment to Postnatal Growth Trajectories in Healthy Preterm Infants. Pediatr. Res. 2016, 79, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Jennifer Moltu, S.; Lapillonne, A.; van den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper From the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Di Nicola, P.; Giuliani, F.; Coscia, A.; Varalda, A.; Occhi, L.; Rossi, C. Evaluation of Postnatal Growth of Preterm Infants. J. Matern. Fetal Neonatal Med. 2011, 24 (Suppl. S2), 9–11. [Google Scholar] [CrossRef]
- Tudehope, D.; Gibbons, K.; Cormack, B.; Bloomfield, F. Growth Monitoring of Low Birthweight Infants: What References to Use? J. Paediatr. Child. Health 2012, 48, 759–767. [Google Scholar] [CrossRef]
- Olsen, I.E.; Groveman, S.A.; Lawson, M.L.; Clark, R.H.; Zemel, B.S. New Intrauterine Growth Curves Based on United States Data. Pediatrics 2010, 125, e214–e224. [Google Scholar] [CrossRef]
- Bertino, E.; Spada, E.; Occhi, L.; Coscia, A.; Giuliani, F.; Gagliardi, L.; Gilli, G.; Bona, G.; Fabris, C.; De Curtis, M.; et al. Neonatal Anthropometric Charts: The Italian Neonatal Study Compared with Other European Studies. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A Systematic Review and Meta-Analysis to Revise the Fenton Growth Chart for Preterm Infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Grummer-Strawn, L.M.; Reinold, C.; Krebs, N.F.; Centers for Disease Control and Prevention (CDC). Use of World Health Organization and CDC Growth Charts for Children Aged 0-59 Months in the United States. MMWR Recomm. Rep. 2010, 59, 1–15. [Google Scholar]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International Standards for Newborn Weight, Length, and Head Circumference by Gestational Age and Sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Patel, A.L.; Engstrom, J.L.; Meier, P.P.; Kimura, R.E. Accuracy of Methods for Calculating Postnatal Growth Velocity for Extremely Low Birth Weight Infants. Pediatrics 2005, 116, 1466–1473. [Google Scholar] [CrossRef]
- Cormack, B.E.; Embleton, N.D.; van Goudoever, J.B.; Hay, W.W.; Bloomfield, F.H. Comparing Apples with Apples: It Is Time for Standardized Reporting of Neonatal Nutrition and Growth Studies. Pediatr. Res. 2016, 79, 810–820. [Google Scholar] [CrossRef]
- Gounaris, A.K.; Sokou, R.; Gounari, E.A.; Panagiotounakou, P.; Grivea, I.N. Extrauterine Growth Restriction and Optimal Growth of Very Preterm Neonates: State of the Art. Nutrients 2023, 15, 3231. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.H.; Thomas, P.; Peabody, J. Extrauterine Growth Restriction Remains a Serious Problem in Prematurely Born Neonates. Pediatrics 2003, 111, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Spada, E.; Giuliani, F.; Maiocco, G.; Raia, M.; Cresi, F.; Bertino, E.; Coscia, A. Extrauterine Growth Restriction: Definitions and Predictability of Outcomes in a Cohort of Very Low Birth Weight Infants or Preterm Neonates. Nutrients 2020, 12, 1224. [Google Scholar] [CrossRef]
- Zozaya, C.; Díaz, C.; Saenz de Pipaón, M. How Should We Define Postnatal Growth Restriction in Preterm Infants? Neonatology 2018, 114, 177–180. [Google Scholar] [CrossRef]
- Shah, P.S.; Wong, K.Y.; Merko, S.; Bishara, R.; Dunn, M.; Asztalos, E.; Darling, P.B. Postnatal Growth Failure in Preterm Infants: Ascertainment and Relation to Long-Term Outcome. J. Perinat. Med. 2006, 34, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Figueras-Aloy, J.; Palet-Trujols, C.; Matas-Barceló, I.; Botet-Mussons, F.; Carbonell-Estrany, X. Extrauterine Growth Restriction in Very Preterm Infant: Etiology, Diagnosis, and 2-Year Follow-Up. Eur. J. Pediatr. 2020, 179, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Feng, H.-M.; Caicike, B.; Zhu, Y.-P. Investigation into the Current Situation and Analysis of the Factors Influencing Extrauterine Growth Retardation in Preterm Infants. Front. Pediatr. 2021, 9, 643387. [Google Scholar] [CrossRef]
- Fenton, T.R.; Chan, H.T.; Madhu, A.; Griffin, I.J.; Hoyos, A.; Ziegler, E.E.; Groh-Wargo, S.; Carlson, S.J.; Senterre, T.; Anderson, D.; et al. Preterm Infant Growth Velocity Calculations: A Systematic Review. Pediatrics 2017, 139, e20162045. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Cormack, B.; Goldberg, D.; Nasser, R.; Alshaikh, B.; Eliasziw, M.; Hay, W.W.; Hoyos, A.; Anderson, D.; Bloomfield, F.; et al. “Extrauterine Growth Restriction” and “Postnatal Growth Failure” Are Misnomers for Preterm Infants. J. Perinatol. 2020, 40, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jiménez, M.D.; Gómez-García, F.J.; Gil-Campos, M.; Pérez-Navero, J.L. Comorbidities in Childhood Associated with Extrauterine Growth Restriction in Preterm Infants: A Scoping Review. Eur. J. Pediatr. 2020, 179, 1255–1265. [Google Scholar] [CrossRef]
- Hack, M.; Merkatz, I.R.; Gordon, D.; Jones, P.K.; Fanaroff, A.A. The Prognostic Significance of Postnatal Growth in Very Low-Birth Weight Infants. Am. J. Obstet. Gynecol. 1982, 143, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Babson, S.G.; Benda, G.I. Growth Graphs for the Clinical Assessment of Infants of Varying Gestational Age. J. Pediatr. 1976, 89, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Tudehope, D.I.; Burns, Y.; O’Callaghan, M.; Mohay, H.; Silcock, A. The Relationship between Intrauterine and Postnatal Growth on the Subsequent Psychomotor Development of Very Low Birthweight (VLBW) Infants. Aust. Paediatr. J. 1983, 19, 3–8. [Google Scholar] [CrossRef]
- Usher, R.; McLean, F. Intrauterine Growth of Live-Born Caucasian Infants at Sea Level: Standards Obtained from Measurements in 7 Dimensions of Infants Born between 25 and 44 Weeks of Gestation. J. Pediatr. 1969, 74, 901–910. [Google Scholar] [CrossRef]
- Kramer, M.S.; Platt, R.W.; Wen, S.W.; Joseph, K.S.; Allen, A.; Abrahamowicz, M.; Blondel, B.; Bréart, G.; Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System. A New and Improved Population-Based Canadian Reference for Birth Weight for Gestational Age. Pediatrics 2001, 108, E35. [Google Scholar] [CrossRef] [PubMed]
- Kan, E.; Roberts, G.; Anderson, P.J.; Doyle, L.W.; Victorian Infant Collaborative Study Group. The Association of Growth Impairment with Neurodevelopmental Outcome at Eight Years of Age in Very Preterm Children. Early Hum. Dev. 2008, 84, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Freeman, J.V.; Preece, M.A. British 1990 Growth Reference Centiles for Weight, Height, Body Mass Index and Head Circumference Fitted by Maximum Penalized Likelihood. Stat. Med. 1998, 17, 407–429. [Google Scholar] [CrossRef]
- Chien, H.-C.; Chen, C.-H.; Wang, T.-M.; Hsu, Y.-C.; Lin, M.-C. Neurodevelopmental Outcomes of Infants with Very Low Birth Weights Are Associated with the Severity of Their Extra-Uterine Growth Retardation. Pediatr. Neonatol. 2018, 59, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.-S.; Wu, H.-C.; Jeng, S.-F.; Liao, H.-F.; Su, Y.-N.; Lin, S.-J.; Hsieh, C.-J.; Chen, P.-C. Nationwide Singleton Birth Weight Percentiles by Gestational Age in Taiwan, 1998–2002. Acta Paediatr. Taiwan. 2006, 47, 25–33. [Google Scholar] [PubMed]
- Maiocco, G.; Migliaretti, G.; Cresi, F.; Peila, C.; Deantoni, S.; Trapani, B.; Giuliani, F.; Bertino, E.; Coscia, A. Evaluation of Extrauterine Head Growth From 14-21 Days to Discharge with Longitudinal Intergrowth-21st Charts: A New Approach to Identify Very Preterm Infants at Risk of Long-Term Neurodevelopmental Impairment. Front. Pediatr. 2020, 8, 572930. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.A.; Bhatia, A.; Carlo, W.A. Postnatal Growth of Preterm Infants 24 to 26 Weeks of Gestation and Cognitive Outcomes at 2 Years of Age. Pediatr. Res. 2021, 89, 1804–1809. [Google Scholar] [CrossRef]
- De Rose, D.U.; Cota, F.; Gallini, F.; Bottoni, A.; Fabrizio, G.C.; Ricci, D.; Romeo, D.M.; Mercuri, E.; Vento, G.; Maggio, L. Extra-Uterine Growth Restriction in Preterm Infants: Neurodevelopmental Outcomes According to Different Definitions. Eur. J. Paediatr. Neurol. 2021, 33, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, L.; Fernández-Baizán, C.; González-García, L.; García-López, E.; González-López, C.; Arias, J.L.; Méndez, M.; Sánchez, G.S. Neuropsychological Development and New Criteria for Extrauterine Growth Restriction in Very Low-Birth-Weight Children. Children 2021, 8, 955. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, E.-K.; Song, H.; Cheon, J.-E.; Kim, B.N.; Kim, H.-S.; Shin, S.H. Association of Brain Microstructure and Functional Connectivity With Cognitive Outcomes and Postnatal Growth Among Early School-Aged Children Born with Extremely Low Birth Weight. JAMA Netw. Open 2023, 6, e230198. [Google Scholar] [CrossRef]
- Frondas-Chauty, A.; Simon, L.; Branger, B.; Gascoin, G.; Flamant, C.; Ancel, P.Y.; Darmaun, D.; Rozé, J.C. Early Growth and Neurodevelopmental Outcome in Very Preterm Infants: Impact of Gender. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F366–F372. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, M.; Lapinleimu, H.; Lind, A.; Matomäki, J.; Lehtonen, L.; Haataja, L.; Rautava, P.; PIPARI Study Group. Antenatal and Postnatal Growth and 5-Year Cognitive Outcome in Very Preterm Infants. Pediatrics 2014, 133, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sorva, R.; Perheentupa, J.; Tolppanen, E.M. A Novel Format for a Growth Chart. Acta Paediatr. Scand. 1984, 73, 527–529. [Google Scholar] [CrossRef]
- Cordova, E.G.; Cherkerzian, S.; Bell, K.; Joung, K.E.; Collins, C.T.; Makrides, M.; Gould, J.; Anderson, P.J.; Belfort, M.B. Association of Poor Postnatal Growth with Neurodevelopmental Impairment in Infancy and Childhood: Comparing the Fetus and the Healthy Preterm Infant References. J. Pediatr. 2020, 225, 37–43.e5. [Google Scholar] [CrossRef]
- Yitayew, M.; Chahin, N.; Rustom, S.; Thacker, L.R.; Hendricks-Muñoz, K.D. Fenton vs. Intergrowth-21st: Postnatal Growth Assessment and Prediction of Neurodevelopment in Preterm Infants. Nutrients 2021, 13, 2841. [Google Scholar] [CrossRef]
- El Rafei, R.; Jarreau, P.H.; Norman, M.; Maier, R.F.; Barros, H.; Van Reempts, P.; Pedersen, P.; Cuttini, M.; Costa, R.; Zemlin, M.; et al. Association between Postnatal Growth and Neurodevelopmental Impairment by Sex at 2 Years of Corrected Age in a Multi-National Cohort of Very Preterm Children. Clin. Nutr. 2021, 40, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- El Rafei, R.; Maier, R.F.; Jarreau, P.H.; Norman, M.; Barros, H.; Van Reempts, P.; Van Heijst, A.; Pedersen, P.; Cuttini, M.; Johnson, S.; et al. Postnatal Growth Restriction and Neurodevelopment at 5 Years of Age: A European Extremely Preterm Birth Cohort Study. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 492–498. [Google Scholar] [CrossRef]
- Strobel, K.M.; Wood, T.R.; Valentine, G.C.; German, K.R.; Gogcu, S.; Hendrixson, D.T.; Kolnik, S.E.; Law, J.B.; Mayock, D.E.; Comstock, B.A.; et al. Contemporary definitions of infant growth failure and neurodevelopmental and behavioral outcomes in extremely premature infants at two years of age. J. Perinatol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Ramel, S.E.; Demerath, E.W.; Gray, H.L.; Younge, N.; Boys, C.; Georgieff, M.K. The Relationship of Poor Linear Growth Velocity with Neonatal Illness and Two-Year Neurodevelopment in Preterm Infants. Neonatology 2012, 102, 19–24. [Google Scholar] [CrossRef]
- Guellec, I.; Lapillonne, A.; Marret, S.; Picaud, J.-C.; Mitanchez, D.; Charkaluk, M.-L.; Fresson, J.; Arnaud, C.; Flamant, C.; Cambonie, G.; et al. Effect of Intra- and Extrauterine Growth on Long-Term Neurologic Outcomes of Very Preterm Infants. J. Pediatr. 2016, 175, 93–99.e1. [Google Scholar] [CrossRef]
- Ong, K.K.; Kennedy, K.; Castañeda-Gutiérrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Latulippe, M.E.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.H.; et al. Postnatal Growth in Preterm Infants and Later Health Outcomes: A Systematic Review. Acta Paediatr. 2015, 104, 974–986. [Google Scholar] [CrossRef]
| Study | Year | EUGR Definition | Growth Chart | Population | Neurodevelopmental Assessment | Outcomes |
|---|---|---|---|---|---|---|
| Hack et al. [27] | 1982 | Weight z-score < −2 at 40 weeks | Babson and Benda [28] | 192 VLBW infants. | BSID-I of neurosensory impairment at 8 months. | No significant association if catch-up occurred by 8 months of corrected age. |
| Tudehope et al. [29] | 1983 | Weight z-score < −2 at discharge | Usher–McLean Intrauterine Growth Chart [30] | 164 VLBW infants. | GMSD at 3 years. | EUGR not predictive of neurodevelopmental outcome if catch-up occurred. |
| Shah et al. [21] | 2006 | Weight < 10th centile and <3rd centile at 36 weeks | Kramer [31] | 221 infants, ≤28 weeks gestational age (GA). | BSID-II at 18–24 months or best clinical estimate of performance. | No significant association with neurodevelopmental outcomes. |
| Kan et al. [32] | 2008 | Weight z-score at discharge | Cole [33] | 401 infants, <28 weeks of GA. | WISC-III, WRAT3 and movement ABC at 8 years. | Weight not related to outcomes. |
| Chien et al. [34] | 2018 | Weight z-score < −2, <−2.5 and <−3 at discharge | Hsieh [35] | 224 VLBW infants. | BSID-II at 24 months cGA. | EUGR associated with MDI < 85, and this association was related to the severity of EUGR. |
| Zozaya et al. [20] | 2018 | Weight z-score < −1.5 at 36 weeks | Fenton | 168 VLBW infants, <34 weeks of GA. | BSID-II at 24 months cGA. | No association with worse BSID-II. |
| Maiocco et al. [36] | 2020 | Weight < 10th percentile at discharge | INTERGROWTH-21st | 195 infants <30 weeks of GA. | GMSD at 24 (+−6) months cGA. | No association with worse outcomes after multivariable analysis. |
| Salas et al. [37] | 2021 | Weight Z-score < −1 at 36 weeks cGA | INTERGROWTH-21st | 359 infants 24–26 weeks of GA. | BSID-III at 24 months cGA. | Significant association with higher risk of cognitive delay. |
| De Rose et al. ** [38] | 2021 | Weight < 10th percentile at discharge or 36 weeks cGA. Weight Z-score <−2 SDS at discharge or 36 weeks | Italian neonatal study charts (INeS) and INTERGROWTH-21st | 254 infants ≤30 weeks. | GMSD at 24 (+−4) months cGA and GMFCS. | Significant association with EUGR definitions by <10th percentile and <−2 z-score and worse GSMD and GMFCS. |
| Alcántara et al. [39] | 2021 | Weight < 10th percentile at discharge. | Fenton and INTERGROWTH-21st | 87 VLBW infants. | RIST and NEPSY-II at 5–7 years | No significant association between EUGR and clinical neurological development disorder. |
| Kim et al. [40] | 2023 | Weight < 3rd percentile at discharge. | Fenton | 82 infants, 21 VLBW with EUGR. | MRI, K-WISC-IV, KEDI-WISC, ATA and executive function at 6–8 years. | Infants with EUGR had significantly lower FSIQ scores and 3 index scores in K-WISC-IV. Higher ATA score (worse function) with EUGR. |
| Study | Year | EUGR Definition | Growth Chart | Population | Neurodevelopmental Assessment | Outcomes |
|---|---|---|---|---|---|---|
| Shah et al. [21] | 2006 | Weight z score difference > 1 and >2 from birth to 36 weeks. | Kramer | 221 infants, ≤28 weeks gestational age (GA). | BSID-II at 18–24 months or best clinical estimate of performance. | Significant association of Z-score difference from birth >2 with PDI but not with MDI. Not significant for Z-score >1. |
| Kan et al. [32] | 2008 | Weight z-score change from birth to discharge | Cole | 401 infants, <28 weeks of GA. | WISC-III, WRAT3 and movement ABC at 8 years. | Weight not related to outcomes. |
| Frondas-Chauty et al. [41] | 2014 | Weight z-score difference from birth to discharge (<−2, −2 to −1.01, −1 to −0.51, −0.50 to 0.01 and ≥0, the reference). | Olsen for infants discharged <41 weeks, WHO for infants discharged >41 weeks. | 2047 infants, <33 weeks of GA. | Physical exam, PY-BL-R and ASQ at 24 months. | Inefficient growth during hospitalization is associated with a non-optimal neurological outcome at 2 years of age. |
| Leppänen et al. [42] | 2014 | Weight z-score change from birth to 36 and 40 weeks. | Sorva [43] | 274 infants, <1501 g or less than 32 weeks of GA. | WPPSI-R at 5 years. | No association with 5-year cognitive outcome. |
| Zozaya et al. [20] | 2018 | Fall in weight z-scores from birth to 36 weeks. | Fenton | 168 VLBW infants, born <34 weeks of GA. | BSID-II at 24 months cGA. | Every 1-point fall in weight z-score was associated with a 5.6-point decrease in the MDI. |
| Cordova et al. [44] | 2020 | Weight z-score decline > 0.8 SD from birth to term-equivalent. | Fenton, Olsen and INTERGROWTH-21st | 613 infants, <33 weeks of GA. | BSID-II at 18 months corrected age. WASI test and WRAT4 at 7 years of corrected age. | EUGR with Fenton and Olsen was associated with low neurodevelopmental scores. EUGR with Fenton was associated with MDI < 85. |
| Maiocco et al. [36] | 2020 | Fall in weight z-score > 1 from birth to discharge | INTERGROWTH-21st | 195 infants < 30 weeks of GA. | GMSD at 24 (+−6) months cGA | No association with worse outcomes after multivariable analysis |
| Yitayew et al. [45] | 2021 | Weight z-score decrease > 1 from birth to discharge. | Fenton and INTERGROWTH-21st | 340 preterms, <33 weeks of GA. | BSID-III at 12 and 24 months of corrected age. | Significant association between growth failure and poor neurodevelopmental outcomes. |
| De Rose et al. ** [38] | 2021 | Weight z-score decrease > 1 from 2 weeks after birth or at 27 weeks cGA to discharge or 36 weeks of cGA | Italian neonatal study charts (INeS) and INTERGROWTH-21st | 254 infants ≤ 30 weeks. | GMSD at 24 (+−4) months cGA and GMFCS. | Association with worse GSMD and GMFCS using INeS but not significant with INTERGROWTH-21st |
| Alcántara et al. [39] | 2021 | Weight z-score difference from birth >1 or >2 from birth to discharge. | Fenton and INTERGROWTH-21st | 87 VLBW infants. | RIST and NEPSY-II at 5–7 years. | No significant association between EUGR and clinical neurological development disorder. |
| El Rafei et al. [46] | 2021 | Weight z-scores difference < −2 (severe) and −2 to −1 (moderate) from birth to discharge. | Fenton | 4197 infants, <32 weeks of GA. | Standardized parental questionnaire at 24 months. | Increased risk of neurodevelopmental impairment with severe EUGR (unadjusted). Increased risk with boys with severe EUGR (adjusted). |
| El Rafei et al. [47] | 2023 | Weight z-scores difference < −2 (severe) and −2 to −1 (moderate) from birth to discharge. | Fenton | 957 infants, <28 weeks of GA. | CP diagnosis, WPPSI-R and Movement ABC-2 at 5 years. | Severe EUGR related to lower IQ. No significant associations were observed between motor function and CP. |
| Strobel et al. [48] | 2024 | Weight z-score decrease ≥ 0.8 from birth to discharge. | Fenton | 590 infants in preterms 24 to 27 + 6 weeks GA. | BSID-III at 20–33 months. CBCL at 1–5 years. | No significant association after adjustments for comorbidities. |
| Study | Year | EUGR Definition | Growth Chart | Population | Neurodevelopmental Assessment | Outcomes |
|---|---|---|---|---|---|---|
| Ramel et al. [49] | 2014 | AGA at birth, weight z-score at discharge. | Fenton | 62 AGA, ≤30 weeks GA. | BSID-III at 24 months of corrected age. | Weight z-score at discharge (when length and head circumference z-score were controlled for) was not associated with 24-month cognitive scores. |
| Guellec et al. [50] | 2016 | AGA at birth with weight z-score difference ≥−1 from birth to 6 months. | WHO | 1493 infants, <32 weeks of GA. | Medical examination, K-ABC and behavioral difficulties at 5 years. School performance at 8 years. | Higher risk of cerebral palsy. No other significant differences in outcomes after adjustment on multivariate analysis. |
| Alcántara et al. [39] | 2021 | Not IUGR infants with weight at discharge < 10th percentile. | Fenton and INTERGROWTH-21st | 87 VLBW infants. | RIST and NEPSY-II at 5–7 years. | No significant association between EUGR and clinical neurological development disorder. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-López, C.; Solís-Sánchez, G.; Lareu-Vidal, S.; Mantecón-Fernández, L.; Ibáñez-Fernández, A.; Rubio-Granda, A.; Suárez-Rodríguez, M. Variability in Definitions and Criteria of Extrauterine Growth Restriction and Its Association with Neurodevelopmental Outcomes in Preterm Infants: A Narrative Review. Nutrients 2024, 16, 968. https://doi.org/10.3390/nu16070968
González-López C, Solís-Sánchez G, Lareu-Vidal S, Mantecón-Fernández L, Ibáñez-Fernández A, Rubio-Granda A, Suárez-Rodríguez M. Variability in Definitions and Criteria of Extrauterine Growth Restriction and Its Association with Neurodevelopmental Outcomes in Preterm Infants: A Narrative Review. Nutrients. 2024; 16(7):968. https://doi.org/10.3390/nu16070968
Chicago/Turabian StyleGonzález-López, Clara, Gonzalo Solís-Sánchez, Sonia Lareu-Vidal, Laura Mantecón-Fernández, Aleida Ibáñez-Fernández, Ana Rubio-Granda, and Marta Suárez-Rodríguez. 2024. "Variability in Definitions and Criteria of Extrauterine Growth Restriction and Its Association with Neurodevelopmental Outcomes in Preterm Infants: A Narrative Review" Nutrients 16, no. 7: 968. https://doi.org/10.3390/nu16070968
APA StyleGonzález-López, C., Solís-Sánchez, G., Lareu-Vidal, S., Mantecón-Fernández, L., Ibáñez-Fernández, A., Rubio-Granda, A., & Suárez-Rodríguez, M. (2024). Variability in Definitions and Criteria of Extrauterine Growth Restriction and Its Association with Neurodevelopmental Outcomes in Preterm Infants: A Narrative Review. Nutrients, 16(7), 968. https://doi.org/10.3390/nu16070968





