Effect of Donated Premature Milk in the Prevention of Bronchopulmonary Dysplasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
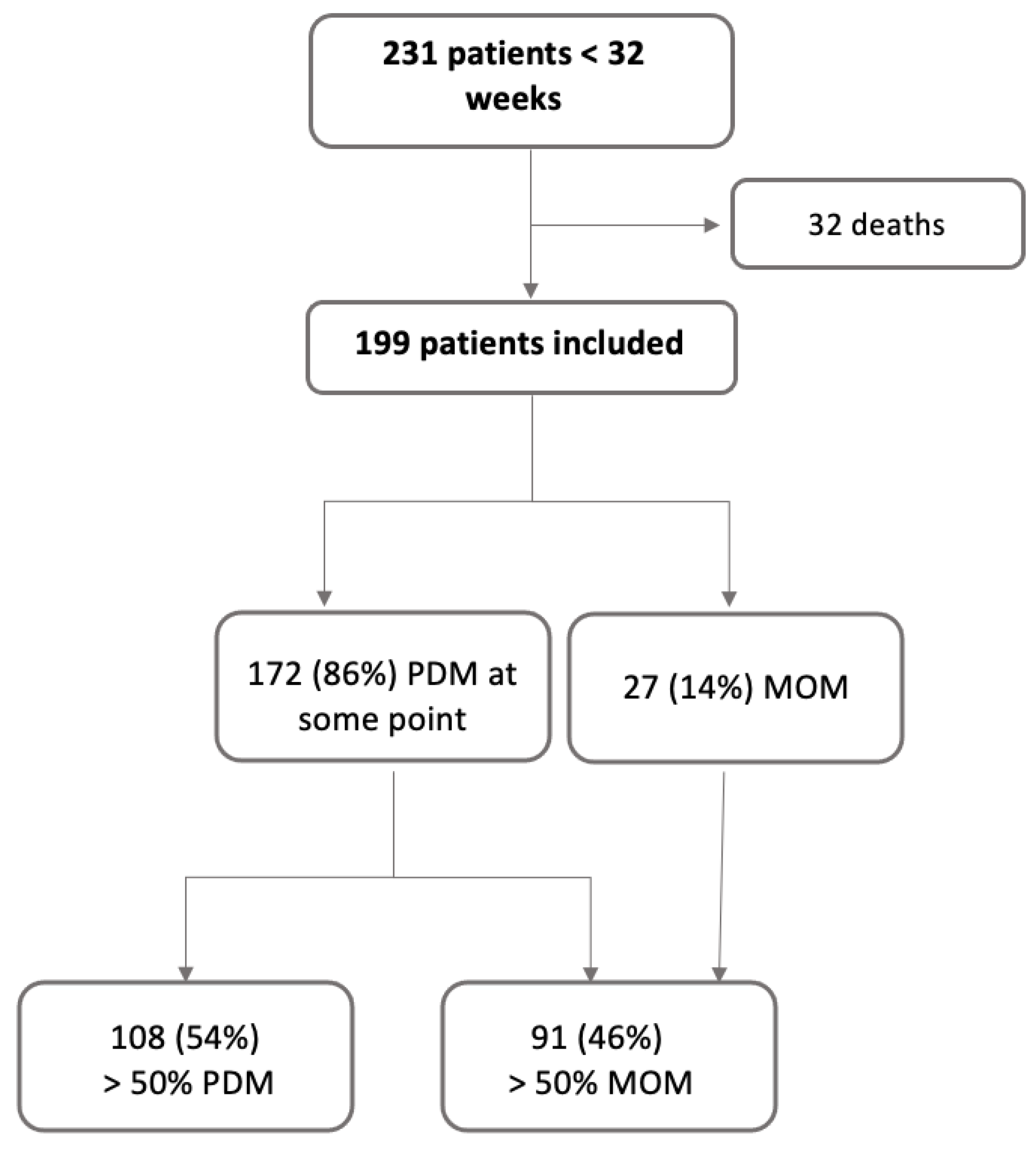
2.2. Participants
2.3. Data Collection
2.4. Grouping
2.5. Fortification
2.6. Premature Donor Milk Bank
2.7. Definition of Bronchopulmonary Dysplasia
2.8. Statistical Analysis
2.9. Approval of the Ethics Committee
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Day, C.L.; Ryan, R.M. Bronchopulmonary dysplasia: New becomes old again! Pediatr. Res. 2017, 81, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Luna, M.; Moreno Hernando, J.; Botet Mussons, F.; Fernández Lorenzo, J.R.; Herranz Carrillo, G.; Rite Gracia, S.; Salguero García, E.; Echaniz Urcelay, I.; Comisión de Estándares de la Sociedad Española de Neonatología. Bronchopulmonary dysplasia: Definitions and classifications. An. Pediatr. Barc. Spain 2013, 79, 262.e1–262.e6. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Tang, J.; Shi, J.; Qu, Y.; Xiong, T.; Mu, D. Human milk as a protective factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F128–F136. [Google Scholar] [PubMed]
- Fonseca, L.T.; Senna, D.C.; Silveira, R.C.; Procianoy, R.S. Association between Breast Milk and Bronchopulmonary Dysplasia: A Single Center Observational Study. Am. J. Perinatol. 2017, 34, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Serrao, F.; Papacci, P.; Costa, S.; Giannantonio, C.; Cota, F.; Vento, G.; Romagnoli, C. Effect of Early Expressed Human Milk on Insulin-Like Growth Factor 1 and Short-Term Outcomes in Preterm Infants. PLoS ONE 2016, 11, e0168139. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, P.; Luo, K.; He, M.; Gong, X. Effects of human milk on short-term outcomes of very/extremely low birth weight preterm infants. Chin. J. Perinat. Med. 2019, 22, 461–466. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Zhu, J.; Jiang, C.; Yu, Z.; Su, A. Effect of First Mother’s Own Milk Feeding Time on the Risk of Moderate and Severe Bronchopulmonary Dysplasia in Infants with Very Low Birth Weight. Front. Pediatr. 2022, 10, 887028. [Google Scholar] [CrossRef] [PubMed]
- Section on Breastfeeding; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef]
- Guidelines on Optimal Feeding of Low Birth-Weight Infants in Low- and Middle-Income Countries; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-4-154836-6.
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Kramer, B.W.; Villamor, E. Donor Human Milk Protects against Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 238. [Google Scholar] [CrossRef]
- ESPGHAN Committee on Nutrition; Arslanoglu, S.; Corpeleijn, W.; Moro, G.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellöf, M.; Fewtrell, M.; et al. Donor human milk for preterm infants: Current evidence and research directions. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Daniels, B.; Schmidt, S.; King, T.; Israel-Ballard, K.; Amundson Mansen, K.; Coutsoudis, A. The Effect of Simulated Flash-Heat Pasteurization on Immune Components of Human Milk. Nutrients 2017, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Moro, G.E.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The Effect of Holder Pasteurization on Nutrients and Biologically-Active Components in Donor Human Milk: A Review. Nutrients 2016, 8, 477. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Patel, A.; Esquerra-Zwiers, A. Donor Human Milk Update: Evidence, Mechanisms, and Priorities for Research and Practice. J. Pediatr. 2017, 180, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Luna, M.; Martin, S.C.; Gómez-de-Orgaz, C.S. Human milk bank and personalized nutrition in the NICU: A narrative review. Eur. J. Pediatr. 2021, 180, 1327–1333. [Google Scholar] [CrossRef]
- Rite Gracia, S.; Fernández Lorenzo, J.R.; Echániz Urcelay, I.; Botet Mussons, F.; Herranz Carrillo, G.; Moreno Hernando, J.; Salguero García, E.; Sánchez Luna, M. Comité de Estándares y la Junta Directiva de la Sociedad Espãnola de Neonatología [Health care levels and minimum recommendations for neonatal care]. An. Pediatr. Barc. Spain 2013, 79, 51.e1–51.e11. [Google Scholar] [CrossRef]
- INTERGROWTH-21st. Available online: http://intergrowth21.ndog.ox.ac.uk/ (accessed on 15 July 2023).
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, S.; Deng, X.; Luo, Z.; Chen, A.; Yu, R. Effects of Antioxidants in Human Milk on Bronchopulmonary Dysplasia Prevention and Treatment: A Review. Front. Nutr. 2022, 9, 924036. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.L.; Johnson, T.J.; Robin, B.; Bigger, H.R.; Buchanan, A.; Christian, E.; Nandhan, V.; Shroff, A.; Schoeny, M.; Engstrom, J.L.; et al. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F256–F261. [Google Scholar] [CrossRef] [PubMed]
- Hair, A. Own mother’s milk significantly decreases the risk of bronchopulmonary dysplasia. Evid. Based Nurs. 2018, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Villamor-Martínez, E.; Pierro, M.; Cavallaro, G.; Mosca, F.; Villamor, E. Mother’s Own Milk and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, J.; Preuß, M.; Gebauer, C.; Bendiks, M.; Herting, E.; Göpel, W. German Neonatal Network (GNN) German Neonatal Network GNN Does Breastmilk Influence the Development of Bronchopulmonary Dysplasia? J. Pediatr. 2016, 169, 76–80.e4. [Google Scholar] [CrossRef]
- Fang, L.-Y.; Chen, D.-M.; Han, S.-P.; Chen, X.-H.; Yu, Z.-B. Association of early nutrition deficiency with the risk of bronchopulmonary dysplasia: A Meta analysis. Zhongguo Dang Dai Er Ke Za Zhi Chin. J. Contemp. Pediatr. 2021, 23, 390–396. [Google Scholar] [CrossRef]
- Thiess, T.; Lauer, T.; Woesler, A.; Neusius, J.; Stehle, S.; Zimmer, K.-P.; Eckert, G.P.; Ehrhardt, H. Correlation of Early Nutritional Supply and Development of Bronchopulmonary Dysplasia in Preterm Infants <1000 g. Front. Pediatr. 2021, 9, 741365. [Google Scholar] [CrossRef]
- Uberos-Fernández, J.; Ruiz-López, A.; Carrasco-Solis, M.; Fernandez-Marín, E.; Garcia-Cuesta, A.; Campos-Martínez, A. Extrauterine growth restriction and low energy intake during the early neonatal period of very low birth weight infants are associated with decreased lung function in childhood. Br. J. Nutr. 2023, 130, 2095–2103. [Google Scholar] [CrossRef]
- Malikiwi, A.I.; Lee, Y.-M.; Davies-Tuck, M.; Wong, F.Y. Postnatal nutritional deficit is an independent predictor of bronchopulmonary dysplasia among extremely premature infants born at or less than 28 weeks gestation. Early Hum. Dev. 2019, 131, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bronchopulmonary Dysplasia (BPD): Management and Outcome—UpToDate. Available online: https://www.uptodate.com/contents/bronchopulmonary-dysplasia-bpd-management-and-outcome (accessed on 1 November 2023).
- Rocha, G.; Guimarães, H.; Pereira-da-Silva, L. The Role of Nutrition in the Prevention and Management of Bronchopulmonary Dysplasia: A Literature Review and Clinical Approach. Int. J. Environ. Res. Public Health 2021, 18, 6245. [Google Scholar] [CrossRef] [PubMed]
- SENEO—Protocolos de la SENEO 2023. Available online: https://www.seneo.es/index.php/publicaciones/protocolos-de-la-seneo-2023 (accessed on 2 November 2023).
- Leaf, A. Introducing enteral feeds in the high-risk preterm infant. Semin. Fetal Neonatal Med. 2013, 18, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Wemhöner, A.; Ortner, D.; Tschirch, E.; Strasak, A.; Rüdiger, M. Nutrition of preterm infants in relation to bronchopulmonary dysplasia. BMC Pulm. Med. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Jennifer Moltu, S.; Lapillonne, A.; van den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Lakshminrusimha, S.; Steinhorn, R.H.; Wedgwood, S. Malnutrition, poor post-natal growth, intestinal dysbiosis and the developing lung. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2021, 41, 1797–1810. [Google Scholar] [CrossRef]
- Natarajan, G.; Johnson, Y.R.; Brozanski, B.; Farrow, K.N.; Zaniletti, I.; Padula, M.A.; Asselin, J.M.; Durand, D.J.; Short, B.L.; Pallotto, E.K.; et al. Postnatal weight gain in preterm infants with severe bronchopulmonary dysplasia. Am. J. Perinatol. 2014, 31, 223–230. [Google Scholar] [CrossRef]
- Huysman, W.A. Growth and body composition in preterm infants with bronchopulmonary dysplasia. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, 46F–51F. [Google Scholar] [CrossRef] [PubMed]
- Giannì, M.L.; Roggero, P.; Colnaghi, M.R.; Piemontese, P.; Amato, O.; Orsi, A.; Morlacchi, L.; Mosca, F. The role of nutrition in promoting growth in pre-term infants with bronchopulmonary dysplasia: A prospective non-randomised interventional cohort study. BMC Pediatr. 2014, 14, 235. [Google Scholar] [CrossRef]
- Lardón-Fernández, M.; Uberos, J.; Molina-Oya, M.; Narbona-López, E. Epidemiological factors involved in the development of bronchopulmonary dysplasia in very low birth-weight preterm infants. Minerva Pediatr. 2017, 69, 42–49. [Google Scholar] [CrossRef]
- Sucasas Alonso, A.; Pértega Diaz, S.; Sáez Soto, R.; Avila-Alvarez, A. Epidemiology and risk factors for bronchopulmonary dysplasia in preterm infants born at or less than 32 weeks of gestation. An. Pediatr. 2022, 96, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Aparici, S.; Riverola, A.; Torre, N.; Alarcon, A.; Moreno, J.; Iriondo, M. PO-0757 Perinatal Factors in the Development of Bronchopulmonary Dysplasia. Arch. Dis. Child. 2014, 99 (Suppl. 2), A502–A503. [Google Scholar] [CrossRef][Green Version]
- Eriksson, L.; Haglund, B.; Odlind, V.; Altman, M.; Ewald, U.; Kieler, H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr. 2015, 104, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Klinger, G.; Sokolover, N.; Boyko, V.; Sirota, L.; Lerner-Geva, L.; Reichman, B. Israel Neonatal Network Perinatal risk factors for bronchopulmonary dysplasia in a national cohort of very-low-birthweight infants. Am. J. Obstet. Gynecol. 2013, 208, 115.e1–115.e9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Shin, J.I.; Kim, S.; Yong-Sung, C.; Shin, Y.H.; Hwang, J.; Shin, J.U.; Koyanagi, A.; Jacob, L.; Smith, L.; et al. Breastfeeding and impact on childhood hospital admissions: A nationwide birth cohort in South Korea. Nat. Commun. 2023, 14, 5819. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Hwang, J.; Kwon, R.; Lee, S.W.; Kim, M.S.; GBD 2019 Allergic Disorders Collaborators; Shin, J.I.; Yon, D.K. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Allergy 2023, 78, 2232–2254. [Google Scholar] [CrossRef] [PubMed]
| NoBPD/1 (n = 159) | BPD 2–3 (n = 40) | Total (n = 199) | p | |
|---|---|---|---|---|
| GA * | 29.25 (1.90) | 26.32 (1.99) | 28.67 (2.24) | <0.01 |
| Sex, % male | 81 (51%) | 22 (55%) | 103 (52%) | 0.65 |
| Weight | 1224.45 (333.32) | 781.28 (218.70) | 1135.37 (360.25) | <0.01 |
| Weight percentile | 46.69 (27.83) | 36.20 (28.25) | 44.50 (28.26) | 0.04 |
| Weight Z Score | −0.17 (1.01) | −0.59 (1.32) | −0.26 (1.09) | 0.03 |
| Length | 37.66 (3.51) | 33.19 (3.29) | 36.76 (3.90) | <0.01 |
| Length percentile | 40.07 (26.97) | 31.41 (27.00) | 38.33 (27.13) | 0.07 |
| Length Z score | −0.34 (0.97) | −0.72 (1.21) | −0.42 (1.03) | 0.04 |
| HC * | 26.75 (2.35) | 23.56 (1.98) | 26.10 (2.61) | <0.01 |
| HC percentile * | 47.19 (27.50) | 35.63 (26.01) | 44.84 (27.54) | 0.02 |
| HC Z Score * | −0.09 (1.06) | −0.52 (1.05) | −0.18 (1.07) | 0.02 |
| IUGR * | 36 (23%) | 8 (20%) | 44 (22%) | 0.72 |
| SGA * | 20 (13%) | 11 (28%) | 31 (16%) | 0.02 |
| Antenatal corticosteroids | 90 (57%) | 17 (43%) | 107 (54%) | 0.11 |
| Chorioamnionitis | 26 (16%) | 9 (23%) | 35 (18%) | 0.36 |
| Cesarean | 90 (57%) | 19 (48%) | 109 (55%) | 0.44 |
| Ventilation at delivery | 98 (62%) | 34 (85%) | 132 (66%) | <0.01 |
| Surfactant administration | 77 (48%) | 29 (73%) | 106 (53%) | <0.01 |
| Postnatal corticosteroids | 6 (38%) | 18 (45%) | 24 (12%) | <0.01 |
| IMV * | 52 (33%) | 35 (88%) | 87 (44%) | <0.01 |
| Hours of IMV * | 38.50 (101.39) | 442.64 (474.74) | 120.15 (281.55) | <0.01 |
| NIMV/days of oxygen * | 30.43 (23.03) | 72.93(27.05) | 39.08 (29.35) | <0.01 |
| PDM * | 142 (89%) | 30 (75%) | 172 (86%) | 0.05 |
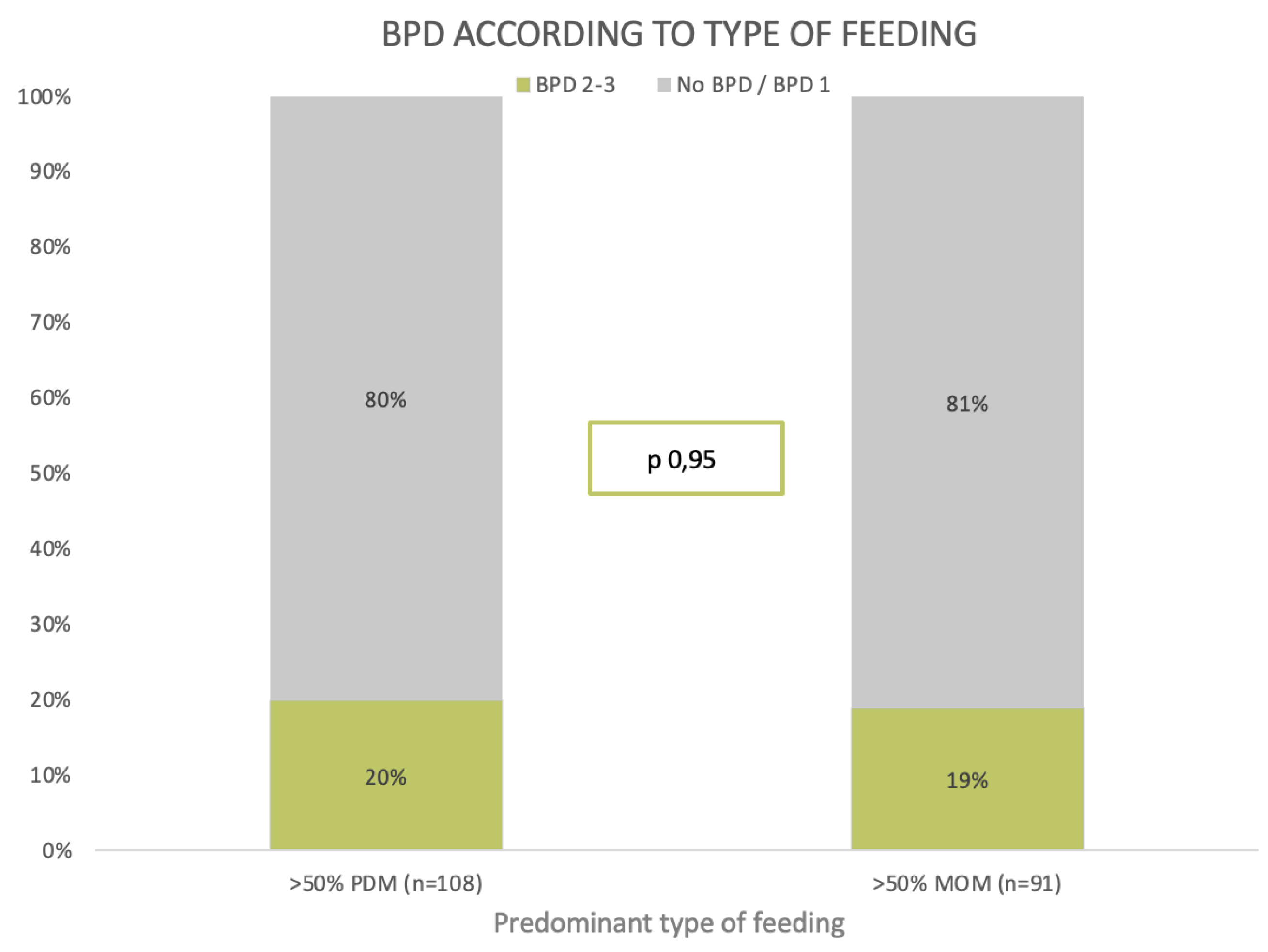
| DM > 50% * | 87 (55%) | 21 (53%) | 108 (54%) | 0.95 |
| NEC * | 8 (5%) | 5 (12%) | 13 (6.5%) | 0.04 |
| IVH > II * | 18 (11%) | 10 (25%) | 28 (14%) | 0.01 |
| PDA * | 49 (31%) | 23 (58%) | 72 (36%) | <0.01 |
| LOS * | 29 (18%) | 21 (53%) | 50 (25%) | <0.01 |
| Oxygen at discharge | 0 (0%) | 10 (25%) | 10 (50%) | <0.01 |
| Calostrum | PDM | 142 (89%) | 30 (75%) | 172 (86%) | 0.05 | |
| NoBPD/1 (n = 142) | BPD 2–3 (n = 30) | Total (n = 172) | p | aOR (IC) | ||
| Lactose | 6.70 (0.34) | 6.65 (0.45) | 6.69 (0.36) | 0.47 | 0.68 (0.23–1.99) | |
| Fat | 2.83 (0.78) | 2.78 (1.01) | 2.82 (0.83) | 0.72 | 0.97 (0.57–1.63) | |
| Protein | 2.02 (0.29) | 2.24 (0.37) | 2.06 (0.32) | <0.01 | 4.16 (1.12–15.47) | |
| K Calories | 60.37 (7.59) | 60.51 (9.61) | 60.40 (7.98) | 0.92 | 1.04 (0.95–1.06) | |
| Mature milk | PDM > 50% | 87 (55%) | 21 (53%) | 108 (54%) | 0.95 | |
| NoBPD/1 (n = 87) | BPD 2–3 (n = 21) | Total (n = 108) | p | aOR (IC) | ||
| Lactose | 7.02 (0.25) | 7.18 (0.23) | 7.05 (0.25) | 0.01 | 7.27 (0.68–78.15) | |
| Fat | 3.44 (0.59) | 3.49 (0.81) | 3.45 (0.64) | 0.78 | 1.04 (0.45–2.41) | |
| Protein | 1.62 (0.23) | 1.73 (0.37) | 1.64 (0.26) | 0.09 | 3.92 (0.49–31.41) | |
| K Calories | 64.81 (8.61) | 67.05 (8.22) | 65.22 (8.55) | 0.31 | 1.03 (0.95–1.11) |
| NoBPD/1 (n = 159) | BPD 2–3 (n = 40) | p | aOR (IC) | |
|---|---|---|---|---|
| FI 3rd day * (mL/kg) | 121.71 (24.12) | 123.10 (22.38) | 0.74 | 1.00 (0.99–1.01) |
| FI 7th day * (mL/kg) | 138.60 (17.60) | 135.00 (32.30) | 0.34 | 0.99 (0.98–1.01) |
| FI 15th day * (mL/kg) | 157.32 (16.88) | 143.60 (27.61) | <0.01 | 0.97 (0.96–0.99) |
| FI 30th day * (mL/kg) | 10.34 (8.69) | 17.28 (14.14) | <0.01 | 1.01 (0.98–1.05) |
| Start fortifying (days of life) | 10.96 (6.91) | 16.62 (8.92) | <0.01 | 1.03 (0.98–1.10) |
| At Admission | ||||
| Weight (g) | 1224.45 (333.32) | 781.28 (218.70) | <0.01 | 0.99 (0.98–0.99) |
| Weight Z score | −0.17 (1.01) | −0.59 (1.32) | 0.03 | 0.47 (0.32–0.69) |
| Length (cm) | 37.66 (3.51) | 33.19 (3.29) | <0.01 | 0.83 (0.72–0.97) |
| Length Z score | −0.34 (0.97) | −0.72 (1.21) | 0.04 | 0.53 (0.35–0.81) |
| HC * (cm) | 26.75 (2.35) | 23.56 (1.98) | <0.01 | 0.66 (0.50–0.88) |
| HC Z score * | −0.09 (1.06) | −0.52 (1.05) | 0.02 | 0.52 (0.33–0.81) |
| At discharge | ||||
| Weight (g) | 2468.26 (456.62) | 3106.75 (622.45) | <0.01 | 1.01 (1.01–1.01) |
| Weight Z score | −1.03 (1.07) | −1.72 (2.59) | 0.01 | 0.80 (0.60–1.08) |
| Length (cm) | 45.40 (5.85) | 47.64 (3.36) | 0.02 | 1.12 (0.97–1.29) |
| Length Z score | −0.75 (1.49) | −2.34 (3.10) | <0.01 | 0.72 (0.56–0.93) |
| HC * (cm) | 32.98 (2.13) | 34.51 (1.75) | <0.01 | 1.31 (1.09–1.56) |
| HC Z score * | 0.01 (1.16) | −0.86 (2.89) | <0.01 | 0.88 (0.69–1.13) |
| aOR (IC) | p | |
|---|---|---|
| Sex, % male | 0.60 (0.25–1.42) | 0.24 |
| Weight Z score | 0.47 (0.33–0.70) | <0.01 |
| Length Z score | 0.53 (0.35–0.81) | <0.01 |
| HC Z score * | 0.52 (0.33–0.81) | <0.01 |
| IUGR * | 1.48 (0.54–4.03) | 0.44 |
| SGA * | 7.25 (2.32–22.69) | <0.01 |
| Antenatal corticosteroids | 0.93 (0.52- 1.66) | 0.81 |
| Corioamnionitis | 1.08 (0.39–3.01) | 0.87 |
| Cesarean | 0.69 (0.29–1.64) | 0.40 |
| Ventilation at delivery | 1.77 (0.62–5.05) | 0.28 |
| Surfactant application | 1.35 (0.55–3.31) | 0.52 |
| Postnatal corticosteroids | 4.55 (1.34–15.53) | 0.01 |
| IMV * | 4.98 (1.64–15.15) | <0.01 |
| IMV Hours * | 1.005 (1.002–1.007) | <0.01 |
| NIMV/Days of oxygen * | 1.06 (1.03–1.08) | <0.01 |
| NEC * | 2.38 (0.57–9.95) | 0.23 |
| IVH III/IV * | 0.71 (0.22–2.31) | 0.57 |
| PDA * | 1.08 (0.43–2.73) | 0.87 |
| LOS * | 0.72 (0.28–1.87) | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino-Hernández, A.; Palacios-Bermejo, A.; Ramos-Navarro, C.; Caballero-Martín, S.; González-Pacheco, N.; Rodríguez-Corrales, E.; Sánchez-Gómez de Orgaz, M.C.; Sánchez-Luna, M. Effect of Donated Premature Milk in the Prevention of Bronchopulmonary Dysplasia. Nutrients 2024, 16, 859. https://doi.org/10.3390/nu16060859
Merino-Hernández A, Palacios-Bermejo A, Ramos-Navarro C, Caballero-Martín S, González-Pacheco N, Rodríguez-Corrales E, Sánchez-Gómez de Orgaz MC, Sánchez-Luna M. Effect of Donated Premature Milk in the Prevention of Bronchopulmonary Dysplasia. Nutrients. 2024; 16(6):859. https://doi.org/10.3390/nu16060859
Chicago/Turabian StyleMerino-Hernández, Amaia, Andrea Palacios-Bermejo, Cristina Ramos-Navarro, Silvia Caballero-Martín, Noelia González-Pacheco, Elena Rodríguez-Corrales, María Carmen Sánchez-Gómez de Orgaz, and Manuel Sánchez-Luna. 2024. "Effect of Donated Premature Milk in the Prevention of Bronchopulmonary Dysplasia" Nutrients 16, no. 6: 859. https://doi.org/10.3390/nu16060859
APA StyleMerino-Hernández, A., Palacios-Bermejo, A., Ramos-Navarro, C., Caballero-Martín, S., González-Pacheco, N., Rodríguez-Corrales, E., Sánchez-Gómez de Orgaz, M. C., & Sánchez-Luna, M. (2024). Effect of Donated Premature Milk in the Prevention of Bronchopulmonary Dysplasia. Nutrients, 16(6), 859. https://doi.org/10.3390/nu16060859





