Comparison of Metabolic Syndrome (MetS) Risk and Nutritional Status According to Menopause Age and the Impact of Socioeconomic Status on MetS Prevalence in Postmenopausal Women: A Cross-Sectional Study Based on the 8th Korea National Health and Nutrition Examination Survey (KNHANES)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Research Contents and Methods
2.3. Data Analysis
3. Results
3.1. Sociodemographic and Anthropometric Characteristics According to Menopausal Status
3.2. Prevalence of Metabolic Syndrome Components Based on Menopausal Status
3.3. Average Daily Nutrient Intake According to Menopausal Status
3.4. Factors Associated with the Risk of Premature Menopause
3.5. Factors Associated with the Risk of Metabolic Syndrome in Postmenopausal Women
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. Research on the Menopause in the 1990s: Report of a WHO Scientific Group. 1996. Available online: https://iris.who.int/handle/10665/41841 (accessed on 6 February 2024).
- Williams, R.E.; Levine, K.B.; Kalilani, L.; Lewis, J.; Clark, R.V. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas 2009, 62, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Shuster, L.T.; Rhodes, D.J.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010, 65, 161–166. [Google Scholar] [CrossRef]
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- Korean Statistical Information Service. Prospective Population Projection: Nationwide Life Expectancy at Birth. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B41&checkFlag=N (accessed on 6 February 2024).
- Choe, S.A.; Sung, J. Trends of premature and early menopause: A comparative study of the US National Health and Nutrition Examination Survey and the Korea National Health and Nutrition Examination Survey. J. Korean Med. Sci. 2020, 35, e97. [Google Scholar] [CrossRef] [PubMed]
- Batool, M.; Kiran, S.; Mazhar, S.B. Socio-Economic Determinants of Age at Menopause. J. Soc. Obstet. Gynaecol. Pak. 2020, 10, 185–189. [Google Scholar]
- Lim, Y.M.; Jeong, K.; Lee, S.R.; Chung, H.W.; Lee, W. Association between premature ovarian insufficiency, early menopause, socioeconomic status in a nationally representative sample from Korea. Maturitas 2019, 121, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Gurzkowska, B.; Kułaga, Z.; Litwin, M.; Grajda, A.; Świąder, A.; Kułaga, K.; Góźdź, M.; Wojtyło, M. The relationship between selected socioeconomic factors and basic anthropometric parameters of school-aged children and adolescents in Poland. Eur. J. Pediatr. 2014, 173, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Morawa, E.; Erim, Y. Health-related lifestyle behavior and religiosity among first-generation immigrants of polish origin in Germany. Int. J. Environ. Res. Public Health 2018, 15, 2545. [Google Scholar] [CrossRef]
- Chan, C.Q.H.; Lee, K.H.; Low, L.L. A systematic review of health status, health seeking behaviour and healthcare utilisation of low socioeconomic status populations in urban Singapore. Int. J. Equity Health 2018, 17, 39. [Google Scholar] [CrossRef]
- Frisch, R.E. The right weight: Body fat, menarche and fertility. Proc. Nutr. Soc. 1994, 53, 113–129. [Google Scholar] [CrossRef]
- Szegda, K.; Whitcomb, B.W.; Purdue-Smithe, A.; Boutot, M.; Manson, J.; Hankinson, S.; Rosner, B.A.; Bertone-Johnson, E.R. Adult adiposity and risk of early menopause. Hum. Reprod. 2017, 32, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.A.; Jackson, C.A.; Rowlands, J.V.; Mishra, G.D. Socioeconomic position, lifestyle factors and age at natural menopause: A systematic review and meta-analyses of studies across six continents. Int. J. Epidemiol. 2014, 43, 1542–1562. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A.; Davis, S.R.; De Villiers, T.; Gompel, A.; Henderson, V.W.; Hodis, H.N.; Lumsden, M.A.; Mack, W.J.; Shapiro, S.; Baber, R.J. Prevention of diseases after menopause. Climacteric 2014, 17, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Marchi, R.; Dell’Agnolo, C.M.; Lopes, T.C.R.; Gravena, A.A.F.; Demitto, M.O.; Brischiliari, S.C.R.; Borghesan, D.H.P.; Carvalho, M.D.B.; Pelloso, S.M. Prevalence of metabolic syndrome in pre-and postmenopausal women. Arch. Endocrinol. Metab. 2017, 61, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi-Oyelere, B.L.; Kruger, M.C. Nutrient and dietary patterns in relation to the pathogenesis of postmenopausal osteoporosis—A literature review. Life 2020, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Han, A.; Park, Y. Association of dietary total antioxidant capacity with bone mass and osteoporosis risk in Korean Women: Analysis of the Korea national health and nutrition examination survey 2008–2011. Nutrients 2021, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Yang, J.H.; Seo, J.S.; Kim, Y.J.; Kang, S.W. Prevalence and diagnosis experience of osteoporosis in postmenopausal women over 50: Focusing on socioeconomic factors. PLoS ONE 2021, 16, e0248020. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Park, E.J.; Cho, Y.H.; Lee, S.Y.; Choi, J.I.; Lee, Y.I.; Kim, Y.J.; Lee, J.G.; Yi, Y.H.; Tak, Y.J.; et al. Association between Weekend Catch-Up Sleep and Metabolic Syndrome with Sleep Restriction in Korean Adults: A Cross-Sectional Study Using KNHANES. Diabetes Metab. Syndr. Obes. 2020, 13, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.; Lynch, J. Trends in socioeconomic inequalities in adult health behaviors among US states, 1990–2004. Public Health Rep. 2007, 122, 177–189. [Google Scholar] [CrossRef]
- Mackenbach, J.P.; Stirbu, I.; Roskam, A.-J.R.; Schaap, M.M.; Menvielle, G.; Leinsalu, M.; Kunst, A.E.; European Union Working Group on Socioeconomic Inequalities in Health. Socioeconomic inequalities in health in 22 European countries. N. Engl. J. Med. 2008, 358, 2468–2481. [Google Scholar] [CrossRef]
- Lee, S.M.; Hong, M.; Park, S.; Kang, W.S.; Oh, I.H. Economic burden of eating disorders in South Korea. J. Eat. Disord. 2021, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L.; Aragaki, A.K.; Howard, B.V.; Chlebowski, R.T.; Thomson, C.A.; Van Horn, L.; Tinker, L.F.; Manson, J.E.; Anderson, G.L.; Kuller, L.E.; et al. Low-fat dietary pattern among postmenopausal women influences long-term cancer, cardiovascular disease, and diabetes outcomes. J. Nutr. 2019, 149, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Noll, P.R.E.S.; Campos, C.A.S.; Leone, C.; Zangirolami-Raimundo, J.; Noll, M.; Baracat, E.; Júnior, J.M.S.; Sorpreso, I.C.E. Dietary intake and menopausal symptoms in postmenopausal women: A systematic review. Climacteric 2021, 24, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Foster-Schubert, K.E.; Alfano, C.M.; Duggan, C.R.; Xiao, L.; Campbell, K.L.; Kong, A.; Bain, C.E.; Wang, C.Y.; Blackburn, G.L.; McTiernan, A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity 2012, 20, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Migliardi, A.; Costa, G. Health inequalities and nutrition in Italy during crisis times. Epidemiol. Prev. 2015, 39, 322–331. [Google Scholar] [PubMed]
- Bruggink, J.W.; de Goeij, M.C.; Otten, F.; Kunst, A.E. Changes between pre-crisis and crisis period in socioeconomic inequalities in health and stimulant use in Netherlands. Eur. J. Public Health 2016, 26, 772–777. [Google Scholar] [CrossRef]
- Hoveling, L.A.; Liefbroer, A.C.; Bültmann, U.; Smidt, N. Socioeconomic differences in metabolic syndrome development: Examining the mediating role of chronic stress using the Lifelines Cohort Study. BMC Public Health 2022, 22, 261. [Google Scholar] [CrossRef]
- Follis, S.L.; Bea, J.; Klimentidis, Y.; Hu, C.; Crandall, C.; Garcia, D.O.; Shadyab, A.H.; Nassir, R.; Chen, Z. Psychosocial stress and bone loss among postmenopausal women: Results from the Women’s Health Initiative. J. Epidemiol. Community Health 2019, 73, 888–892. [Google Scholar] [CrossRef]
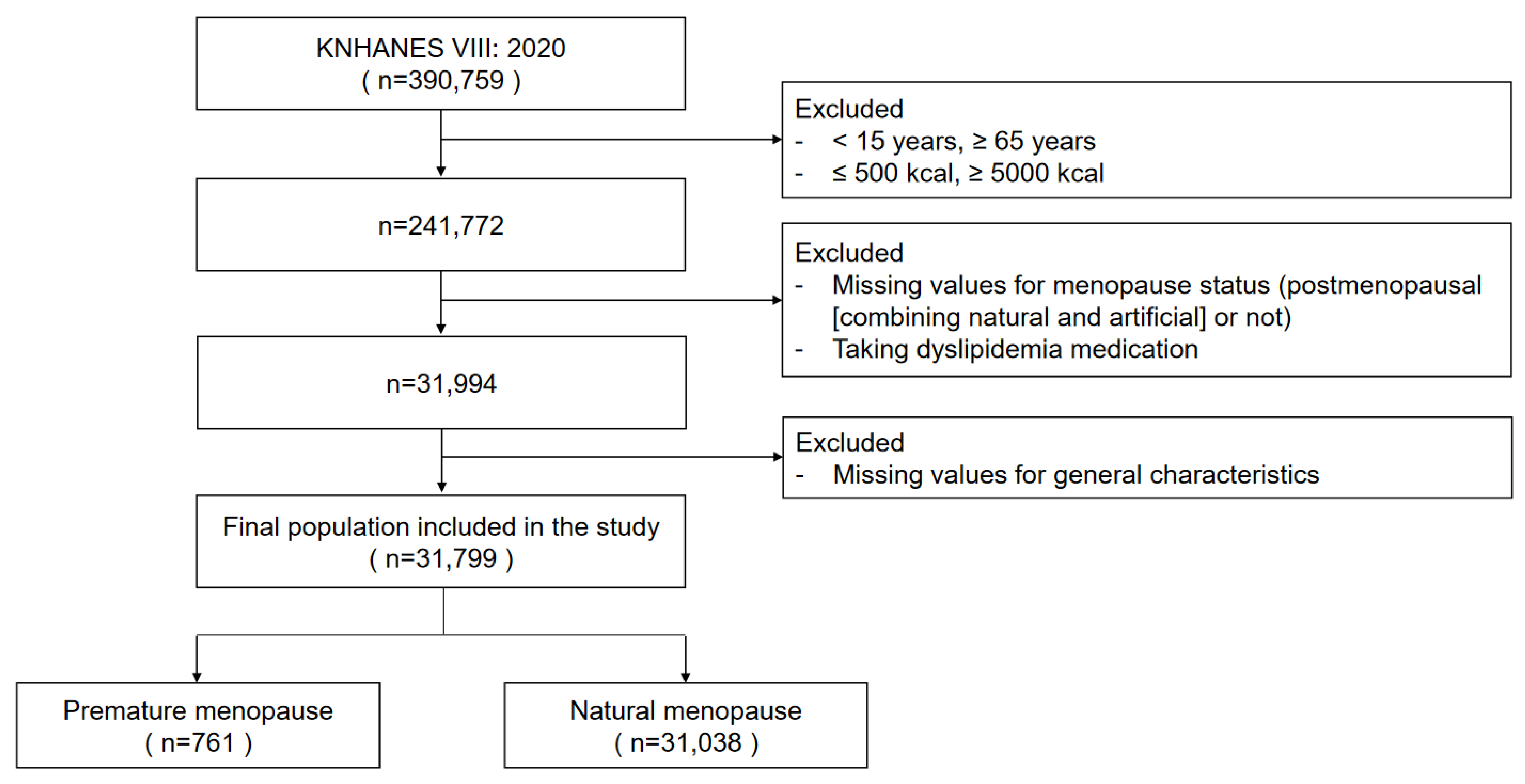
| Premature Menopause (N = 761) | Natural Menopause (N = 31,038) | p (1) | |
|---|---|---|---|
| Age at current (2) | 56.114 ± 9.850 | 57.276 ± 4.365 | <0.0001 |
| Age at menopause | 36.223 ± 2.709 | 50.622 ± 3.252 | <0.0001 |
| N (%) | |||
| Age at current | <0.0001 | ||
| 15 ≤ age < 30 | 22 (2.89%) | 0 (0.00%) | |
| 30 ≤ age < 40 | 53 (6.96%) | 0 (0.00%) | |
| 40 ≤ age < 50 | 104 (13.67%) | 1675 (5.40%) | |
| 50 ≤ age < 65 | 582 (76.48%) | 29,363 (94.60%) | |
| Age at menopause | <0.0001 | ||
| age < 50 | 761 (100.00%) | 9761 (31.45%) | |
| 50 ≤ age < 60 | 0 (0.00%) | 21,277 (68.55%) | |
| Income level | 0.0003 | ||
| Low | 23 (3.02%) | 3446 (11.10%) | |
| Low-middle | 183 (24.05%) | 5385 (17.35%) | |
| Middle-high | 390 (51.25%) | 8308 (26.77%) | |
| High | 165 (21.68%) | 13,899 (44.78%) | |
| Education | <0.0001 | ||
| ≤Elementary school | 311 (40.87%) | 2622 (8.45%) | |
| Middle school | 86 (11.30%) | 4453 (14.35%) | |
| High school | 252 (33.11%) | 13,345 (43.00%) | |
| ≥College | 112 (14.72%) | 10,618 (34.21%) | |
| Heavy alcohol drinking | 0.0019 | ||
| Yes | 37 (4.86%) | 909 (2.93%) | |
| No | 724 (95.14%) | 30,129 (97.07%) | |
| Current smoking | <0.0001 | ||
| Yes | 71 (9.33%) | 679 (2.19%) | |
| No | 690 (90.67%) | 30,359 (97.81%) | |
| Walking | <0.0001 | ||
| <5 days/week | 534 (70.17%) | 17,293 (55.86%) | |
| ≥5 days/week | 227 (29.83%) | 13,664 (44.14%) | |
| Leisure-related physical activities (moderate-intensity) | <0.0001 | ||
| Yes | 261 (34.30%) | 7810 (25.16%) | |
| No | 500 (65.70%) | 23,228 (74.84%) | |
| Self-assessment of health | <0.0001 | ||
| Good | 130 (17.08%) | 8609 (27.74%) | |
| Moderate | 382 (50.20%) | 16889 (54.41%) | |
| Poor | 249 (32.72%) | 5540 (17.85%) | |
| Premature Menopause (N = 761) | Natural Menopause (N = 31,038) | p (1) | |
|---|---|---|---|
| Height (cm) (2) | 157.493 ± 6.793 | 157.070 ± 5.644 | 0.0424 |
| Weight (kg) | 57.500 ± 8.087 | 58.177 ± 8.169 | 0.0238 |
| Waist circumference (cm) | 78.627 ± 6.851 | 80.999 ± 8.238 | <0.0001 |
| Body mass index (kg/m2) | 23.171 ± 2.967 | 23.584 ± 3.153 | 0.0003 |
| Premature Menopause (N = 761) | Natural Menopause (N = 31,038) | ||||
|---|---|---|---|---|---|
| N (%) | p (1) | ||||
| Obesity | <0.0001 | ||||
| Yes | 149 (19.58%) | 9564 (30.81%) | |||
| No | 612 (80.42%) | 21,474 (69.19%) | |||
| HyperTG (2) | <0.0001 | ||||
| Yes | 260 (34.17%) | 7441 (23.97%) | |||
| No | 501 (65.83%) | 23,597 (76.03%) | |||
| Diabetes | <0.0001 | ||||
| Yes | 198 (26.02%) | 11,027 (35.53%) | |||
| No | 563 (73.98%) | 20,011 (64.47%) | |||
| HyperCHL (3) | <0.0001 | ||||
| Yes | 100 (13.14%) | 6889 (22.20%) | |||
| No | 661 (86.86%) | 24,149 (77.80%) | |||
| Hypertension | 0.0145 | ||||
| Yes | 238 (31.27%) | 8466 (27.28%) | |||
| No | 523 (68.73%) | 22,572 (72.72%) | |||
| HDL-cholesterol | <0.0001 | ||||
| ≥50 | 379 (49.80%) | 19,633 (63.25%) | |||
| <50 | 382 (50.20%) | 11,405 (36.75%) | |||
| Metabolic diagnosis | <0.0001 | ||||
| ≤3 | 609 (80.03%) | 28,052 (90.38%) | |||
| >3 | 152 (19.97%) | 2986 (9.62%) | |||
| Premature Menopause (N = 761) | Natural Menopause (N = 31,038) | p (1) | |
|---|---|---|---|
| Energy (Kcal) (2) | 1874.628 ± 21.788 | 1738.942 ± 3.413 | <0.0001 |
| Water (g) | 1113.319 ± 14.807 | 1146.723 ± 2.318 | 0.0258 |
| Carbohydrate (g) | 281.359 ± 1.687 | 267.280 ± 0.264 | <0.0001 |
| Protein (g) | 60.367 ± 0.556 | 66.557 ± 0.087 | <0.0001 |
| Fat (g) | 43.600 ± 0.602 | 44.299 ± 0.094 | 0.2519 |
| SFA (g) (3) | 14.309 ± 0.246 | 13.247 ± 0.038 | <0.0001 |
| MUSFA (g) (4) | 13.594 ± 0.244 | 14.001 ± 0.038 | 0.0995 |
| PUSFA (g) (5) | 11.621 ± 0.194 | 12.315 ± 0.030 | 0.0004 |
| n-3 Fatty acid (g) | 1.698 ± 0.054 | 2.150 ± 0.009 | <0.0001 |
| n-6 Fatty acid (g) | 9.905 ± 0.168 | 10.139 ± 0.026 | 0.1673 |
| Cholesterol (mg) | 233.549 ± 5.412 | 242.466 ± 0.847 | 0.1036 |
| Fiber (g) | 34.776 ± 0.365 | 29.790 ± 0.057 | <0.0001 |
| Sugar (g) | 81.072 ± 1.093 | 63.613 ± 0.171 | <0.0001 |
| Calcium (mg) | 540.839 ± 7.113 | 517.873 ± 1.114 | 0.0014 |
| Phosphorus (mg) | 1151.231 ± 7.961 | 1067.583 ± 1.245 | <0.0001 |
| Iron (mg) | 9.275 ± 0.147 | 9.958 ± 0.023 | <0.0001 |
| Sodium (mg) | 2173.107 ± 49.323 | 3320.482 ± 7.723 | <0.0001 |
| Potassium (mg) | 3043.785 ± 33.109 | 3075.978 ± 5.184 | 0.3368 |
| Vitamin A (μg RAE (6)) | 385.004 ± 18.250 | 477.881 ± 2.857 | <0.0001 |
| β-Carotene (μg) | 2403.778 ± 118.102 | 3887.748 ± 18.491 | <0.0001 |
| Retinol (μg) | 184.655 ± 14.029 | 153.783 ± 2.196 | 0.0297 |
| Thiamin (mg) | 1.185 ± 0.014 | 1.053 ± 0.002 | <0.0001 |
| Riboflavin (mg) | 1.449 ± 0.018 | 1.552 ± 0.003 | <0.0001 |
| Niacin (mg) | 11.440 ± 0.141 | 11.475 ± 0.022 | 0.8106 |
| Folate (μg DFE (7)) | 352.823 ± 4.556 | 354.893 ± 0.713 | 0.6536 |
| Vitamin C (mg) | 77.730 ± 2.486 | 80.035 ± 0.389 | 0.3597 |
| Energy distribution | |||
| %Carbohydrate | 63.909 ± 0.356 | 61.960 ± 0.056 | <0.0001 |
| %Protein | 13.981 ± 0.130 | 15.395 ± 0.020 | <0.0001 |
| %Fat | 22.110 ± 0.296 | 22.645 ± 0.046 | 0.0741 |
| Variables | Premature Menopause (N = 761) | p | |
|---|---|---|---|
| Adjusted OR (1) | 95% CI | ||
| Body mass index (kg/m2) | 0.954 | 0.932–0.978 | 0.0002 |
| Income level | |||
| Lower-middle | 1 | ||
| Middle-high | 0.209 | 0.122–0.359 | <0.0001 |
| High | 0.009 | 0.003–0.023 | <0.0001 |
| Education | |||
| ≤Middle school | 1 | ||
| ≥High school | 2.454 | 1.726–3.489 | <0.0001 |
| Heavy alcohol drinking | |||
| Yes | 1.375 | 0.953–1.984 | 0.0885 |
| No | 1 | ||
| Current smoking | |||
| Yes | 4.230 | 3.175–5.636 | <0.0001 |
| No | 1 | ||
| Walking | |||
| <5 days/week | 1 | ||
| ≥5 days/week | 0.675 | 0.572–0.798 | <0.0001 |
| Leisure-related physical activities (moderate-intensity) | |||
| Yes | 2.292 | 1.946–2.698 | <0.0001 |
| No | 1 | ||
| Self-assessment of health | |||
| Good or moderate | 1 | ||
| Poor | 1.175 | 0.854–1.617 | 0.3216 |
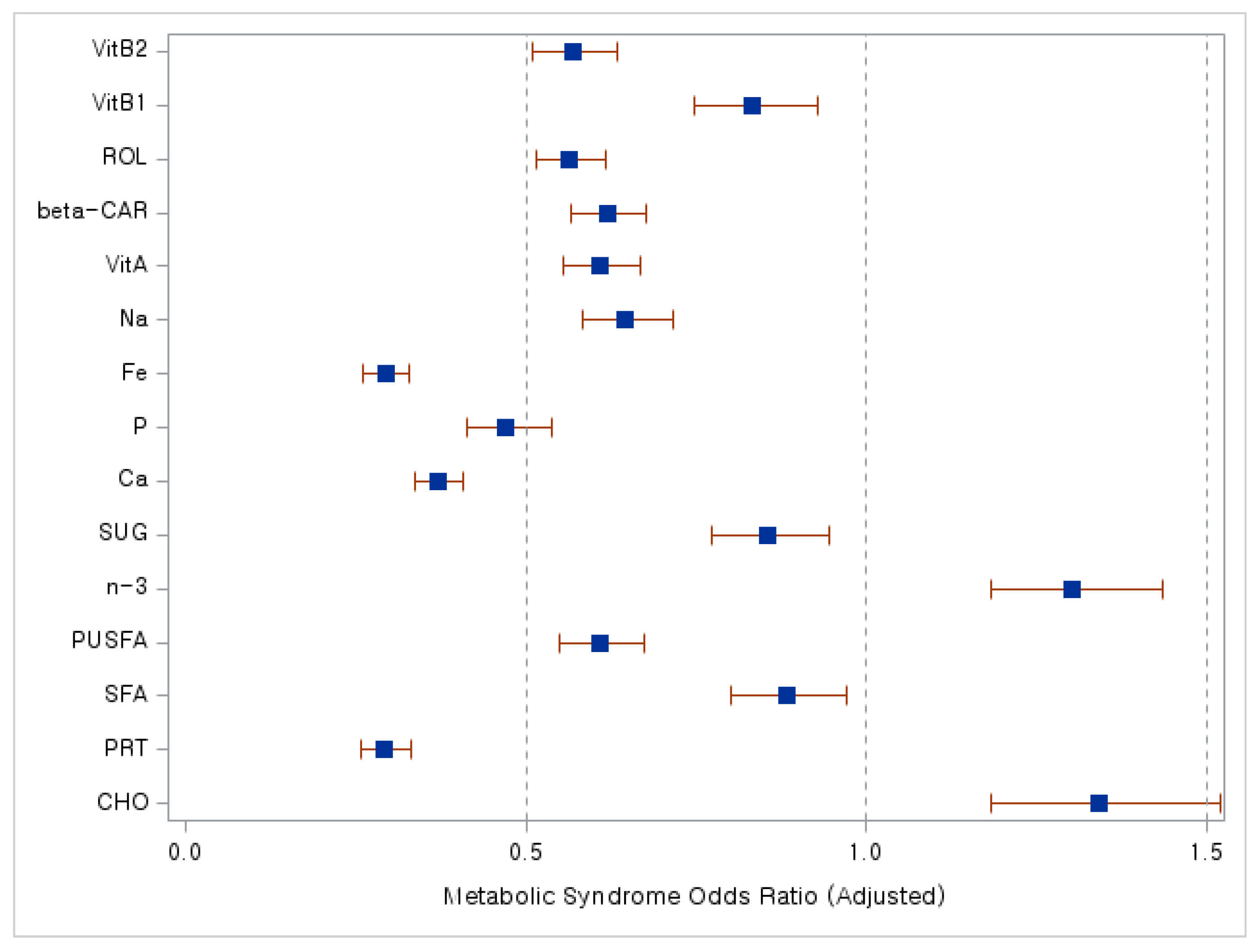
| Variables | Metabolic Syndrome (N = 3138) | p | |
|---|---|---|---|
| Adjusted OR (1) | 95% CI | ||
| Body mass index (kg/m2) | 1.398 | 1.380–1.417 | <0.0001 |
| Income level | |||
| Lower-middle | 1 | ||
| Middle-high | 1.816 | 1.417–2.327 | 0.0470 |
| High | 2.679 | 1.816–3.951 | <0.0001 |
| Education | |||
| ≤Middle school | 1 | ||
| ≥High school | 0.764 | 0.637–0.915 | 0.0034 |
| Heavy alcohol drinking | |||
| Yes | 2.684 | 2.148–3.353 | <0.0001 |
| No | 1 | ||
| Current smoking | |||
| Yes | 0.168 | 0.115–0.245 | <0.0001 |
| No | 1 | ||
| Walking | |||
| <5 days/week | 1 | ||
| ≥5 days/week | 0.524 | 0.479–0.573 | <0.0001 |
| Leisure-related physical activities (moderate-intensity) | |||
| Yes | 0.525 | 0.464–0.594 | <0.0001 |
| No | 1 | ||
| Self-assessment of health | |||
| Good or moderate | 1 | ||
| Poor | 1.246 | 1.050–1.479 | 0.0120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, A.; Choi, Y.-J. Comparison of Metabolic Syndrome (MetS) Risk and Nutritional Status According to Menopause Age and the Impact of Socioeconomic Status on MetS Prevalence in Postmenopausal Women: A Cross-Sectional Study Based on the 8th Korea National Health and Nutrition Examination Survey (KNHANES). Nutrients 2024, 16, 967. https://doi.org/10.3390/nu16070967
Han A, Choi Y-J. Comparison of Metabolic Syndrome (MetS) Risk and Nutritional Status According to Menopause Age and the Impact of Socioeconomic Status on MetS Prevalence in Postmenopausal Women: A Cross-Sectional Study Based on the 8th Korea National Health and Nutrition Examination Survey (KNHANES). Nutrients. 2024; 16(7):967. https://doi.org/10.3390/nu16070967
Chicago/Turabian StyleHan, Anna, and Yean-Jung Choi. 2024. "Comparison of Metabolic Syndrome (MetS) Risk and Nutritional Status According to Menopause Age and the Impact of Socioeconomic Status on MetS Prevalence in Postmenopausal Women: A Cross-Sectional Study Based on the 8th Korea National Health and Nutrition Examination Survey (KNHANES)" Nutrients 16, no. 7: 967. https://doi.org/10.3390/nu16070967
APA StyleHan, A., & Choi, Y.-J. (2024). Comparison of Metabolic Syndrome (MetS) Risk and Nutritional Status According to Menopause Age and the Impact of Socioeconomic Status on MetS Prevalence in Postmenopausal Women: A Cross-Sectional Study Based on the 8th Korea National Health and Nutrition Examination Survey (KNHANES). Nutrients, 16(7), 967. https://doi.org/10.3390/nu16070967






