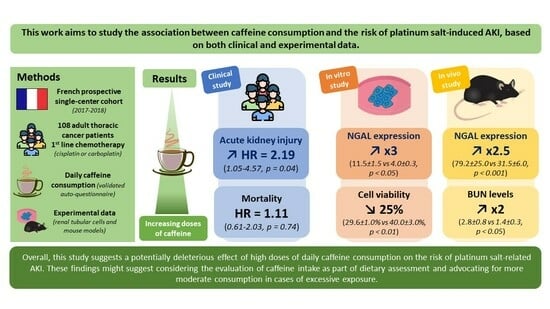
Daily Caffeine Consumption May Increase the Risk of Acute Kidney Injury Related to Platinum-Salt Chemotherapy in Thoracic Cancer Patients: A Translational Study
Abstract
1. Introduction
2. Methods
2.1. Clinical Study
2.2. In Vitro Model
2.3. Mouse Model of Cisplatin-Induced Nephrotoxicity
2.4. Gene Expression
2.5. Statistical Analyses
3. Results
3.1. Study Population
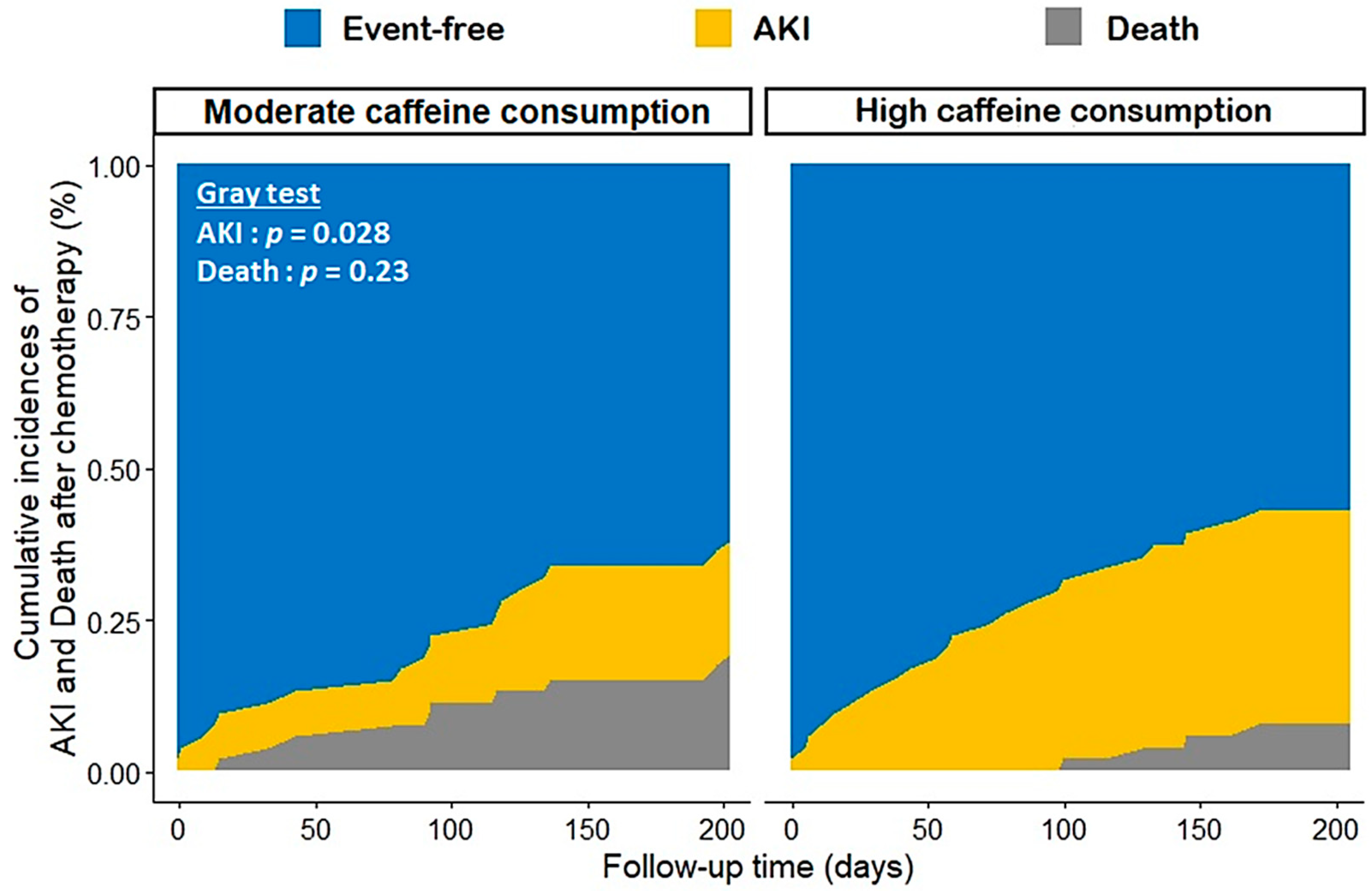
3.2. Platinum-Salt-Related AKI and Mortality
3.3. Secondary Outcomes
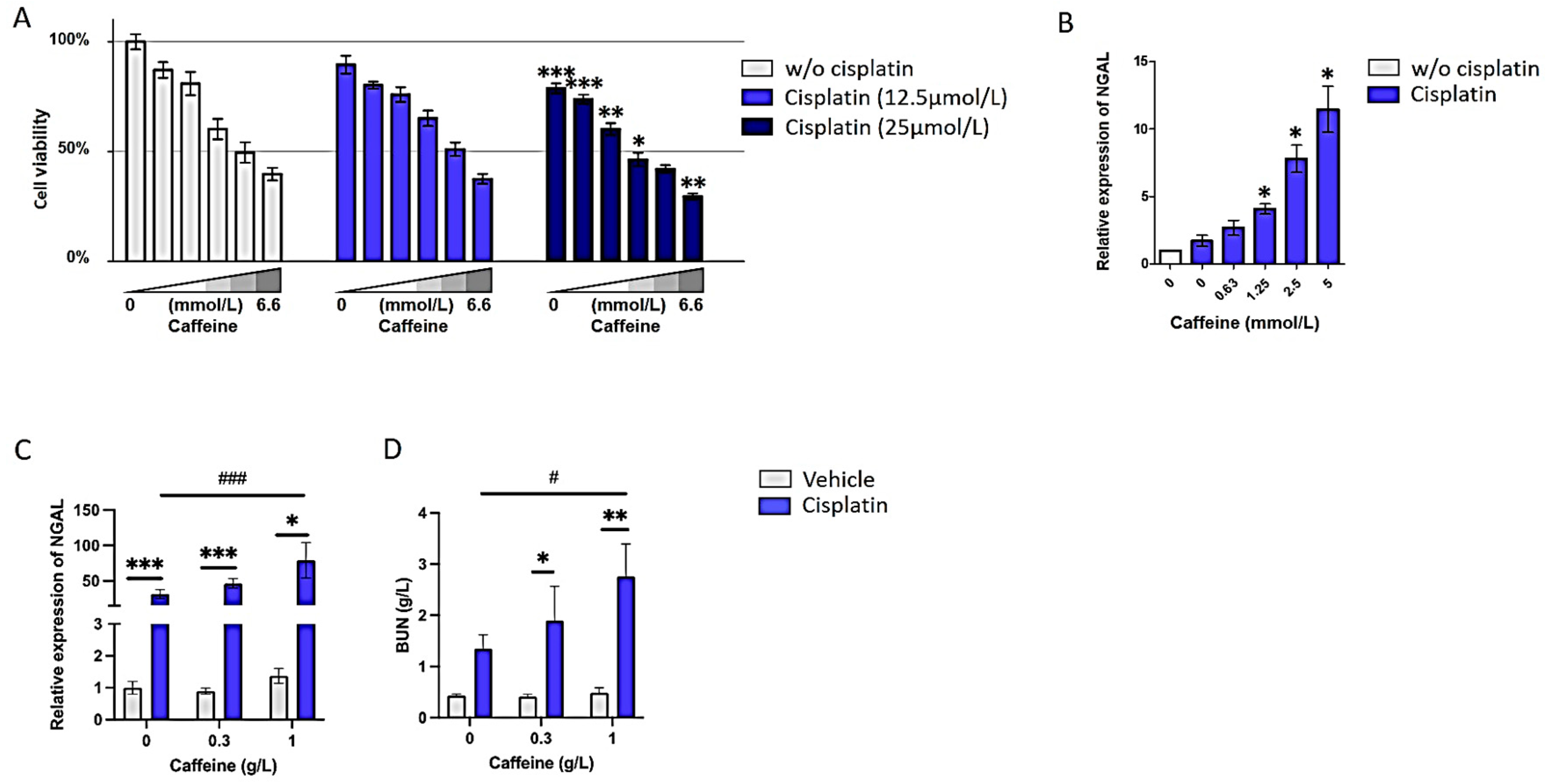
3.4. High Doses of Caffeine Promote Cisplatin-Induced Nephrotoxicity Both In Vitro and In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Ho, G.Y.; Woodward, N.; Coward, J.I.G. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol. Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef]
- dos Santos, N.A.G.; Carvalho Rodrigues, M.A.; Martins, N.M.; dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef]
- Arany, I.; Safirstein, R.L. Cisplatin nephrotoxicity. Semin. Nephrol. 2003, 23, 460–464. [Google Scholar] [CrossRef]
- de Jongh, F.E.; van Veen, R.N.; Veltman, S.J.; De Wit, R.; Van Der Burg, M.E.L.; Bent, M.V.D.; Planting, A.; Graveland, W.J.; Stoter, G.; Verweij, J. Weekly high-dose cisplatin is a feasible treatment option: Analysis on prognostic factors for toxicity in 400 patients. Br. J. Cancer 2003, 88, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Mitra, P.; Dutta, A.; Khatun, A.; Das, T.K.; Pradhan, S.; Nandi, D.K.; Roy, S. Early diagnostic biomarkers for acute kidney injury using cisplatin-induced nephrotoxicity in rat model. Curr. Res. Toxicol. 2023, 5, 100135. [Google Scholar] [CrossRef]
- Hamroun, A.; Lenain, R.; Bigna, J.J.; Speyer, E.; Bui, L.; Chamley, P.; Pottier, N.; Cauffiez, C.; Dewaeles, E.; Dhalluin, X.; et al. Prevention of Cisplatin-Induced Acute Kidney Injury: A Systematic Review and Meta-Analysis. Drugs 2019, 79, 1567–1582. [Google Scholar] [CrossRef]
- Casanova, A.G.; Hernández-Sánchez, M.T.; López-Hernández, F.J.; Martínez-Salgado, C.; Prieto, M.; Vicente-Vicente, L.; Morales, A.I. Systematic review and meta-analysis of the efficacy of clinically tested protectants of cisplatin nephrotoxicity. Eur. J. Clin. Pharmacol. 2020, 76, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hamano, H.; Ikeda, Y.; Goda, M.; Fukushima, K.; Kishi, S.; Chuma, M.; Yamashita, M.; Niimura, T.; Takechi, K.; Imanishi, M.; et al. Diphenhydramine may be a preventive medicine against cisplatin-induced kidney toxicity. Kidney Int. 2021, 99, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Launay-Vacher, V.; Rey, J.B.; Isnard-Bagnis, C.; Deray, G.; Daouphars, M. Prevention of cisplatin nephrotoxicity: State of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother. Pharmacol. 2008, 61, 903–909. [Google Scholar] [CrossRef]
- Dewaeles, E.; Carvalho, K.; Fellah, S.; Sim, J.; Boukrout, N.; Caillierez, R.; Ramakrishnan, H.; Van der Hauwaert, C.; Shankara, J.V.; Martin, N.; et al. Istradefylline protects from cisplatin-induced nephrotoxicity and peripheral neuropathy while preserving cisplatin antitumor effects. J. Clin. Investig. 2022, 132, e152924. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Wortham, K.; Costa, D.; Davis, W.; Ticho, B.; Whalley, E. Protective effect of tonapofylline (BG9928), an adenosine A1 receptor antagonist, against cisplatin-induced acute kidney injury in rats. Am. J. Nephrol. 2009, 30, 521–526. [Google Scholar] [CrossRef]
- Benoehr, P.; Krueth, P.; Bokemeyer, C.; Grenz, A.; Osswald, H.; Hartmann, J.T. Nephroprotection by theophylline in patients with cisplatin chemotherapy: A randomized, single-blinded, placebo-controlled trial. J. Am. Soc. Nephrol. 2005, 16, 452–458. [Google Scholar] [CrossRef]
- Rochat, C.; Eap, C.B.; Bochud, M.; Chatelan, A. Caffeine Consumption in Switzerland: Results from the First National Nutrition Survey MenuCH. Nutrients 2019, 12, 28. [Google Scholar] [CrossRef]
- Treur, J.L.; Taylor, A.E.; Ware, J.J.; McMahon, G.; Hottenga, J.-J.; Baselmans, B.M.L.; Willemsen, G.; Boomsma, D.I.; Munafò, M.R.; Vink, J.M. Associations between smoking and caffeine consumption in two European cohorts. Addiction 2016, 111, 1059–1068. [Google Scholar] [CrossRef]
- Bolignano, D.; Coppolino, G.; Barillà, A.; Campo, S.; Criseo, M.; Tripodo, D.; Buemi, M. Caffeine and the kidney: What evidence right now? J. Ren. Nutr. 2007, 17, 225–234. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sebastião, A.M. Caffeine and adenosine. J. Alzheimers Dis. 2010, 20 (Suppl. S1), S3–S15. [Google Scholar] [CrossRef]
- Nieber, K. The Impact of Coffee on Health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef]
- Dranoff, J.A. Coffee, adenosine, and the liver. Purinergic Signal. 2023, 20, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tej, G.N.V.C.; Nayak, P.K. Mechanistic considerations in chemotherapeutic activity of caffeine. Biomed. Pharmacother. 2018, 105, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Selvin, E.; Grams, M.E.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Coffee Consumption and Incident Kidney Disease: Results from the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. 2018, 72, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Jhee, J.H.; Nam, K.H.; An, S.Y.; Cha, M.-U.; Lee, M.; Park, S.; Kim, H.; Yun, H.-R.; Kee, Y.K.; Park, J.T.; et al. Effects of Coffee Intake on Incident Chronic Kidney Disease: A Community-Based Prospective Cohort Study. Am. J. Med. 2018, 131, 1482–1490.e3. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.J.; Pirastu, N.; Poole, R.; Fallowfield, J.A.; Hayes, P.C.; Grzeszkowiak, E.J.; Taal, M.W.; Wilson, J.F.; Parkes, J.; Roderick, P.J. Coffee Consumption and Kidney Function: A Mendelian Randomization Study. Am. J. Kidney Dis. 2020, 75, 753–761. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Simonin, C.; Duru, C.; Salleron, J.; Hincker, P.; Charles, P.; Delval, A.; Youssov, K.; Burnouf, S.; Azulay, J.-P.; Verny, C.; et al. Association between caffeine intake and age at onset in Huntington’s disease. Neurobiol. Dis. 2013, 58, 179–182. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 20 August 2023).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Aschauer, L.; Carta, G.; Vogelsang, N.; Schlatter, E.; Jennings, P. Expression of xenobiotic transporters in the human renal proximal tubule cell line RPTEC/TERT1. Toxicol. In Vitro 2015, 30 Pt A, 95–105. [Google Scholar] [CrossRef]
- Lemaire, J.; Van der Hauwaert, C.; Savary, G.; Dewaeles, E.; Perrais, M.; Guidice, J.M.L.; Pottier, N.; Glowacki, F.; Cauffiez, C. Cadmium-Induced Renal Cell Toxicity Is Associated with MicroRNA Deregulation. Int. J. Toxicol. 2020, 39, 103–114. [Google Scholar] [CrossRef]
- Vandenbussche, C.; Van der Hauwaert, C.; Dewaeles, E.; Franczak, J.; Hennino, M.-F.; Gnemmi, V.; Savary, G.; Tavernier, Q.; Nottet, N.; Paquet, A.; et al. Tacrolimus-induced nephrotoxicity in mice is associated with microRNA deregulation. Arch. Toxicol. 2018, 92, 1539–1550. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.; Zacharia, L.; Cracchiolo, J.; Shippy, D.; Tan, J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience 2006, 142, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Vomelová, I.; Vanícková, Z.; Sedo, A. Methods of RNA purification. All ways (should) lead to Rome. Folia Biol. 2009, 55, 243–251. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Benson, S.M.; Unice, K.M.; Glynn, M.E. Hourly and daily intake patterns among U.S. caffeinated beverage consumers based on the National Health and Nutrition Examination Survey (NHANES, 2013–2016). Food Chem. Toxicol. 2019, 125, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Rehm, C.D.; Ratliff, J.C.; Riedt, C.S.; Drewnowski, A. Coffee Consumption among Adults in the United States by Demographic Variables and Purchase Location: Analyses of NHANES 2011–2016 Data. Nutrients 2020, 12, 2463. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; Freedman, N.D.; Dodd, K.W.; Vogtmann, E.; Xiao, Q.; Sinha, R.; I Graubard, B. Coffee Drinking Is Widespread in the United States, but Usual Intake Varies by Key Demographic and Lifestyle Factors. J. Nutr. 2016, 146, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Di Maio, M.; Chiodini, P.; Rudd, R.M.; Okamoto, H.; Skarlos, D.V.; Früh, M.; Qian, W.; Tamura, T.; Samantas, E.; et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: The COCIS meta-analysis of individual patient data. J. Clin. Oncol. 2012, 30, 1692–1698. [Google Scholar] [CrossRef]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109 Pt 1, 585–648. [Google Scholar] [CrossRef]
- Willson, C. The clinical toxicology of caffeine: A review and case study. Toxicol. Rep. 2018, 5, 1140–1152. [Google Scholar] [CrossRef]
- Zhou, A.; Hyppönen, E. Long-term coffee consumption, caffeine metabolism genetics, and risk of cardiovascular disease: A prospective analysis of up to 347,077 individuals and 8368 cases. Am. J. Clin. Nutr. 2019, 109, 509–516. [Google Scholar] [CrossRef] [PubMed]
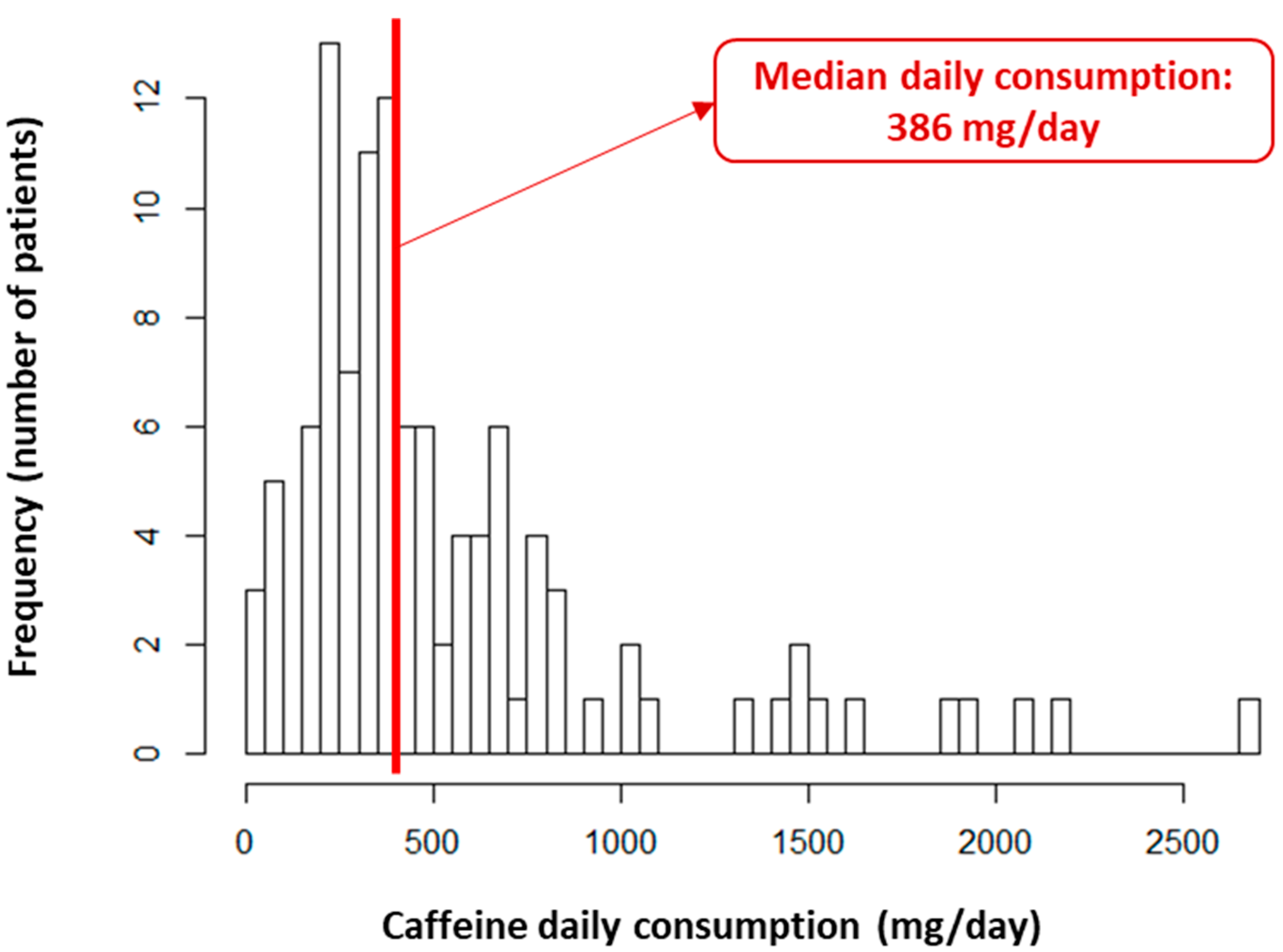
| Overall N = 108 | Daily Caffeine Consumption (</≥386 mg/Day) | |||
|---|---|---|---|---|
| Moderate N = 54 | High N = 54 | p-Value | ||
| Demographic characteristics | ||||
| Age (years) | 61.7 (±9.44) | 61.4 (±9.96) | 62.1 (±8.98) | 0.686 |
| Male sex | 71 (65.7%) | 30 (55.6%) | 41 (75.9%) | 0.043 |
| Body mass index (kg/m2) | 24.2 [21.3; 27.0] | 23.9 [20.3; 26.9] | 24.2 [21.4; 27.0] | 0.381 |
| Performance status (≥1) | 63 (58.3%) | 29 (53.7%) | 34 (63.0%) | 0.435 |
| Medical history | ||||
| Diabetes | 15 (13.9%) | 8 (14.8%) | 7 (13.0%) | 1.000 |
| Hypertension | 55 (50.9%) | 26 (48.1%) | 29 (53.7%) | 0.700 |
| Heart failure | 7 (6.48%) | 4 (7.41%) | 3 (5.56%) | 1.000 |
| Cardiovascular disease | 30 (27.8%) | 12 (22.2%) | 18 (33.3%) | 0.283 |
| Tobacco use | 0.337 | |||
| Never | 22 (20.4%) | 14 (25.9%) | 8 (14.8%) | |
| Former | 49 (45.4%) | 22 (40.7%) | 27 (50.0%) | |
| Active | 37 (34.3%) | 18 (33.3%) | 19 (35.2%) | |
| Tobacco quantity (Pack-Year) | 32.4 (±25.3) | 31.2 (±27.2) | 33.5 (±23.5) | 0.645 |
| Malignancy characteristics | ||||
| Histological type | 0.395 | |||
| Adenocarcinoma | 62 (57.4%) | 29 (53.7%) | 33 (61.1%) | |
| Squamous cell carcinoma | 7 (6.48%) | 2 (3.70%) | 5 (9.26%) | |
| Pleural mesothelioma | 13 (12.0%) | 7 (13.0%) | 6 (11.1%) | |
| Other | 26 (24.1%) | 16 (29.6%) | 10 (18.5%) | |
| Stage | 0.589 | |||
| I | 3 (2.78%) | 2 (3.70%) | 1 (1.85%) | |
| II | 14 (13.0%) | 5 (9.26%) | 9 (16.7%) | |
| III | 17 (15.7%) | 11 (20.4%) | 6 (11.1%) | |
| IV | 57 (52.8%) | 28 (51.9%) | 29 (53.7%) | |
| Unclassified | 17 (15.7%) | 8 (14.8%) | 9 (16.7%) | |
| Metastatic status | 63 (60.0%) | 33 (61.1%) | 30 (58.8%) | 0.968 |
| Baseline biological findings | ||||
| Creatinine (mg/L) | 0.77 (±0.23) | 0.75 (±0.23) | 0.79 (±0.23) | 0.272 |
| eGFR (MDRD, mL/min/1.73 m2) | 86.1 [67.2; 109] | 88.0 [73.4; 110] | 79.1 [65.8; 102] | 0.212 |
| Albumin (g/dL) | 3.82 (±0.60) | 3.83 (±0.62) | 3.82 (±0.59) | 0.979 |
| C-reactive protein (mg/L) | 15.7 [3.23; 45.4] | 18.9 [4.18; 66.1] | 13.2 [3.23; 38.0] | 0.305 |
| Prealbumin (g/L) | 0.23 (±0.08) | 0.21 (±0.09) | 0.25 (±0.08) | 0.020 |
| Hemoglobin (g/dL) | 12.9 (±1.74) | 13.0 (±1.70) | 12.8 (±1.78) | 0.539 |
| Chemotherapy treatment | ||||
| Chemotherapy regimen | 0.700 | |||
| Cisplatin | 51 (47.2%) | 24 (44.4%) | 27 (50.0%) | |
| Carboplatin | 57 (52.8% | 30 (55.6%) | 27 (50.0%) | |
| High-dose chemotherapy | 55 (50.9%) | 29 (53.7%) | 26 (48.1%) | 0.700 |
| Co-administered drug | 0.410 | |||
| Etoposide | 16 (14.8%) | 10 (18.5%) | 6 (11.1%) | |
| Navelbine | 20 (18.5%) | 7 (13.0%) | 13 (24.1%) | |
| Pemetrexed | 57 (52.8%) | 29 (53.7%) | 28 (51.9%) | |
| Taxol | 15 (13.9%) | 8 (14.8%) | 7 (13.0%) | |
| Other treatments | ||||
| Dopamine antagonist | 77 (71.3%) | 35 (64.8%) | 42 (77.8%) | 0.202 |
| Setron | 17 (15.9%) | 10 (18.5%) | 7 (13.2%) | 0.626 |
| Aprepitant | 96 (89.7%) | 48 (88.9%) | 48 (90.6%) | 1.000 |
| Diuretic | 15 (13.9%) | 5 (9.26%) | 10 (18.5%) | 0.266 |
| RAAS inhibitor | 39 (36.1%) | 17 (31.5%) | 22 (40.7%) | 0.423 |
| Beta-blocker | 22 (20.4%) | 13 (24.1%) | 9 (16.7%) | 0.474 |
| Statin | 35 (32.4%) | 16 (29.6%) | 19 (35.2%) | 0.681 |
| Biguanide | 5 (4.63%) | 3 (5.56%) | 2 (3.70%) | 1.000 |
| NSAID | 2 (1.85%) | 1 (1.85%) | 1 (1.85%) | 1.000 |
| Steroid | 104 (96.3%) | 52 (96.3%) | 52 (96.3%) | 1.000 |
| Folate | 53 (49.1%) | 23 (42.6%) | 30 (55.6%) | 0.248 |
| Proton pump inhibitors | 32 (29.9%) | 12 (22.2%) | 20 (37.7%) | 0.123 |
| AKI HR [95% CI] | p-Value | Death HR [95% CI] | p-Value | |
|---|---|---|---|---|
| Daily caffeine consumption | ||||
| moderate (<386 mg/day) | ref | - | ref | - |
| high (≥386 mg/day) | 2.19 (1.05; 4.57) | 0.04 | 1.11 (0.61; 2.03) | 0.74 |
| Chemotherapy regimen | ||||
| Cisplatin | ref | - | - | - |
| Carboplatin | 0.50 (0.23; 1.10) | 0.11 | - | - |
| High-dose chemotherapy | 3.17 (1.44; 6.99) | 0.003 | 0.37 (0.18; 0.77) | 0.008 |
| Tobacco-user (former or current exposition) | 1.04 (0.41; 2.65) | 0.58 | 1.10 (0.55; 2.18) | 0.80 |
| Performance status (≥1 vs. 0) | 2.24 (1.04; 4.82) | 0.03 | 1.24 (0.67; 2.30) | 0.49 |
| Baseline eGFR (per ml/min/1.73 m2 increase) | 0.98 (0.96; 0.99) | 0.004 | 0.99 (0.98; 1.01) | 0.35 |
| Baseline serum albumin level (per g/dl increase) | 0.98 (0.92; 1.04) | 0.18 | - | - |
| Overall N = 108 | Daily Caffeine Consumption (</≥386 mg/Day) | |||
|---|---|---|---|---|
| Moderate N = 54 | High N = 54 | p-Value * | ||
| Chemotherapy withdrawal | 0.396 | |||
| AKI | 13 (12.0%) | 7 (13.0%) | 6 (11.1%) | |
| Death | 8 (7.4%) | 4 (7.4%) | 4 (7.4%) | |
| Other | 21 (19.4%) | 7 (13.0%) | 14 (25.9%) | |
| Chemotherapy response | 0.568 | |||
| Progression | 60 (55.6%) | 28 (51.9%) | 32 (59.3%) | |
| Partial response | 31 (28.7%) | 18 (33.3%) | 13 (24.1%) | |
| Complete response | 17 (15.7%) | 8 (14.8%) | 9 (16.7%) | |
| Digestive toxicity | 22 (20.4%) | 6 (11.1%) | 16 (29.6%) | 0.032 |
| Ototoxicity | 4 (3.7%) | 3 (5.6%) | 1 (1.9%) | 0.618 |
| Neurotoxicity | 13 (12.0%) | 5 (9.3%) | 8 (14.8%) | 0.554 |
| Hematotoxicity | 26 (24.1%) | 13 (24.1%) | 13 (24.1%) | 1.000 |
| Death | 47 (43.5%) | 20 (42.6%) | 27 (57.4%) | 0.487 |
| Cause of death | 0.470 | |||
| Progression | 36 (76.6%) | 15 (75.0%) | 21 (77.8%) | |
| Sepsis | 6 (12.8%) | 2 (10.0%) | 4 (14.8%) | |
| Other/unknown | 5 (10.6%) | 3 (15.0%) | 2 (7.4%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamroun, A.; Decaestecker, A.; Larrue, R.; Fellah, S.; Blum, D.; Van der Hauwaert, C.; Scherpereel, A.; Cortot, A.; Lenain, R.; Maanaoui, M.; et al. Daily Caffeine Consumption May Increase the Risk of Acute Kidney Injury Related to Platinum-Salt Chemotherapy in Thoracic Cancer Patients: A Translational Study. Nutrients 2024, 16, 889. https://doi.org/10.3390/nu16060889
Hamroun A, Decaestecker A, Larrue R, Fellah S, Blum D, Van der Hauwaert C, Scherpereel A, Cortot A, Lenain R, Maanaoui M, et al. Daily Caffeine Consumption May Increase the Risk of Acute Kidney Injury Related to Platinum-Salt Chemotherapy in Thoracic Cancer Patients: A Translational Study. Nutrients. 2024; 16(6):889. https://doi.org/10.3390/nu16060889
Chicago/Turabian StyleHamroun, Aghiles, Antoine Decaestecker, Romain Larrue, Sandy Fellah, David Blum, Cynthia Van der Hauwaert, Arnaud Scherpereel, Alexis Cortot, Rémi Lenain, Mehdi Maanaoui, and et al. 2024. "Daily Caffeine Consumption May Increase the Risk of Acute Kidney Injury Related to Platinum-Salt Chemotherapy in Thoracic Cancer Patients: A Translational Study" Nutrients 16, no. 6: 889. https://doi.org/10.3390/nu16060889
APA StyleHamroun, A., Decaestecker, A., Larrue, R., Fellah, S., Blum, D., Van der Hauwaert, C., Scherpereel, A., Cortot, A., Lenain, R., Maanaoui, M., Pottier, N., Cauffiez, C., & Glowacki, F. (2024). Daily Caffeine Consumption May Increase the Risk of Acute Kidney Injury Related to Platinum-Salt Chemotherapy in Thoracic Cancer Patients: A Translational Study. Nutrients, 16(6), 889. https://doi.org/10.3390/nu16060889