Betanin Attenuates Epigenetic Mechanisms and UV-Induced DNA Fragmentation in HaCaT Cells: Implications for Skin Cancer Chemoprevention
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. UV Radiation and Betanin Treatment
2.3. Comet Assay Evaluation
2.4. Cell Viability/XTT Assay
2.5. RNA Extraction and Quantitative Real-Time PCR Analysis
2.6. Statistical Methods
3. Results
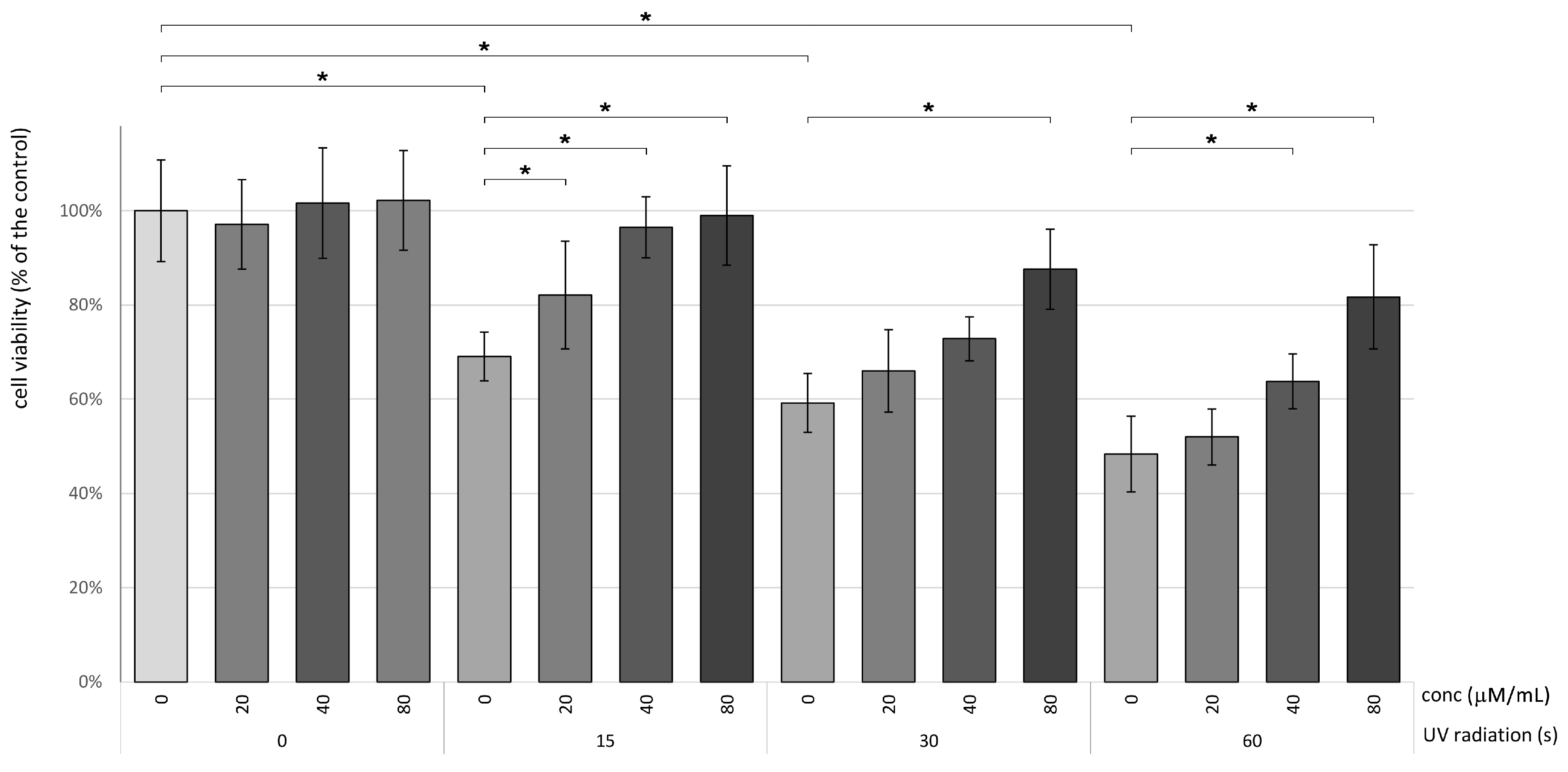
3.1. Betanin Enhances the Viability of HaCaT Subsequent to Exposure to UVB Radiation
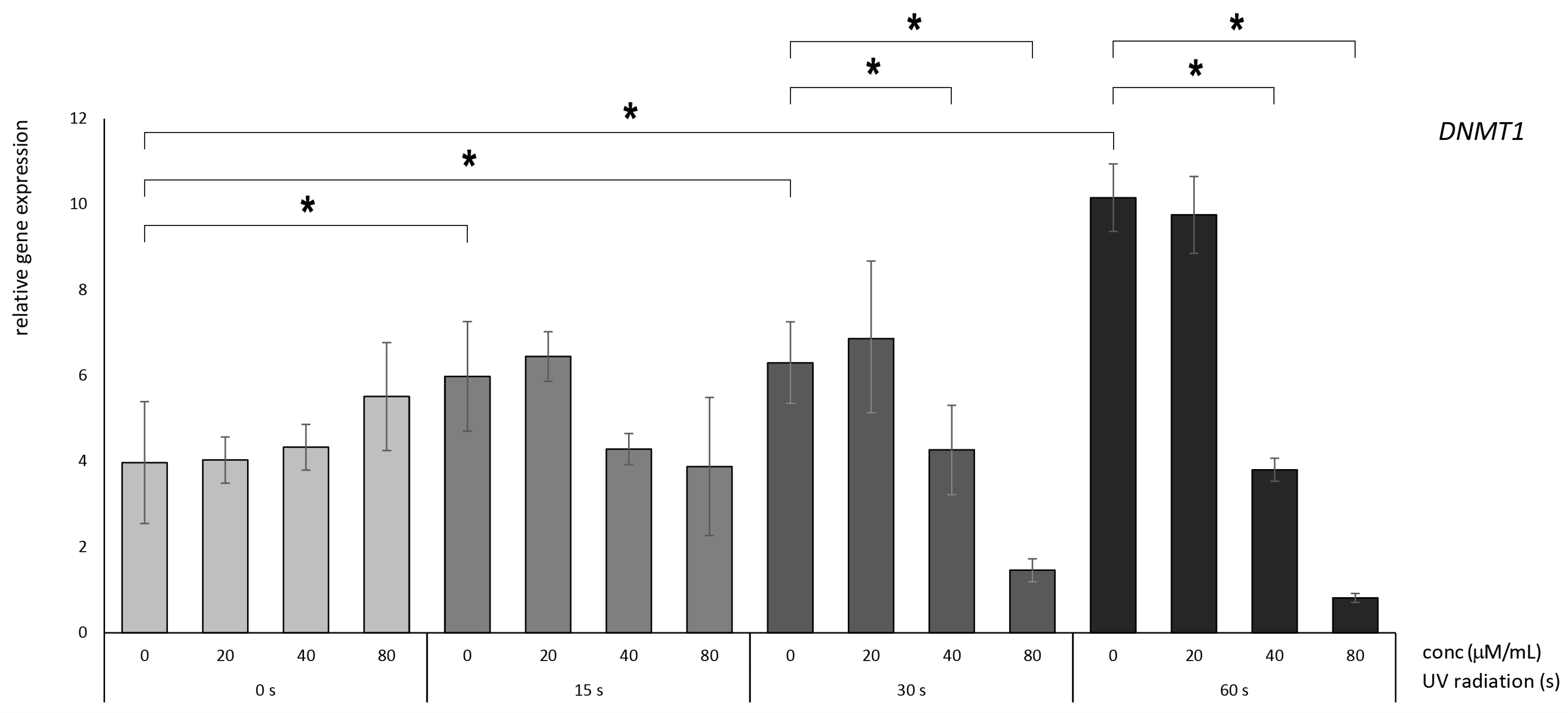
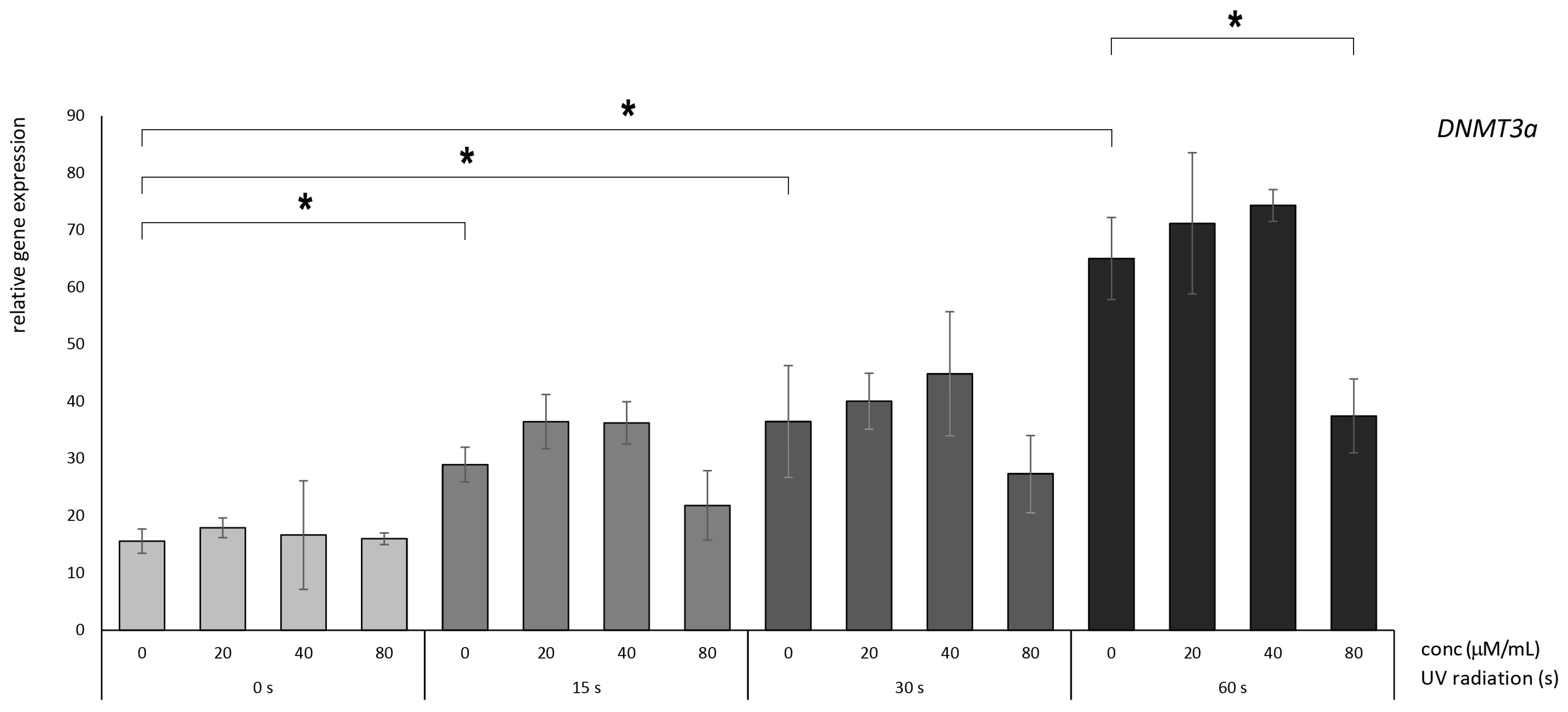
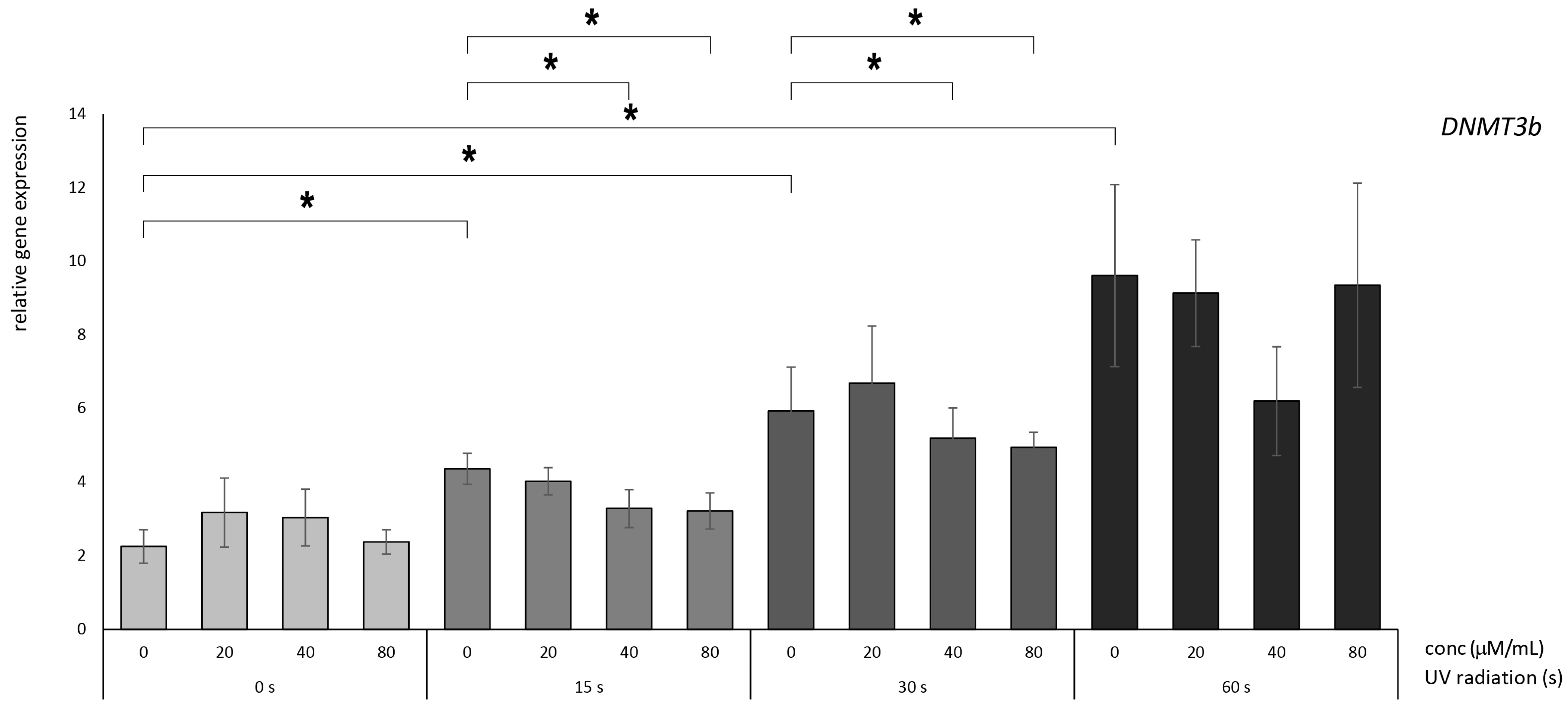
3.2. Betanin Suppresses the Impact of UVB Radiation on DNMT Gene Expression on HaCaT
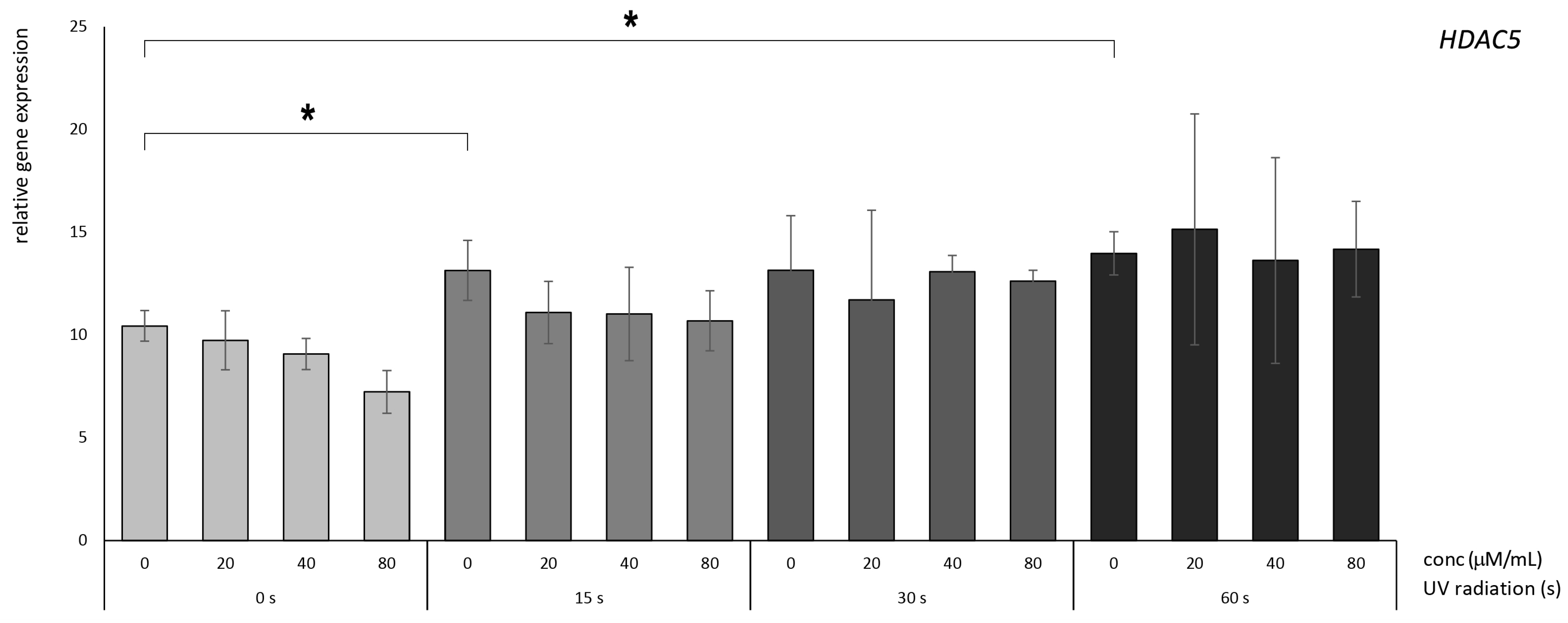
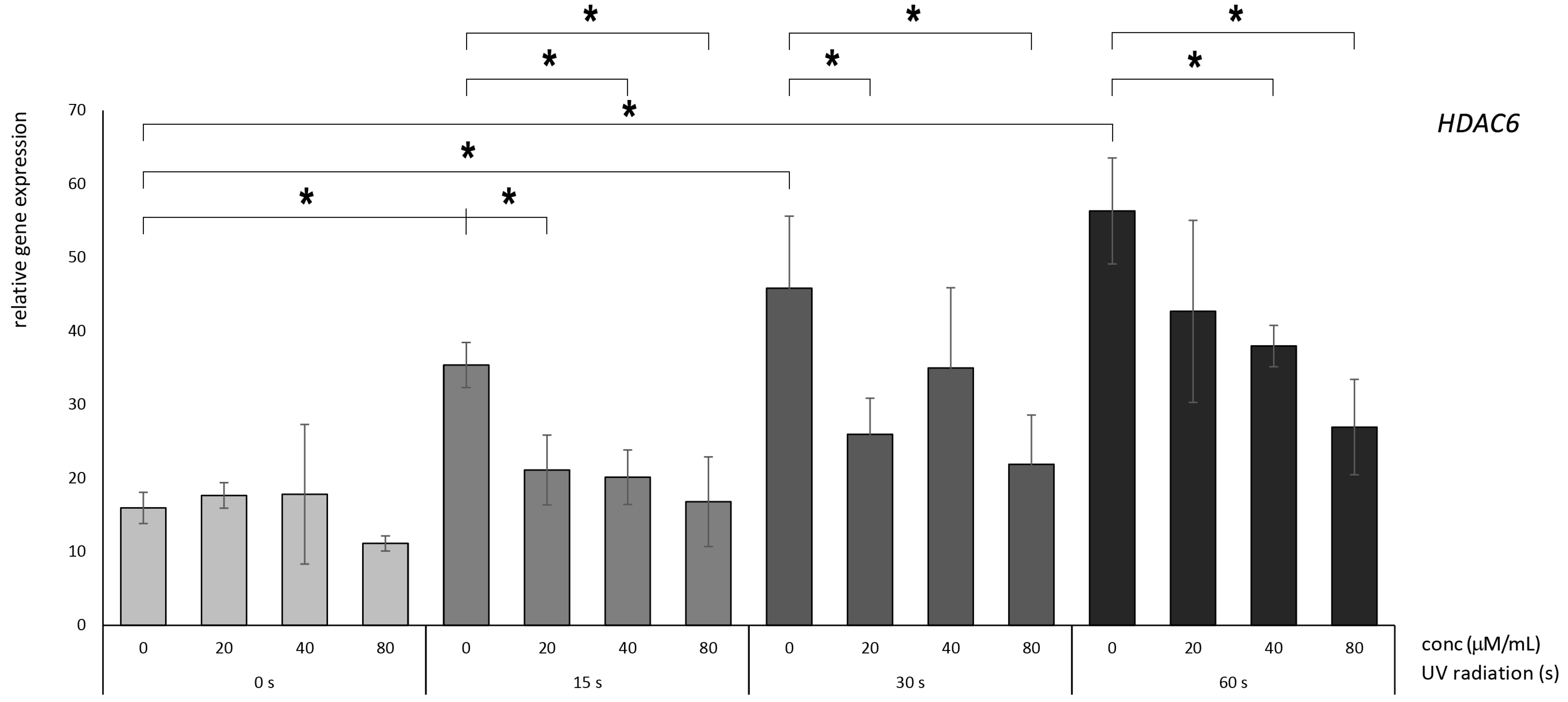
3.3. Betanin Exhibits the Potential to Mitigate the UVB-Radiation-Induced Overexpression of HDAC Genes in HaCaT
3.4. The Protective Impact of Betanin against DNA Fragmentation
4. Discussion
4.1. Betanin Modulation of the DNMT1, DNMT3A, and DNMT3B Genes
4.2. Molecular Effects of Betanin on the HDAC5 and HDAC6 Genes
4.3. Betanin Ameliorates UV-Related DNA Fragmentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet Radiation and Skin Cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Battie, C.; Verschoore, M. Cutaneous Solar Ultraviolet Exposure and Clinical Aspects of Photodamage. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 9. [Google Scholar] [CrossRef]
- Marais, T.L.D.; Kluz, T.; Xu, D.; Zhang, X.; Gesumaria, L.; Matsui, M.S.; Costa, M.; Sun, H. Transcription Factors and Stress Response Gene Alterations in Human Keratinocytes Following Solar Simulated Ultra Violet Radiation. Sci. Rep. 2017, 7, 13622. [Google Scholar] [CrossRef]
- Kim, S.J.; Na, H.W.; Jang, Y.; Shin, D.Y.; Choi, H.; Kim, H.J.; Seo, Y.R. Correction to: Network Analysis to Understand Side Effects of UVB on Skin through Transcriptomic Approach. Mol. Cell Toxicol. 2022, 18, 647. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Grootveld, M.; Bahorun, T. Free Radicals in Biology and Medicine: From Inflammation to Biotechnology. BioFactors 2006, 27, 1–3. [Google Scholar] [CrossRef]
- Li, Y.; Yin, R.; Liang, M.; Chen, C. Nrf2 Suppresses Erastin-Induced Ferroptosis through Activating System Xc(-) in Ovarian Cancer. Mol. Cell Toxicol. 2022, 20, 85–95. [Google Scholar] [CrossRef]
- Begum, R.; Kim, C.S.; Fadriquela, A.; Bajgai, J.; Jing, X.; Kim, D.H.; Kim, S.K.; Lee, K.J. Molecular Hydrogen Protects against Oxidative Stress-Induced RAW 264.7 Macrophage Cells through the Activation of Nrf2 and Inhibition of MAPK Signaling Pathway. Mol. Cell Toxicol. 2020, 16, 103–118. [Google Scholar] [CrossRef]
- McArdle, F.; Rhodes, L.E.; Parslew, R.; Jack, C.I.A.; Friedmann, P.S.; Jackson, M.J. UVR-Induced Oxidative Stress in Human Skin in Vivo: Effects of Oral Vitamin C Supplementation. Free Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, K.; Jia, X.; Fu, C.; Yu, H.; Wang, Y. Antioxidant Peptides, the Guardian of Life from Oxidative Stress. Med. Res. Rev. 2023, 44, 275–364. [Google Scholar] [CrossRef] [PubMed]
- Baião, D.D.S.; da Silva, D.V.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, Bioactive and Physicochemical Characteristics of Different Beetroot Formulations. In Food Additives; IntechOpen: London, UK, 2017; Chapter 6. [Google Scholar]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant Activity and Phenolic Content of Betalain Extracts from Intact Plants and Hairy Root Cultures of the Red Beetroot Beta vulgaris Cv. Detroit Dark Red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Comparative Effect of Red Yeast Rice (Monascus purpureus), Red Beet Root (Beta vulgaris) and Betanin (E-162) on Colour and Consumer Acceptability of Fresh Pork. J. Sci. Food Agric. 2006, 86, 500–508. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, M.A.; Rao, G.S.; Arai, T.; Iida, A.; Tokuda, H. Cytotoxic Effect of the Red Beetroot (Beta vulgaris L.) Extract Compared to Doxorubicin (Adriamycin) in the Human Prostate (PC-3) and Breast (MCF-7) Cancer Cell Lines. Anticancer. Agents Med. Chem. 2011, 11, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Stintzing, F.C.; Carle, R.; Bitsch, I.; Quaas, D.; Straß, G.; Bitsch, R.; Netzel, M. Urinary Pharmacokinetics of Betalains Following Consumption of Red Beet Juice in Healthy Humans. Pharmacol. Res. 2005, 52, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Carle, R. Functional Properties of Anthocyanins and Betalains in Plants, Food, and in Human Nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, J.; Wang, Y.; Lubet, R.; You, M. Beetroot Red (Betanin) Inhibits Vinyl Carbamate-and Benzo (a) Pyrene-induced Lung Tumorigenesis through Apoptosis. Mol. Carcinog. 2013, 52, 686–691. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, M.A.; Sridhar, R.; Okuda, Y.; Tsuruta, A.; Ichiishi, E.; Mukainake, T.; Takasaki, M.; Konoshima, T.; Nishino, H.; et al. Chemoprevention of DMBA-Induced UV-B Promoted, NOR-1-Induced TPA Promoted Skin Carcinogenesis, and DEN-Induced Phenobarbital Promoted Liver. Pharmacol. Res. 2003, 47, 141–148. [Google Scholar] [CrossRef]
- Kujawska, M.; Ignatowicz, E.; Murias, M.; Ewertowska, M.; Mikołajczyk, K.; Jodynis-Liebert, J. Protective Effect of Red Beetroot against Carbon Tetrachloride- and N-Nitrosodiethylamine-Induced Oxidative Stress in Rats. J. Agric. Food Chem. 2009, 57, 2570–2575. [Google Scholar] [CrossRef]
- Saber, A.; Abedimanesh, N.; Somi, M.H.; Khosroushahi, A.Y.; Moradi, S. Anticancer Properties of Red Beetroot Hydro-Alcoholic Extract and Its Main Constituent; Betanin on Colorectal Cancer Cell Lines. BMC Complement. Med. Ther. 2023, 23, 246. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free Radical Scavenging and Antioxidant Activity of Betanin: Electron Spin Resonance Spectroscopy Studies and Studies in Cultured Cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the Nature-Inspired Pigments, in Health and Diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ma, D.; Zhang, M.; Yang, X.; Tan, D. Natural Antioxidant Betanin Protects Rats from Paraquat-Induced Acute Lung Injury Interstitial Pneumonia. BioMed Res. Int. 2015, 2015, 608174. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, Z.; Yang, S.; Wang, J.; Yang, X.; Tan, D. Betanin Attenuates Paraquat-Induced Liver Toxicity through a Mitochondrial Pathway. Food Chem. Toxicol. 2014, 70, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Bai, B.; Yang, X.; Han, J. Betanin Attenuates Oxidative Stress and Inflammatory Reaction in Kidney of Paraquat-Treated Rat. Food Chem. Toxicol. 2015, 78, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Szaefer, H.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Beetroot Juice Protects against N-Nitrosodiethylamine-Induced Liver Injury in Rats. Food Chem. Toxicol. 2012, 50, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Ladeira, C.; Giovannelli, L.; Boutet-Robinet, E.; Bonassi, S.; Neri, M.; Gajski, G.; Duthie, S.; Del Bo’, C.; Riso, P.; et al. Application of the Comet Assay in Human Biomonitoring: An HCOMET Perspective. Mutat. Res. Rev. Mutat. Res. 2020, 783, 108288. [Google Scholar] [CrossRef] [PubMed]
- Mobarakeh, K.M.; Etebari, M.; Zolfaghari, B.; Jafarian-Dehkordi, A. Evaluation of Genoprotective Effects of Hydroalcoholic and Polyphenolic Extracts of Quince by Comet Assay. J. Rep. Pharm. Sci. 2015, 4, 141–147. [Google Scholar]
- Nasiri, M.; Etebari, M.; Jafarian-Dehkordi, A.; Moradi, S. Lovastatin Prevents Bleomycin-Induced DNA Damage to HepG2 Cells. Res. Pharm. Sci. 2016, 11, 470–475. [Google Scholar] [CrossRef]
- Taylor, R.W.; Turnbull, D.M. Mitochondrial DNA Mutations in Human Disease. Nat. Rev. Genet. 2005, 6, 389–402. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in Cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef]
- Poh, W.J.; Wee, C.P.P.; Gao, Z. DNA Methyltransferase Activity Assays: Advances and Challenges. Theranostics 2016, 6, 369–391. [Google Scholar] [CrossRef]
- De Ruijter, A.J.M.; Van Gennip, A.H.; Caron, H.N.; Kemp, S.; Van Kuilenburg, A.B.P. Histone Deacetylases (HDACs): Characterization of the Classical HDAC Family. Biochem. J. 2003, 370, 737. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.S. A Short Guide to Histone Deacetylases Including Recent Progress on Class II Enzymes. Exp. Mol. Med. 2020, 52, 204–212. [Google Scholar] [CrossRef]
- Glozak, M.A.; Seto, E. Histone Deacetylases and Cancer. Oncogene 2007, 26, 5420–5432. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Gerencsér, G.; Szabó, I.; Szendi, K.; Hanzel, A.; Raposa, B.; Gyöngyi, Z.; Varga, C. Effects of Medicinal Waters on the UV-Sensitivity of Human Keratinocytes—A Comparative Pilot Study. Int. J. Biometeorol. 2019, 63, 1417–1423. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural Plant Pigments with Potential Application in Functional Foods. LWT 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.; Szaefer, H.; Baer-Dubowska, W. Betanin, a Beetroot Component, Induces Nuclear Factor Erythroid-2-Related Factor 2-Mediated Expression of Detoxifying/Antioxidant Enzymes in Human Liver Cell Lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J. DNA Methyltransferases and Their Roles in Tumorigenesis. Biomark. Res. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Li, E.; Zhang, Y. DNA Methylation in Mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef]
- Smith, Z.D.; Meissner, A. DNA Methylation: Roles in Mammalian Development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef]
- de Oliveira, N.F.P.; de Souza, B.F.; de Castro Coêlho, M. Uv Radiation and Its Relation to Dna Methylation in Epidermal Cells: A Review. Epigenomes 2020, 4, 23. [Google Scholar] [CrossRef]
- Greene, R.; Pisano, M.M. Palate morphogenesis: Current understanding and future direction. Birth Defects Res. C Embryo Today 2010, 90, 133–154. [Google Scholar] [CrossRef]
- El Omari, N.; Bakrim, S.; Bakha, M.; Lorenzo, J.M.; Rebezov, M.; Shariati, M.A.; Aboulaghras, S.; Balahbib, A.; Khayrullin, M.; Bouyahya, A. Natural Bioactive Compounds Targeting Epigenetic Pathways in Cancer: A Review on Alkaloids, Terpenoids, Quinones, and Isothiocyanates. Nutrients 2021, 13, 3714. [Google Scholar] [CrossRef]
- Zhao, S.L.; Zhu, S.T.; Hao, X.; Li, P.; Zhang, S.T. Effects of DNA Methyltransferase 1 Inhibition on Esophageal Squamous Cell Carcinoma. Dis. Esophagus 2011, 24, 601–610. [Google Scholar] [CrossRef]
- Ibrahim, A.E.K.; Arends, M.J.; Silva, A.L.; Wyllie, A.H.; Greger, L.; Ito, Y.; Vowler, S.L.; Huang, T.H.M.; Tavaré, S.; Murrell, A.; et al. Sequential DNA Methylation Changes Are Associated with DNMT3B Overexpression in Colorectal Neoplastic Progression. Gut 2011, 60, 499–508. [Google Scholar] [CrossRef]
- Nosho, K.; Shima, K.; Irahara, N.; Kure, S.; Baba, Y.; Kirkner, G.J.; Chen, L.; Gokhale, S.; Hazra, A.; Spiegelman, D.; et al. DNMT3B Expression Might Contribute to CpG Island Methylator Phenotype in Colorectal Cancer. Clin. Cancer Res. 2009, 15, 3663–3671. [Google Scholar] [CrossRef]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Verza, F.A.; Das, U.; Fachin, A.L.; Dimmock, J.R.; Marins, M. Roles of Histone Deacetylases and Inhibitors in Anticancer Therapy. Cancers 2020, 12, 1664. [Google Scholar] [CrossRef]
- Li, Y.; Shin, D.; Kwon, S.H. Histone Deacetylase 6 Plays a Role as a Distinct Regulator of Diverse Cellular Processes. FEBS J. 2013, 280, 775–793. [Google Scholar] [CrossRef]
- McGee, S.L.; Van Denderen, B.J.W.; Howlett, K.F.; Mollica, J.; Schertzer, J.D.; Kemp, B.E.; Hargreaves, M. AMP-Activated Protein Kinase Regulates GLUT4 Transcription by Phosphorylating Histone Deacetylase 5. Diabetes 2008, 57, 860–867. [Google Scholar] [CrossRef]
- Nowacki, L.; Vigneron, P.; Rotellini, L.; Cazzola, H.; Merlier, F.; Prost, E.; Ralanairina, R.; Gadonna, J.P.; Rossi, C.; Vayssade, M. Betanin-Enriched Red Beetroot (Beta vulgaris L.) Extract Induces Apoptosis and Autophagic Cell Death in MCF-7 Cells. Phytother. Res. 2015, 29, 1964–1973. [Google Scholar] [CrossRef]
- Sreekanth, D.; Arunasree, M.K.; Roy, K.R.; Chandramohan Reddy, T.; Reddy, G.V.; Reddanna, P. Betanin a Betacyanin Pigment Purified from Fruits of Opuntia Ficus-Indica Induces Apoptosis in Human Chronic Myeloid Leukemia Cell Line-K562. Phytomedicine 2007, 14, 739–746. [Google Scholar] [CrossRef]
- Ninfali, P.; Angelino, D. Nutritional and Functional Potential of Beta vulgaris Cicla and Rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef]
- Tan, M.L.; Hamid, S.B.S. Beetroot as a Potential Functional Food for Cancer Chemoprevention, a Narrative Review. J. Cancer Prev. 2021, 26, 1–17. [Google Scholar] [CrossRef]
- Clement, Y.N.; Mahase, V.; Jagroop, A.; Kissoon, K.; Maharaj, A.; Mathura, P.; Quan, C.M.; Ramadhin, D.; Mohammed, C. Herbal Remedies and Functional Foods Used by Cancer Patients Attending Specialty Oncology Clinics in Trinidad. BMC Complement. Altern. Med. 2016, 16, 399. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.S.; Chang, H.; Hamm, F.; Elsner, P.; Thiele, J.J.; Jens Thiele, C. Role of Oxidative Stress and the Antioxidant Network in Cutaneous Carcinogenesis. Int. J. Dermatol. 2004, 43, 326–335. [Google Scholar] [CrossRef]
- Wu, Y.; Antony, S.; Meitzler, J.L.; Doroshow, J.H. Molecular Mechanisms Underlying Chronic Inflammation-Associated Cancers. Cancer Lett. 2014, 345, 164–173. [Google Scholar] [CrossRef]
- Sakihama, Y.; Maeda, M.; Hashimoto, M.; Tahara, S.; Hashidoko, Y. Beetroot Betalain Inhibits Peroxynitrite-Mediated Tyrosine Nitration and DNA Strand Cleavage. Free Radic. Res. 2012, 46, 93–99. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A New Class of Dietary Cationized Antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
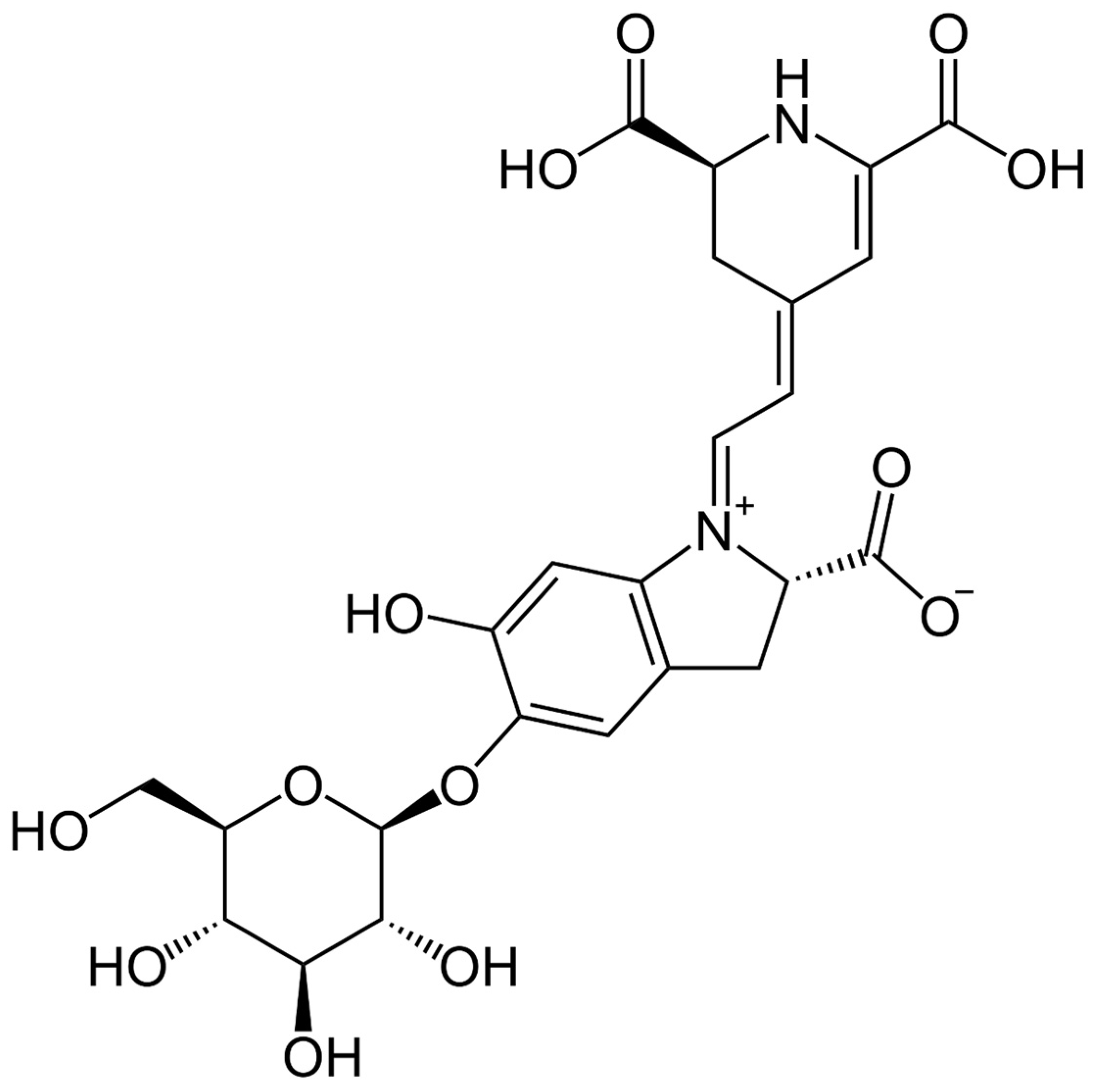

| Name of the Groups | UV Radiation (s) | Food Colorant (µM) | |
|---|---|---|---|
| 1 | neg. control | 0 | 0 |
| 2 | treated #1 | 0 | 20 |
| 3 | treated #2 | 0 | 40 |
| 4 | treated #3 | 0 | 80 |
| 5 | treated #4 | 15 | 20 |
| 6 | treated #5 | 15 | 40 |
| 7 | treated #6 | 15 | 80 |
| 8 | treated #7 | 30 | 20 |
| 9 | treated #8 | 30 | 40 |
| 10 | treated #9 | 30 | 80 |
| 11 | treated #10 | 60 | 20 |
| 12 | treated #11 | 60 | 40 |
| 13 | treated #12 | 60 | 80 |
| 14 | pos. Control #1 | 15 | 0 |
| 15 | pos. Control #2 | 30 | 0 |
| 16 | pos. Control #3 | 60 | 0 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| DNMT1 | 5′-AGGTGGAGAGTTATGACGAGGC-3′ | 5′-GGTAGAATGCCTGATGGTCTGC-3′ |
| DNMT3A | 5′-GCA GCG TCA CAC AGA AG-3′ | 5′-GGC GGT AGA ACT CAA AGA AG-3′ |
| DNMT3B | 5′-GAA CGA CGT GAG GAA CAT C-3′ | 5′-GGC CTG TAC CCT CAT ACA-3′ |
| HDAC5 | 5′-CAG CAC CAT CGG TTC ATA G-3′ | 5′-CAG GGA GAG AGT GGG TAA G-3′ |
| HDAC6 | 5′-GCC CAG GCT TCA GTT TC-3′ | 5′-CCT CGC TCT CCT CTA CAT T-3′ |
| HPRT1 | 5′-TGC TTC TCC TCA GCT TCA-3′ | 5′-CTC AGG AGG AGG AAG CC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zand, A.; Enkhbilguun, S.; Macharia, J.M.; Varajti, K.; Szabó, I.; Gerencsér, G.; Tisza, B.B.; Raposa, B.L.; Gyöngyi, Z.; Varjas, T. Betanin Attenuates Epigenetic Mechanisms and UV-Induced DNA Fragmentation in HaCaT Cells: Implications for Skin Cancer Chemoprevention. Nutrients 2024, 16, 860. https://doi.org/10.3390/nu16060860
Zand A, Enkhbilguun S, Macharia JM, Varajti K, Szabó I, Gerencsér G, Tisza BB, Raposa BL, Gyöngyi Z, Varjas T. Betanin Attenuates Epigenetic Mechanisms and UV-Induced DNA Fragmentation in HaCaT Cells: Implications for Skin Cancer Chemoprevention. Nutrients. 2024; 16(6):860. https://doi.org/10.3390/nu16060860
Chicago/Turabian StyleZand, Afshin, Sodbuyan Enkhbilguun, John M. Macharia, Krisztina Varajti, Istvan Szabó, Gellért Gerencsér, Boglárka Bernadett Tisza, Bence L. Raposa, Zoltán Gyöngyi, and Timea Varjas. 2024. "Betanin Attenuates Epigenetic Mechanisms and UV-Induced DNA Fragmentation in HaCaT Cells: Implications for Skin Cancer Chemoprevention" Nutrients 16, no. 6: 860. https://doi.org/10.3390/nu16060860
APA StyleZand, A., Enkhbilguun, S., Macharia, J. M., Varajti, K., Szabó, I., Gerencsér, G., Tisza, B. B., Raposa, B. L., Gyöngyi, Z., & Varjas, T. (2024). Betanin Attenuates Epigenetic Mechanisms and UV-Induced DNA Fragmentation in HaCaT Cells: Implications for Skin Cancer Chemoprevention. Nutrients, 16(6), 860. https://doi.org/10.3390/nu16060860









