The Effects of Olive Oil Consumption on Biochemical Parameters and Body Mass Index of People with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
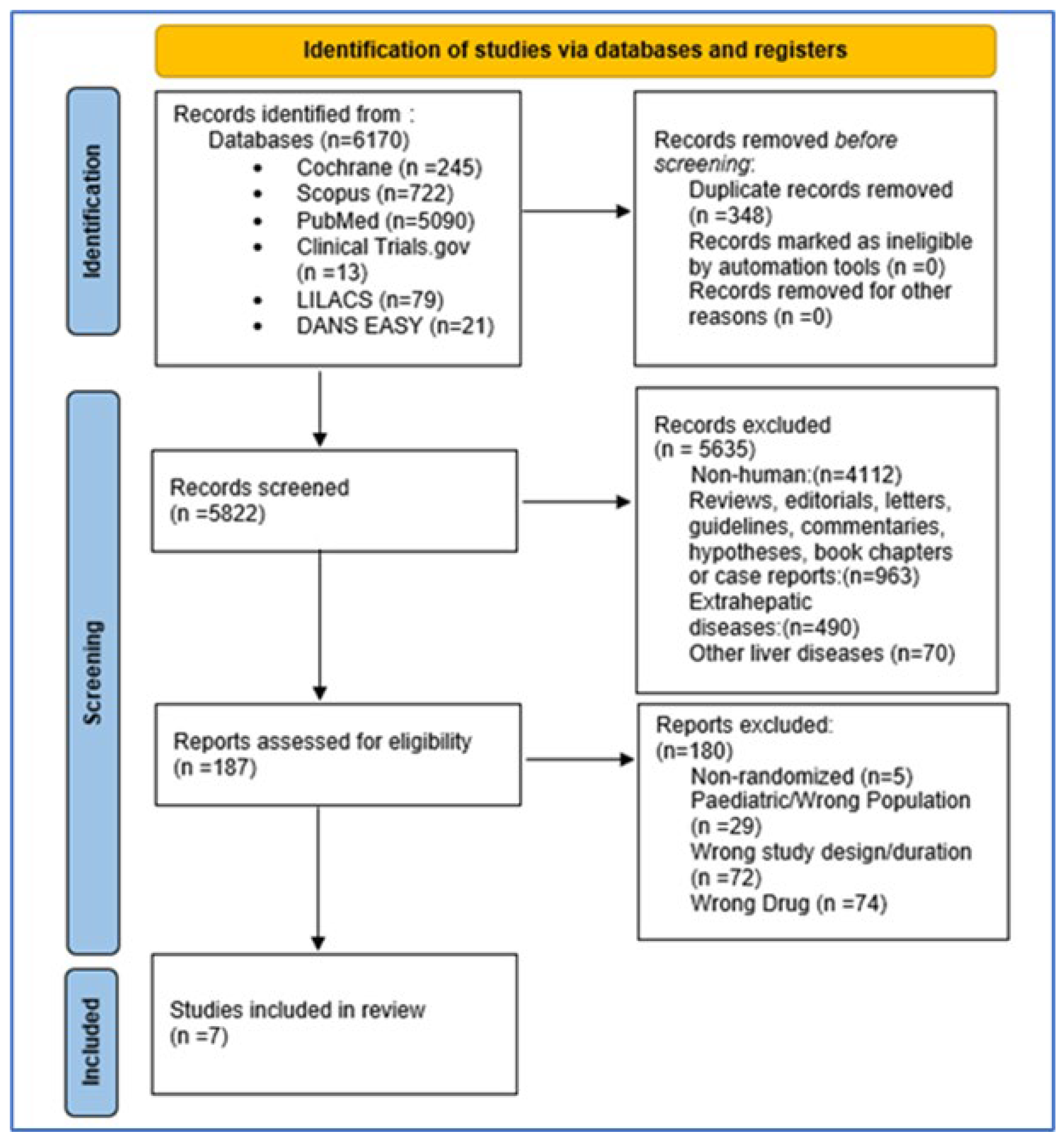
2.2. Search Strategy and Study Selection
2.3. Article Selection and Data Extraction
2.4. Assessment of Quality
2.5. Statistical Analysis, Meta-Analysis, and Meta-Regression
3. Results
3.1. Study Characteristics
3.2. Design of Eligible Studies and Outcome Measures
3.3. Intervention and Comparison Arms
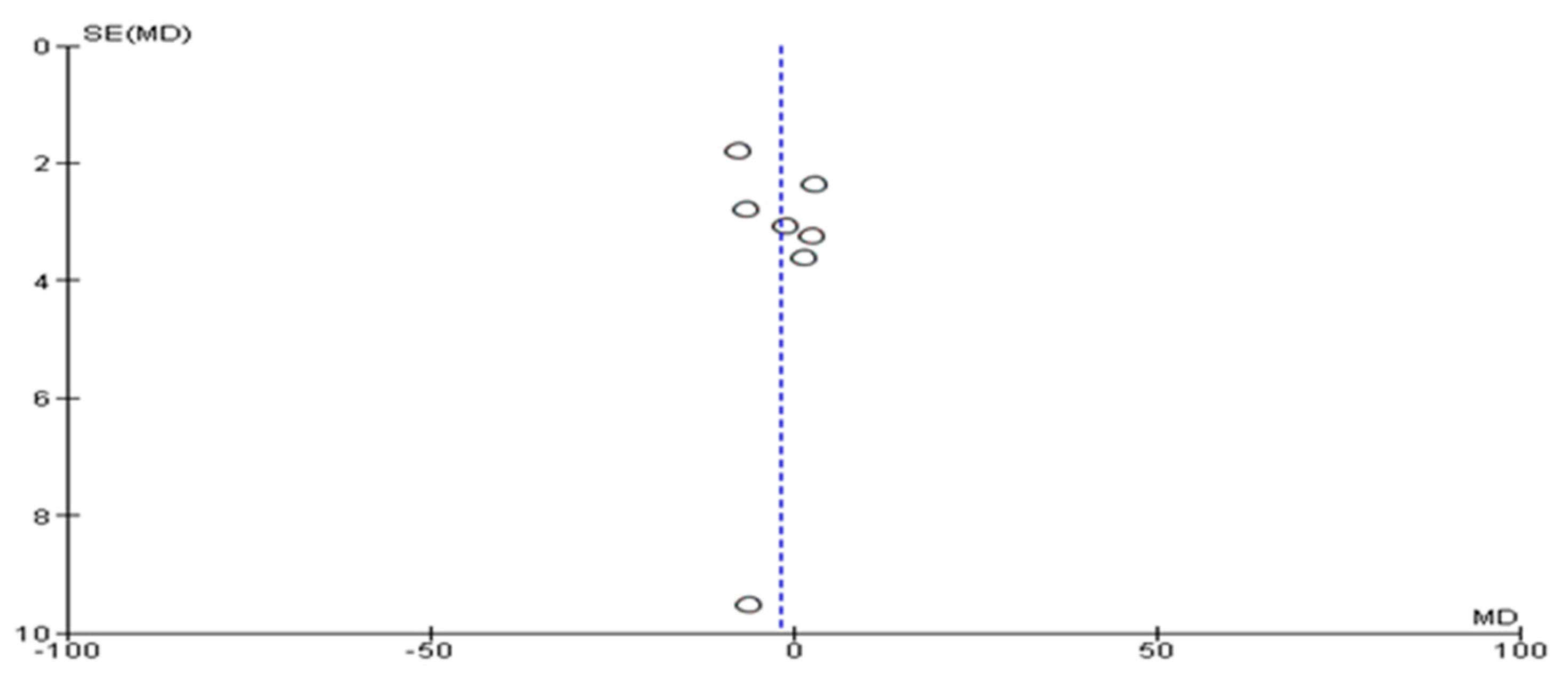
3.4. Assessment of Risk of Bias and Publication Bias
3.5. Effects of Olive Oil on Primary and Secondary Outcomes
3.6. Rates of Dropout and Attrition
3.7. Meta-Analysis and Meta-Regression of the Effects of Olive Oil on NAFLD
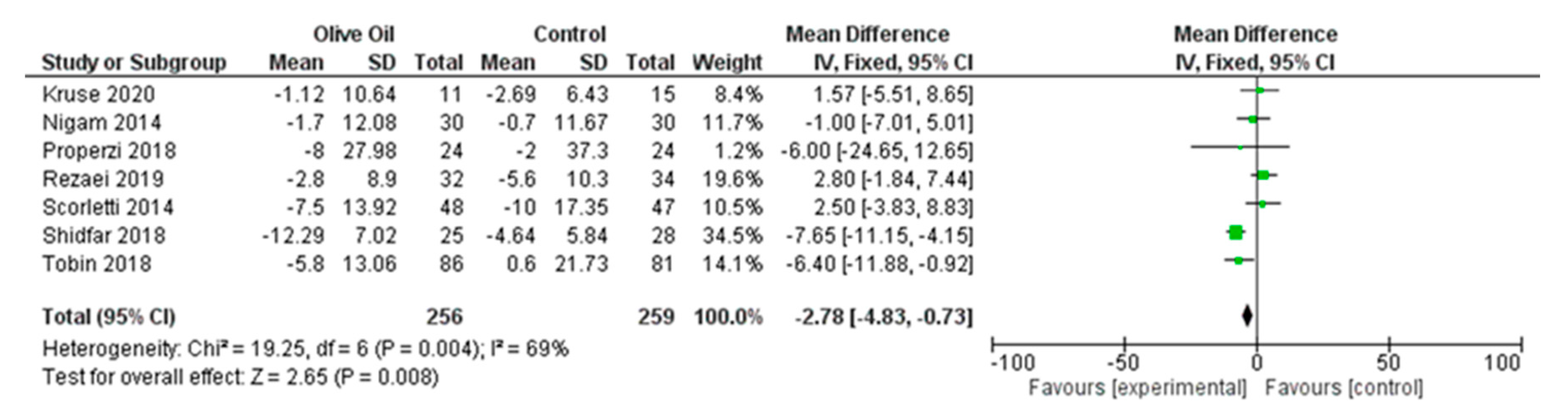
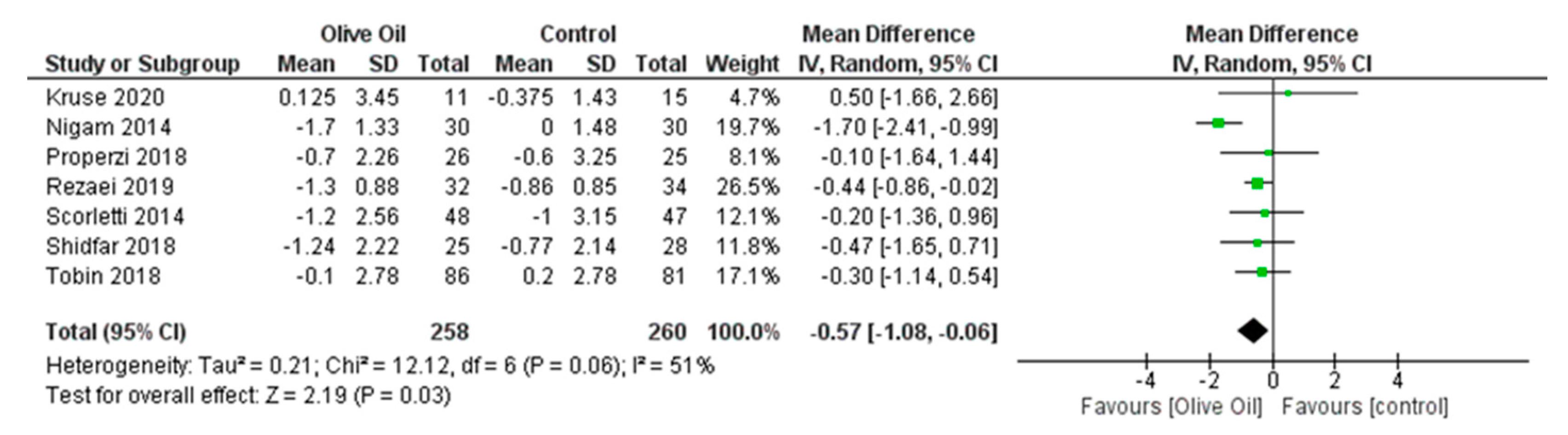
3.7.1. Primary Outcomes
Impact of Olive Oil on ALT
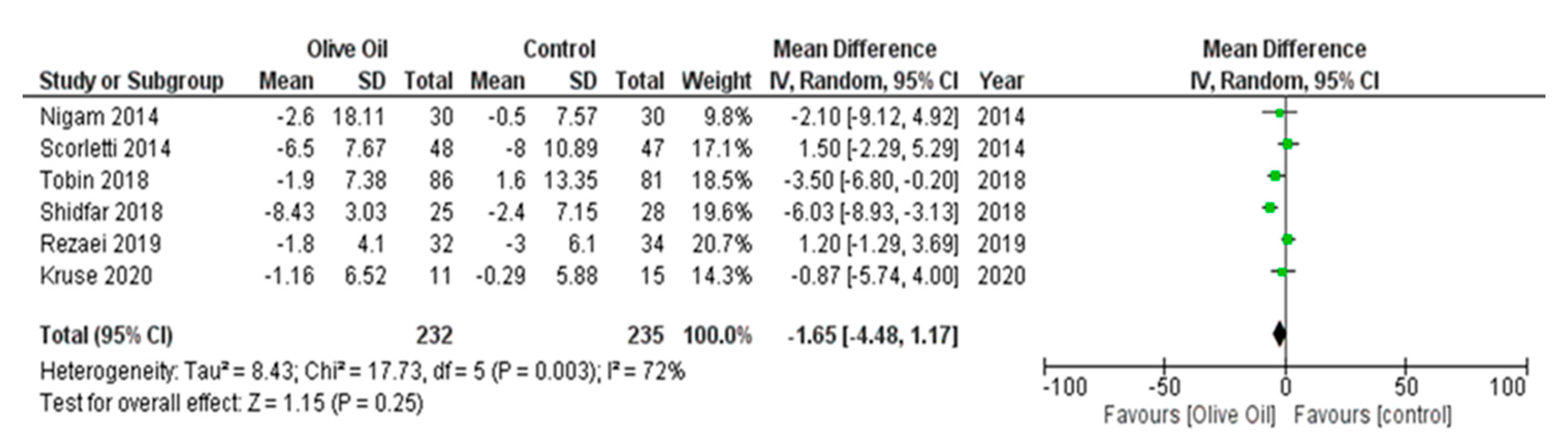
3.7.2. Secondary Outcomes
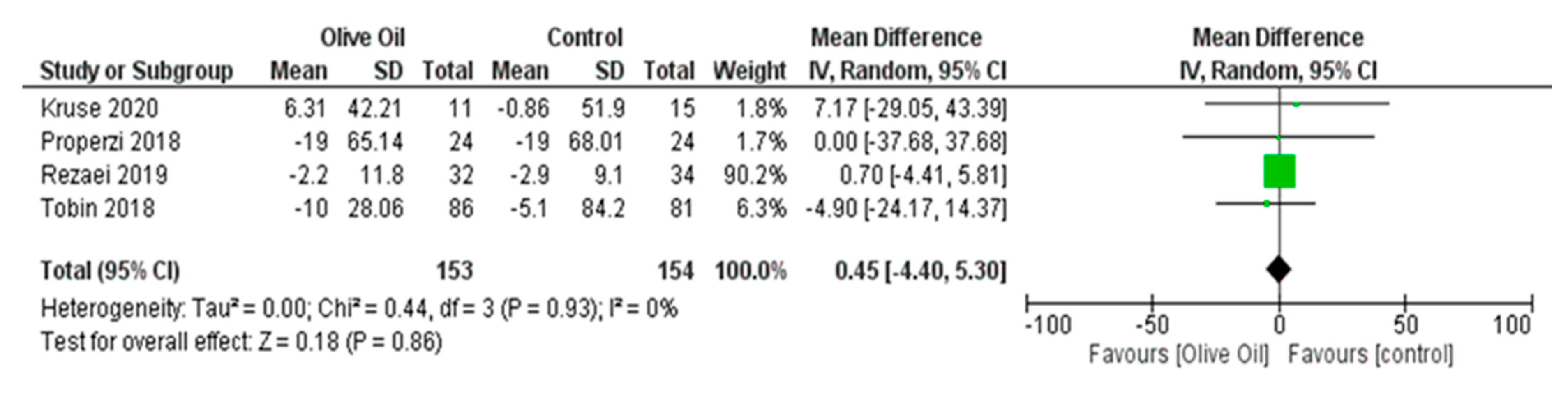
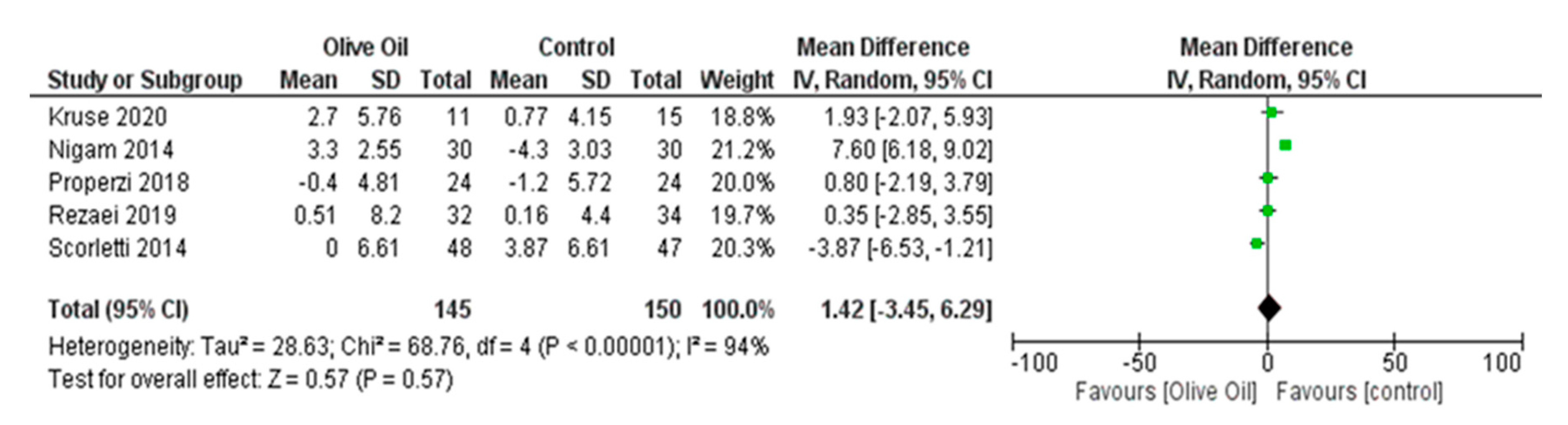
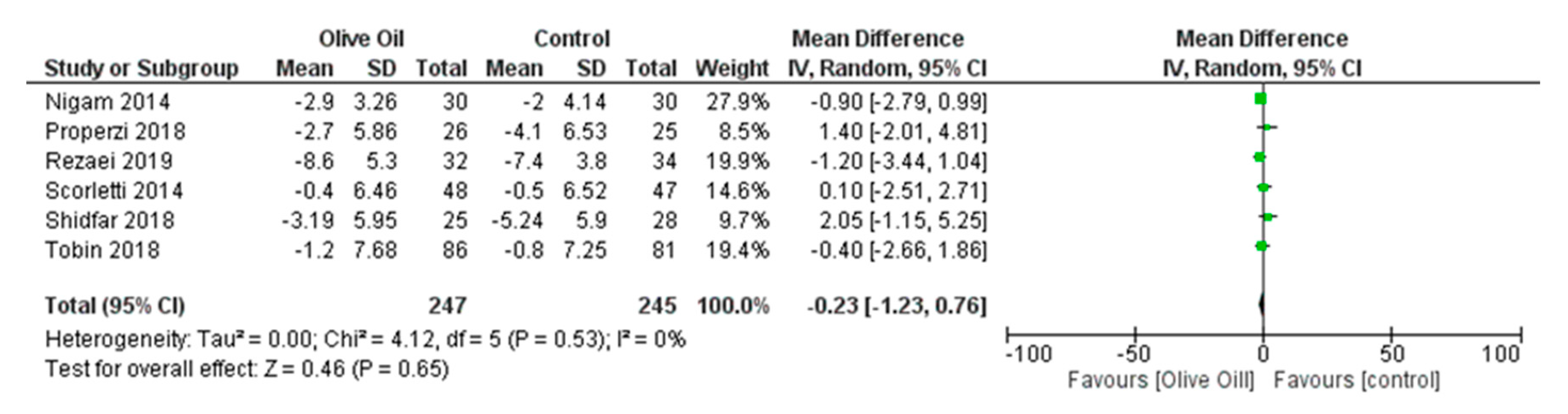
Impact of Olive Oil on Other NAFLD Surrogate Markers
Effect of Olive Oil on Anthropometric Measures
3.8. Sensitivity Analysis
4. Discussion
Strengths and Limitations and Areas for Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Brea, Á.; Pintó, X.; Ascaso, J.F.; Blasco, M.; Díaz, Á.; González-Santos, P.; Mijares, A.H.; Mantilla, T.; Millán, J.; Pedro-Botet, J. Nonalcoholic fatty liver disease, association with cardiovascular disease and treatment. (I). Nonalcoholic fatty liver disease and its association with cardiovascular disease. Clin. Investig. Arterioscler. 2017, 29, 141–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu. Rev. Pathol. 2010, 5, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Kahen, T.; Lavine, J.E.; Stanley, C.; Behling, C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006, 118, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Cortez-Pinto, H. Management of fatty liver disease with the metabolic syndrome. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 487–500. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Akhlaghi, M.; Sasani, M.R.; Barati Boldaji, R. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Nutrition 2019, 57, 154–161. [Google Scholar] [CrossRef] [PubMed]
- FDA Completes Review of Qualified Health Claim Petition for Oleic Acid and the Risk of Coronary Heart Disease|FDA. Available online: https://www.fda.gov/food/cfsan-constituent-updates/fda-completes-review-qualified-health-claim-petition-oleic-acid-and-risk-coronary-heart-disease (accessed on 25 September 2023).
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Belfort, R.; Harrison, S.A.; Brown, K.; Darland, C.; Finch, J.; Hardies, J.; Balas, B.; Gastaldelli, A.; Tio, F.; Pulcini, J.; et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 2006, 355, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Peta, V.; Alfieri, F.; De Lorenzo, A.; Bellentani, S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J. Gastroenterol. 2014, 20, 16831–16840. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Di Renzo, L.; Boccuto, L.; Alwardat, N.; Gratteri, S.; De Lorenzo, A. Health benefits of Mediterranean diet in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Tauriainen, M.M.; Männistö, V.; Kaminska, D.; Vaittinen, M.; Kärjä, V.; Käkelä, P.; Venesmaa, S.; Gylling, H.; Pihlajamäki, J. Serum, liver and bile sitosterol and sitostanol in obese patients with and without NAFLD. Biosci. Rep. 2018, 38, BSR20171274. [Google Scholar] [CrossRef]
- Sánchez-Calvo, B.; Cassina, A.; Mastrogiovanni, M.; Santos, M.; Trias, E.; Kelley, E.E.; Rubbo, H.; Trostchansky, A. Olive oil-derived nitro-fatty acids: Protection of mitochondrial function in non-alcoholic fatty liver disease. J. Nutr. Biochem. 2021, 94, 108646. [Google Scholar] [CrossRef]
- Lama, A.; Pirozzi, C.; Mollica, M.P.; Trinchese, G.; Di Guida, F.; Cavaliere, G.; Calignano, A.; Raso, G.M.; Canani, R.B.; Meli, R. Polyphenol-rich virgin olive oil reduces insulin resistance and liver inflammation and improves mitochondrial dysfunction in high-fat diet fed rats. Mol. Nutr. Food Res. 2017, 61, 1600418. [Google Scholar] [CrossRef] [PubMed]
- Soto-Alarcon, S.A.; Valenzuela, R.; Valenzuela, A.; Videla, L.A. Liver Protective Effects of Extra Virgin Olive Oil: Interaction between Its Chemical Composition and the Cell-signaling Pathways Involved in Protection. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Amor, L.; Sierra, A.L.; Cárdenas, A.; López-Bermudo, L.; López-Beas, J.; Andújar, E.; Pérez-Alegre, M.; Gallego-Durán, R.; Varela, L.M.; Martin-Montalvo, A.; et al. Extra virgin olive oil improved body weight and insulin sensitivity in high fat diet-induced obese LDLr−/−.Leiden mice without attenuation of steatohepatitis. Sci. Rep. 2021, 11, 8250. [Google Scholar] [CrossRef] [PubMed]
- Rajcic, D.; Brandt, A.; Jin, C.J.; Sánchez, V.; Engstler, A.J.; Jung, F.; Nier, A.; Baumann, A.; Bergheim, I. Exchanging dietary fat source with extra virgin olive oil does not prevent progression of diet-induced non-alcoholic fatty liver disease and insulin resistance. PLoS ONE 2020, 15, e0237946. [Google Scholar] [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef]
- Pipitone, R.M.; Zito, R.; Gambino, G.; Di Maria, G.; Javed, A.; Lupo, G.; Giglia, G.; Sardo, P.; Ferraro, G.; Rappa, F.; et al. Red and golden tomato administration improves fat diet-induced hepatic steatosis in rats by modulating HNF4α, Lepr, and GK expression. Front. Nutr. 2023, 10, 1221013. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training. Version 6.2. 2021. Available online: https://training.cochrane.org/handbook/archive/v6.2 (accessed on 25 September 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tacconelli, E. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Kruse, M.; Kemper, M.; Gancheva, S.; Osterhoff, M.; Dannenberger, D.; Markgraf, D.; Machann, J.; Hierholzer, J.; Roden, M.; Pfeiffer, A.F.H. Dietary Rapeseed Oil Supplementation Reduces Hepatic Steatosis in Obese Men-A Randomized Controlled Trial. Mol. Nutr. Food Res. 2020, 64, 2000419. [Google Scholar] [CrossRef]
- Nigam, P.; Bhatt, S.; Misra, A.; Chadha, D.S.; Vaidya, M.; Dasgupta, J.; Pasha, Q.M. Effect of a 6-month intervention with cooking oils containing a high concentration of monounsaturated fatty acids (olive and canola oils) compared with control oil in male Asian Indians with nonalcoholic fatty liver disease. Diabetes Technol. Ther. 2014, 16, 255–261. [Google Scholar] [CrossRef]
- Scorletti, E.; Bhatia, L.; Mccormick, K.G.; Clough, G.F.; Nash, K.; Hodson, L.; Moyses, H.E.; Calder, P.C.; Byrne, C.D.; WELCOME Study. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: Results from the Welcome* study. Hepatology 2014, 60, 1211–1221. [Google Scholar] [CrossRef]
- Properzi, C.; O’Sullivan, T.A.; Sherriff, J.L.; Ching, H.L.; Jeffrey, G.P.; Buckley, R.F.; Tibballs, J.; MacQuillan, G.C.; Garas, G.; Adams, L.A. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology 2018, 68, 1741–1754. [Google Scholar] [CrossRef]
- Tobin, D.; Brevik-Andersen, M.; Qin, Y.; Innes, J.K.; Calder, P.C. Evaluation of a High Concentrate Omega-3 for Correcting the Omega-3 Fatty Acid Nutritional Deficiency in Non-Alcoholic Fatty Liver Disease (CONDIN). Nutrients 2018, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Shidfar, F.; Bahrololumi, S.S.; Doaei, S.; Mohammadzadeh, A.; Gholamalizadeh, M.; Mohammadimanesh, A. The Effects of Extra Virgin Olive Oil on Alanine Aminotransferase, Aspartate Aminotransferase, and Ultrasonographic Indices of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Patients Undergoing Low Calorie Diet. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1053710. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Charlton, M.; Kawaguchi, A.; Yamamura, S.; Nakano, D.; Tsutsumi, T.; Zafer, M.; Torimura, T. Effects of Mediterranean Diet in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Randomized Controlled Trials. Semin. Liver Dis. 2021, 41, 225–234. [Google Scholar] [CrossRef]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Hassani Zadeh, S.; Mozaffari-Khosravi, H.; Hosseinzadeh, M. Effect of Mediterranean diet on liver enzymes: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2022, 128, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ding, X.; Gu, J.; Zhou, S.; Jiang, Y. Effects of olive oil on hepatic steatosis and liver enzymes: A systematic review. J. Funct. Foods 2023, 109, 105815. [Google Scholar] [CrossRef]
- Uylaşer, V.; Yildiz, G. The historical development and nutritional importance of olive and olive oil constituted an important part of the Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2014, 54, 1092–1101. [Google Scholar] [CrossRef]
- Uli, G.B.; Asyahir, S.R.; Harti, L.B. Literature Review: The Effect of Mediterranean Diet on Lipid Profile and Fasting Blood Glucose in Overweight or Obese. Amerta Nutr. 2023, 7, 139–146. [Google Scholar] [CrossRef]
- Milano, A.; Kabbaha, S.; Thorlund, K. Effects of the mediterranean diet versus low-fat diet on metabolic syndrome outcomes: A systematic review and meta-analysis of randomized controlled trials. Hum. Nutr. Metab. 2022, 30, 200175. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, J.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, e6–e17. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef]
- Mancini, J.G.; Filion, K.B.; Atallah, R.; Eisenberg, M.J. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am. J. Med. 2016, 129, 407–415.e4. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Arab, A.; Paknahad, Z. The effect of a Mediterranean diet on metabolic parameters in patients with non-alcoholic fatty liver disease: A systematic review of randomized controlled trials. Clin. Nutr. ESPEN 2020, 35, 40–46. [Google Scholar] [CrossRef]
- Marakis, G.; Gaitis, F.; Mila, S.; Papadimitriou, D.; Tsigarida, E.; Mousia, Z.; Karpouza, A.; Magriplis, E.; Zampelas, A. Attitudes towards olive oil usage, domestic storage, and knowledge of quality: A consumers’ survey in Greece. Nutrients 2021, 13, 3709. [Google Scholar] [CrossRef]
- de Bock, M.; Derraik, J.G.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.S.; Cordeiro Simões-Ambrosio, L.M.; Ovídio, P.P.; Ramalho, L.Z.; Jordao, A.A. Effects of Different Vegetable Oils on the Nonalcoholic Fatty Liver Disease in C57/BL Mice. Evid. Based Complement. Alternat. Med. 2023, 2023, 4197955. [Google Scholar] [CrossRef]
- Patti, A.M.; Carruba, G.; Cicero, A.F.G.; Banach, M.; Nikolic, D.; Giglio, R.V.; Terranova, A.; Soresi, M.; Giannitrapani, L.; Montalto, G.; et al. Daily Use of Extra Virgin Olive Oil with High Oleocanthal Concentration Reduced Body Weight, Waist Circumference, Alanine Transaminase, Inflammatory Cytokines and Hepatic Steatosis in Subjects with the Metabolic Syndrome: A 2-Month Intervention Study. Metabolites 2020, 10, 392. [Google Scholar] [CrossRef]
- Zamora, F.Z.; Galiano, J.M.M.; Martínez, J.J.G.; Rodríguez, M.D. Olive Oil and Body Weight. Systematic Review and Meta-Analysis of Randomized Controlled Trials. Rev. Esp. Salud Publica 2018, 92, e201811083. Available online: https://pubmed.ncbi.nlm.nih.gov/30461730/ (accessed on 1 March 2024).
- National Guideline Centre (UK). Non-Alcoholic Fatty Liver Disease: Assessment and Management. In National Guideline Centre (UK)-National Institute for Health and Clinical Excellence NG49. Overview. 2016. Available online: https://pubmed.ncbi.nlm.nih.gov/27441333/ (accessed on 25 September 2023).
- Long, M.T.; Noureddin, M.; Lim, J.K. AGA Clinical Practice Update: Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Lean Individuals: Expert Review. Gastroenterology 2022, 163, 764–774.e1. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Jou, J.H. Nonalcoholic Fatty Liver Disease and Recent Guideline Updates. Clin. Liver Dis. 2021, 17, 23–28. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef]
- Biolato, M.; Manca, F.; Marrone, G.; Cefalo, C.; Racco, S.; Miggiano, G.A.; Valenza, V.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal permeability after Mediterranean diet and low-fat diet in non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 509–520. [Google Scholar] [CrossRef]
- Marjot, T.; Tomlinson, J.W.; Hodson, L.; Ray, D.W. Timing of energy intake and the therapeutic potential of intermittent fasting and time-restricted eating in NAFLD. Gut 2023, 72, 1607–1619. [Google Scholar] [CrossRef]
- Ristic-Medic, D.; Bajerska, J.; Vucic, V. Crosstalk between dietary patterns, obesity and nonalcoholic fatty liver disease. World J. Gastroenterol. 2022, 28, 3314–3333. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Li, Y.; Willett, W.C.; Sun, Q.; Sampson, L.; Salas-Salvadó, J.; Martínez-González, M.A.; Stampfer, M.J.; Hu, F.B. Consumption of Olive Oil and Risk of Total and Cause-Specific Mortality among U.S. Adults. J. Am. Coll. Cardiol. 2022, 79, 101–112. [Google Scholar] [CrossRef]
- Di Majo, D.; Sardo, P.; Giglia, G.; Di Liberto, V.; Zummo, F.P.; Zizzo, M.G.; Caldara, G.F.; Rappa, F.; Intili, G.; van Dijk, R.M.; et al. Correlation of Metabolic Syndrome with Redox Homeostasis Biomarkers: Evidence from High-Fat Diet Model in Wistar Rats. Antioxidants 2023, 12, 89. [Google Scholar] [CrossRef]







| Unique ID | Study ID | Experimental | Comparator | Outcome | W | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kruse 2020 [27] | DOI: 10.1002/mnfr.202000419 | Olive Oil (OL) | Rapeseed Oil (RA) | ALT | 1 |  |  |  |  |  |  |
| Nigam 2014 [28] | DOI: 10.1089/dia.2013.0178 | Olive Oil or Canola | Control Oil | ALT | 1 |  |  |  |  |  |  |
| Scorletti 2014 [29] | DOI: 10.1002/hep.27289 | Omacor | Olive Oil | ALT | 1 |  |  |  |  |  |  |
| Properzi 2018 [30] | DOI: 10.1002/hep.30076 | MED diet | Low-Fat Diet | ALT | 1 |  |  |  |  |  |  |
| Rezai 2019 [9] | DOI: 10.1016/j.nut.2018.02.021 | Olive Oil | Sunflower Oil | ALT | 1 |  |  |  |  |  |  |
| Tobin 2018 [31] | DOI: 10.3390/nu10081126 | MF4637 | Olive Oil | ALT | 1 |  |  |  |  |  |  |
| Shidfar 2018 [32] | DOI: 10.1155/2018/1053710 | Olive Oil | Control | ALT | 1 |  |  |  |  |  |  |
 High Risk High Risk Low Risk Low Risk Some Concerns Some Concerns | D1: Randomization process D2: Deviations from the intended interventions D3: Missing outcome data | D4: Measurement of the outcome D5: Selection of the reported result W: Weight | |||||||||
| Study | Design | Clinical Group | Follow-Up (weeks) | Intervention/Control Groups | Number of Patients, Male/Female (%) | Age—Mean (years) | BMI—Mean (kg/m2) | Intervention | Intervention Features |
|---|---|---|---|---|---|---|---|---|---|
| Properzi 2018, Australia [30] | RCT | NAFLD | 12 | Intervention group ** | 26, (57.7%)/(42.3%) | 51 | 31.5 | MD—based on the traditional Cretan diet (40% CHO, 20% PRO, 35–40% FAT, and <10% SFA). | 750 g nuts, 750 mL olive oil (MD), 1 kg of natural muesli, and 200 g of low-fat snack bars every 4 weeks (LFD). |
| Control group | 25, (44%)/(56%) | 53 | 30.2 | Low-fat/high-CHO (LFD)—based on National Health and Medical Research Council and AHA guidelines (50% CHO, 20% PRO, 30% FAT, and <10% SFA). | |||||
| Rezaei 2019, Iran [9] | RCT | NAFLD | 12 | Intervention group ** | 32, (37.5%)/(62.5%) | 46.3 | 30.6 | MD component—increased olive oil intake (20 g/day). | Cal restriction: 500 cal/d deficit (both). Physical activity: Moderate intensity 30–40 min/d (both). Olive oil and sunflower oil dosages are provided (both). 50–55% CHO, 10–15% PRO and 30–35% FAT (both). |
| Control group | 34, (50%)/(50%) | 40.8 | 29.6 | Increased sunflower oil intake (20 g/day). | |||||
| Nigam 2014, India [28] | RCT | NAFLD | 24 | Intervention group ** | 30, (100%)/0 | 37.2 | 27.2 | Olive oil (not exceeding 20 g/day). | Cal intake: 15–21% PRO, 55–70 CHO, 20% FAT (both). Physical activity: Moderate intensity 40–45 min/d (both). Therapeutic lifestyle changes were given (both). |
| Intervention group | 33, (100%)/0 | 38 | 27.4 | Canola oil (not exceeding 20 g/day). | |||||
| Control group | 30, (100%)/0 | 36.2 | 27.4 | Refined oil (not exceeding 20 g/day). | |||||
| Scorletti 2014, United Kingdom [29] | RCT | NAFLD | 72 | Intervention group | 51, (49%)/(51%) | 48.6 | 34.3 | Omacor (gel capsules contained 460 mg EPA and 380 mg DHA)—4 g/day | General dietary advice and education information were provided without advice on calorie allowances, physical activity, or behavior changes (both). |
| Control group ** | 52, (67.3%)/(32.7%) | 54 | 32 | Olive oil (gel capsules contained 1 g of olive oil)—4 g/day | |||||
| Kruse 2020, Germany [27] | RCT | NAFLD | 8 | Intervention group ** | 11, (100%)/0 | 54 | 33.1 | Increased olive oil intake (50 g/day). | Modification of energy intake according to the weight (>1 kg from the initial body weight). No advice given on physical activity or behavior changes (both). |
| Control group | 16, (100%)/0 | 58 | 32 | Increased rapeseed oil intake (50 g/day). | |||||
| Tobin 2018, United States [31] | RCT | NAFLD | 24 | Intervention group | 87, (44.4%)/(55.6%) | 55.3 | 32.1 | Omega-3 MF46367 (gel capsules containing 460 mg EPA and 380 mg DHA)—3 caps daily. | Cal restriction: Reduced regular caloric intake. Consumption of 2 meals of omega-3-rich fish per week and reduction in consumption of foods rich in trans and omega-6 fatty acids. Physical activity: Stable levels. |
| Control group ** | 89, (51.1%)/(48.9%) | 55.1 | 32.4 | Olive oil (gel capsules containing 1 g of olive oil)—3 caps daily. | |||||
| Shidfar 2018, Iran [32] | RCT | NAFLD | 12 | Intervention group ** | 25, (61.9%)/(38.1%) * | 46.14 | 29.64 | MD component—increased olive oil intake (20% of total fat). | Cal restriction: Personalized Cal deficit (both). Olive oil dosage supplied. 50% CHO, 20% PRO and 30% FAT (both). |
| Control group | 25, (59.1%)/(40.9%) * | 45.68 | 29.9 | Healthy diet lifestyle (sunflower oil). |
| Study | Outcome Measures in Study | Attrition/Dropout | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|
| Properzi 2018, Australia [30] | HS (MRS-PDFF), LSM [TE FibroScan™ (Echocens, Paris, France)], HepaScore, ALT, cardiometabolics, anthropometry, and QoL | Intervention: 2/26, 7.7% Control: 1/25, 4.0% | ↓ HS and ↓ ALT (both) HS RR: −32.4% ± 25.5% vs. −255.0% ± 25.3% (intervention vs. control) | ↓ gGT, HbA1c, TC, TG and FRS (intervention) ↓ WC and ↑ QoL (both) ↓ BW (both) BW: −2.3% vs. −2.1% (intervention vs. control) |
| Rezaei 2019, Iran [9] | HS (US), ALT, AST, cardiometabolics, anthropometry and diet record diaries | Intervention: 6/32, 18.8% Control: 6/34, 17.6% | ↓ HS (both) ↓ ALT (control) ↓ HS (intervention vs. control) | ↓ AST (both) ↓ TG (intervention) ↓ BW, WC, and systolic/diastolic BP (both) BW: −4.1% vs. −2.9% (intervention vs. control) |
| Nigam 2014, India [28] | LF (US), AST, ALT, anthropometry, cardiometabolics, and food questionnaire | Intervention: 0/30, 0% Intervention: 0/30, 0% Control: 3/33, 9.1% | ↓ LF (intervention) ↓ ALT (intervention) | ↓ AST, TC, TG (intervention) ↑ HDL-C (intervention) ↓ FBG (intervention vs. control) ↓ FBG (olive oil vs. canola oil) ↓ BW (olive vs. control) |
| Scorletti 2014, United Kingdom [29] | HS (MRS), diet questionnaire, ALT, AST, cardiometabolics, and anthropometry | Intervention: 4/51, 7.8% Control: 4/52, 7.7% | ↓ ALT (control) | ↓ HS and TG (intervention) ↓ AST (control) ↓ HDL-C (intervention) |
| Kruse 2020, Germany [27] | HS (H-MRS), ALT, AST, anthropometry, cardiometabolics, and diet record diaries | Intervention: 0/11, 0% Control: 1/16, 6.3% | ↓ ALT (both) ↑ IHL (intervention vs. control) | ↓ LDL, AST (intervention) ↑ HDL (intervention) No changes BW, TG (both) |
| Tobin 2018, United States [31] | LF (MRI-PDFF), ALT, AST, anthropometry, and cardiometabolics | Intervention: 6/87, 6.9% Control: 3/89, 3.4% | ↓ ALT (control) | ↓AST and gGT (control) ↓ WC (control) ↓ in LF percentage (both) ↓ TG 18% vs. 7% (intervention vs. control) |
| Shidfar 2018, Iran [32] | HS (US), ALT, AST, anthropometry, and diet questionnaire | Intervention: 4/25, 16.0% Control: 3/25, 12.0% | ↓ ALT (both) ↓ ALT and HS (intervention vs. control) | ↓ AST (intervention) ↓ WC (both) ↓ BW (both) BW: −4.3% vs.−3.5% (intervention vs. control) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsamos, G.; Kalopitas, G.; Evripidou, K.; Vasdeki, D.; Koufakis, T.; Kanavas, V.; Antza, C.; Germanidis, G.; Chourdakis, M. The Effects of Olive Oil Consumption on Biochemical Parameters and Body Mass Index of People with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2024, 16, 857. https://doi.org/10.3390/nu16060857
Tsamos G, Kalopitas G, Evripidou K, Vasdeki D, Koufakis T, Kanavas V, Antza C, Germanidis G, Chourdakis M. The Effects of Olive Oil Consumption on Biochemical Parameters and Body Mass Index of People with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2024; 16(6):857. https://doi.org/10.3390/nu16060857
Chicago/Turabian StyleTsamos, Georgios, Georgios Kalopitas, Kleo Evripidou, Dimitra Vasdeki, Theocharis Koufakis, Vasileios Kanavas, Christina Antza, Georgios Germanidis, and Michail Chourdakis. 2024. "The Effects of Olive Oil Consumption on Biochemical Parameters and Body Mass Index of People with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 16, no. 6: 857. https://doi.org/10.3390/nu16060857
APA StyleTsamos, G., Kalopitas, G., Evripidou, K., Vasdeki, D., Koufakis, T., Kanavas, V., Antza, C., Germanidis, G., & Chourdakis, M. (2024). The Effects of Olive Oil Consumption on Biochemical Parameters and Body Mass Index of People with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 16(6), 857. https://doi.org/10.3390/nu16060857






