An Evaluation of the Usefulness of Selected Screening Methods in Assessing the Risk of Malnutrition in Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. The Characteristics of the Study Group
2.2. GLIM Criteria
2.3. Screening Tests
2.3.1. MNA
2.3.2. MUST
2.3.3. SASKIBD-NR
2.3.4. MST
2.4. Statistical Analysis
3. Results
Characteristics of the Study Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borowitz, S.M. The epidemiology of inflammatory bowel disease: Clues to pathogenesis? Front. Pediatr. 2023, 10, 1103713. [Google Scholar] [CrossRef]
- Ng, S.; Shi, H.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan Sung, J.J.Y.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Zagórowicz, E.; Walkiewicz, D.; Kucha, P.; Perwieniec, J.; Maluchnik, M.; Wieszczy, P.; Reguła, J. Nationwide data on epidemiology of inflammatory bowel disease in Poland between 2009 and 2020. Pol. Arch. Intern. Med. 2022, 132, 16194. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The rising burden of inflammatory bowel disease in Poland. Pol. Arch. Intern. Med. 2022, 132, 16257. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, F.; Bouin, M.; D’Aoust, L.; Lemoyne, M.; Presse, N. Food avoidance in patients with inflammatory bowel disease: What, when and who? Clin. Nutr. 2018, 37, 884–889. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef]
- Opstelten, J.L.; de Vries, J.H.M.; Wools, A.; Siersema, P.D.; Oldenburg, B.; Witteman, B.J.M. Dietary intake of patients with inflammatory bowel disease: A comparison with individuals from a general population and associations with relapse. Clin. Nutr. 2019, 38, 1892–1898. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2016, 36, 321–347. [Google Scholar] [CrossRef]
- Li, S.; Ney, M.; Eslamparast, T.; Vandermeer, B.; Ismond, K.P.; Kroeker, K.; Halloran, B.; Raman, M.; Tandon, P. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 3823–3837. [Google Scholar] [CrossRef]
- Vidarsdottir, J.B.; Johannsdottir, S.E.; Thorsdottir, I.; Bjornsson, E.; Ramel, A. A cross-sectional study on nutrient intake and -status in inflammatory bowel disease patients. Nutr. J. 2016, 15, 61. [Google Scholar] [CrossRef]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): An open-label, pilot, randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59. [Google Scholar] [CrossRef]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of malnutrition and nutritional characteristics of patients with Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Krznaric, Z.; Pirlich, M. Diagnosis of malnutrition in patients with gastrointestinal diseases: Recent observations from a Global Leadership Initiative on Malnutrition perspective. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 361–366. [Google Scholar] [CrossRef]
- Chávez-Tostado, M.; Cervantes-Guevara, G.; López-Alvarado, S.E.; Cervantes-Pérez, G.; Barbosa-Camacho, F.J.; Fuentes-Orozco, C.; Hernández-Corona, D.M.; González-Heredia, T.; Cervantes-Cardona, G.A.; González-Ojeda, A. Comparison of nutritional screening tools to assess nutritional risk and predict clinical outcomes in Mexican patients with digestive diseases. BMC Gastroenterol. 2020, 20, 79. [Google Scholar] [CrossRef]
- Haskey, N.; Peña-Sánchez, J.N.; Jones, J.L.; Fowler, S.A. Development of a screening tool to detect nutrition risk in patients with inflammatory bowel disease. Asia Pac. J. Clin. Nutr. 2018, 27, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Gao, X.; Dai, C.; Huang, Y.; Wu, Y.; Zhou, W.; Cao, Q.; Jing, X.; Jiang, H.; et al. Validation of the GLIM criteria for diagnosis of malnutrition and quality of life in patients with inflammatory bowel disease: A multicenter, prospective, observational study. Clin. Nutr. 2022, 41, 1297–1306. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Gao, X.; Dai, C.; Huang, Y.; Wu, Y.; Zhou, W.; Cao, Q.; Jing, X.; Jiang, H.; et al. Impact of malnutrition and sarcopenia on quality of life in patients with inflammatory bowel disease: A multicentre study. J. Cachexia Sarcopenia Muscle 2023, 14, 2663–2675. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Glinkowski, S.; Marcinkowska, D. Ulcerative colitis: Assessment of disease activity based on contemporary scales. Nowa Med. 2018, 25, 123–137. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stos´, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i ich Zastosowanie; Instytut Żywności i Żywienia: Warsaw, Poland, 2020. [Google Scholar]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–487. [Google Scholar] [PubMed]
- Green, S.M.; Watson, R. Nutritional screening and assessment tools for older adults: Literature review. J. Adv. Nurs. 2006, 54, 477–490. [Google Scholar] [CrossRef]
- Rozporządzenie Ministra Zdrowia z dnia 22 Listopada 2013 r. w Sprawie Świadczeń Gwarantowanych z Zakresu Leczenia Szpitalnego (Dz.U. 2013 poz. 1520). Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20130001520 (accessed on 30 June 2018).
- Salvi, F.; Giorgi, R.; Grilli, A.; Morichi, V.; Espinosa, E.; Spazzafumo, L.; Marinozzi, M.L.; Dessì-Fulgheri, P. Mini Nutritional Assessment (Short Form) and Functional Decline in Older Patients Admitted to an Acute Medical Ward Aging Clinical and Experimental Research. Aging Clin. Exp. Res. 2008, 20, 322–328. [Google Scholar] [CrossRef]
- Dent, E.; Chapman, I.; Piantadosi, C.; Visvanathan, R. Nutritional Screening Tools and Anthropometric Measures Associate with Hospital Discharge Outcomes in Older People. Australas. J. Ageing 2015, 34, E1–E6. [Google Scholar] [CrossRef]
- Dent, E.; Chapman, I.M.; Piantadosi, C.; Visvanathan, R. Performance of Nutritional Screening Tools in Predicting Poor Six-Month Outcome in Hospitalised Older Patients. Asia Pac. J. Clin. Nutr. 2014, 23, 394–399. [Google Scholar] [CrossRef]
- Raslan, M.; Gonzalez, M.C.; Gonçalves Dias, M.C.; Nascimento, M.; Castro, M.; Marques, P.; Segatto, S.; Torrinhas, R.S.; Cecconello, I.; Waitzberg, D.L. Comparison of Nutritional Risk Screening Tools for Predicting Clinical Outcomes in Hospitalized Patients. Nutrition 2010, 26, 721–726. [Google Scholar] [CrossRef]
- Poulia, K.A.; Klek, S.; Doundoulakis, I.; Bouras, E.; Karayiannis, D.; Baschali, A.; Passakiotou, M.; Chourdakis, M. The Two Most Popular Malnutrition Screening Tools in the Light of the New ESPEN Consensus Definition of the Diagnostic Criteria for Malnutrition. Clin. Nutr. 2017, 36, 1130–1135. [Google Scholar] [CrossRef]
- Lomivorotov, V.V.; Efremov, S.M.; Boboshko, V.A.; Nikolaev, D.A.; Vedernikov, P.E.; Deryagin, M.N.; Lomivorotov, V.N.; Karaskov, A.M. Prognostic Value of Nutritional Screening Tools for Patients Scheduled for Cardiac Surgery. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Calleja Fernández, A.; Vidla Casariego, A.; Cano Rodríguez, I.; Ballesteros Pomar, M.D. Eficacia y Efectividad de Las Distintas Herramientas de Cribado Nutricional En Un Hospital de Tercer Nivel. Nutr. Hosp. 2015, 31, 2240–2246. [Google Scholar] [CrossRef]
- Fiol-Martínez, L.; Calleja-Fernández, A.; Pintor de la Maza, B.; Vidal-Casariego, A.; Villar-Taibo, R.; Urioste-Fondo, A.; Cuervo, M.; Cano-Rodríguez, I.; Ballesteros-Pomar, M.D. Comparison of Two Nutritional Screening Tools to Detect Nutritional Risk in Hematologic Inpatients. Nutrition 2017, 34, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN Guidelines for Nutrition Screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a Valid and Reliable Malnutrition Screening Tool for Adult Acute Hospital Patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef] [PubMed]
- De Ulibarri Perez, J.I.; Giménez, A.G.M.; Pérez, P.G.; Fernandez, G.; Salvanés, F.R.; Estrada, A.M.A.; Diaz, A.; Travé, T.D.; Romero, C.D.; Sánchez, P.H.; et al. New Procedure for the Early Detection and Control of Under-Nourishment in Hospitals. Nutr. Hosp. 2002, 17, 179–188. [Google Scholar] [PubMed]
- Nguyen, G.C.; Munsell, M.; Harris, M.L. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm. Bowel Dis. 2008, 14, 1105–1111. [Google Scholar] [CrossRef]
- Valentini, L.; Schaper, L.; Buning, C.; Hengstermann, S.; Koernicke, T.; Tillinger, W.; Guglielmi, F.W.; Norman, K.; Buhner, S.; Ockenga, J.; et al. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition 2008, 24, 694–702. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Zhang, Q.; Rao, S.; Wu, X.; Zhang, J.; Li, J. Comparison of the Suitability Between NRS2002 and MUST as the First-Step Screening Tool for GLIM Criteria in Hospitalized Patients With GIST. Front. Nutr. 2022, 9, 864024. [Google Scholar] [CrossRef]
- Jabłońska, B.; Mrowiec, S. Nutritional Status and Its Detection in Patients with Inflammatory Bowel Diseases. Nutrients 2023, 15, 1991. [Google Scholar] [CrossRef]
- Huang, S.; Niu, Y.; Liu, X.; Gu, Z.; Huang, A.; Wu, J. Characteristics of malnutrition according to Global Leadership Initiative on Malnutrition criteria in non-surgical patients with inflammatory bowel disease. Nutrition 2022, 94, 111514. [Google Scholar] [CrossRef]
- Fiorindi, C.; Dragoni, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. Relationship between Nutritional Screening Tools and GLIM in Complicated IBD Requiring Surgery. Nutrients 2021, 13, 3899. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Pieczyńska, J.; Prescha, A.; Zabłocka-Słowińska, K.; Neubauer, K.; Smereka, A.; Grajeta, H.; Biernat, J.; Paradowski, L. Occurrence of dietary risk factors in inflammatory bowel disease: Influence on the nutritional status of patients in clinical remission. Adv. Clin. Exp. Med. 2019, 28, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Prescha, A.; Pieczyńsk, J.; Biernat, K.; Neubauer, K.; Smereka, A.; Ilow, R.; Grajeta, H.; Biernat, J.; Paradowski, L. Nutritional status assessment of patients with inflammatory bowel disease. Gastroenterol. Pol. 2010, 17, 57–63. [Google Scholar]
- Fiorindi, C.; Luceri, C.; Dragoni, G.; Piemonte, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. GLIM Criteria for Malnutrition in Surgical IBD Patients: A Pilot Study. Nutrients 2020, 12, 2222. [Google Scholar] [CrossRef]
- Einav, L.; Hirsch, A.; Ron, Y.; Cohen, N.A.; Lahav, S.; Kornblum, J.; Anbar, R.; Maharshak, N.; Fliss-Isakov, N. Risk Factors for Malnutrition among IBD Patients. Nutrients 2021, 13, 4098. [Google Scholar] [CrossRef] [PubMed]
- Csontos, A.A.; Molnár, A.; Piri, Z.; Pálfi, E.; Miheller, P. Malnutrition risk questionnaire combined with body composition measurement in malnutrition screening in inflammatory bowel disease. Rev. Esp. Enferm. Dig. 2017, 109, 26–32. [Google Scholar] [CrossRef][Green Version]
- Ostrowska, J.; Sulz, I.; Tarantino, S.; Hiesmayr, M.; Szostak-Węgierek, D. Hospital Malnutrition, Nutritional Risk Factors, and Elements of Nutritional Care in Europe: Comparison of Polish Results with All European Countries Participating in the nDay Survey. Nutrients 2021, 13, 263. [Google Scholar] [CrossRef]
- Jahnsen, J.; Falch, J.A.; Mowinckel, P.; Aadland, E. Body composition in patients with inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2003, 98, 1556–1562. [Google Scholar] [CrossRef]
- Cosnes, J. Smoking, physical activity, nutrition and lifestyle: Environmental factors and their impact on IBD. Dig. Dis. 2010, 28, 411–417. [Google Scholar] [CrossRef]
- NicSuibhne, T.; Raftery, T.C.; McMahon, O.; Walsh, C.; O’Morain, C.; O’Sullivan, M. High prevalence of overweight and obesity in adults with Crohn’s disease: Associations with disease and lifestyle factors. J. Crohns Colitis 2013, 7, e241–e248. [Google Scholar] [CrossRef] [PubMed]
- Hass, D.J.; Brensinger, C.M.; Lewis, J.D.; Lichtenstein, G.R. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Steed, H.; Walsh, S.; Reynolds, N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes. Facts 2009, 2, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Ungar, B.; Kopylov, U.; Goitein, D.; Lahat, A.; Bardan, E.; Avidan, B.; Lang, A.; Maor, Y.; Eliakim, R.; Ben-Horin, S. Severe and morbid obesity in Crohn’s disease patients: Prevalence and disease associations. Digestion 2013, 88, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.M.; Eslamparast, T.; Farhat, K.; Kroeker, K.; Halloran, B.; Shommu, N.; Kumar, A.; Fitzgerald, Q.; Gramlich, L.; Abraldes, J.G.; et al. Using Patient Completed Screening Tools to Predict Risk of Malnutrition in Patients With Inflammatory Bowel Disease. Crohns Colitis 360 2021, 3, otab043. [Google Scholar] [CrossRef]
- Losurdo, G.; La Fortezza, R.F.; Iannone, A.; Contaldo, A.; Barone, M.; Ierardi, E.; Di Leo, A.; Principi, M. Prevalence and associated factors of obesity in inflammatory bowel disease: A case-control study. World J. Gastroenterol. 2020, 26, 7528–7537. [Google Scholar] [CrossRef]
- Bramuzzo, M.; Grazian, F.; Grigoletto, V.; Daidone, A.; Martelossi, S.; Mario, F.; Maurel, E.; Lega, S.; Giudici, F.; Di Leo, G.; et al. Dietary Beliefs in Children and Adolescents with Inflammatory Bowel Disease and their Parents. J. Pediatr. Gastroenterol. Nutr. 2022, 75, e43–e48. [Google Scholar] [CrossRef]
- Campmans-Kuijpers, M.J.E.; Dijkstra, G. Food and Food Groups in Inflammatory Bowel Disease (IBD): The Design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients 2021, 13, 1067. [Google Scholar] [CrossRef]
| IBD n (%), Mean ± SD | CD n (%), Mean ± SD | UC n (%), Mean ± SD | p | |
|---|---|---|---|---|
| Patients | 82 | 48 (58.5) | 34 (41.5) | |
| Age [years] | 38.14 ± 11.65 | 34.66 ± 10.24 | 43.06 ± 11.87 | 0.0010 |
| Female | 42 (51.22) | 28 (58.33) | 14 (41.18) | 0.1257 |
| Duration of the disease [years] | 8.39 ± 5.71 | 8.08 ± 5.87 | 8.82 ± 4.56 | 0.7234 |
| Past intestinal partial resection | 22 (26.83) | 17 (35.42) | 5 (14.71) | 0.0315 |
| Disease activity | ||||
| CDAI [0/1/2/3] | 17(35.4)/10(20.9)/17(35.4)/4 (8.3) | |||
| Partial Mayo Score (0/1/2/3) | 17 (50.0)/0 (0)/11 (32.4)/6 (17.6) | |||
| BMI [kg/m2] | 24.25 ± 4.76 | 23.81 ± 4.88 | 24.81 ± 4.59 | 0.4705 |
| ≤18.5 | 10 (12.20) | 6 (12.5) | 4 (11.76) | 0.6745 |
| 18.5–24.9 | 38 (46.34) | 24 (50) | 14 (41.18) | |
| ≥25.0 | 34 (41.46) | 18 (37.5) | 16 (47.06) | |
| UWL [% body weight] | 16.50 ± 8.23 | 16.20 ± 7.85 | 16.95 ± 8.97 | 0.5812 |
| ≤5% | 6 (7.32) | 4 (8.33) | 2 (5.88) | 0.8640 |
| 5–10% | 6 (7.32) | 4 (8.33) | 2 (5.88) | |
| ≥10% | 38 (46.34) | 22 (45.83) | 16 (47.06) | |
| FFM % | 75.14 ± 11.23 | 74.55 ± 12.6 | 75.98 ± 9.72 | 0.7896 |
| FFM % (M) | 80.08 ± 11.54 | 78.96 ± 14.38 | 81.20 ± 7.98 | <0.0001 |
| FFM % (F) | 70.44 ± 8.73 | 71.39 ± 9.55 | 68.54 ± 6.71 | <0.0001 |
| FFMI kg/m2 | 17.97 ± 3.26 | 17.51 ± 3.43 | 18.62 ± 2.95 | 0.6596 |
| FFMI kg/m2(M) | 19.77 ± 3.26 | 19.32 ± 4.02 | 20.21 ± 2.29 | <0.0001 |
| FFMI kg/m2 (F) | 16.26 ± 2.19 | 15.22 ± 2.22 | 16.34 ± 2.21 | <0.0001 |
| FFMI < 17 (M) or <16 (F) | 18 (21.95) | 12 (25.00) | 6 (17.65) | 0.4281 |
| Food group elimination | 72 (87.8) | 44 (91.67) | 28 (82.35) | 0.5743 |
| Energy intake [kcal] | 1575 ± 302 | 1540 ± 282 | 1626 ± 320 | 0.7658 |
| <50% energy requirements | 30 (36.59) | 20 (41.67) | 10 (29.41) | 0.0321 |
| IBD n (%) | CD n (%) | UC n (%) | Active n (%) | Remission n (%) | p *,** | |
|---|---|---|---|---|---|---|
| Malnutrition diagnosis | ||||||
| GLIM stage 1 | 38 (46.34) | 23 (47.92) | 15 (44.12) | 24 (50) | 14 (41.18) | 0.9696 0.8745 |
| GLIM stage 2 | 22 (27.5) | 13 (27.66) | 9 (27.27) | 19 (45.24) | 3 (7.89) | 0.9853 0.0005 |
| Nutritional screening tools | ||||||
| MNA | 34 (43.59) | 22 (48.89) | 12 (36.36) | 24 (60) | 10 (26.32) | 0.2704 0.0027 |
| MUST | 30 (37.5) | 17 (36.17) | 13 (39.39) | 23 (54.76) | 7 (18.42) | 0.7694 0.0008 |
| SASKIBD-NR | 16 (20.25) | 10 (21.28) | 6 (18.75) | 13 (30.95) | 3 (8.11) | 0.7838 0.0250 |
| MST | 20 (25) | 14 (29.79) | 6 (18.18) | 16 (38.10) | 4 (10.53) | 0.2380 0.0097 |
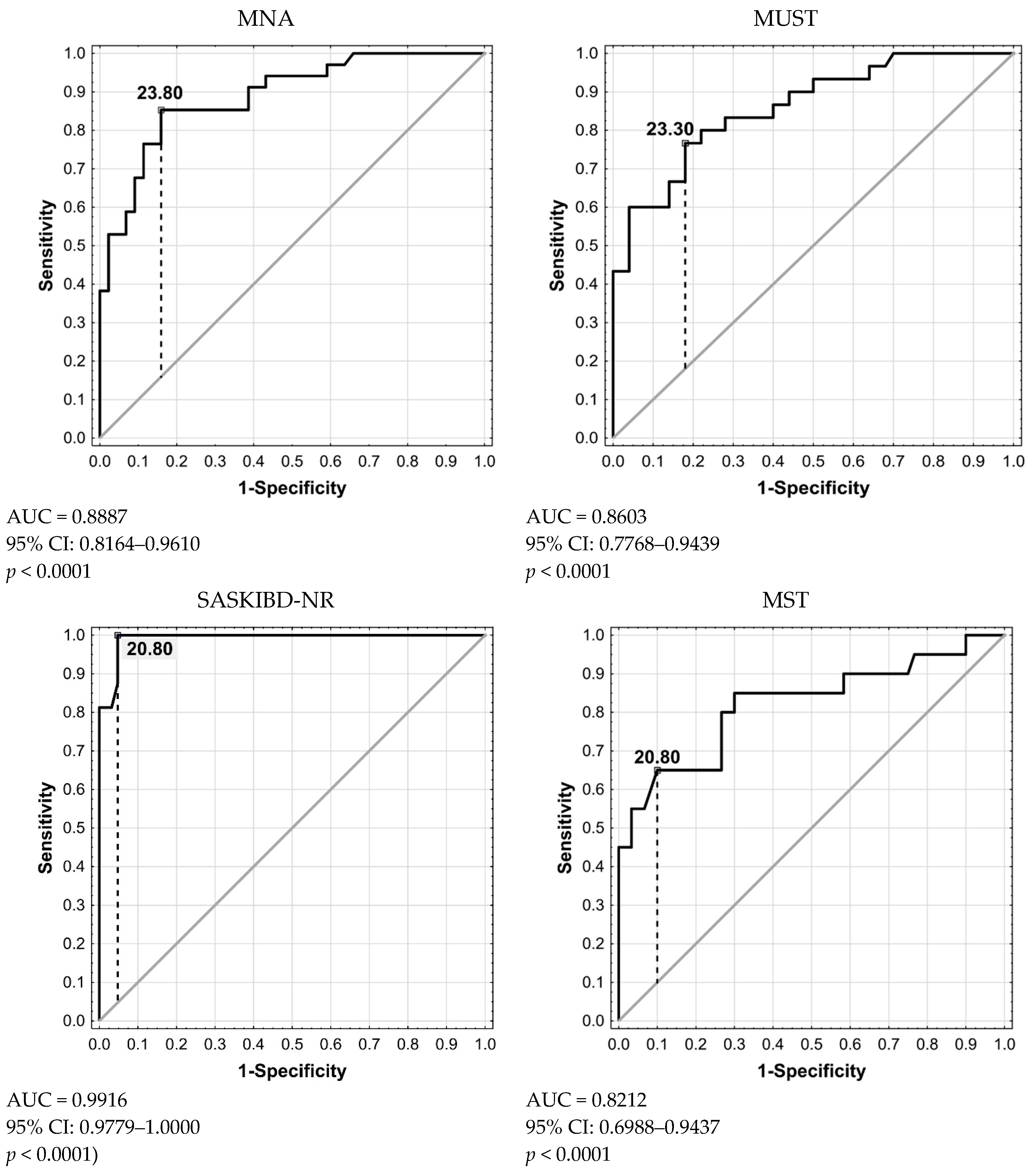
| Value | 95% CI | |
|---|---|---|
| MNA | ||
| Sensitivity [%] | 100 | 83.89–100 |
| Specificity [%] | 77.19 | 64.16–87.26 |
| Positive Likelihood Ratio | 4.38 | 2.72–7.07 |
| Negative Likelihood Ratio | 0 | |
| Positive Predictive Value [%] | 61.76 | 50.05–72.26 |
| Negative Predictive Value [%] | 100 | 91.96–100 |
| Accuracy [%] | 83.33 | 73.19–90.82 |
| Youden’s Index J | 0.77 | |
| MUST | ||
| Sensitivity [%] | 100 | 84.56–100 |
| Specificity [%] | 86.21 | 74.62–93.85 |
| Positive Likelihood Ratio | 7.25 | 3.81–13.80 |
| Negative Likelihood Ratio | 0 | |
| Positive Predictive Value [%] | 73.33 | 59.10–83.96 |
| Negative Predictive Value [%] | 100 | 92.89–100 |
| Accuracy [%] | 90 | 81.24–95.58 |
| Youden’s Index J | 0.86 | |
| SASKIBD-NR | ||
| Sensitivity [%] | 68.18 | 45.13–86.14 |
| Specificity [%] | 98.25 | 90.61–99.96 |
| Positive Likelihood Ratio | 38.86 | 5.45–276.90 |
| Negative Likelihood Ratio | 0.32 | 0.18–0.60 |
| Positive Predictive Value [%] | 93.75 | 67.80–99.07 |
| Negative Predictive Value [%] | 88.89 | 81.26–93.66 |
| Accuracy [%] | 89.87 | 81.02–95.53 |
| Youden’s Index J | 0.66 | |
| MST | ||
| Sensitivity [%] | 81.82 | 59.72–94.81 |
| Specificity [%] | 96.55 | 88.09–99.58 |
| Positive Likelihood Ratio | 23.73 | 5.99–93.94 |
| Negative Likelihood Ratio | 0.19 | 0.08–0.46 |
| Positive Predictive Value [%] | 90 | 69.45–97.27 |
| Negative Predictive Value [%] | 93.33 | 85.21–97.14 |
| Accuracy [%] | 92.5 | 84.39–97.20 |
| Youden’s Index J | 0.78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godala, M.; Gaszyńska, E.; Walczak, K.; Małecka-Wojciesko, E. An Evaluation of the Usefulness of Selected Screening Methods in Assessing the Risk of Malnutrition in Patients with Inflammatory Bowel Disease. Nutrients 2024, 16, 814. https://doi.org/10.3390/nu16060814
Godala M, Gaszyńska E, Walczak K, Małecka-Wojciesko E. An Evaluation of the Usefulness of Selected Screening Methods in Assessing the Risk of Malnutrition in Patients with Inflammatory Bowel Disease. Nutrients. 2024; 16(6):814. https://doi.org/10.3390/nu16060814
Chicago/Turabian StyleGodala, Małgorzata, Ewelina Gaszyńska, Konrad Walczak, and Ewa Małecka-Wojciesko. 2024. "An Evaluation of the Usefulness of Selected Screening Methods in Assessing the Risk of Malnutrition in Patients with Inflammatory Bowel Disease" Nutrients 16, no. 6: 814. https://doi.org/10.3390/nu16060814
APA StyleGodala, M., Gaszyńska, E., Walczak, K., & Małecka-Wojciesko, E. (2024). An Evaluation of the Usefulness of Selected Screening Methods in Assessing the Risk of Malnutrition in Patients with Inflammatory Bowel Disease. Nutrients, 16(6), 814. https://doi.org/10.3390/nu16060814









