Nutritional and Morphofunctional Assessment of Post-ICU Patients with COVID-19 at Hospital Discharge: NutriEcoMuscle Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Variables
2.3. Statistical Analysis
3. Results
3.1. Nutritional Evaluation
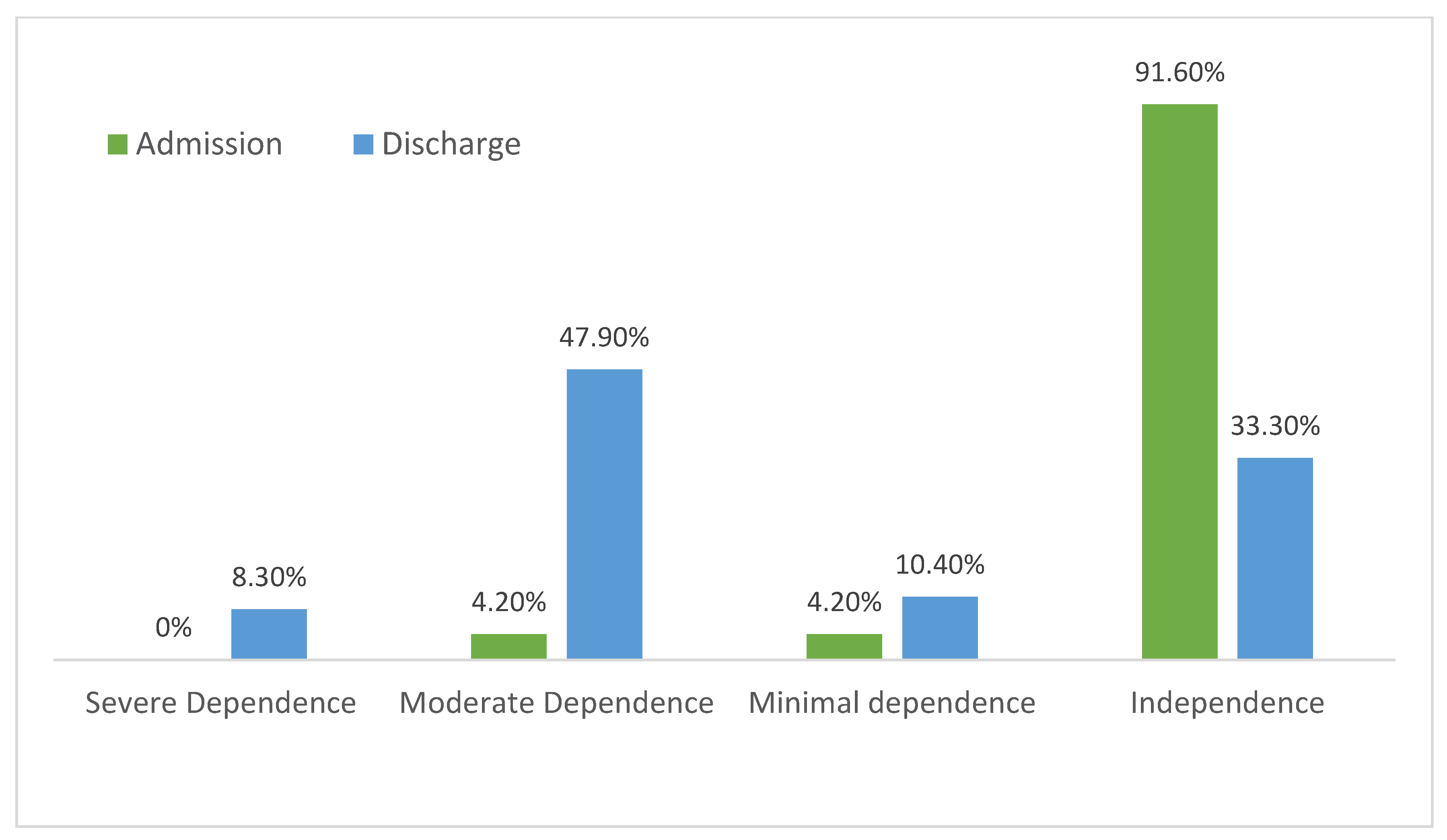
3.2. Functional Assessment
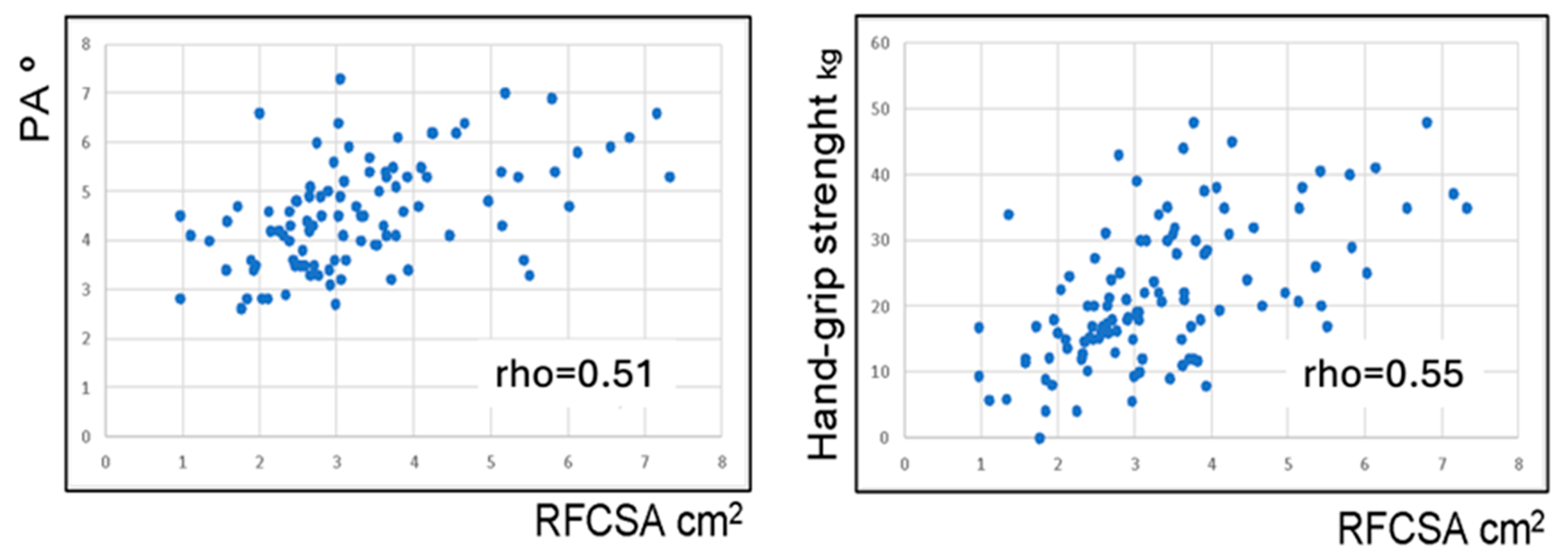
3.3. Body Composition Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Abate, S.M.; Ali, S.A.; Mantfardo, B.; Basu, B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: A systematic review and Meta-analysis. PLoS ONE 2020, 15, e0235653. [Google Scholar] [CrossRef]
- Singer, P. Preserving the quality of life: Nutrition in the ICU. Crit. Care 2019, 23, 139. [Google Scholar] [CrossRef]
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; Pearson, C.A.B.; CMMID Working Group; Jombart, T.; Procter, S.R.; Knight, G.M. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020, 18, 270. [Google Scholar] [CrossRef]
- Junek, M.L.; Jones, A.; Heckman, G.; Demers, C.; Griffith, L.E.; Costa, A.P. The predictive utility of functional status at discharge: A population-level cohort analysis. BMC Geriatr. 2022, 22, 8. [Google Scholar] [CrossRef]
- James, P.T.; Ali, Z.; Armitage, A.E.; Bonell, A.; Cerami, C.; Drakesmith, H.; Jobe, M.; Jones, K.S.; Liew, Z.; Moore, S.E.; et al. The Role of Nutrition in COVID-19 Susceptibility and Severity of Disease: A Systematic Review. J. Nutr. 2021, 151, 1854–1878. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Guerrero, D.B.; Lesmes, I.B.; Tinahones Madueño, F.J. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70 (Suppl. S1), 74–84. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Soler-Beunza, A.G.; Vegas-Aguilar, I.M.; Fernández-Jiménez, R.; Tinahones, F.J.; García-Almeida, J.M. Predictors of Sarcopenia in Outpatients with Post-Critical SARS-CoV2 Disease. Nutritional Ultrasound of Rectus Femoris Muscle, a Potential Tool. Nutrients 2022, 14, 4988. [Google Scholar] [CrossRef] [PubMed]
- da Silva Fink, J.; Daniel de Mello, P.; Daniel de Mello, E. Subjective global assessment of nutritional status—A systematic review of the literature. Clin. Nutr. 2015, 34, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; Crivelli, A.; Evans, D.; Gramlich, L.; Fuchs-Tarlovsky, V.; Keller, H.; Llido, L.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 207–217. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points [Internet]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed on 31 January 2024).
- American-Society-of-Hand-Therapists-Clinical-Assessment-Recommendations.pdf [Internet]. Available online: https://www.researchgate.net/profile/Elaine-Fess/publication/303400806_American_Society_of_Hand_Therapists_Clinical_Assessment_Recommendations/links/57409a6208aea45ee847b254/American-Society-of-Hand-Therapists-Clinical-Assessment-Recommendations.pdf (accessed on 31 January 2024).
- Bahat, G.; Kilic, C.; Altinkaynak, M.; Akif Karan, M. Comparison of standard versus population-specific handgrip strength cut-off points in the detection of probable sarcopenia after launch of EWGSOP2. Aging Male 2020, 23, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Kear, B.M.; Guck, T.P.; McGaha, A.L. Timed Up and Go (TUG) Test: Normative Reference Values for Ages 20 to 59 Years and Relationships with Physical and Mental Health Risk Factors. J. Prim. Care Community Health 2017, 8, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Chumlea, W.C.; Heymsfield, S.B.; Lukaski, H.C.; Schoeller, D.; Friedl, K.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Hubbard, V.S. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am. J. Clin. Nutr. 2003, 77, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R.; Charlier, R.; Caspers, M.; Knaeps, S.; Mertens, E.; Lambrechts, D.; Lefevre, J.; et al. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.; Genton, L.; Hans, D.; Pichard, C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin. Nutr. 2003, 22, 537–543. [Google Scholar] [CrossRef]
- Sergi, G.; De Rui, M.; Veronese, N.; Bolzetta, F.; Berton, L.; Carraro, S.; Bano, G.; Coin, A.; Manzato, E.; Perissinotto, E. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin. Nutr. 2015, 34, 667–673. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Vegas-Aguilar, I.M.; García-Almeida, J.M.; Bellido-Guerrero, D.; Talluri, A.; Lukaski, H.; Tinahones, F.J. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: A longitudinal cohort study. Clin. Nutr. 2022, 41, 3106–3114. [Google Scholar] [CrossRef]
- Nakamura, K.; Liu, K.; Katsukawa, H.; Nydahl, P.; Ely, E.W.; Kudchadkar, S.R.; Inoue, S.; Lefor, A.K.; Nishida, O. Nutrition therapy in the intensive care unit during the COVID-19 pandemic: Findings from the ISIIC point prevalence study. Clin. Nutr. 2022, 41, 2947–2954. [Google Scholar] [CrossRef]
- Eden, T.; McAuliffe, S.; Crocombe, D.; Neville, J.; Ray, S. Nutritional parameters and outcomes in patients admitted to intensive care with COVID-19: A retrospective single-centre service evaluation. BMJ Nutr. Prev. Health 2021, 4, 416. [Google Scholar] [CrossRef] [PubMed]
- Cuerda, C.; López, I.S.; Gil Martínez, C.; Viveros, M.M.; Velasco, C.; Peñafiel, V.C.; Jiménez, M.M.; Gonzalo, I.; González-Sánchez, V.; Carrasco, A.R.; et al. Impact of COVID-19 in nutritional and functional status of survivors admitted in intensive care units during the first outbreak. Preliminary results of the NUTRICOVID study. Clin. Nutr. 2021, 41, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 41, 1115–1127. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin. Cell Dev. Biol. 2016, 54, 42–52. [Google Scholar] [CrossRef]
- Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Horiguchi, H.; Brakenridge, S.C.; Gardner, A.; Efron, P.A.; Bihorac, A.; Segal, M.; Moore, F.A.; et al. Chronic Critical Illness and the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Front. Immunol. 2018, 9, 1511. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; D’Amico, M.; Sofia, V.; Roveri, L.; Mele, R.; Saibene, A.; Rovere-Querini, P.; Conte, C. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin. Nutr. 2021, 40, 2420–2426. [Google Scholar] [CrossRef]
- Shahbazi, S.; Hajimohammadebrahim-Ketabforoush, M.; Shariatpanahi, M.V.; Shahbazi, E.; Shariatpanahi, Z.V. The validity of the global leadership initiative on malnutrition criteria for diagnosing malnutrition in critically ill patients with COVID-19: A prospective cohort study. Clin. Nutr. ESPEN 2021, 43, 377–382. [Google Scholar] [CrossRef]
- Allard, J.P.; Keller, H.; Gramlich, L.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R. GLIM criteria has fair sensitivity and specificity for diagnosing malnutrition when using SGA as comparator. Clin. Nutr. 2020, 39, 2771–2777. [Google Scholar] [CrossRef]
- Ramos, A.; Joaquin, C.; Ros, M.; Martin, M.; Cachero, M.; Sospedra, M.; Martínez, E.; Migallón, J.M.S.; Sendrós, M.-J.; Soldevila, B.; et al. Impact of COVID-19 on nutritional status during the first wave of the pandemic. Clin. Nutr. 2022, 41, 3032–3037. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yang, Y.; Zhang, J. Obesity is associated with severe disease and mortality in patients with coronavirus disease 2019 (COVID-19): A meta-analysis. BMC Public Health 2021, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Kassir, R. Risk of COVID-19 for patients with obesity. Obes. Rev. 2020, 21, e13034. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [PubMed]
- Peckham, H.; De Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Sawadogo, W.; Tsegaye, M.; Gizaw, A.; Adera, T. Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: Systematic review and meta-analysis. BMJ Nutr. Prev. Health 2022, 5, 10–18. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, T.; Wang, Y.; Xia, L. The Centrality of Obesity in the Course of Severe COVID-19. Front. Endocrinol. 2021, 12, 620566. [Google Scholar] [CrossRef]
- Huang, M.S.; Chan, K.S.; Zanni, J.M.P.; Parry, S.M.; Neto, S.-C.G.B.; Neto, J.A.A.M.; da Silva, V.Z.M.; Kho, M.E.; Needham, D.M.F. Functional Status Score for the ICU: An International Clinimetric Analysis of Validity, Responsiveness, and Minimal Important Difference. Crit. Care Med. 2016, 44, e1155–e1164. [Google Scholar] [CrossRef]
- Costa, A.; Gonçalves, A.F.; Rodrigues, M.; Santos, R.; Almeida, M.P.; Lima, A. Post-intensive Care Unit COVID-19 Survivors: Functional Status and Respiratory Function Three Months After an Inpatient Rehabilitation Program. Cureus 2022, 14, e31281. [Google Scholar] [CrossRef] [PubMed]
- Cavalleri, J.; Treguier, D.; Deliège, T.; Gurdebeke, C.; Ernst, M.; Lambermont, B.; Misset, B.; Rousseau, A.-F. One-Year Functional Decline in COVID-19 and Non-COVID-19 Critically Ill Survivors: A Prospective Study Incorporating a Pre-ICU Status Assessment. Healthcare 2022, 10, 2023. [Google Scholar] [CrossRef] [PubMed]
- Leite, L.C.; Carvalho, L.; de Queiroz, D.M.; Farias, M.D.S.Q.; Cavalheri, V.; Edgar, D.W.; Nery, B.R.D.A.; Barros, N.V.; Maldaner, V.; Campos, N.G.; et al. Can the post-COVID-19 functional status scale discriminate between patients with different levels of fatigue, quality of life and functional performance? Pulmonology 2022, 28, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.J.; Barco, S.; Endres, M.; Geelhoed, J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef] [PubMed]
- Ciudin, A.; Simó-Servat, A.; Palmas, F.; Barahona, M.J. Sarcopenic obesity: A new challenge in the clinical practice. Endocrinol. Diabetes Nutr. 2020, 67, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007, 297, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Correa-De-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; El Behi, H.; Boizeau, P.; Verdonk, F.; Alberti, C.; Lescot, T. Reliability of ultrasound measurements of quadriceps muscle thickness in critically ill patients. BMC Anesthesiol. 2018, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Tsutsumi, R.; Okayama, Y.; Takashima, T.; Ueno, Y.; Itagaki, T.; Tsutsumi, Y.; Sakaue, H.; Oto, J. Monitoring of muscle mass in critically ill patients: Comparison of ultrasound and two bioelectrical impedance analysis devices. J. Intensive Care 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Padilla, I.A.; Rodríguez-Moguel, N.C.; Rodríguez-Llamazares, S.; Aguilar-Vargas, A.; Casas-Aparicio, G.A.; Ríos-Ayala, M.A.; Hernández-Cardenas, C.M. Low phase angle is associated with 60-day mortality in critically ill patients with COVID-19. J. Parenter. Enter. Nutr. 2022, 46, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Rijk, J.M.; Roos, P.R.; Deckx, L.; Akker, M.v.D.; Buntinx, F. Prognostic value of handgrip strength in people aged 60 years and older: A systematic review and meta-analysis. Geriatr. Gerontol. Int. 2016, 16, 5–20. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Pucci, G.; D’abbondanza, M.; Curcio, R.; Alcidi, R.; Campanella, T.; Chiatti, L.; Gandolfo, V.; Veca, V.; Casarola, G.; Leone, M.C.; et al. Handgrip strength is associated with adverse outcomes in patients hospitalized for COVID-19-associated pneumonia. Intern. Emerg. Med. 2022, 17, 1997–2004. [Google Scholar] [CrossRef]
- Mueller, N.; Murthy, S.; Tainter, C.R.M.; Lee, J.; Riddell, K.; Fintelmann, F.J.; Grabitz, S.D.; Timm, F.P.; Levi, B.; Kurth, T.M.; et al. Can Sarcopenia Quantified by Ultrasound of the Rectus Femoris Muscle Predict Adverse Outcome of Surgical Intensive Care Unit Patients as well as Frailty? A Prospective, Observational Cohort Study. Ann. Surg. 2016, 264, 1116–1124. [Google Scholar] [CrossRef]
- de Andrade-Junior, M.C.; de Salles, I.C.D.; de Brito, C.M.M.; Pastore-Junior, L.; Righetti, R.F.; Yamaguti, W.P. Skeletal Muscle Wasting and Function Impairment in Intensive Care Patients with Severe COVID-19. Front. Physiol. 2021, 12, 640973. [Google Scholar] [CrossRef]
- Umbrello, M.; Guglielmetti, L.; Formenti, P.; Antonucci, E.; Cereghini, S.; Filardo, C.; Montanari, G.; Muttini, S. Qualitative and quantitative muscle ultrasound changes in patients with COVID-19–related ARDS. Nutrition 2021, 91–92, 111449. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diabetes Rep. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Martínez, J.J.; Solís García Del Pozo, J.; Quílez Toboso, R.P.; García Blasco, L.; Rosa Felipe, C. Study of incidence of COVID-19 in Spain and its relationship to geographical province distribution. J. Healthc. Qual. Res. 2023, 38, 299–303. [Google Scholar] [CrossRef] [PubMed]
| Total | Men | Women | p-Value | |
|---|---|---|---|---|
| n (%) | 96 (100.0) | 69 (71.9) | 27 (28.1) | - |
| Age, mean (SD), y | 58.8 (8.5) | 58.5 (8.8) | 59.7 (7.7) | NS |
| Age ≥ 65 years, n (%) | 29 (30.2) | 20 (29.0) | 9 (33.3) | NS |
| BMI, mean (SD), kg/m2 | 28.8 (5.8) | 27.9 (5.1) | 30.9 (6.2) | NS |
| BI scores upon admission | 99.0 (4.9) | 98.8 (5.6) | 99.6 (1.9) | NS |
| Comorbidities, n (%): | ||||
| Obesity | 40 (41.7) | 24 (34.8) | 16 (59.3) | 0.0386 * |
| HBP | 34 (35.4) | 22 (31.9) | 12 (44.4) | NS |
| Diabetes mellitus | 19 (19.8) | 13 (18.8) | 6 (22.2) | NS |
| COPD | 6 (6.3) | 5 (7.2) | 1 (3.7) | NS |
| CKD | 2 (2.1) | 2 (2.9) | 0 (0.0) | NS |
| CHF | 3 (3.1) | 3 (4.3) | 0 (0.0) | NS |
| Active oncologic pathology | 2 (2.1) | 2 (2.9) | 0 (0.0) | NS |
| Length of stay, mean (SD), days | 48.2 (37.6) | 49.7 (40.9) | 44.6 (27.6) | NS |
| Pre-ICU hospital stay, mean (SD), days | 2.3 (3.2) | 2.0 (2.4) | 3.1 (4.7) | NS |
| ICU stay, mean (SD), days | 28.7 (7.5) | 30.3 (29.7) | 24.4 (20.4) | NS |
| ICU characteristics, mean (SD): | ||||
| SOFA score | 4.1 (2.4) | 4.2 (2.7) | 4.0 (2.3) | NS |
| Mechanical ventilation, n (%): | 58 (60.4) | 40 (58.0) | 18 (66.7) | NS |
| NIMV | 5 (5.2) | 4 (5.8) | 1 (3.7) | NS |
| HFNC | 33 (34.4) | 25 (36.2) | 8 (29.6) | NS |
| CRP, median (IQR), mg/dL | 2.9 (0.5–9.0) | 2.9 (0.4–11.3) | 2.9 (0.7–8.4) | NS |
| Total | SGA-B | SGA-C | p-Value | |
|---|---|---|---|---|
| n (%) | 96 | 50 (52.1) | 46 (47.9) | - |
| Weight lost during last 6 months: | ||||
| Mean (SD), kg | 11.0 (7.1) | 7.3 (4.7) | 14.9 (7.3) | <0.0001 |
| Mean (SD), % | 11.6 (6.7) | 8.2 (5.2) | 15.3 (6.1) | <0.0001 |
| Physical examination, n (%): | ||||
| Subcutaneous fat loss | 79 (82.3) | 34 (68.0) | 45 (97.8) | <0.0001 |
| Loss of muscle mass | 80 (83.3) | 35 (70.0) | 45 (97.8) | 0.0002 |
| Malleolar edema | 15 (15.6) | 7 (14.0) | 8 (17.4) | NS |
| Sacral edema | 4 (4.2) | 2 (4.3) | 2 (4.3) | NS |
| Ascites | 1 (1.0) | 0 (0.0) | 1 (2.2) | NS |
| Gastrointestinal symptoms, n (%): | ||||
| None | 62 (64.6) | 37 (74.0) | 25 (54.3) | NS |
| Nausea | 4 (4.2) | 0 (0.0) | 4 (8.7) | 0.0491 |
| Vomiting | 1 (1.0) | 0 (0.0) | 1 (2.2) | NS |
| Diarrhea | 10 (10.4) | 2 (4.0) | 8 (17.4) | 0.0447 |
| Dysphagia | 4 (4.2) | 3 (6.0) | 1 (2.2) | NS |
| Abdominal pain | 6 (6.3) | 3 (6.0) | 3 (6.5) | NS |
| Anorexia | 19 (19.8) | 7 (14.0) | 12 (26.1) | NS |
| Total | Moderate | Severe | p-Value | |
|---|---|---|---|---|
| n (%) | 96 | 44 (45.8) | 52 (54.2) | - |
| Weight loss within the past 6 months: | ||||
| Mean (SD), kg | 11.1 (7.1) | 5.9 (3.0) | 15.2 (6.9) | <0.0001 |
| Mean (SD), % | 11.6 (6.7) | 6.3 (2.9) | 16.1 (5.6) | <0.0001 |
| Weight loss: | ||||
| 5–10% within the last six months or 10-20% beyond six months, n (%) | 36 (37.5) | 34 (77.3) | 2 (3.8) | <0.0001 |
| >10% within the last six months or >20% beyond 6 months, n (%) | 49 (51.0) | 0 (0.0) | 49 (94.2) | <0.0001 |
| Low BMI (kg/m2): <20 in <70 years or <22 in ≥70 years, n (%) | 3 (3.1) | 1 (2.3) | 2 (3.8) | NS |
| Reduced muscle mass *: | ||||
| Mild to moderate deficit, n (%) | 30 (31.3) | 20 (45.5) | 10 (19.2) | 0.0079 |
| Severe deficit, n (%) | 18 (18.8) | 0 (0.0) | 18 (34.6) | <0.0001 |
| Reduced dietary intake (or absorption), n (%) | 58 (60.4) | 27 (61.4) | 31 (59.6) | NS |
| Inflammation, n (%) ** | 88 (91.7) | 39 (88.6) | 49 (94.2) | NS |
| Total | Men | Women | p-Value | |
|---|---|---|---|---|
| n (%) | 96 (100.0) | 69 (71.9) | 27 (28.1) | |
| BI score, median (IQR) | 90 (65–100) | 90 (65–100) | 85 (70–95) | NS |
| BI < 100, n (%) | 64 (66.7) | 42 (60.9) | 22 (81.5) | NS |
| Total dependency | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Severe dependency | 8 (8.3) | 6 (8.7) | 2 (7.4) | |
| Moderate dependency | 20 (20.8) | 15 (21.7) | 5 (18.5) | |
| Media dependency | 26 (27.1) | 15 (21.7) | 11 (40.7) | |
| Minimal dependency | 10 (10.4) | 6 (8.7) | 4 (14.8) |
| Total | Men * | Women | p-Value | |
|---|---|---|---|---|
| Handgrip strength: | ||||
| Mean (SD), kg | 21.7 (11.0) | 25.0 (10.9) | 13.1 (5.0) | <0.0001 |
| <27 men or <16 women; n (%) | 60 (62.5) | 39 (56.5) | 21 (77.8) | NS |
| TUG test: | ||||
| Mean (SD), seconds | 20.0 (17.3) | 16.7 (14.2) | 28.1 (21.5) | 0.0004 |
| >20 s, n (%) | 26 (27.1) | 14 (20.3) | 12 (44.4) | 0.0224 |
| Total | Men | Women | p-Value | |
|---|---|---|---|---|
| n (%) | 96 (100.0) | 69 (71.9) | 27 (28.1) | |
| BIA *: | ||||
| FFMI, mean (SD) FFMI < 17 men or <15 kg/m2 women; n (%) | 17.4 (3.9) 33.3 | 17.7 (4.1) 35.8 | 16.3 (3.3) 26.1 | 0.0341 NS |
| SMMI, mean (SD), kg/m2 | 8.5 (2.4) | 8.9 (2.1) | 7.5 (2.9) | 0.0007 |
| PA, mean (SD), o PA < 3.95 (%) | 4.5 (1.1) 29.5 | 4.6 (1.1) 30.8 | 4.4 (0.9) 27.3 | NS NS |
| Nutritional US: | ||||
| Subcutaneous abdominal adipose tissue, mean (SD), cm | 2.11 (0.9) | 1.87 (0.8) | 2.72 (0.9) | <0.0001 |
| Preperitoneal adipose tissue, mean (SD), cm | 0.9 (0.5) | 0.8 (0.4) | 1.0 (0.5) | NS |
| RF-CSA, mean (SD), cm2 | 3.4 (1.3) | 3.7 (1.4) | 2.6 (0.7) | <0.0001 |
| RF thickness, mean ± (SD), cm | 1.0 (0.6) | 1.2 (0.5) | 0.9 (0.2) | <0.0001 |
| RF circumference, mean (SD), cm | 8.7 (1.4) | 9.0 (1.4) | 7.9 (1.1) | <0.0001 |
| Myosteatosis (%) ** | 83.7 | 78.1 | 100.0 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joaquín, C.; Bretón, I.; Ocón Bretón, M.J.; Burgos, R.; Bellido, D.; Matía-Martín, P.; Martínez Olmos, M.Á.; Zugasti, A.; Riestra, M.; Botella, F.; et al. Nutritional and Morphofunctional Assessment of Post-ICU Patients with COVID-19 at Hospital Discharge: NutriEcoMuscle Study. Nutrients 2024, 16, 886. https://doi.org/10.3390/nu16060886
Joaquín C, Bretón I, Ocón Bretón MJ, Burgos R, Bellido D, Matía-Martín P, Martínez Olmos MÁ, Zugasti A, Riestra M, Botella F, et al. Nutritional and Morphofunctional Assessment of Post-ICU Patients with COVID-19 at Hospital Discharge: NutriEcoMuscle Study. Nutrients. 2024; 16(6):886. https://doi.org/10.3390/nu16060886
Chicago/Turabian StyleJoaquín, Clara, Irene Bretón, María Julia Ocón Bretón, Rosa Burgos, Diego Bellido, Pilar Matía-Martín, Miguel Ángel Martínez Olmos, Ana Zugasti, María Riestra, Francisco Botella, and et al. 2024. "Nutritional and Morphofunctional Assessment of Post-ICU Patients with COVID-19 at Hospital Discharge: NutriEcoMuscle Study" Nutrients 16, no. 6: 886. https://doi.org/10.3390/nu16060886
APA StyleJoaquín, C., Bretón, I., Ocón Bretón, M. J., Burgos, R., Bellido, D., Matía-Martín, P., Martínez Olmos, M. Á., Zugasti, A., Riestra, M., Botella, F., & García Almeida, J. M. (2024). Nutritional and Morphofunctional Assessment of Post-ICU Patients with COVID-19 at Hospital Discharge: NutriEcoMuscle Study. Nutrients, 16(6), 886. https://doi.org/10.3390/nu16060886






