Supplementing Ryegrass Ameliorates Commercial Diet-Induced Gut Microbial Dysbiosis-Associated Spleen Dysfunctions by Gut–Microbiota–Spleen Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval Statement
2.2. Animals, Diets, and Management
2.3. Sample Collection
2.4. Determination of LPS, ROS, IgA, IgG, and IgM
2.5. Determination of Antioxidant Activity
2.6. RNA Extraction and Gene Expression Analysis
2.7. Determination of Short-Chain Fatty Acids
2.8. DNA Extraction and 16S rRNA Gene Sequencing
2.9. Illumina MiSeq Sequencing
2.10. Bioinformatics Analysis of Sequencing Data
2.11. Statistical Analysis
3. Results
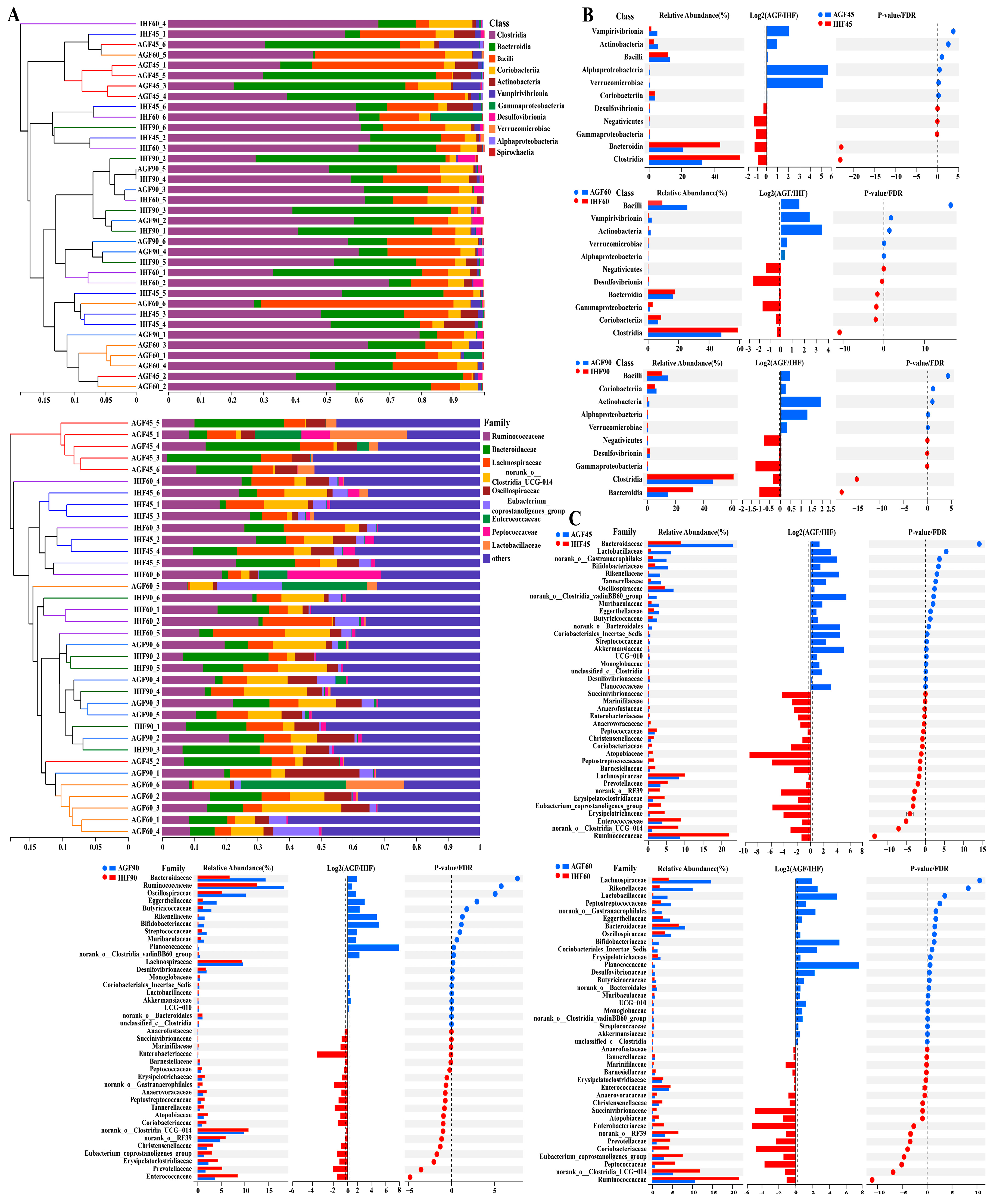
3.1. Commercial Diet Caused Gut Microbial Alterations in Geese
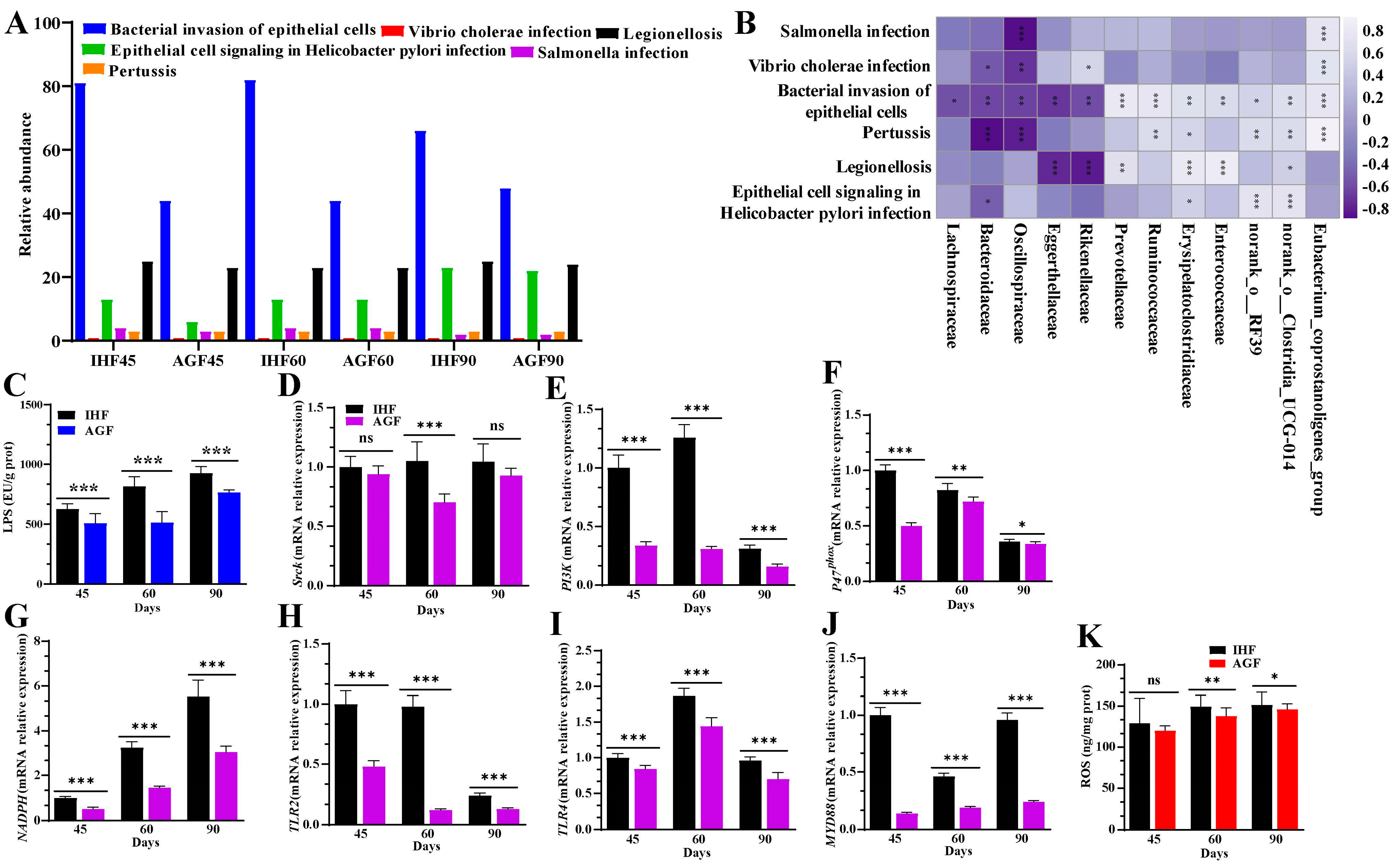
3.2. Commercial diet-Dependent Gut Microbial Alterations Are Paralleled by LPS-Induced Oxidative Stress Production
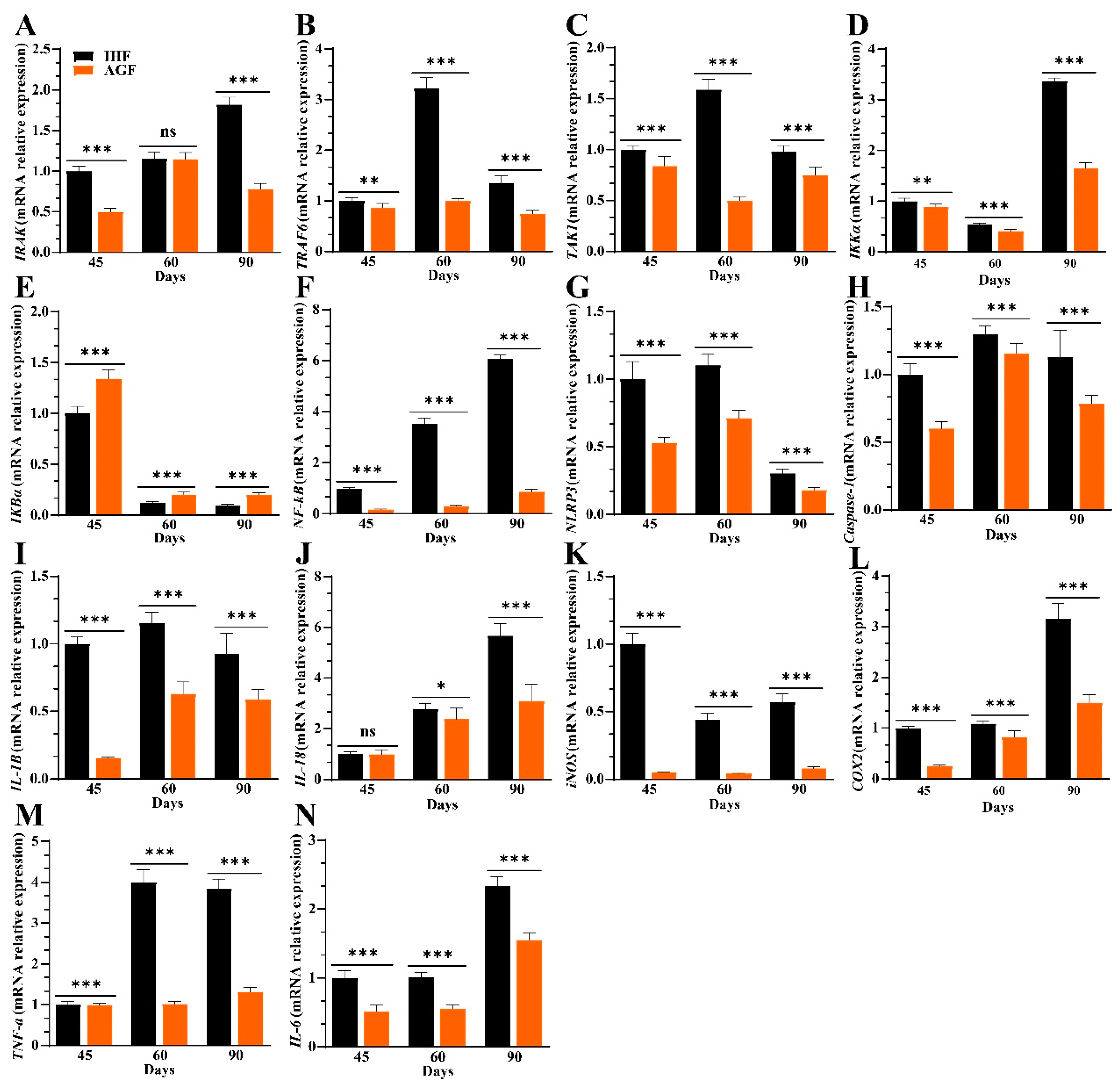
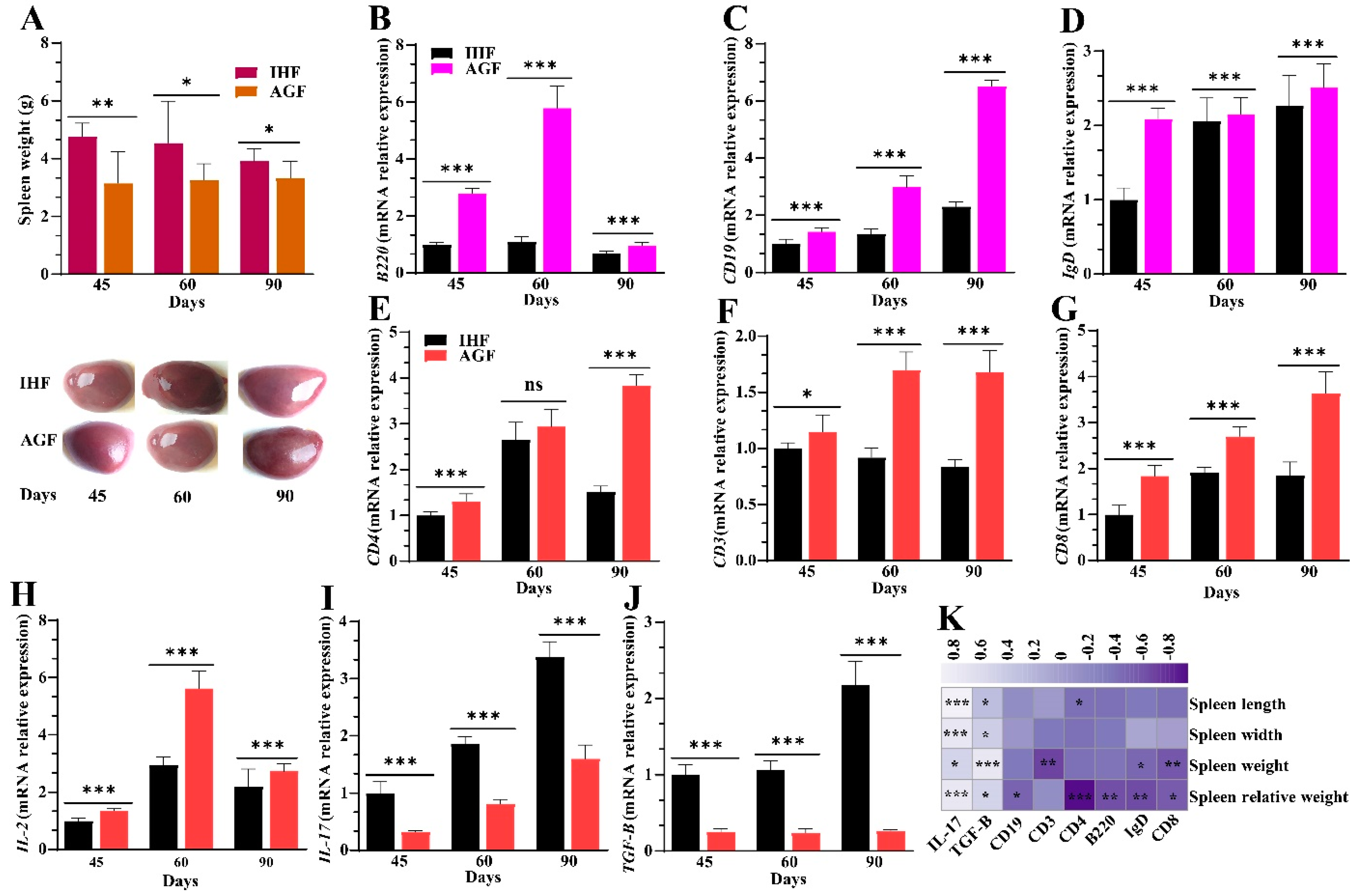
3.3. Commercial Diet-Induced Oxidative Stress Modulates Spleen Weight and Splenocyte Populations via NF-κB/NLRP3 Pathway-Induced Systemic Inflammation
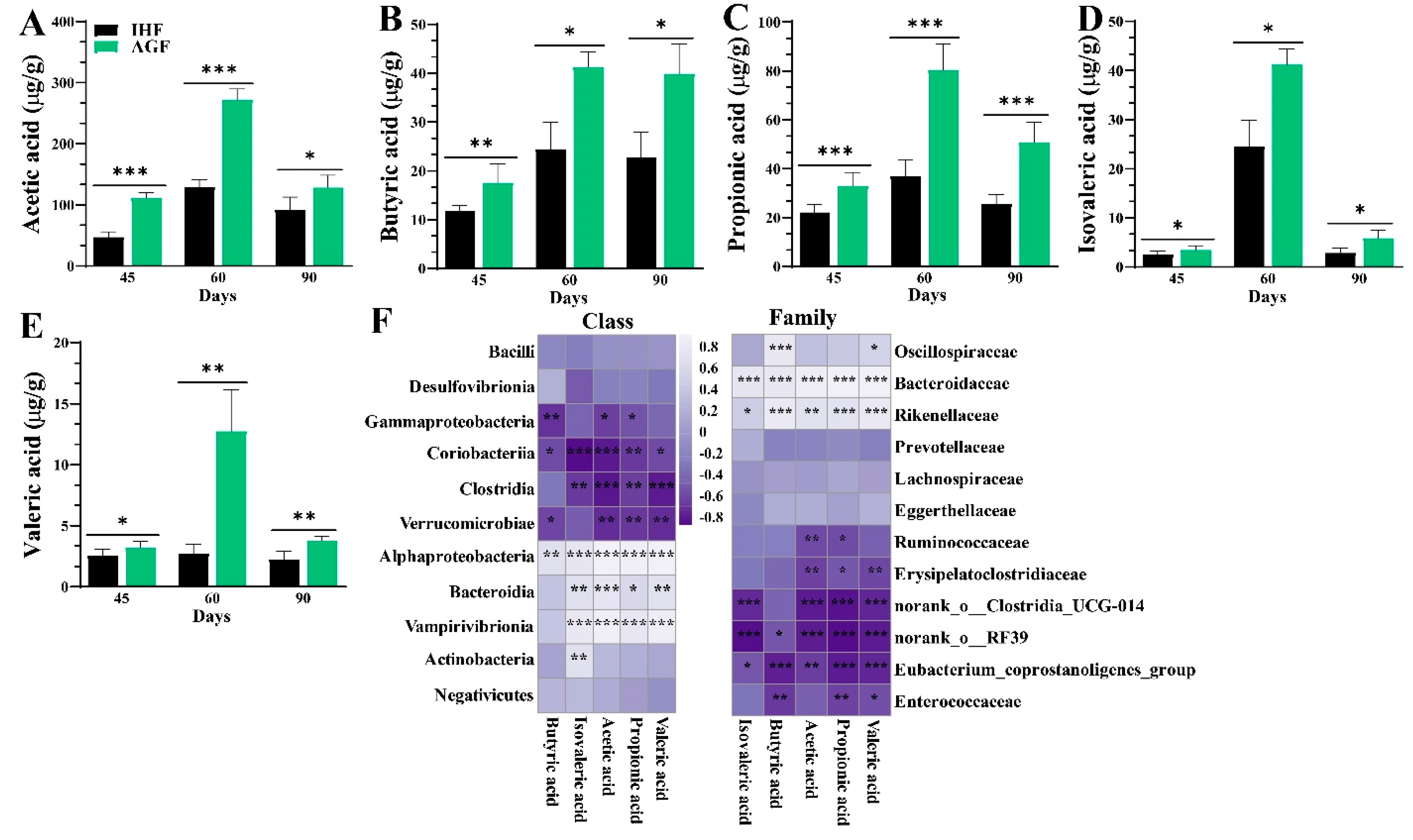
3.4. Long-Term Ryegrass Intake Causes Gut Microbial Short-Chain Fatty Acid Enrichment
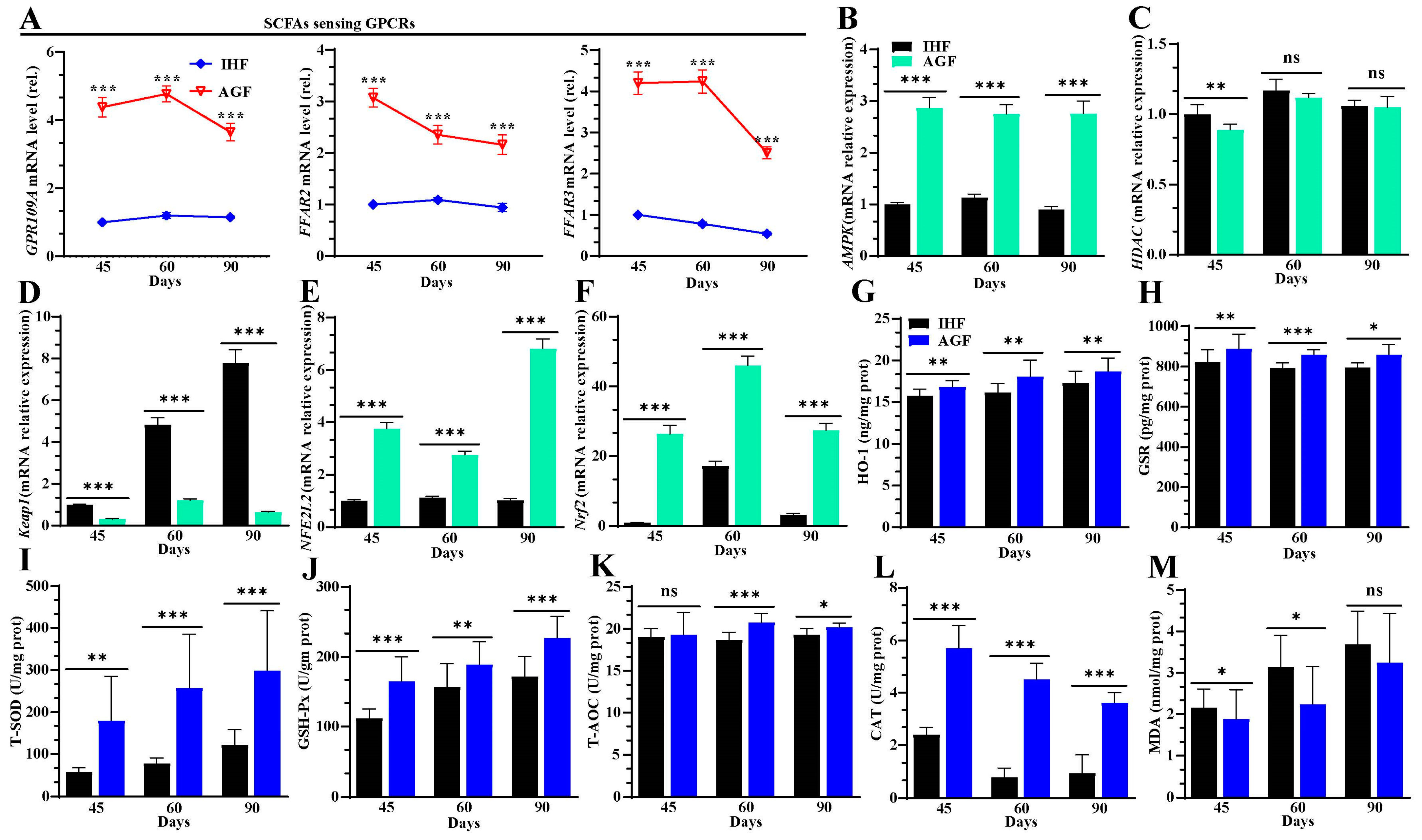
3.5. Ryegrass-Dependent SCFA Regulation Improved Keap1-Nrf2 Pathway Activation
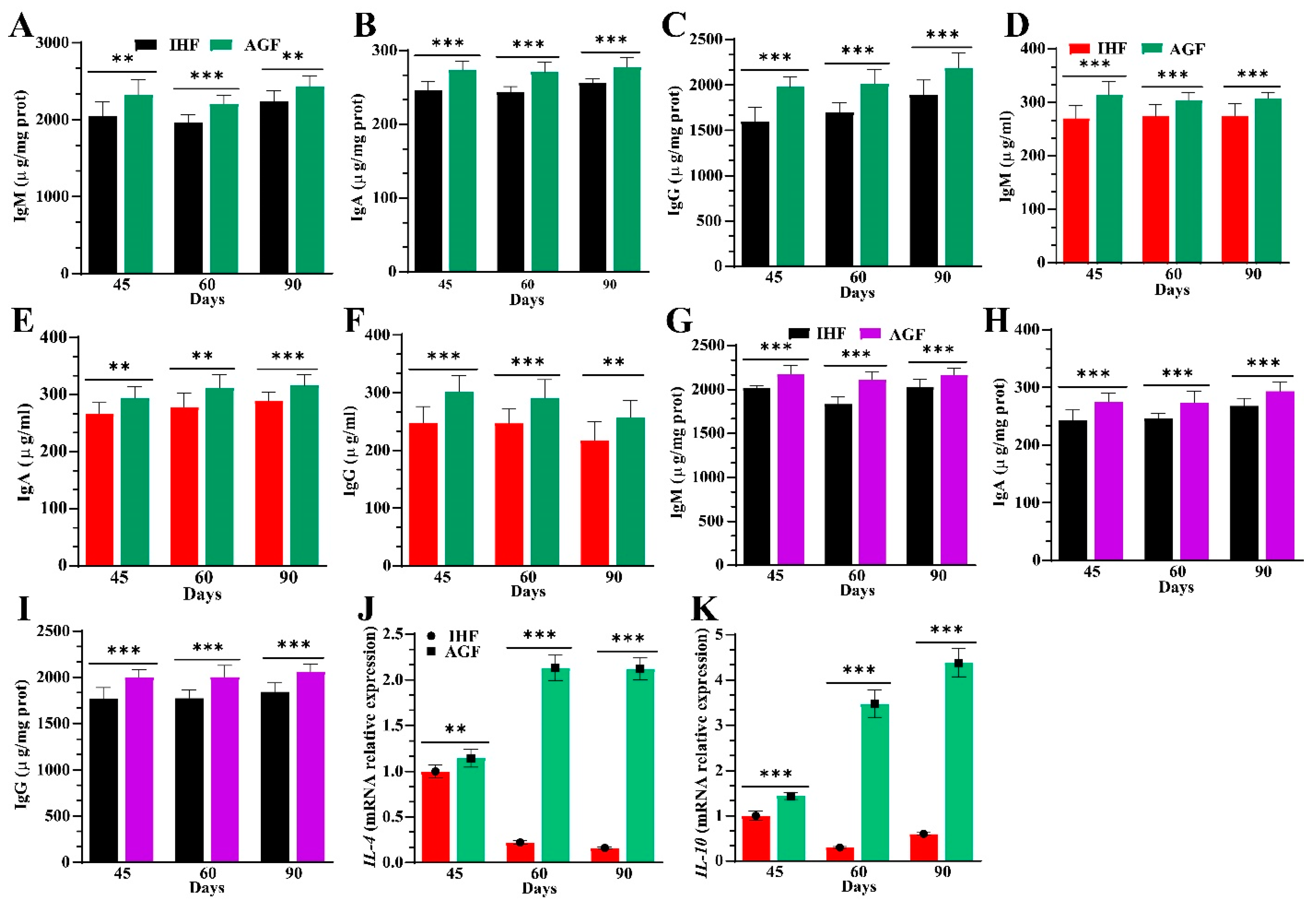
3.6. Ryegrass-Dependent Keap1-Nrf2 Pathway Activation Impedes Endotoxemia, Systemic Inflammation, and Spleen Dysfunctions
3.7. Long-Term Ryegrass Supplementation Impedes Splenic Dysfunctions by Maintaining the Gut Microbial Microenvironment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Zhou, M.; Zhou, Y.; Guan, X. Dietary components regulate chronic diseases through gut microbiota: A review. J. Sci. Food Agric. 2023, 103, 6752–6766. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ma, S.; Farooq, U.; Niu, J.; Li, F.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; Shi, Y. Pasture intake protects against commercial diet-induced lipopolysaccharide production facilitated by gut microbiota through activating intestinal alkaline phosphatase enzyme in meat geese. Front. Immunol. 2022, 13, 1041070. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ma, S.; La, S.; Guo, Z.; Liu, B.; Gao, Z.; Farooq, U.; Wang, Z.; Zhu, X.; Cui, Y.; et al. Microbial short-chain fatty acids: A bridge between dietary fibers and poultry gut health—A review. Anim. Biosci. 2022, 35, 1461–1478. [Google Scholar] [CrossRef] [PubMed]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Di Somma, C.; Muscogiuri, G.; Tarantino, G.; Tenore, G.C.; Orio, F.; Colao, A.; Savastano, S. Nutrition, inflammation and liver-spleen axis. Crit. Rev. Food Sci. Nutr. 2018, 58, 3141–3158. [Google Scholar] [CrossRef]
- Sun, L.; Jia, H.; Li, J.; Yu, M.; Yang, Y.; Tian, D.; Zhang, H.; Zou, Z. Cecal Gut Microbiota and Metabolites Might Contribute to the Severity of Acute Myocardial Ischemia by Impacting the Intestinal Permeability, Oxidative Stress, and Energy Metabolism. Front. Microbiol. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Sahin, G.S.; Lee, H.; Engin, F. An accomplice more than a mere victim: The impact of β-cell ER stress on type 1 diabetes pathogenesis. Mol. Metab. 2021, 54, 101365. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Ishii, T.; Miyazawa, M.; Takanashi, Y.; Tanigawa, M.; Yasuda, K.; Onouchi, H.; Kawabe, N.; Mitsushita, J.; Hartman, P.S.; Ishii, N. Genetically induced oxidative stress in mice causes thrombocytosis, splenomegaly and placental angiodysplasia that leads to recurrent abortion. Redox Biol. 2014, 2, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sakamoto, A.; Chang, L.; Qu, Y.; Wang, S.; Pu, Y.; Tan, Y.; Wang, X.; Fujita, Y.; Ishima, T.; et al. Splenic NKG2D confers resilience versus susceptibility in mice after chronic social defeat stress: Beneficial effects of (R)-ketamine. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, L.; Chang, L.; Pu, Y.; Qu, Y.; Hashimoto, K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Weledji, E.P. Benefits and risks of splenectomy. Int. J. Surg. 2014, 12, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, Y.O.; Mueller, S.N. Splenic stromal niches in homeostasis and immunity. Nat. Rev. Immunol. 2023, 23, 705–719. [Google Scholar] [CrossRef]
- Rosado, M.M.; Aranburu, A.; Scarsella, M.; Cascioli, S.; Giorda, E.; Del Chierico, F.; Mortera, S.L.; Mortari, E.P.; Petrini, S.; Putignani, L.; et al. Spleen development is modulated by neonatal gut microbiota. Immunol. Lett. 2018, 199, 1–15. [Google Scholar] [CrossRef]
- Carsetti, R.; Di Sabatino, A.; Rosado, M.M.; Cascioli, S.; Piano Mortari, E.; Milito, C.; Grimsholm, O.; Aranburu, A.; Giorda, E.; Tinozzi, F.P.; et al. Lack of Gut Secretory Immunoglobulin A in Memory B-Cell Dysfunction-Associated Disorders: A Possible Gut-Spleen Axis. Front. Immunol. 2019, 10, 2937. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, B.; Chen, X.; Zhang, J.; Yuan, S. A key role of gut microbiota-vagus nerve/spleen axis in sleep deprivation-mediated aggravation of systemic inflammation after LPS administration. Life Sci. 2021, 265, 118736. [Google Scholar] [CrossRef]
- Fang, H.; Feng, X.; Xu, T.; Zhong, R.; Lu, D.; Zhang, H.; Shen, W.; Zhao, Y.; Chen, L.; Wang, J. Gut-Spleen Axis: Microbiota via Vascular and Immune Pathways Improve Busulfan-Induced Spleen Disruption. mSphere 2023, 8, e0058122. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Song, Y.; Yang, Z.; Cai, Z. Exposure to ambient fine particulate matter impedes the function of spleen in the mouse metabolism of high-fat diet. J. Hazard. Mater. 2022, 423, 127129. [Google Scholar] [CrossRef]
- Fang, T.; Wu, X.; Cao, W.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Tang, J.; Wang, J.; Liu, G. Effects of dietary fiber on the antioxidant capacity, immune status, and antioxidant-relative signaling molecular gene expression in rat organs. RSC Adv. 2017, 7, 19611–19620. [Google Scholar] [CrossRef]
- Schäfer, A.L.; Eichhorst, A.; Hentze, C.; Kraemer, A.N.; Amend, A.; Sprenger, D.T.L.; Fluhr, C.; Finzel, S.; Daniel, C.; Salzer, U.; et al. Low Dietary Fiber Intake Links Development of Obesity and Lupus Pathogenesis. Front. Immunol. 2021, 12, 696810. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, L.; Zaman, M.H.; Dong, W.; He, G.; Deng, G.M. Predominant Role of Immunoglobulin G in the Pathogenesis of Splenomegaly in Murine Lupus. Front. Immunol. 2019, 10, 3020. [Google Scholar] [CrossRef]
- Das, Q.; Islam, M.R.; Lepp, D.; Tang, J.; Yin, X.; Mats, L.; Liu, H.; Ross, K.; Kennes, Y.M.; Yacini, H.; et al. Gut Microbiota, Blood Metabolites, and Spleen Immunity in Broiler Chickens Fed Berry Pomaces and Phenolic-Enriched Extractives. Front. Vet. Sci. 2020, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, X.; Yin, Z.; Guo, W.; Wu, S.; Yang, X. Transgenerational effects of paternal dietary Astragalus polysaccharides on spleen immunity of broilers. Int. J. Biol. Macromol. 2018, 115, 90–97. [Google Scholar] [CrossRef]
- Błaszczyk, K.; Wilczak, J.; Harasym, J.; Gudej, S.; Suchecka, D.; Królikowski, T.; Lange, E.; Gromadzka-Ostrowska, J. Impact of low and high molecular weight oat beta-glucan on oxidative stress and antioxidant defense in spleen of rats with LPS induced enteritis. Food Hydrocoll. 2015, 51, 272–280. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. CVI 2010, 17, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.R.; Jerome, F.N.; Reinhart, B.S. Degree of Splenomegaly in Domestic Chickens Selected for Resistance and Susceptibility to Marek’s Disease. Poult. Sci. 1969, 48, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.-c.; Huang, J. Effects of soybean meal replacement with fermented alfalfa meal on the growth performance, serum antioxidant functions, digestive enzyme activities, and cecal microflora of geese. J. Integr. Agric. 2016, 15, 2077–2086. [Google Scholar] [CrossRef]
- Mourão, J.L.; Pinheiro, V.M.; Prates, J.A.; Bessa, R.J.; Ferreira, L.M.; Fontes, C.M.; Ponte, P.I. Effect of dietary dehydrated pasture and citrus pulp on the performance and meat quality of broiler chickens. Poult. Sci. 2008, 87, 733–743. [Google Scholar] [CrossRef]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef]
- Wan, X.; Eguchi, A.; Sakamoto, A.; Fujita, Y.; Yang, Y.; Qu, Y.; Hatano, M.; Mori, C.; Hashimoto, K. Impact of broad-spectrum antibiotics on the gut-microbiota-spleen-brain axis. Brain Behav. Immun.—Health 2023, 27, 100573. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, B.; Cui, Y.; Ali, Q.; Zhu, X.; Li, D.; Ma, S.; Wang, Z.; Wang, C.; Shi, Y. Gut Microbiota Modulate Rabbit Meat Quality in Response to Dietary Fiber. Front. Nutr. 2022, 9, 849429. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Zhao, X.; Shang, C.; Xiang, M.; Li, L.; Cui, X. Microbiota-derived short-chain fatty acids: Implications for cardiovascular and metabolic disease. Front. Cardiovasc. Med. 2022, 9, 900381. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Yoon, B.I.; Hunter, C.A.; Kwon, H.M.; Sung, H.W.; Park, J. Short chain fatty acids facilitate protective immunity by macrophages and T cells during acute fowl adenovirus-4 infection. Sci. Rep. 2023, 13, 17999. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, X.; Kim, Y.; Li, J.; Huang, S.; Saleem, S.; Li, R.C.; Xu, Y.; Dore, S.; Cao, W. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic. Biol. Med. 2012, 52, 928–936. [Google Scholar] [CrossRef] [PubMed]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8(+) T Cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Y.; Su, Y.; Gao, Z.; Diao, X.; Li, J.; Zhu, X.; Li, D.; Li, Z.; Wang, C.; et al. Dietary Fiber Ameliorates Lipopolysaccharide-Induced Intestinal Barrier Function Damage in Piglets by Modulation of Intestinal Microbiome. mSystems 2021, 6, e01374-20. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Y.; Li, S.; Jin, Y.; Zhao, L.; Zhao, F.; Feng, J.; Yan, W.; Wei, Y. Altered gut microbiota after traumatic splenectomy is associated with endotoxemia. Emerg. Microbes Infect. 2018, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Ofek, T.; Lalzar, M.; Izhaki, I.; Halpern, M. Intestine and spleen microbiota composition in healthy and diseased tilapia. Anim. Microbiome 2022, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Patil, N.K.; Stothers, C.L.; Luan, L.; McBride, M.A.; Owen, A.M.; Burelbach, K.R.; Williams, D.L.; Sherwood, E.R.; Bohannon, J.K. Immunobiology and application of toll-like receptor 4 agonists to augment host resistance to infection. Pharmacol. Res. 2019, 150, 104502. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y.; Xie, B.; Yao, H.; Yuan, Y.; Yuan, S.; Zhang, J. The spleen mediates chronic sleep restriction-mediated enhancement of LPS-induced neuroinflammation, cognitive deficits, and anxiety-like behavior. Aging 2020, 12, 15446–15461. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef]
- Ichikawa, S.; Miyake, M.; Fujii, R.; Konishi, Y. MyD88 associated ROS generation is crucial for Lactobacillus induced IL-12 production in macrophage. PLoS ONE 2012, 7, e35880. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A. Effect of Omega-3 or Omega-6 Dietary Supplementation on Testicular Steroidogenesis, Adipokine Network, Cytokines, and Oxidative Stress in Adult Male Rats. Oxidative Med. Cell. Longev. 2021, 2021, 5570331. [Google Scholar] [CrossRef]
- Ali, Q.; Ma, S.; Liu, B.; Mustafa, A.; Wang, Z.; Sun, H.; Cui, Y.; Li, D.; Shi, Y. Artificial Pasture Grazing System Attenuates Lipopolysaccharide-Induced Gut Barrier Dysfunction, Liver Inflammation, and Metabolic Syndrome by Activating ALP-Dependent Keap1-Nrf2 Pathway. Animals 2023, 13, 3574. [Google Scholar] [CrossRef]
- Check, J.; Byrd, C.L.; Menio, J.; Rippe, R.A.; Hines, I.N.; Wheeler, M.D. Src kinase participates in LPS-induced activation of NADPH oxidase. Mol. Immunol. 2010, 47, 756–762. [Google Scholar] [CrossRef]
- Kong, X.; Thimmulappa, R.; Kombairaju, P.; Biswal, S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J. Immunol. 2010, 185, 569–577. [Google Scholar] [CrossRef]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Okorji, U.P.; Velagapudi, R.; El-Bakoush, A.; Fiebich, B.L.; Olajide, O.A. Antimalarial Drug Artemether Inhibits Neuroinflammation in BV2 Microglia Through Nrf2-Dependent Mechanisms. Mol. Neurobiol. 2016, 53, 6426–6443. [Google Scholar] [CrossRef] [PubMed]
- Jangula, A.; Murphy, E.J. Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci. Lett. 2013, 551, 23–27. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Sato, S.; Sanjo, H.; Takeda, K.; Ninomiya-Tsuji, J.; Yamamoto, M.; Kawai, T.; Matsumoto, K.; Takeuchi, O.; Akira, S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005, 6, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, A.; Johansson, C.; Persson, K.; Demirel, I. The role of caspase-1, caspase-4 and NLRP3 in regulating the host cell response evoked by uropathogenic Escherichia coli. Sci. Rep. 2022, 12, 2005. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Zhang, R.; Han, S.; Zhang, Z.; Zhang, W.; Yang, J.; Wan, Z.; Qin, L. Cereal Fiber Ameliorates High-Fat/Cholesterol-Diet-Induced Atherosclerosis by Modulating the NLRP3 Inflammasome Pathway in ApoE−/− Mice. J. Agric. Food Chem. 2018, 66, 4827–4834. [Google Scholar] [CrossRef]
- Inoue, M.; Gotoh, K.; Seike, M.; Masaki, T.; Honda, K.; Kakuma, T.; Yoshimatsu, H. Role of the spleen in the development of steatohepatitis in high-fat-diet-induced obese rats. Exp. Biol. Med. 2012, 237, 461–470. [Google Scholar] [CrossRef]
- Wu, L.; Parekh, V.V.; Hsiao, J.; Kitamura, D.; Van Kaer, L. Spleen supports a pool of innate-like B cells in white adipose tissue that protects against obesity-associated insulin resistance. Proc. Natl. Acad. Sci. USA 2014, 111, E4638–E4647. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Becker, C.; Barbulescu, K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut 1998, 43, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Kulthinee, S.; Yano, N.; Zhuang, S.; Wang, L.; Zhao, T.C. Critical Functions of Histone Deacetylases (HDACs) in Modulating Inflammation Associated with Cardiovascular Diseases. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2022, 29, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, Y.; Yin, J.; Huang, M.; Luo, F. Targeting AMPK signaling by polyphenols: A novel strategy for tackling aging. Food Funct. 2023, 14, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Song, D.; Su, Y.; Chen, J.; Wu, L.; Lian, H.; Hai, N.; Li, J.; Jiang, J.; Zhao, J.; et al. Pharmacology-based molecular docking of 4-methylcatechol and its role in RANKL-mediated ROS/Keap1/Nrf2 signalling axis and osteoclastogenesis. Biomed. Pharmacother. 2023, 159, 114101. [Google Scholar] [CrossRef] [PubMed]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef]
- Noel, S.; Martina, M.N.; Bandapalle, S.; Racusen, L.C.; Potteti, H.R.; Hamad, A.R.; Reddy, S.P.; Rabb, H. T Lymphocyte-Specific Activation of Nrf2 Protects from AKI. J. Am. Soc. Nephrol. 2015, 26, 2989–3000. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Wu, M.-R.; Chou, T.; Huang, C.-Y.; Hsiao, J.-K. A potential probiotic—Lachnospiraceae NK4A136 group: Evidence from the restoration of the dietary pattern from a high-fat diet. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Chen, J.H.; Zeng, L.Y.; Zhao, Y.F.; Tang, H.X.; Lei, H.; Wan, Y.F.; Deng, Y.Q.; Liu, K.X. Causal effects of gut microbiota on sepsis: A two-sample Mendelian randomization study. Front. Microbiol. 2023, 14, 1167416. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Agudelo Higuita, N.I.; Huycke, M.M. Enterococcal Disease, Epidemiology, and Implications for Treatment. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, Q.; Ma, S.; Liu, B.; Niu, J.; Liu, M.; Mustafa, A.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; et al. Supplementing Ryegrass Ameliorates Commercial Diet-Induced Gut Microbial Dysbiosis-Associated Spleen Dysfunctions by Gut–Microbiota–Spleen Axis. Nutrients 2024, 16, 747. https://doi.org/10.3390/nu16050747
Ali Q, Ma S, Liu B, Niu J, Liu M, Mustafa A, Li D, Wang Z, Sun H, Cui Y, et al. Supplementing Ryegrass Ameliorates Commercial Diet-Induced Gut Microbial Dysbiosis-Associated Spleen Dysfunctions by Gut–Microbiota–Spleen Axis. Nutrients. 2024; 16(5):747. https://doi.org/10.3390/nu16050747
Chicago/Turabian StyleAli, Qasim, Sen Ma, Boshuai Liu, Jiakuan Niu, Mengqi Liu, Ahsan Mustafa, Defeng Li, Zhichang Wang, Hao Sun, Yalei Cui, and et al. 2024. "Supplementing Ryegrass Ameliorates Commercial Diet-Induced Gut Microbial Dysbiosis-Associated Spleen Dysfunctions by Gut–Microbiota–Spleen Axis" Nutrients 16, no. 5: 747. https://doi.org/10.3390/nu16050747
APA StyleAli, Q., Ma, S., Liu, B., Niu, J., Liu, M., Mustafa, A., Li, D., Wang, Z., Sun, H., Cui, Y., & Shi, Y. (2024). Supplementing Ryegrass Ameliorates Commercial Diet-Induced Gut Microbial Dysbiosis-Associated Spleen Dysfunctions by Gut–Microbiota–Spleen Axis. Nutrients, 16(5), 747. https://doi.org/10.3390/nu16050747






