Quercetin in the Prevention of Induced Periodontal Disease in Animal Models: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Focus Question
2.2. Bias Risk Assessment
2.3. Statistical Analysis
3. Results
3.1. Risk of Bias in Studies
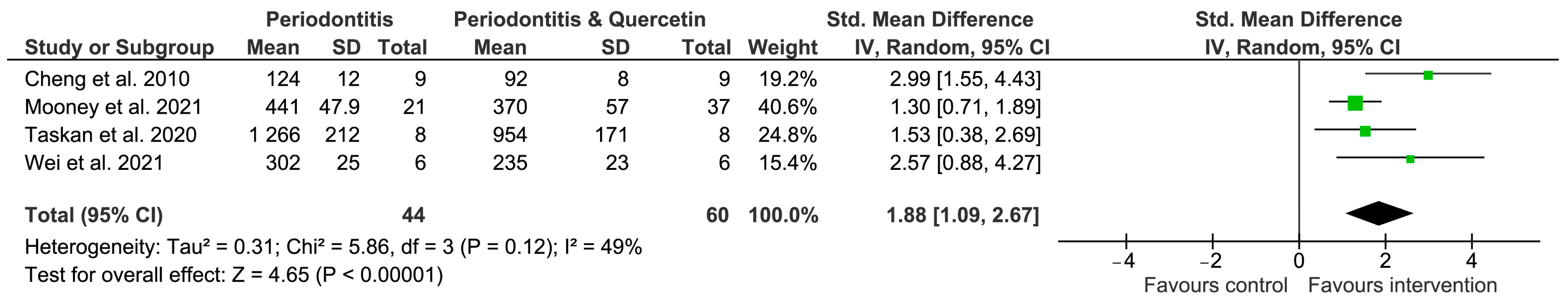
3.2. Meta-Analysis Results
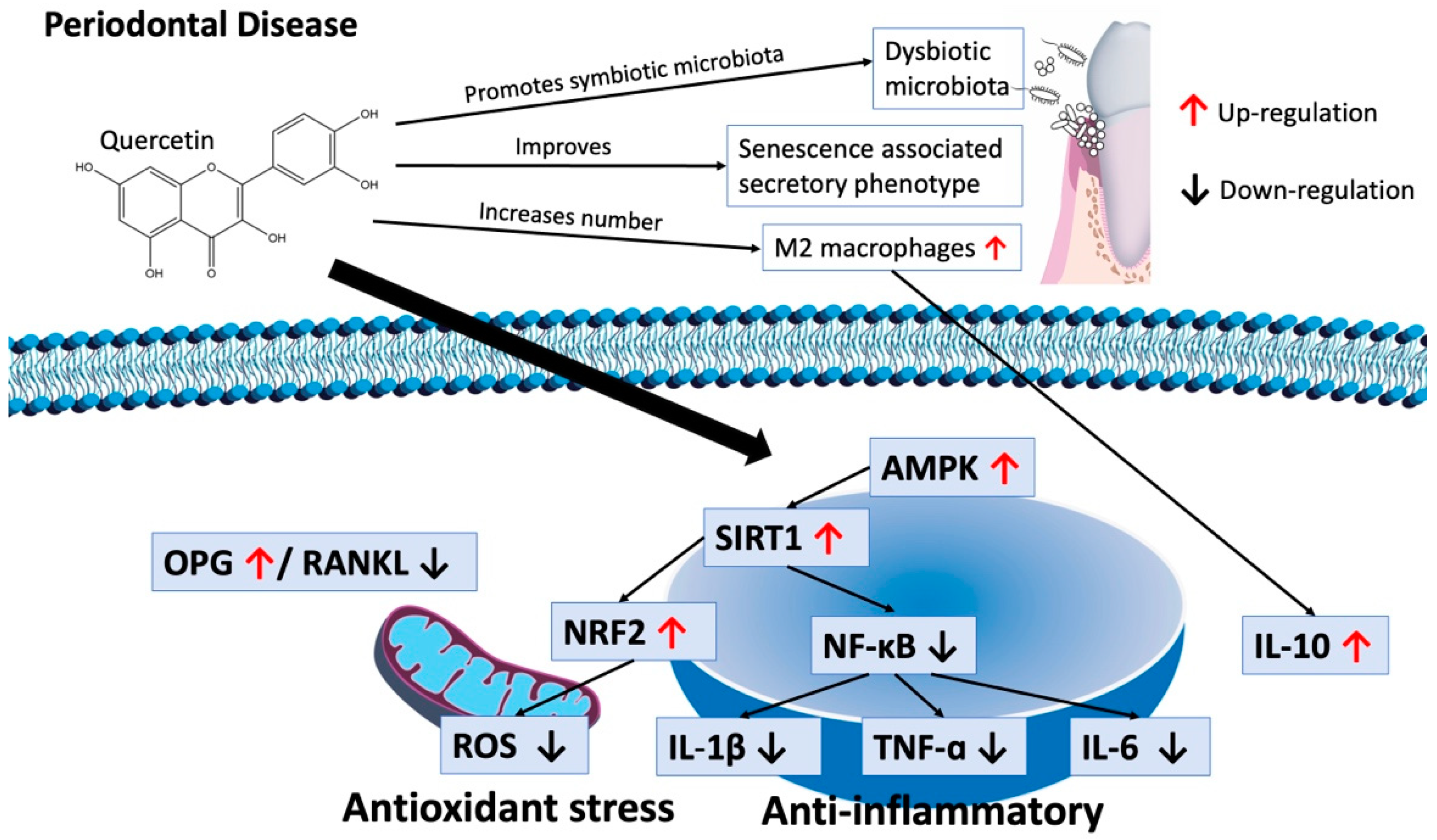
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Almohamad, M.; Kaye, E.K.; Mofleh, D.; Spartano, N.L. The association of sedentary behaviour and physical activity with periodontal disease in NHANES 2011–2012. J. Clin. Periodontol. 2022, 49, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Isola, G. The Impact of Diet, Nutrition and Nutraceuticals on Oral and Periodontal Health. Nutrients 2020, 12, 2724. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Clark, D. Periodontal disease as a model to study chronic inflammation in aging. Geroscience 2023. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontology 2021, 87, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Mooney, E.C.; Holden, S.E.; Xia, X.J.; Li, Y.; Jiang, M.; Banson, C.N.; Zhu, B.; Sahingur, S.E. Quercetin Preserves Oral Cavity Health by Mitigating Inflammation and Microbial Dysbiosis. Front. Immunol. 2021, 12, 774273. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Augusco, M.A.C.; Sarri, D.A.; Panontin, J.F.; Rodrigues, M.A.M.; Fernandes, R.M.N.; Silva, J.; Cardoso, C.A.L.; Rambo, M.K.D.; Scapin, E. Extracts from the Leaf of Couroupita guianensis (Aubl.): Phytochemical, Toxicological Analysis and Evaluation of Antioxidant and Antimicrobial Activities against Oral Microorganisms. Plants 2023, 12, 2327. [Google Scholar] [CrossRef]
- De Sousa Lages, A.; Lopes, V.; Horta, J.; Espregueira-Mendes, J.; Andrade, R.; Rebelo-Marques, A. Therapeutics That Can Potentially Replicate or Augment the Anti-Aging Effects of Physical Exercise. Int. J. Mol. Sci. 2022, 23, 9957. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and Quercetin: Promising Flavonoids with Chemopreventive Potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Nikitkova, A.; Abdelsalam, H.; Li, J.; Xiao, J. Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch. Oral. Biol. 2019, 98, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bureau, G.; Longpre, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Taskan, M.M.; Gevrek, F. Quercetin Decreased Alveolar Bone Loss and Apoptosis in Experimentally Induced Periodontitis Model in Wistar Rats. Antiinflamm Antiallergy Agents Med. Chem. 2020, 19, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Dagher, O.; Mury, P.; Thorin-Trescases, N.; Noly, P.E.; Thorin, E.; Carrier, M. Therapeutic Potential of Quercetin to Alleviate Endothelial Dysfunction in Age-Related Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 658400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, Y.; Yan, F.; Dong, M.; Ren, Y. Research progress of quercetin in cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1203713. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Napimoga, M.H.; Clemente-Napimoga, J.T.; Macedo, C.G.; Freitas, F.F.; Stipp, R.N.; Pinho-Ribeiro, F.A.; Casagrande, R.; Verri, W.A., Jr. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J. Nat. Prod. 2013, 76, 2316–2321. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, J.; Wu, W.; Ma, P.; Ren, L.; Yi, Z.; Wu, J. Quercetin Prevents Oxidative Stress-Induced Injury of Periodontal Ligament Cells and Alveolar Bone Loss in Periodontitis. Drug Des. Devel Ther. 2021, 15, 3509–3522. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.C.; Huang, R.Y.; Chiang, C.Y.; Chen, J.K.; Liu, C.H.; Chu, C.L.; Fu, E. Ameliorative effect of quercetin on the destruction caused by experimental periodontitis in rats. J. Periodontal Res. 2010, 45, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Baima, G.; Romandini, M.; Citterio, F.; Romano, F.; Aimetti, M. Periodontitis and Accelerated Biological Aging: A Geroscience Approach. J. Dent. Res. 2022, 101, 125–132. [Google Scholar] [CrossRef]
- Erdogan, K.; Sanlier, N.T.; Sanlier, N. Are epigenetic mechanisms and nutrition effective in male and female infertility? J. Nutr. Sci. 2023, 12, e103. [Google Scholar] [CrossRef]
- Shah, M.A.; Faheem, H.I.; Hamid, A.; Yousaf, R.; Haris, M.; Saleem, U.; Shah, G.M.; Alhasani, R.H.; Althobaiti, N.A.; Alsharif, I.; et al. The entrancing role of dietary polyphenols against the most frequent aging-associated diseases. Med. Res. Rev. 2023, 44, 235–274. [Google Scholar] [CrossRef]
- Wicinski, M.; Erdmann, J.; Nowacka, A.; Kuzminski, O.; Michalak, K.; Janowski, K.; Ohla, J.; Biernaciak, A.; Szambelan, M.; Zabrzynski, J. Natural Phytochemicals as SIRT Activators-Focus on Potential Biochemical Mechanisms. Nutrients 2023, 15, 3578. [Google Scholar] [CrossRef]
- Andrade, E.F.; Orlando, D.R.; Araújo, A.M.S.; de Andrade, J.N.B.M.; Azzi, D.V.; de Lima, R.R.; Lobo-Júnior, A.R.; Pereira, L.J. Can Resveratrol Treatment Control the Progression of Induced Periodontal Disease? A Systematic Review and Meta-Analysis of Preclinical Studies. Nutrients 2019, 11, 953. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Corsetti, G. Natural Compounds and Autophagy: Allies Against Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 555409. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef] [PubMed]
- Scarfo, G.; Daniele, S.; Chelucci, E.; Rizza, A.; Fusi, J.; Freggia, G.; Costa, B.; Taliani, S.; Artini, P.; Martini, C.; et al. Regular exercise delays microvascular endothelial dysfunction by regulating antioxidant capacity and cellular metabolism. Sci. Rep. 2023, 13, 17671. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, N.O.; Pereira, G.J.S.; Silva, V.O.; de Molon, R.S.; Morari, J.; Velloso, L.A.; Andrade, E.F.; Pereira, L.J.; Moura, R.F. Voluntary physical activity mitigates alveolar bone loss in mice with ligature-induced experimental periodontitis. Arch. Oral. Biol. 2022, 140, 105451. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.F.; Silva, V.O.; Moura, N.O.; Foureaux, R.C.; Orlando, D.R.; Moura, R.F.; Pereira, L.J. Physical Exercise Improves Glycemic and Inflammatory Profile and Attenuates Progression of Periodontitis in Diabetic Rats (HFD/STZ). Nutrients 2018, 10, 1702. [Google Scholar] [CrossRef]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef]
- Lee, M.J.; Ryu, H.H.; Hwang, J.W.; Kim, J.R.; Cho, E.S.; Choi, J.K.; Moon, Y.J. Sirt6 Activation Ameliorates Inflammatory Bone Loss in Ligature-Induced Periodontitis in Mice. Int. J. Mol. Sci. 2023, 24, 10714. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Zheng, W.; Weiss, S.; Chua, K.F.; Steegborn, C. Structural basis for the activation and inhibition of Sirtuin 6 by quercetin and its derivatives. Sci. Rep. 2019, 9, 19176. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, P.; Rahnasto-Rilla, M.; Mellini, P.; Jarho, E.; Lahtela-Kakkonen, M.; Kokkola, T. Studying SIRT6 regulation using H3K56 based substrate and small molecules. Eur. J. Pharm. Sci. 2014, 63, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, M.; Naud, P.; Abu-Taha, I.H.; Hiram, R.; Xiong, F.; Xiao, J.; Saljic, A.; Kamler, M.; Vuong-Robillard, N.; Thorin, E.; et al. The role of cellular senescence in profibrillatory atrial remodeling associated with cardiac pathology. Cardiovasc. Res. 2024. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, J.; Liu, G.H.; Belmonte, J.C.I. The ageing epigenome and its rejuvenation. Nat. Rev. Mol. Cell Biol. 2020, 21, 137–150. [Google Scholar] [CrossRef]
- Geng, L.; Liu, Z.; Zhang, W.; Li, W.; Wu, Z.; Wang, W.; Ren, R.; Su, Y.; Wang, P.; Sun, L.; et al. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell 2019, 10, 417–435. [Google Scholar] [CrossRef]
- Yu, J.; Jing, Z.; Shen, D.; Yang, M.; Liu, K.; Xiang, K.; Zhou, C.; Gong, X.; Deng, Y.; Li, Y.; et al. Quercetin promotes autophagy to alleviate cigarette smoke-related periodontitis. J. Periodontal Res. 2023, 58, 1082–1095. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.K.; Hussain, A.; Haque, S.; Raina, R.; Afroze, N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell Biochem. 2019, 120, 18357–18369. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Lehoux, J.; Desmarty, C.; Moine, E.; Legrand, P.; Dorandeu, C.; Simon, L.; Durand, T.; Brabet, P.; Crauste, C.; et al. A novel lipophenol quercetin derivative to prevent macular degeneration: Intravenous and oral formulations for preclinical pharmacological evaluation. Int. J. Pharm. 2023, 651, 123740. [Google Scholar] [CrossRef]
- Yang, N.; Nakagawa, M.; Nishiura, A.; Yamada, M.; Morikuni, H.; Honda, Y.; Matsumoto, N. Identification of Senescent Cells in Peri-Implantitis and Prevention of Mini-Implant Loss Using Senolytics. Int. J. Mol. Sci. 2023, 24, 2507. [Google Scholar] [CrossRef]
- Wang, X.; Honda, Y.; Zhao, J.; Morikuni, H.; Nishiura, A.; Hashimoto, Y.; Matsumoto, N. Enhancement of Bone-Forming Ability on Beta-Tricalcium Phosphate by Modulating Cellular Senescence Mechanisms Using Senolytics. Int. J. Mol. Sci. 2021, 22, 12415. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, Z.; Yang, S.; Zhou, Y.; Zhu, H.; Zhu, Y.; Zhou, J.; Lin, K.; Xu, Y. A multifunctional quercetin/polycaprolactone electrospun fibrous membrane for periodontal bone regeneration. Mater. Today Bio. 2024, 24, 100906. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Graves, C.L.; Gonzalez, O.A.; Dawson, D., 3rd; Morford, L.A.; Huja, P.E.; Hartsfield, J.K., Jr.; Huja, S.S.; Pandruvada, S.; Wallet, S.M. Aging, inflammation, immunity and periodontal disease. Periodontology 2016, 72, 54–75. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Dal-Fabbro, R.; Cosme-Silva, L.; de Oliveira, F.R.S.M.; Capalbo, L.C.; Plazza, F.A.; Ervolino, E.; Cintra, L.T.A.; Gomes-Filho, J.E. Effect of red wine or its polyphenols on induced apical periodontitis in rats. Int. Endod. J. 2021, 54, 2276–2289. [Google Scholar] [CrossRef]
- Ge, Y.W.; Feng, K.; Liu, X.L.; Zhu, Z.A.; Chen, H.F.; Chang, Y.Y.; Sun, Z.Y.; Wang, H.W.; Zhang, J.W.; Yu, D.G.; et al. Quercetin inhibits macrophage polarization through the p-38alpha/beta signalling pathway and regulates OPG/RANKL balance in a mouse skull model. J. Cell Mol. Med. 2020, 24, 3203–3216. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.; Slobodnikova, L.; Trajcikova, E.; Rendekova, K.; Mucaji, P.; Sychrova, A.; Fialova, S.B. Therapeutic Potential of Flavonoids and Tannins in Management of Oral Infectious Diseases-A Review. Molecules 2022, 28, 158. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Lin, Q.; Yao, J.; Zhang, G.; Peng, X.; Tian, J. In vitro outcomes of quercetin on Candida albicans planktonic and biofilm cells and in vivo effects on vulvovaginal candidiasis. Evidences of its mechanisms of action. Phytomedicine 2023, 114, 154800. [Google Scholar] [CrossRef] [PubMed]
- Debinska, A.; Sozanska, B. Dietary Polyphenols-Natural Bioactive Compounds with Potential for Preventing and Treating Some Allergic Conditions. Nutrients 2023, 15, 4823. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, X.; Song, Z.; Li, L.; Chang, H.; Li, S.; Zhou, W. Quercetin inhibits virulence properties of Porphyromas gingivalis in periodontal disease. Sci. Rep. 2020, 10, 18313. [Google Scholar] [CrossRef] [PubMed]
- Di Cristo, F.; Valentino, A.; De Luca, I.; Peluso, G.; Bonadies, I.; Calarco, A.; Di Salle, A. PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment. Molecules 2022, 27, 2205. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, H.; Ishfaq, M.; Han, Y.; Zhang, X.; Li, X.; Wang, B.; Lu, X.; Gao, B. Quercetin and AMPK: A Dynamic Duo in Alleviating MG-Induced Inflammation via the AMPK/SIRT1/NF-kappaB Pathway. Molecules 2023, 28, 7388. [Google Scholar] [CrossRef]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Dunnick, J.K.; Hailey, J.R. Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundam. Appl. Toxicol. 1992, 19, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Z.; Chen, F.; Chai, Y. Polyphenols in Oral Health: Homeostasis Maintenance, Disease Prevention, and Therapeutic Applications. Nutrients 2023, 15, 4384. [Google Scholar] [CrossRef] [PubMed]
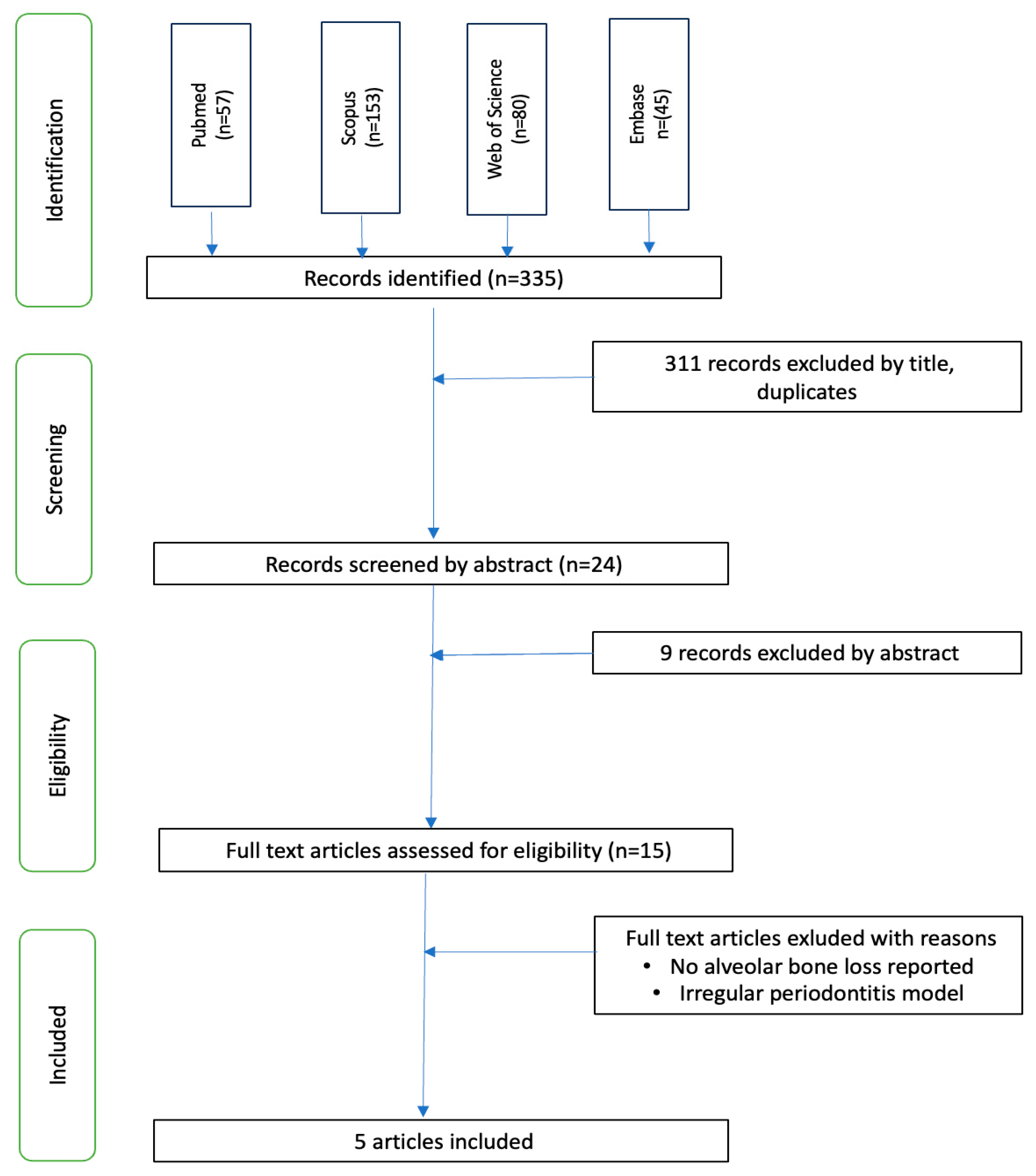
| Database | Search Strategy |
|---|---|
| Pubmed | (periodontitis”[MeSH Terms] OR “periodontitis”[Title/Abstract] OR “periodontal diseases” [MeSH Terms] OR “periodontal diseases”[Title/Abstract] OR “gingivitis”[Title/Abstract]) AND (“quercetin” [Title/Abstract] OR “cyanidanol”[Title/Abstract] OR “sophoretin”[Title/Abstract] OR “pentahydroxyflavone”[Title/Abstract]) |
| Scopus | (TITLE-ABS-KEY (periodontitis) OR TITLE-ABS-KEY (gingivitis) OR TITLE-ABS-KEY (periodontal AND disease)) AND (TITLE-ABS-KEY (quercetin) OR TITLE-ABS-KEY (cyanidanol) OR TITLE-ABS-KEY (sophoretin) OR TITLE-ABS-KEY (pentahydroxyflavone)) |
| Web of Science | (TS = (“periodontitis”) OR TS = (“periodontal disease”) OR TS = (“gingivitis”)) AND (TS = (“quercetin”) OR TS = (“cyanidanol”) OR TS = (“sophoretin”) OR TS = (“pentahydroxyflavone”)) |
| Embase | (periodontitis:ti,ab,kw OR ‘periodontal disease’:ti,ab,kw OR gingivitis:ti,ab,kw AND (quercetin:ti,ab,kw OR sophoretin:ti,ab,kw OR cyanidanol: ti,ab,kw OR pentahy- droxyflavone:ti,ab,kw) |
| Author, Year | Animal Model | Periodontitis Model | Time of Ligature | Groups | Quercetin Administration | Dose | Main Outcome |
|---|---|---|---|---|---|---|---|
| Cheng et al., 2010 [22] | Male 6 weeks old Sprague Dawley rats n = 9 per group | 3 O silk ligature | 12 days | Control Periodontitis Periodontitis + quercetin | Oral feeding | 75 mg/kg | Bone loss: 124 ± 12 µm (P) vs. 92 ± 8 µm (P + Q) |
| Mooney et al., 2021 [6] | Male, 10–12 week C57B/6J mice n = 37 Quercetin n = 21 vehicle | Silk ligature | 7 days | Control, Periodontitis, Periodontitis + quercetin | Oral administration | 40 mg/kg/twice per d | Bone loss 441 ± 47.9 µm (P) vs. 370 ± 57 µm (P + Q) |
| Napimoga et al., 2013 [20] | 5 mice per group | Aggregatibacter actinomycetemcomitans JP2 periodontitis model 3 times (0, 48 h, 96 h) | No ligature | Sham Aa infected Aa infected + quercetin | Subcutaneous injection | 100 mg/kg 15 days | Bone loss: Aa: 170 µm ± 15 µm Aa + Q: 99 ± 7.6 µm |
| Taskan et al., 2020 [14] | Female Wistar rats (weighing 230–250 gr.) 8 per group | 4–0 silk ligature | 15 days | Control Periodontitis Periodontitis + 75 mg/kg/d Periodontitis + 150 mg/kg/d | Intraperitoneal injection 14 days | 75 mg/kg 150 mg/kg | Bone loss: P: 1266 ± 212 µm P + 75 mg/kg: 1059 ± 107 µm P + 150 mg/kg: 954 ± 171 µm |
| Wei et al., 2021 [21] | Male C57BL/6J mice 8 weeks n = 6 per group | Silk ligature | 10 days | Control Periodontitis Periodontitis + quercetin | Oral gavage | 50 mg/kg | Bone loss bucc.: 302 ± 25 µm (P) vs. 235 ± 23 µm (P + Q) Bone loss pal.: 282 ± 14.5 (P) vs. 216 ± 14.5 mm (P + Q) |
| Studies | I | II | III | IV | V | VI | VII | VIII | IX | X |
|---|---|---|---|---|---|---|---|---|---|---|
| Cheng et al., 2010 [22] | + | + | ? | ? | ? | + | ? | + | + | + |
| Mooney et al., 2021 [6] | + | + | ? | ? | − | + | ? | + | + | + |
| Napimoga et al., 2013 [20] | ? | ? | ? | ? | ? | ? | ? | ? | + | ? |
| Taskan et al., 2020 [14] | ? | + | ? | ? | + | + | + | + | + | + |
| Wei et al., 2021 [21] | + | + | ? | ? | ? | + | ? | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laky, M.; Arslan, M.; Zhu, X.; Rausch-Fan, X.; Moritz, A.; Sculean, A.; Laky, B.; Ramseier, C.A.; Stähli, A.; Eick, S. Quercetin in the Prevention of Induced Periodontal Disease in Animal Models: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 735. https://doi.org/10.3390/nu16050735
Laky M, Arslan M, Zhu X, Rausch-Fan X, Moritz A, Sculean A, Laky B, Ramseier CA, Stähli A, Eick S. Quercetin in the Prevention of Induced Periodontal Disease in Animal Models: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(5):735. https://doi.org/10.3390/nu16050735
Chicago/Turabian StyleLaky, Markus, Muazzez Arslan, Xilei Zhu, Xiaohui Rausch-Fan, Andreas Moritz, Anton Sculean, Brenda Laky, Christoph A. Ramseier, Alexandra Stähli, and Sigrun Eick. 2024. "Quercetin in the Prevention of Induced Periodontal Disease in Animal Models: A Systematic Review and Meta-Analysis" Nutrients 16, no. 5: 735. https://doi.org/10.3390/nu16050735
APA StyleLaky, M., Arslan, M., Zhu, X., Rausch-Fan, X., Moritz, A., Sculean, A., Laky, B., Ramseier, C. A., Stähli, A., & Eick, S. (2024). Quercetin in the Prevention of Induced Periodontal Disease in Animal Models: A Systematic Review and Meta-Analysis. Nutrients, 16(5), 735. https://doi.org/10.3390/nu16050735







