It’s Dead! Can Postbiotics Really Help Performance and Recovery? A Systematic Review
Abstract
1. Introduction
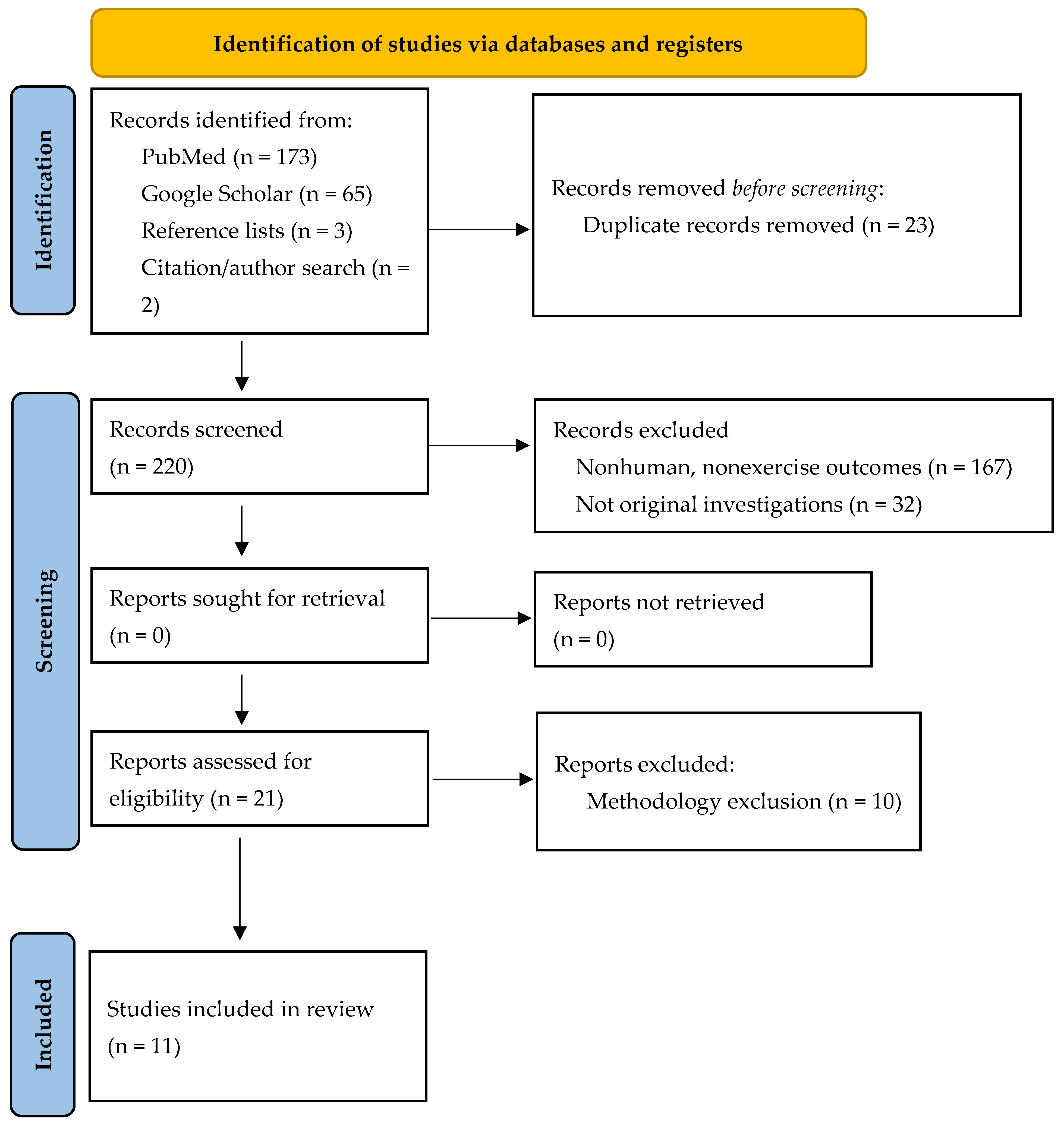
2. Materials and Methods
2.1. Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
4. Discussion
4.1. Exercise + Postbiotics: Exercise Performance + Recovery
4.2. Exercise + Postbiotics: Muscle Damage and Recovery
4.3. Exercise + Postbiotics: Immunity, Oxidative Stress, and Inflammation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Salminen, S.; Sanz, Y. The impact of probiotic on gut health. Curr. Drug Metab. 2009, 10, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Marotta, A.; Sarno, E.; Del Casale, A.; Pane, M.; Mogna, L.; Amoruso, A.; Felis, G.E.; Fiorio, M. Effects of Probiotics on Cognitive Reactivity, Mood, and Sleep Quality. Front. Psychiatry 2019, 10, 164. [Google Scholar] [CrossRef]
- Walden, K.E.; Moon, J.M.; Hagele, A.M.; Allen, L.E.; Gaige, C.J.; Krieger, J.M.; Jäger, R.; Mumford, P.W.; Pane, M.; Kerksick, C.M. A randomized controlled trial to examine the impact of a multi-strain probiotic on self-reported indicators of depression, anxiety, mood, and associated biomarkers. Front. Nutr. 2023, 10, 1219313. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.E.; Pyne, D.B.; McKune, A.J.; Penm, J.; Pumpa, K.L. Probiotic supplementation elicits favourable changes in muscle soreness and sleep quality in rugby players. J. Sci. Med. Sport 2021, 24, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Javanshir, N.; Hosseini, G.N.G.; Sadeghi, M.; Esmaeili, R.; Satarikia, F.; Ahmadian, G.; Allahyari, N. Evaluation of the Function of Probiotics, Emphasizing the Role of their Binding to the Intestinal Epithelium in the Stability and their Effects on the Immune System. Biol. Proced. Online 2021, 23, 23. [Google Scholar] [CrossRef]
- Allaker, R.P.; Stephen, A.S. Use of Probiotics and Oral Health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; De Leonardis, N.; Sardano, R.; Pezzolla, C.; de Ruvo, E.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; et al. The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 2023, 16, 1313. [Google Scholar] [CrossRef]
- Eslami, M.; Bahar, A.; Keikha, M.; Karbalaei, M.; Kobyliak, N.M.; Yousefi, B. Probiotics function and modulation of the immune system in allergic diseases. Allergol. Immunopathol. 2020, 48, 771–788. [Google Scholar] [CrossRef]
- Pavlidou, E.; Fasoulas, A.; Mantzorou, M.; Giaginis, C. Clinical Evidence on the Potential Beneficial Effects of Probiotics and Prebiotics in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 15898. [Google Scholar] [CrossRef]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Muñoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013, 109 (Suppl. S2), S35–S50. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Fontana, L.; Gil, A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J. Gastroenterol. 2014, 20, 15632–15649. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef]
- Di Dio, M.; Calella, P.; Cerullo, G.; Pelullo, C.P.; Di Onofrio, V.; Gallè, F.; Liguori, G. Effects of Probiotics Supplementation on Risk and Severity of Infections in Athletes: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11534. [Google Scholar] [CrossRef] [PubMed]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J. Cachexia Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef] [PubMed]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Chantler, S.; Griffiths, A.; Matu, J.; Davison, G.; Holliday, A.; Jones, B. A systematic review: Role of dietary supplements on markers of exercise-associated gut damage and permeability. PLoS ONE 2022, 17, e0266379. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef] [PubMed]
- Santibañez-Gutierrez, A.; Fernández-Landa, J.; Calleja-González, J.; Delextrat, A.; Mielgo-Ayuso, J. Effects of Probiotic Supplementation on Exercise with Predominance of Aerobic Metabolism in Trained Population: A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.D.K.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Eur. J. Appl. Physiol. 2014, 114, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.; Santiago, M.; Krieger, J.M.; Hagele, A.M.; Zielinska, K.; Scheiman, J.; Jäger, R.; Kostic, A.; Kerksick, C.M. Impact of probiotic Veillonella atypica FB0054 supplementation on anaerobic capacity and lactate. iScience 2024, 27, 108643. [Google Scholar] [CrossRef]
- Huang, W.-C.; Hsu, Y.-J.; Li, H.; Kan, N.-W.; Chen, Y.-M.; Lin, J.-S.; Hsu, T.-K.; Tsai, T.-Y.; Chiu, Y.-S.; Huang, C.-C. Effect of Lactobacillus plantarum TWK10 on Improving Endurance Performance in Humans. Chin. J. Physiol. 2018, 61, 163–170. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lee, M.-C.; Lee, C.-C.; Ng, K.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef]
- Heimer, M.; Teschler, M.; Schmitz, B.; Mooren, F.C. Health Benefits of Probiotics in Sport and Exercise—Non-existent or a Matter of Heterogeneity? A Systematic Review. Front. Nutr. 2022, 9, 804046. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J. Probiotic viability—Does it matter? Microb. Ecol. Health Dis. 2012, 23, 18567. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.-M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. Reply to: Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Sanders, M.E.; Salminen, S.; Szajewska, H. Postbiotics: The concept and their use in healthy populations. Front. Nutr. 2022, 9, 1002213. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-J.; Park, J.M.; Kwon, Y.J.; Kim, K.; Park, S.Y.; Kim, I.; Lim, J.H.; Kim, B.K.; Kim, B.-Y. Immunostimulatory Effect of Heat-Killed Probiotics on RAW264.7 Macrophages. J. Microbiol. Biotechnol. 2022, 32, 638–644. [Google Scholar] [CrossRef]
- Asama, T.; Kimura, Y.; Kono, T.; Tatefuji, T.; Hashimoto, K.; Benno, Y. Effects of heat-killed Lactobacillus kunkeei YB38 on human intestinal environment and bowel movement: A pilot study. Benef. Microbes 2016, 7, 337–344. [Google Scholar] [CrossRef]
- Sawada, D.; Kuwano, Y.; Tanaka, H.; Hara, S.; Uchiyama, Y.; Sugawara, T.; Fujiwara, S.; Rokutan, K.; Nishida, K. Daily intake of Lactobacillus gasseri CP2305 relieves fatigue and stress-related symptoms in male university Ekiden runners: A double-blind, randomized, and placebo-controlled clinical trial. J. Funct. Foods 2019, 57, 465–476. [Google Scholar] [CrossRef]
- Warda, A.K.; Rea, K.; Fitzgerald, P.; Hueston, C.; Gonzalez-Tortuero, E.; Dinan, T.G.; Hill, C. Heat-killed lactobacilli alter both microbiota composition and behaviour. Behav. Brain Res. 2019, 362, 213–223. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kataria, J.; Li, N.; Wynn, J.L.; Neu, J. Probiotic microbes: Do they need to be alive to be beneficial? Nutr. Rev. 2009, 67, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Hu, A.; Shu, X.; Huang, W.; Liu, J.; Wang, B.; Zhang, R.; Yue, M.; Yang, C. Lactobacillus plantarum-derived postbiotics prevent Salmonella-induced neurological dysfunctions by modulating gut-brain axis in mice. Front. Nutr. 2022, 9, 946096. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.-C.; Lan, C.-C.E.; Huang, T.-Y.; Chen, K.-W.; Chai, C.-Y.; Chen, W.-T.; Fang, A.-H.; Chen, Y.-H.; Wu, C.-S. Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Song, M.W.; Lee, N.-K.; Paik, H.-D. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. J. Food Sci. Technol. 2018, 55, 3174–3180. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Kondo, J.; Iwabuchi, N.; Takahashi, S.; Yamauchi, K.; Abe, F.; Miura, K. Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Benef. Microbes 2018, 9, 855–864. [Google Scholar] [CrossRef]
- Sugawara, T.; Sawada, D.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function. Microb. Ecol. Health Dis. 2016, 27, 30259. [Google Scholar] [CrossRef]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kawai, T.; Kuwano, Y.; Fujiwara, S.; Rokutan, K. Para-psychobiotic Lactobacillus gasseri CP2305 ameliorates stress-related symptoms and sleep quality. J. Appl. Microbiol. 2017, 123, 1561–1570. [Google Scholar] [CrossRef]
- Deshpande, G.; Athalye-Jape, G.; Patole, S. Para-probiotics for Preterm Neonates-The Next Frontier. Nutrients 2018, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Cash, H.A.; Farmer, S.; Keller, D. Inactivated probiotic Bacillus coagulans GBI-30 induces complex immune activating, anti-inflammatory, and regenerative markers in vitro. J. Inflamm. Res. 2017, 10, 107–117. [Google Scholar] [CrossRef]
- Fiore, W.; Arioli, S.; Guglielmetti, S. The Neglected Microbial Components of Commercial Probiotic Formulations. Microorganisms 2020, 8, 1177. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Yoon, S.; Baek, J.; Kim, J.-H.; Park, M.; Hwang, K.; Kim, W. Heat-Killed Lactiplantibacillus plantarum LRCC5314 Mitigates the Effects of Stress-Related Type 2 Diabetes in Mice via Gut Microbiome Modulation. J. Microbiol. Biotechnol. 2022, 32, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Clin. Res. Ed. 2021, 372, n71. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Hoffman, M.W.; Zelicha, H.; Gepner, Y.; Willoughby, D.S.; Feinstein, U.; Ostfeld, I. The Effect of 2 Weeks of Inactivated Probiotic Bacillus coagulans on Endocrine, Inflammatory, and Performance Responses During Self-Defense Training in Soldiers. J. Strength Cond. Res. 2019, 33, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Ho, C.-S.; Hsu, Y.-J.; Huang, C.-C. Live and Heat-Killed Probiotic Lactobacillus paracasei PS23 Accelerated the Improvement and Recovery of Strength and Damage Biomarkers after Exercise-Induced Muscle Damage. Nutrients 2022, 14, 4563. [Google Scholar] [CrossRef]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sports Nutr. 2018, 15, 39. [Google Scholar] [CrossRef]
- Hagele, A.; Moon, J.M.; Blumkaitis, J.C.; Ratliff, K.; Boring, J.; Gaige, C.; Orr, L.; Walden, K.; Stecker, R.A.; Jager, R.; et al. Performance, soreness, and recovery changes in response to a high-volume dose of resistance exercise after supplementation of inactive and active Bacillus coagulans GBI-30 6086 (Abstract, p. 78). In Proceedings of the Nineteenth International Society of Sports Nutrition (ISSN) Conference and Expo, Westin Fort Lauderdale, FL, USA, 16–18 June 2022. [Google Scholar]
- Holley, K.; Mumford, P.W.; Viers, A.; Moon, J.M.; Blumkaitis, J.C.; Hagele, A.; Ratliff, K.; Boring, J.; Gaige, C.; Orr, L.; et al. Inflammatory, Muscle Damage, and Immune Changes After Supplementation with Inactivated and Activated Bacillus coagulans GBI-30 6086 (Abstract, p. 25). In Proceedings of the Nineteenth International Society of Sports Nutrition (ISSN) Conference and Expo, Westin Fort Lauderdale, FL, USA, 16–18 June 2022. [Google Scholar]
- Kalman, D.S.; Hewlings, S. Inactivated Probiotic Bacillus coagulans GBI-30 Demonstrates Immunosupportive Properties in Healthy Adults Following Stressful Exercise. J. Probiotics Health 2018, 6, 100019. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Lee, C.-C.; Lee, M.-C.; Hsu, H.-Y.; Lin, J.-S.; Huang, C.-C.; Watanabe, K. Effects of heat-killed Lactiplantibacillus plantarum TWK10 on exercise performance, fatigue, and muscle growth in healthy male adults. Physiol. Rep. 2023, 11, e15835. [Google Scholar] [CrossRef]
- Lee, C.-C.; Liao, Y.-C.; Lee, M.-C.; Cheng, Y.-C.; Chiou, S.-Y.; Lin, J.-S.; Huang, C.-C.; Watanabe, K. Different Impacts of Heat-Killed and Viable Lactiplantibacillus plantarum TWK10 on Exercise Performance, Fatigue, Body Composition, and Gut Microbiota in Humans. Microorganisms 2022, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
- Sashihara, T.; Nagata, M.; Mori, T.; Ikegami, S.; Gotoh, M.; Okubo, K.; Uchida, M.; Itoh, H. Effects of Lactobacillus gasseri OLL2809 and α-lactalbumin on university-student athletes: A randomized, double-blind, placebo-controlled clinical trial. Appl. Physiol. Nutr. Metab. 2013, 38, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Komano, Y.; Fukao, K.; Shimada, K.; Naito, H.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Effects of Ingesting Food Containing Heat-Killed Lactococcus lactis Strain Plasma on Fatigue and Immune-Related Indices after High Training Load: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Study. Nutrients 2023, 15, 1754. [Google Scholar] [CrossRef] [PubMed]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and Supplementation Strategies to Prevent and Attenuate Exercise-Induced Muscle Damage: A Brief Review. Sports Med. Open 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Fujii, N.; Suzuki, K. Dietary Supplementation for Attenuating Exercise-Induced Muscle Damage and Delayed-Onset Muscle Soreness in Humans. Nutrients 2021, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Pyne, D.B. Respiratory inflammation and infections in high-performance athletes. Immunol. Cell Biol. 2016, 94, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Delgado, L.; Moreira, P.; Haahtela, T. Does exercise increase the risk of upper respiratory tract infections? Br. Med. Bull. 2009, 90, 111–131. [Google Scholar] [CrossRef]
- Shibata, T.; Kanayama, M.; Haida, M.; Fujimoto, S.; Oroguchi, T.; Sata, K.; Mita, N.; Kutsuzawa, T.; Ikeuchi, M.; Kondo, M.; et al. Lactococcus lactis JCM5805 activates anti-viral immunity and reduces symptoms of common cold and influenza in healthy adults in a randomized controlled trial. J. Funct. Foods 2016, 24, 492–500. [Google Scholar] [CrossRef]
- Sato, S.; Arai, S.; Iwabuchi, N.; Tanaka, M.; Hase, R.; Sakane, N. Effects of Heat-Killed Lacticaseibacillus paracasei MCC1849 on the Maintenance of Physical Condition in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2023, 15, 3450. [Google Scholar] [CrossRef]
- Nieman, D.C. Is infection risk linked to exercise workload? Med. Sci. Sports Exerc. 2000, 32, S406–S411. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Mohr, A.E.; Pugh, J.; O’Sullivan, O.; Black, K.; Townsend, J.R.; Pyne, D.B.; Wardenaar, F.C.; West, N.P.; Whisner, C.M.; McFarland, L.V. Best Practices for Probiotic Research in Athletic and Physically Active Populations: Guidance for Future Randomized Controlled Trials. Front. Nutr. 2022, 9, 809983. [Google Scholar] [CrossRef]
| Reference | Participants (n, Age, Sex) | Study Design | Supplementation (Duration) | Exercise Intervention | Endpoints | Findings |
|---|---|---|---|---|---|---|
| Hoffman, 2019 [56] | 16 male soldiers | RAN DB PLA PAR | 1.0 × 109 CFU of inactivated Weizmannia coagulans GBI-30 6086 or PLA (14 days) | Same daily protocol as part of soldiers’ self-defense training course |
|
|
| Lee, 2022 [57] | 105 healthy males and females between (avg. age of 21.6 years) | RAN DB PLA PAR | PLA vs. 2 × 1010 CFU/day live Lacticaseibacillus paracasei PS23 (DSM 32322) cells vs. heat-killed L. paracasei PS23 (DSM 32322). Equivalent to 1 × 1010 cells/day (6 weeks) | 100 maximal vertical jumps |
|
|
| Komano, 2018 [58] | 51 male sports club athletes | RAN DB PLA PAR | PLA vs. 1 × 1011 heat-killed Lactococcus lactis JCM 5805 (13 days) | High-intensity exercise as part of sport training program |
| |
| Hagele, 2023 (Abstract) [59] | 76 healthy resistance-trained men (29.9 ± 9.3 years) | RAN DB PLA PAR | PLA vs. 1 × 109 CFU/day live W. cogulans GBI-30 6086 vs. 1 × 109 inactivated W. coagulans GBI-30 6086 cells/day (14 days) | 30 min cycling intervals followed by leg press (6 × 10 reps) and hex bar deadlifts (5 × 10 reps) at 65% 1RM and 5 × 20 reps of drop jumps |
|
|
| Holley, 2023 (Abstract) [60] | 76 healthy resistance-trained men (29.9 ± 9.3 years) | RAN DB PLA PAR | PLA vs. 1 × 109 CFU/day live W. coagulans GBI-30 6086 vs. 1 × 109 inactivated W. coagulans GBI-30 6086 cells/day (14 days) | 30 min cycling intervals followed by leg press (6 × 10 reps) and hex bar deadlifts (5 × 10 reps) at 65% 1RM and 5 × 20 reps of drop jumps |
|
|
| Kalman, 2018 [61] | 16 healthy males (22.6 ± 3.4 years) | RAN DB PLA PAR | PLA vs. W. coagulans GBI-30 6086 (28 days) | In vitro bacterial challenge 60 min of treadmill running at 60–80% HRR |
|
|
| Cheng, 2023 [62] | 30 healthy males (20–25 years) | RAN DB PLA PAR | PLA vs. 3 × 1010 heat-killed Lactiplantibaccilus plantarum TWK10 cells/day (6 weeks) | No exercise intervention 30 min run at 60% VO2Max Run to exhaustion at 85% VO2Max |
|
|
| Lee, 2022 [63] | 53 healthy adults (20–30 years) | RAN DB PLA PAR | PLA vs. 3 × 1011 CFU/day live Lactiplantibaccilus plantarum TWK10 cells vs. 3 × 1011 heat-killed Lactiplantibaccilus plantarum TWK10 cells/day (6 weeks) | No exercise intervention Standardized bout of exercise was completed before and after supplementation |
|
|
| Sawada, 2019 [39] | 49 male collegiate long-distance relay runners (18–22 years) | RAN DB PLA PAR | Daily intake of 200 mL beverage containing either PLA or 1 × 1010 Lactobacillus gasseri CP2305 (12 weeks) | Daily intake while training for and competing in All-Japan university championships |
|
|
| Sashihara, 2013 [64] | 44 healthy male university football club members (<30 years) | RAN DB PLA PAR | PLA vs. 100 mg/day of 1 × 1010 heat-killed Lactobacillus gasseri LG2809 cells vs. 100 mg/day heat-killed L. gasseri LG2809 cells + 900 mg/day α-lactalbumin (4 weeks) | 60 min of strenuous cycle ergometer exercise at 70% heart rate reserve Completed before and after supplementation |
|
|
| Komano, 2023 [65] | 37 university long-distance track and field athletes (>18 years) | RAN PLA DB PAR | PLA vs. heat-killed dry Lactococcus lactis Plasma 1 × 1011 cells/day (14 days) | Days 1 to 14: performed sport-specific training under the supervision of the track and field coach Day 15: single 2 h exercise bout using a cycle ergometer at 70–80% of heart rate maximum |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerksick, C.M.; Moon, J.M.; Jäger, R. It’s Dead! Can Postbiotics Really Help Performance and Recovery? A Systematic Review. Nutrients 2024, 16, 720. https://doi.org/10.3390/nu16050720
Kerksick CM, Moon JM, Jäger R. It’s Dead! Can Postbiotics Really Help Performance and Recovery? A Systematic Review. Nutrients. 2024; 16(5):720. https://doi.org/10.3390/nu16050720
Chicago/Turabian StyleKerksick, Chad M., Jessica M. Moon, and Ralf Jäger. 2024. "It’s Dead! Can Postbiotics Really Help Performance and Recovery? A Systematic Review" Nutrients 16, no. 5: 720. https://doi.org/10.3390/nu16050720
APA StyleKerksick, C. M., Moon, J. M., & Jäger, R. (2024). It’s Dead! Can Postbiotics Really Help Performance and Recovery? A Systematic Review. Nutrients, 16(5), 720. https://doi.org/10.3390/nu16050720







