Association between Preconception Dietary Fiber Intake and Preterm Birth: The Japan Environment and Children’s Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Determination of Preconception Dietary Fiber Intake, Obstetric Outcomes, and Confounding Factors
2.4. Statistical Analysis
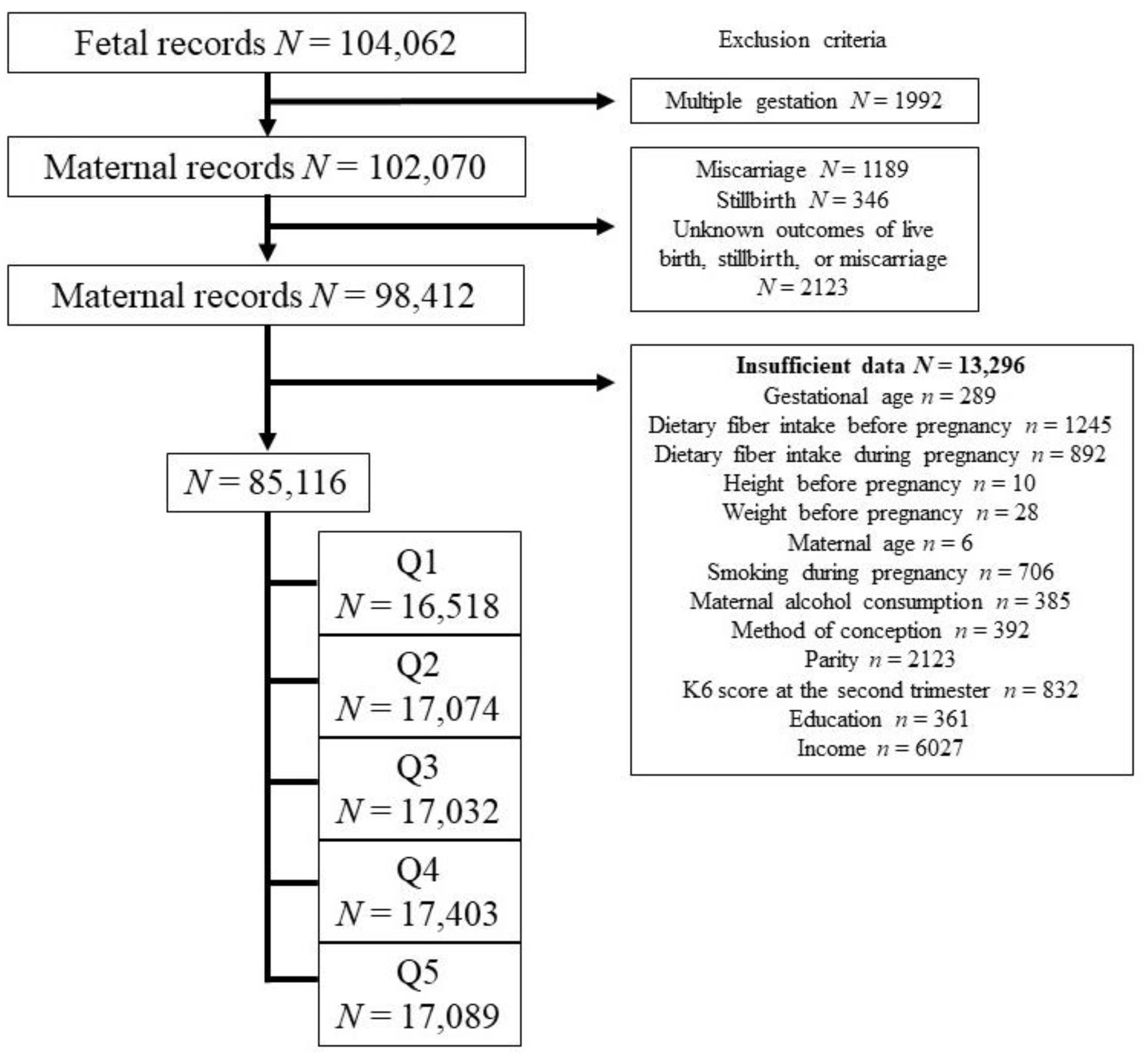
3. Results
Maternal Medical Characteristics and Obstetric Outcomes
4. Discussion
4.1. Main Findings
4.2. Interpretation
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moster, D.; Lie, R.T.; Markestad, T. Long-term medical and social consequences of preterm birth. N. Engl. J. Med. 2008, 359, 262–273. [Google Scholar] [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- Shah, P.S.; Lui, K.; Sjörs, G.; Mirea, L.; Reichman, B.; Adams, M.; Modi, N.; Darlow, B.A.; Kusuda, S.; Feliciano, L.S.; et al. Neonatal outcomes of very low birth weight and very preterm neonates: An international comparison. J. Pediatr. 2016, 177, 144–152.e6. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Sakata, S.; Konishi, S.; Ng, C.F.S.; Watanabe, C. Preterm birth rates in Japan from 1979 to 2014: Analysis of national vital statistics. J. Obstet. Gynaecol. Res. 2018, 44, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, J.; Szamotulska, K.; Drewniak, N.; Mohangoo, A.; Chalmers, J.; Sakkeus, L.; Irgens, L.; Gatt, M.; Gissler, M.; Blondel, B.; et al. Preterm birth time trends in Europe: A study of 19 countries. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 1356–1365. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; May, M.D.; Loveman, E.; Colquitt, J.L.; Rees, K. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2016, 2016, CD011472. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Santangelo, O.E.; Provenzano, S.; Fatigoni, C.; Nardi, M.; Ferrara, P.; Gianfredi, V. Dietary Fiber Intake and Risk of Pancreatic Cancer: Systematic Review and Meta-Analysis of Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 11556. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. J. Nutr. 2021, 151, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Saccone, G.; Pellegrino, R.; Vaccarisi, S.; Taranto, L.; Mazzulla, R.; Bernardo, S.; Venturella, R.; Di Carlo, C.; Morelli, M. Incidental diagnosis of a pancreatic adenocarcinoma in a woman affected by gestational diabetes mellitus: Case report and literature review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100471. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Di Simone, N.; Santamaria Ortiz, A.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; D’Ippolito, S. Recent insights on the maternal microbiota: Impact on pregnancy outcomes. Front. Immunol. 2020, 11, 528202. [Google Scholar] [CrossRef]
- Shiozaki, A.; Yoneda, S.; Yoneda, N.; Yonezawa, R.; Matsubayashi, T.; Seo, G.; Saito, S. Intestinal microbiota is different in women with preterm birth: Results from terminal restriction fragment length polymorphism analysis. PLoS ONE 2014, 9, e111374. [Google Scholar] [CrossRef]
- Othman, M.; Alfirevic, Z.; Neilson, J.P. Probiotics for preventing preterm labour. Cochrane Database Syst. Rev. 2007, 2007, CD005941. [Google Scholar] [CrossRef]
- Jarde, A.; Lewis-Mikhael, A.-M.; Moayyedi, P.; Stearns, J.C.; Collins, S.M.; Beyene, J.; McDonald, S.D. Pregnancy outcomes in women taking probiotics or prebiotics: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2018, 18, 14. [Google Scholar] [CrossRef]
- Kirihara, N.; Kamitomo, M.; Tabira, T.; Hashimoto, T.; Taniguchi, H.; Maeda, T. Effect of probiotics on perinatal outcome in patients at high risk of preterm birth. J. Obstet. Gynaecol. Res. 2018, 44, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Barrett, H.L.; Gomez-Arango, L.F.; Wilkinson, S.A.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. A vegetarian diet is a major determinant of gut microbiota composition in early pregnancy. Nutrients 2018, 10, 890. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Clifton, V.L. Preconception dietary patterns in human pregnancies are associated with preterm delivery. J. Nutr. 2014, 144, 1075–1080. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of short and long self-administered food frequency questionnaires in ranking dietary intake in middle-aged and elderly Japanese in the Japan public health center-based prospective study for the next generation (JPHC-NEXT) protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.A.; Kawakami, N.; Saitoh, M.; Ono, Y.; Nakane, Y.; Nakamura, Y.; Tachimori, H.; Iwata, N.; Uda, H.; Nakane, H.; et al. The performance of the Japanese version of the K6 and K10 in the World Mental Health Survey Japan. Int. J. Methods Psychiatr. Res. 2008, 17, 152–158. [Google Scholar] [CrossRef]
- Hanson, M.A.; Bardsley, A.; De-Regilc, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Purandare, C.N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int. J. Gynaecol. Obstet. 2015, 128 (Suppl. S4), S213–S253. [Google Scholar] [CrossRef]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Schoenaker, D.A.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: Results from the Australian Longitudinal Study on Women’s Health. Am. J. Clin. Nutr. 2015, 102, 94–101. [Google Scholar] [CrossRef]
- Kyozuka, H.; Fukusda, T.; Murata, T.; Yamaguchi, A.; Kanno, A.; Yasuda, S.; Sato, A.; Ogata, Y.; Kuse, M.; Hosoya, M.; et al. Impact of preconception sodium intake on hypertensive disorders of pregnancy: The Japan Environment and Children’s study. Pregnancy Hypertens. 2021, 23, 66–72. [Google Scholar] [CrossRef]
- Kyozuka, H.; Murata, T.; Fukuda, T.; Yamaguchi, A.; Yasuda, S.; Suzuki, D.; Kanno, A.; Sato, A.; Ogata, Y.; Hosoya, M.; et al. Preconception dietary inflammatory index and hypertension disorders of pregnancy: The Japan environment and children’s study. Pregnancy Hypertens. 2022, 28, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Omoto, T.; Kyozuka, H.; Murata, T.; Imaizumi, K.; Yamaguchi, A.; Fukuda, T.; Isogami, H.; Yasuda, S.; Sato, A.; Ogata, Y.; et al. Influence of preconception carbohydrate intake on hypertensive disorders of pregnancy: The Japan Environment and Children’s Study. J. Obstet. Gynaecol. Res. 2023, 49, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Zhang, C.; Chavarro, J.; Bowers, K.; Rich-Edwards, J.; Rosner, B.; Mozaffarian, D.; Hu, F.B. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2012, 96, 289–295. [Google Scholar] [CrossRef]
- Bao, W.; Bowers, K.; Tobias, D.K.; Olsen, S.F.; Chavarro, J.; Vaag, A.; Kiely, M.; Zhang, C. Prepregnancy low-carbohydrate dietary pattern and risk of gestational diabetes mellitus: A prospective cohort study. Am. J. Clin. Nutr. 2014, 99, 1378–1384. [Google Scholar] [CrossRef]
- Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: Results from an Australian population-based prospective cohort study. Diabetologia 2015, 58, 2726–2735. [Google Scholar] [CrossRef]
- Kyozuka, H.; Murata, T.; Isogami, H.; Imaizumi, K.; Fukuda, T.; Yamaguchi, A.; Yasuda, S.; Sato, A.; Ogata, Y.; Hosoya, M.; et al. Preconception dietary inflammatory index and risk of gestational diabetes mellitus based on maternal body mass index: Findings from a Japanese birth cohort study. Nutrients 2022, 14, 4100. [Google Scholar] [CrossRef]
- Kyozuka, H.; Nishigori, H.; Murata, T.; Fukuda, T.; Yamaguchi, A.; Kanno, A.; Yasuda, S.; Sato, A.; Ogata, Y.; Kuse, M.; et al. Prepregnancy antiinflammatory diet in pregnant women with endometriosis: The Japan Environment and Children’s Study. Nutrition 2021, 85, 111129. [Google Scholar] [CrossRef] [PubMed]
- Kyozuka, H.; Murata, T.; Fukuda, T.; Yamaguchi, A.; Kanno, A.; Yasuda, S.; Suzuki, D.; Takahashi, T.; Go, H.; Maeda, H.; et al. Association between preconception dietary inflammatory index and neurodevelopment of offspring at 3 years of age: The Japan Environment and Children’s Study. Nutrition 2022, 102, 111708. [Google Scholar] [CrossRef]
- Rogozińska, E.; Marlin, N.; Jackson, L.; Rayanagoudar, G.; Ruifrok, A.E.; Dodds, J.; Molyneaux, E.; van Poppel, M.N.; Poston, L.; Vinter, C.A.; et al. Effects of antenatal diet and physical activity on maternal and fetal outcomes: Individual patient data meta-analysis and health economic evaluation. Health Technol. Assess. 2017, 21, 1–158. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Ernerudh, J.; Berg, G.; Mjösberg, J. Regulatory T Helper Cells in Pregnancy and their Roles in Systemic versus Local Immune Tolerance. Am. J. Reprod. Immunol. 2011, 66 (Suppl. S1), 31–43. [Google Scholar] [CrossRef]
- Schober, L.; Radnai, D.; Schmitt, E.; Mahnke, K.; Sohn, C.; Steinborn, A. Term and preterm labor: Decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol. Cell Biol. 2012, 90, 935–944. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Arenas-Hernandez, M.; Romero, R.; Miller, D.; Garcia-Flores, V.; Leng, Y.; Xu, Y.; Galaz, J.; Hassan, S.S.; Hsu, C.-D.; et al. Regulatory T cells play a role in a subset of idiopathic preterm labor/birth and adverse neonatal outcomes. Cell Rep. 2020, 32, 107874. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, L.M.; Hull, M.L.; Foyle, K.L.; McCormack, C.D.; Robertson, S.A. Immune-metabolic interactions and T cell tolerance in pregnancy. J. Immunol. 2022, 209, 1426–1436. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Voltolini, C.; Battersby, S.; Etherington, S.L.; Petraglia, F.; Norman, J.E.; Jabbour, H.N. A novel antiinflammatory role for the short-chain fatty acids in human labor. Endocrinology 2012, 153, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Nickodem, C.A.; Menon, R.; McDonald, T.; Taylor, B.D. Circulating short-chain fatty acids in preterm birth: A pilot case-control study. Reprod. Sci. 2020, 27, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113 (Suppl. S3), 17–42. [Google Scholar] [CrossRef] [PubMed]
- The Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, R.; Tuddenham, S.; Caulfield, L.E.; Murphy, C.; Robinson, C.; Ravel, J.; Ghanem, K.G.; Brotman, R.M. Dietary macronutrient intake and molecular-bacterial vaginosis: Role of fiber. Clin. Nutr. 2020, 39, 3066–3071. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Dmochowski, R.; Cash, B.D.; Kopp, Z.S.; Berriman, S.J.; Khullar, V. Systematic review of the relationship between bladder and bowel function: Implications for patient management. Int. J. Clin. Pract. 2013, 67, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. LaW. Dietary Reference Intakes for Japanese. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000061795.html (accessed on 20 May 2023).
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
| Quintile for Dietary Fiber | |||||
|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 | Q5 (High) | |
| Variable | n = 16,518 | n = 17,074 | n = 17,032 | n = 17,403 | n = 17,089 |
| Maternal medical background | |||||
| Preconception dietary fiber intake, g/day median (IQR) | 5.5 (4.5–6.2) | 8.0 (7.4–8.5) | 10.1 (9.6–10.7) | 12.8 (12.0–13.7) | 18.4 (16.3–22.1) |
| Maternal age, mean year (SD) | 29.8 (5.1) | 31.1 (4.9) | 31.6 (4.8) | 32.0 (4.8) | 32.3 (4.7) |
| Maternal age category, % | |||||
| ≤19 | 1.3 | 0.5 | 0.3 | 0.4 | 0.3 |
| 20–29 | 48.1 | 38.0 | 33.6 | 30.7 | 28.4 |
| 30–39 | 47.7 | 57.2 | 61.5 | 63.3 | 65.3 |
| ≥40 | 2.9 | 4.3 | 4.5 | 5.6 | 5.9 |
| BMI, % | |||||
| <18.5 | 16.5 | 16.5 | 16.2 | 15.4 | 15.0 |
| 18.5–24.9 | 71.5 | 72.8 | 74.0 | 74.1 | 74.5 |
| ≥25.0 | 12.0 | 10.7 | 9.8 | 10.5 | 10.6 |
| Maternal education, years, % | |||||
| <10 | 8.0 | 4.5 | 3.7 | 3.2 | 3.3 |
| 10 to 12 | 38.7 | 31.8 | 29.1 | 27.2 | 27.3 |
| 13 to 16 | 52.6 | 62.4 | 65.6 | 67.9 | 67.3 |
| ≥17 | 0.8 | 1.3 | 1.7 | 1.7 | 2.0 |
| Household income, JPY, % | |||||
| <2,000,000 | 8.5 | 5.5 | 4.6 | 4.5 | 5.3 |
| 2,000,000–5,999,999 | 70.5 | 68.3 | 67.1 | 66.1 | 66.3 |
| 6,000,000–9,999,999 | 18.1 | 22.5 | 24.0 | 24.2 | 23.2 |
| ≥10,000,000 | 2.9 | 3.8 | 4.2 | 5.2 | 5.2 |
| Preconception total calorie intake, kcal/day median (IQR) | 1198.0 (995.0–1411.0) | 1483.0 (1306.0–1695.0) | 1686.0 (1479.0–1923.0) | 1928.0 (1683.0–2208.0) | 2410.0 (2041.0–2942.0) |
| Preconception carbohydrate energy ratio, mean % (SD) | 57.5 (9.9) | 55.5 (7.6) | 54.7 (7.3) | 54.4 (7.0) | 53.9 (7.5) |
| Preconception protein energy ratio, mean % (SD) | 12.7 (2.3) | 13.3 (1.9) | 13.6 (1.9) | 13.9 (1.9) | 14.3 (2.1) |
| Preconception fat energy ratio, mean % (SD) | 27.3 (8.3) | 29.1 (6.4) | 29.9 (6.1) | 30.3 (5.8) | 30.9 (6.1) |
| Total calorie intake during pregnancy, kcal/day median (IQR) | 1274.0 (1036.0–1548.0) | 1479.0 (1243.0–1755.0) | 1620.0 (1365.0–1920.0) | 1783.0 (1502.0–2126.0) | 2064.0 (1695.0–2546.0) |
| Carbohydrate energy ratio during pregnancy, mean % (SD) | 57.5 (9.2) | 55.8 (7.8) | 54.9 (7.5) | 54.5 (7.4) | 53.8 (7.8) |
| Protein energy ratio during pregnancy, mean % (SD) | 12.8 (2.2) | 13.3 (2.0) | 13.6 (1.9) | 13.9 (1.9) | 14.2 (2.1) |
| Fat energy ratio during pregnancy, mean % (SD) | 27.9 (7.9) | 29.3 (6.6) | 30.0 (6.3) | 30.4 (6.2) | 31.1 (6.4) |
| Dietary fiber intake during pregnancy, g/day median (IQR) | 6.1 (4.7–7.8) | 8.0 (6.6–9.8) | 9.6 (7.9–11.6) | 11.6 (9.5–14.0) | 15.0 (11.9–19.1) |
| Primipara, % | 49.4 | 42.4 | 39.4 | 36.0 | 32.9 |
| Smoking, % | 7.6 | 4.8 | 3.9 | 3.3 | 3.8 |
| Alcohol, % | 8.2 | 10.1 | 10.7 | 10.8 | 11.0 |
| ART, % | 2.1 | 2.9 | 3.1 | 3.4 | 3.4 |
| K6 score ≥ 13 at the second or third trimester, % | 3.6 | 2.8 | 2.7 | 3.0 | 3.7 |
| Obstetric outcomes | |||||
| HDP, % | 3.3 | 3.2 | 2.9 | 3.1 | 3.0 |
| GDM, % | 2.9 | 2.6 | 2.9 | 2.7 | 2.8 |
| PTB < 37 weeks, % | 4.7 | 4.5 | 4.5 | 4.3 | 4.5 |
| PTB < 34 weeks, % | 1.0 | 0.9 | 0.8 | 0.8 | 0.8 |
| Quintile for Dietary Fiber | |||||
|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 | Q5 (High) | |
| n = 16,518 | n = 17,074 | n = 17,032 | n = 17,403 | n = 17,089 | |
| PTB < 37 weeks | |||||
| OR (95% CI) | 1 (Ref) | 0.96 (0.87–1.06) | 0.96 (0.87–1.07) | 0.92 (0.83–1.02) | 0.96 (0.87–1.06) |
| aOR (95% CI) | 1 (Ref) | 0.94 (0.85–1.05) | 0.95 (0.85–1.06) | 0.90 (0.80–1.01) | 0.92 (0.80–1.06) |
| PTB < 34 weeks | |||||
| OR (95% CI) | 1 (Ref) | 0.85 (0.69–1.06) | 0.79 (0.63–0.99) * | 0.78 (0.62–0.97) * | 0.74 (0.59–0.94) * |
| aOR (95% CI) | 1 (Ref) | 0.83 (0.67–1.05) | 0.78 (0.62–0.997) * | 0.74 (0.57–0.95) * | 0.68 (0.50–0.92) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omoto, T.; Kyozuka, H.; Murata, T.; Fukuda, T.; Isogami, H.; Okoshi, C.; Yasuda, S.; Yamaguchi, A.; Sato, A.; Ogata, Y.; et al. Association between Preconception Dietary Fiber Intake and Preterm Birth: The Japan Environment and Children’s Study. Nutrients 2024, 16, 713. https://doi.org/10.3390/nu16050713
Omoto T, Kyozuka H, Murata T, Fukuda T, Isogami H, Okoshi C, Yasuda S, Yamaguchi A, Sato A, Ogata Y, et al. Association between Preconception Dietary Fiber Intake and Preterm Birth: The Japan Environment and Children’s Study. Nutrients. 2024; 16(5):713. https://doi.org/10.3390/nu16050713
Chicago/Turabian StyleOmoto, Takahiro, Hyo Kyozuka, Tsuyoshi Murata, Toma Fukuda, Hirotaka Isogami, Chihiro Okoshi, Shun Yasuda, Akiko Yamaguchi, Akiko Sato, Yuka Ogata, and et al. 2024. "Association between Preconception Dietary Fiber Intake and Preterm Birth: The Japan Environment and Children’s Study" Nutrients 16, no. 5: 713. https://doi.org/10.3390/nu16050713
APA StyleOmoto, T., Kyozuka, H., Murata, T., Fukuda, T., Isogami, H., Okoshi, C., Yasuda, S., Yamaguchi, A., Sato, A., Ogata, Y., Nagasaka, Y., Hosoya, M., Yasumura, S., Hashimoto, K., Nishigori, H., Fujimori, K., & The Japan Environment and Children’s Study Group. (2024). Association between Preconception Dietary Fiber Intake and Preterm Birth: The Japan Environment and Children’s Study. Nutrients, 16(5), 713. https://doi.org/10.3390/nu16050713






