Polyphenols Influence the Development of Endometrial Cancer by Modulating the Gut Microbiota
Abstract
1. Introduction
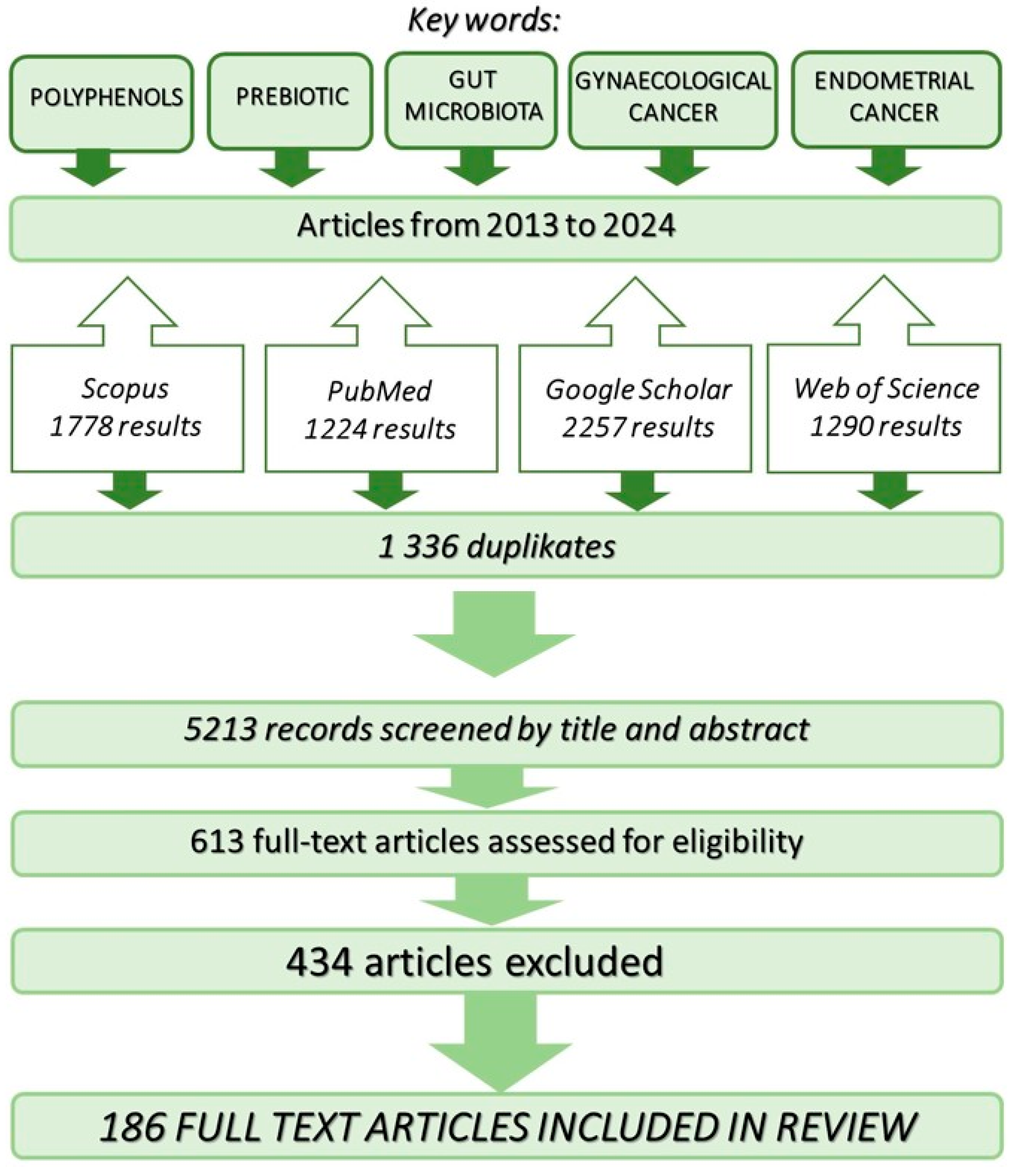
2. Research Strategy Employed in the Review of Available Literature
3. Pathogenesis of Endometrial Cancer
4. The Impact of Gut Microbiota on Carcinogenesis
5. The Impact of Gut Microbiota on Oestrogen Synthesis
6. Prebiotics
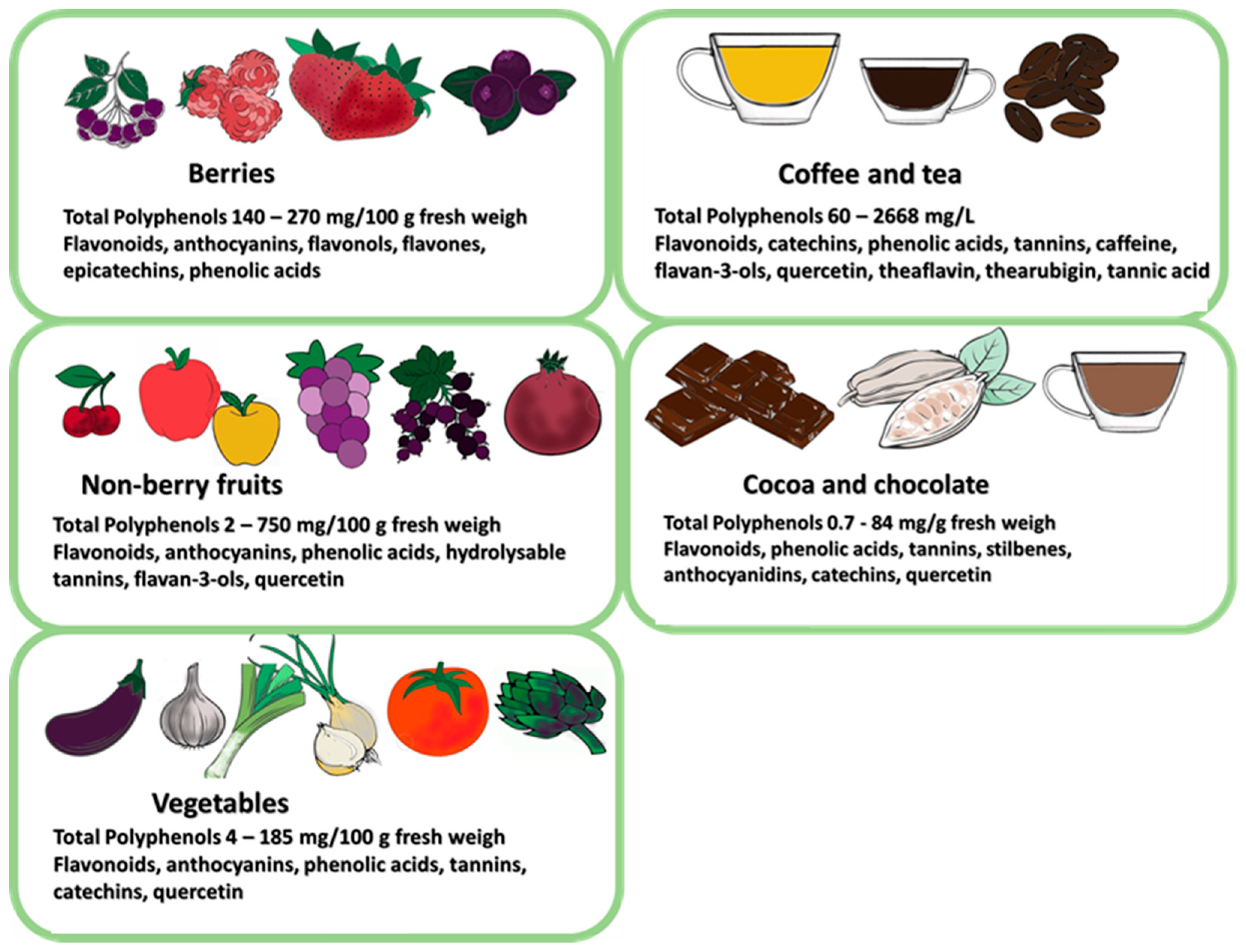
6.1. Prebiotic Properties of Polyphenols
6.1.1. Prebiotic Action of Tea Polyphenols—A Review of Studies
6.1.2. Prebiotic Effects of Coffee Polyphenols—A Review of Studies
6.1.3. Prebiotic Effects of Cocoa Polyphenols—A Review of Studies
6.1.4. Prebiotic Effects of Fruit Polyphenols—A Review of Studies
6.1.5. Prebiotic Effects of Vegetable Polyphenols—A Review of Studies
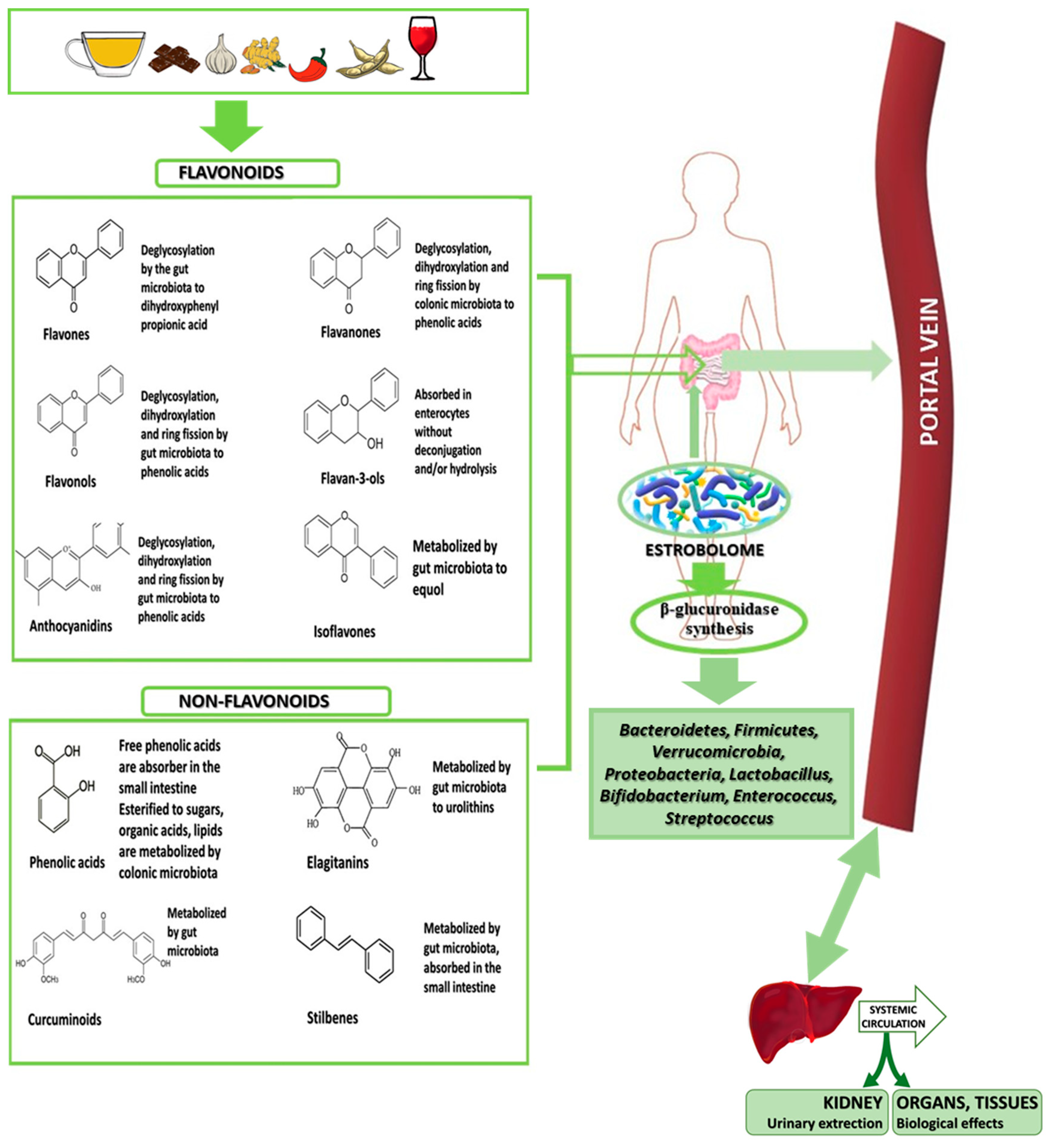
6.1.6. Metabolism of Phenolic Compounds by Estrobolome
7. The Microbiota Structure and Diet-Related Factors in Oestrogen-Dependent Gynaecological Cancers
8. The Effect of Polyphenols on Endometrial Cancer
9. Plant-Based Diets Show Anti-Cancer Effects through Their Polyphenol Content
10. Supplements or Food—Dietary Prevention of Oestrogen-Dependent Gynaecological Cancers
11. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bresser, L.R.F.; de Goffau, M.C.; Levin, E.; Nieuwdorp, M. Gut Microbiota in Nutrition and Health with a Special Focus on Specific Bacterial Clusters. Cells 2022, 11, 3091. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Tomaszewska, E.; Donaldson, J.; Jachimowicz, K. The role of nutritional factors in the modulation of the composition of the gut microbiota in people with autoimmune diabetes. Nutrients 2022, 14, 2498. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, N.; Han, W.; Ban, M.; Sun, T.; Xu, J. Vaginal and tumor microbiomes in gynecological cancer (Review). Oncol. Lett. 2023, 25, 153. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wen, X.S.; Xian, C.J. Chemotherapy-induced intestinal microbiota dysbiosis impairs mucosal homeostasis by modulating toll-like receptor signaling pathways. Int. J. Mol. Sci. 2021, 22, 9474. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, H.; Shokri, R.; Nami, Y.; Khandaghi, J.; Panahi, B. Potential probiotic characterization of lactic acid bacteria isolated from Duimaj, an Iranian traditional snack food, using biochemical, molecular and computational approaches. LWT 2023, 184, 115091. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, S.K. Role of Microbial Flora and Probiotics in Host Immune Homeostasis. J. Appl. Pharm. Sci. 2018, 8, 136–149. [Google Scholar]
- Dahiya, D.; Nigam, P.S. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- Bogusławska-Tryk, M.; Ziółkowska, E.; Sławińska, A.; Siwek, M.; Bogucka, J. Modulation of Intestinal Histology by Probiotics, Prebiotics and Synbiotics Delivered In Ovo in Distinct Chicken Genotypes. Animals 2021, 11, 3293. [Google Scholar] [CrossRef]
- Koleva, P.T.; Bridgman, S.L.; Kozyrskyj, A.L. The infant gut microbiome: Evidence for obesity risk and dietary intervention. Nutrients 2015, 7, 2237–2260. [Google Scholar] [CrossRef]
- Winiaska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Muszyński, S.; Tomszewska, E. Bioactive compounds, antibiotics and heavy metals: Effects on the intestinal structure and microbiome of monogastric animals—A non-systematic review. Ann. Anim. Sci. 2023, 23, 289–313. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- ACS. American Cancer Society: Key Statistics for Endometrial Cancer. Available online: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html (accessed on 10 March 2023).
- Ito, K.; Utsunomiya, H.; Yaegashi, N.; Sasano, H. Biological roles of estrogen and progesterone in human endometrial carcinoma—New development in potential endocrine therapy for endometrial cancer. Endocr. J. 2007, 54, 667–679. [Google Scholar] [CrossRef]
- Berg, A.; Hoivik, E.A.; Mjos, S.; Holst, F.; Werner, H.M.; Tangen, I.L.; Taylor-Weiner, A.; Gibson, W.J.; Kusonmano, K. Molecular profiling of endometrial carcinoma precursor, primary and metastatic lesions suggests different targets for treatment in obese compared to non-obese patients. Oncotarget 2015, 6, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Onstand, M.A.; Schmandt, R.E.; Lu, K.H. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 2016, 34, 4225–4230. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut 2018, 67, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, A.; Jänne, O.; Kujansuu, E.; Vihko, R. Treatment of advanced endometrial adenocarcinoma with a combined cytotoxic therapy. Predictive value of cytosol estrogen and progestin receptor levels. Cancer 1980, 46, 2162–2167. [Google Scholar] [CrossRef] [PubMed]
- Maček, P.; Molinari, N.; Sobočan, M.; Knez, J. What Role do Androgens Play in Endometrial Cancer? J. Pers. Med. 2023, 13, 341. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The intestinal microbiome and estrogen receptor–positive female breast cancer. JNCI 2016, 108, djw029. [Google Scholar]
- Michels, K.A.; Brinton, L.A.; Wentzensen, N.; Pan, K.; Chen, C.; Anderson, G.L.; Pfeiffer, R.M.; Xu, K.; Rohan, T.; Trabert, B. Postmenopausal androgen metabolism and endometrial cancer risk in the women’s health initiative observational study. JNCI Cancer Spectr. 2019, 3, pkz029. [Google Scholar] [CrossRef]
- Plaza-Parrochia, F.; Romero, C.; Valladares, L.; Vega, M. Endometrium and steroids, a pathologic overview. Steroids 2017, 126, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, G.M.; Webb, M.J.; Li, H.; Keeney, G.L. Hyperestrogenism: A relevant risk factor for the development of cancer from endometriosis. Gynecol. Oncol. 2000, 79, 18–22. [Google Scholar] [CrossRef] [PubMed]
- La Marca, A.; Morgante, G.; Palumbo, M.; Cianci, A.; Petraglia, F.; De Leo, V. Insulin-lowering treatment reduces aromatase activity in response to follicle-stimulating hormone in women with polycystic ovary syndrome. Fertil. Steril. 2002, 78, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, L.; Shangguan, A.J.; Bulun, S.E. Aromatase expression and regulation in breast and endometrial cancer. J. Mol. Endocrinol. 2016, 57, R19–R33. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, Y.; Lin, H. Estrogen disorders: Interpreting the abnormal regulation of aromatase in granulosa cells. Int. J. Mol. Med. 2021, 47, 73. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yan, C.; Zhao, Q.; Zhao, B.; Liao, Y.; Chen, Y.; Wang, D.; Tang, D. The Association Between Gut Microbiota, Toll-Like Receptors, and Colorectal Cancer. Clin. Med. Insights Oncol. 2022, 16, 11795549221130549. [Google Scholar] [CrossRef] [PubMed]
- Di Tucci, C.; De Vito, I.; Muzii, L. Immune-Onco-Microbiome: A New Revolution for Gynecological Cancers. Biomedicines 2023, 11, 782. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, X.; Guo, Y.; Yan, J.; Abuduwaili, A.; Aximujiang, K.; Yan, J.; Wu, M. Gut microbiota influence tumor development and Alter interactions with the human immune system. J. Exp. Clin. Cancer Res. 2021, 40, 42. [Google Scholar] [CrossRef]
- Boutriq, S.; González-González, A.; Plaza-Andrades, I.; Laborda-Illanes, A.; Sánchez-Alcoholado, L.; Peralta-Linero, J.; Domínguez-Recio, M.E.; Bermejo-Perez, M.J.; Lavado-Valenzuela, R.; Alba, E.; et al. Gut and endometrial microbiome dysbiosis: A new emergent risk factor for endometrial cancer. J. Pers. Med. 2021, 11, 659. [Google Scholar] [CrossRef]
- Cojocaru, M. Endometrial cancer and the microbiome-Review. J. Clin. Sexol. 2021, 4, 151. [Google Scholar]
- Witkowska, A.M.; Mirończuk-Chodakowska, I.; Terlikowska, K.M.; Kulesza, K.; Zujko, M.E. Coffee and its biologically active components: Is there a connection to breast, endometrial, and ovarian cancer?—A review. Pol. J. Food Nutr. Sci. 2020, 70, 207–222. [Google Scholar] [CrossRef]
- Miao, S.; Yang, F.; Wang, Y.; Shao, C.; Zava, D.T.; Ding, Q.; Shi, Y.E. 4-Hydroxy estrogen metabolite, causing genomic instability by attenuating the function of spindle-assembly checkpoint, can serve as a biomarker for breast cancer. Am. J. Transl. Res. 2019, 11, 4992. [Google Scholar] [PubMed]
- Hevir, N.; Šinkovec, J.; Rižner, T.L. Disturbed expression of phase I and phase II estrogen-metabolizing enzymes in endometrial cancer: Lower levels of CYP1B1 and increased expression of S-COMT. Mol. Cell. Endocrinol. 2011, 331, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wu, J.; Chen, J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef]
- Parida, S.; Sharma, D. The microbiome–estrogen connection and breast cancer risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef] [PubMed]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef] [PubMed]
- Sobstyl, M.; Brecht, P.; Sobstyl, A.; Mertowska, P.; Grywalska, E. The role of microbiota in the immunopathogenesis of endometrial cancer. Int. J. Mol. Sci. 2022, 23, 5756. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Prakash, A.; Nourianpour, M.; Senok, A.; Atiomo, W. Polycystic Ovary Syndrome and Endometrial Cancer: A Scoping Review of the Literature on Gut Microbiota. Cells 2022, 11, 3038. [Google Scholar] [CrossRef]
- Mróz, A.; Mazerska, Z. Glucuronidation of antitumour therapeutics–detoxification, mechanism of resistance or prodrug formation? PHMD 2015, 69, 1462–1477. [Google Scholar]
- Besse, H.C.; Chen, Y.; Scheeren, H.W.; Metselaar, J.M.; Lammers, T.; Moonen, C.T.; Hennink, W.E.; Deckers, R. A doxorubicin-glucuronide prodrug released from nanogels activated by high-intensity focused ultrasound liberated β-glucuronidase. Pharmaceutics 2020, 12, 536. [Google Scholar] [CrossRef] [PubMed]
- Pollet, R.M.; D’Agostino, E.H.; Walton, W.G.; Xu, Y.; Little, M.S.; Biernat, K.A.; Pellock, S.J.; Petterson, L.M.; Creekmore, B.C.; Isenberg, H.N.; et al. An atlas of β-glucuronidases in the human intestinal microbiome. Structure 2017, 25, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Lamuel-Raventos, R.M.; Onge, M.P.S. Prebiotic nut compounds and human microbiota. Crit. Rev. Food Sci. Nutr. 2017, 57, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ding, Q.; Zhang, W.; Kang, M.; Ma, J.; Zhao, L. Gut microbial beta-glucuronidase: A vital regulator in female estrogen metabolism. Gut Microbes. 2023, 15, 2236749. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Murga, M.L.; Gil-Ortiz, F.; Serrano-García, L.; Llombart-Cussac, A. A New Paradigm in the Relationship between Gut Microbiota and Breast Cancer: β-glucuronidase Enzyme Identified as Potential Therapeutic Target. Pathogens 2023, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Filippone, A.; Rossi, C.; Rossi, M.M.; Di Micco, A.; Maggiore, C.; Forcina, L.; Natale, M.; Costantini, L.; Merendino, N.; Di Leone, A.; et al. Endocrine Disruptors in Food, Estrobolome and Breast Cancer. J. Clin. Med. 2023, 12, 3158. [Google Scholar] [CrossRef]
- Wang, K.; Hu, S. The synergistic effects of polyphenols and intestinal microbiota on osteoporosis. Front. Immunol. 2023, 14, 1285621. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Salemi, F.; Small, S.; Syed, S.; Salari, F.; Alam, W.; Cheang, W.S.; Saso, L.; Khan, H. Resveratrol regulates inflammation and improves oxidative stress via Nrf2 signaling pathway: Therapeutic and biotechnological prospects. Phytother. Res. 2023, 37, 1590–1605. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, C.C.; Chen, C.C.; Yang, K.J.; Huang, C.Y. Inhibition of Staphylococcus aureus PriA helicase by flavonol kaempferol. J. Nutr. 2015, 34, 169–172. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cui, Y.; Wang, X.; Liu, F.; Li, X. Apple polyphenol extract improves highfat diet-induced hepatic steatosis by regulating bile acid synthesis and gut microbiota in C57BL/6 male mice. J. Agric. Food. Chem. 2021, 69, 6829–6841. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, J.; Yin, M.; Liu, Y.; You, Y.; Zhan, J.; Huang, W. Grape extract activates brown adipose tissue through pathway involving the regulation of gut microbiota and bile acid. Mol. Nutr. Food. Res. 2020, 64, e2000149. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Green Pea (Pisum sativum L.) hull polyphenol extracts ameliorate DSS-induced colitis through Keap1/Nrf2 pathway and gut microbiota modulation. Foods 2021, 10, 2765. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Morissette, A.; Kropp, C.; Songpadith, J.P.; Junges Moreira, R.; Costa, J.; Marin´e- Casad´o, R.; Pilon, G.; Varin, T.V.; Dudonn´e, S.; Boutekrabt, L.; et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E965–E980. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cheng, N.; Zhou, W.; Chen, S.; Wang, Q.; Gao, H.; Xue, X.; Wu, L.; Cao, W. Honey polyphenols ameliorate DSS-induced ulcerative colitis via modulating gut microbiota in rats. Mol. Nutr. Food. Res. 2019, 63, e1900638. [Google Scholar] [CrossRef]
- Kong, Y.; Yan, T.; Tong, Y.; Deng, H.; Tan, C.; Wan, M.; Wang, M.; Meng, X.; Wang, Y. Gut microbiota modulation by polyphenols from Aronia melanocarpa of LPS-induced liver diseases in rats. J. Agric. Food. Chem. 2021, 69, 3312–3325. [Google Scholar] [CrossRef]
- Wu, Y.; Mo, R.; Zhang, M.; Zhou, W.; Li, D. Grape seed proanthocyanidin alleviates intestinal inflammation through gut microbiota-bile acid crosstalk in mice. Front. Nutr. 2022, 8, 786682. [Google Scholar] [CrossRef]
- Casanova-Martí, A.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ard´evol, A.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food. Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef]
- Zhang, N.N.; Guo, W.H.; Hu, H.; Zhou, A.R.; Liu, Q.P.; Zheng, B.D.; Zeng, S.X. Effect of a polyphenol-rich Canarium album extract on the composition of the gut microbiota of mice fed a high-fat diet. Molecules 2018, 23, 2188. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Meng, K.; Gu, Z.; Yun, Y.; Zhang, W.; Zhang, C.; Zhong, Q.; Pan, F.; Shen, X.; Xia, G.; et al. Arecanut (Areca catechu L.) seed polyphenol-ameliorated osteoporosis by altering gut microbiome via LYZ and the immune system in estrogen-deficient rats. J. Agric. Food. Chem. 2021, 69, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, Y.; Ming, J.; Chen, J.; Zhao, G.; Chen, Z.Y.; Wang, Y.; Lei, L. Polyphenol extract and essential oil of Amomum tsao-ko equally alleviate hypercholesterolemia and modulate gut microbiota. Food. Funct. 2021, 12, 12008–12021. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Yang, H.; Yang, X. Tea polyphenols regulate gut microbiota dysbiosis induced by antibiotic in mice. Food. Res. Int. 2021, 141, 110153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.B.; Hou, M.X.; Han, W.F. Anti-fatigue activity of parsley (Petroselinum crispum) flavonoids via regulation of oxidative stress and gut microbiota in mice. J. Funct. Foods 2022, 89, 104963. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium ruthenicum anthocyanins attenuate high-fat diet-induced colonic barrier dysfunction and inflammation in mice by modulating the gut microbiota. Mol. Nutr. Food. Res. 2021, 65, e2000745. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xu, Y.; Geng, R.; Qiu, J.; He, X. Curcumin alleviates dextran sulfate sodium-induced colitis in mice through regulating gut microbiota. Mol. Nutr. Food. Res. 2022, 66, e2100943. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Wang, Y.; Si, C.; Wang, X.; Wang, R.T.; Lv, Z. Baicalin ameliorates neuropathology in repeated cerebral ischemia-reperfusion injury model mice by remodeling the gut microbiota. Aging 2020, 12, 3791–3806. [Google Scholar] [CrossRef]
- Xu, J.; Ge, J.B.; He, X.Y.; Sheng, Y.; Zheng, S.J.; Zhang, C.H.; Xu, W.T.; Huang, K.L. Caffeic acid reduces body weight by regulating gut microbiota in diet-induced-obese mice. J. Funct. Foods 2020, 74, 104061. [Google Scholar] [CrossRef]
- Ning, K.; Lu, K.; Chen, Q.; Guo, Z.; Du, X.; Riaz, F.; Feng, L.; Fu, Y.; Yin, C.; Zhang, F.; et al. Epigallocatechin gallate protects mice against methionine-choline-deficient-diet-induced nonalcoholic steatohepatitis by improving gut microbiota to attenuate hepatic injury and regulate metabolism. ACS. Omega 2020, 5, 20800–20809. [Google Scholar] [CrossRef]
- Song, Y.; Wu, M.S.; Tao, G.; Lu, M.W.; Lin, J.; Huang, J.Q. Feruloylated oligosaccharides and ferulic acid alter gut microbiome to alleviate diabetic syndrome. Food. Res. Int. 2020, 137, 109410. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jia, Q.; Mehmood, S.; Ma, S.; Liu, X. Genistein ameliorates inflammation and insulin resistance through mediation of gut microbiota composition in type 2 diabetic mice. Eur. J. Nutr. 2021, 60, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Khan, I.; Huang, G.; Lu, Y.; Wang, L.; Liu, Y.; Lu, L.; Hsiao, W.L.W.; Liu, Z. Kaempferol acts on bile acid signaling and gut microbiota to attenuate the tumor burden in ApcMin/+ mice. Eur. J. Pharmacol. 2022, 918, 174773. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Yang, J.W.; Dou, H.Y.; Li, G.Q.; Li, X.Y.; Shen, L.; Ji, H.F. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorg. Chem. 2021, 112, 104966. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Li, X.Y.; Dou, H.Y.; Wang, X.D.; Li, J.D.; Shen, L.; Ji, H.F. Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Rep. 2021, 36, 109641. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.J.; Huang, K.L.; Zhao, C.H.; Xu, W.T.; Sheng, Y.; Luo, Y.B.; He, X.Y. Procyanidin attenuates weight gain and modifies the gut microbiota in high fat diet induced obese mice. J. Funct. Foods 2018, 49, 362–368. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef]
- Zhuang, Y.; Huang, H.; Liu, S.; Liu, F.; Tu, Q.; Yin, Y.; He, S. Resveratrol improves growth performance, intestinal morphology, and microbiota composition and metabolism in mice. Front. Microbiol. 2021, 12, 726878. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Zhao, D.; Du, B.; Wang, M. Protective effect of rosmarinic acid and carnosic acid against streptozotocin-induced oxidation, glycation, inflammation and microbiota imbalance in diabetic rats. Food. Funct. 2018, 9, 851–860. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Zhan, J.; You, Y.; Huang, W. Vanillin alleviates high fat diet-induced obesity and improves the gut microbiota composition. Front. Microbiol. 2018, 9, 2733. [Google Scholar] [CrossRef]
- Olcha, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Nowakowski, Ł.; Miturski, A.; Semczuk, A.; Kiczorowska, B.; Gałczyński, K. Antioxidative, anti-inflammatory, anti-obesogenic, and antidiabetic properties of tea polyphenols—The positive impact of regular tea consumption as an element of prophylaxis and pharmacotherapy support in endometrial cancer. Int. J. Mol. Sci. 2022, 23, 6703. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure—A rat model study. RTP 2015, 73, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Jaiswal, A.K.; Abu-Ghannam, N. Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas: A kinetic study. Food Res. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.P. Green and black tea phenolics: Bioavailability, transformation by colonic microbiota, and modulation of colonic microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.; Penders, J.; Hursel, R.; Budding, A.E.; Savelkoul, P.H.; Westerterp-Plantenga, M.S. Long-term green tea supplementation does not change the human gut microbiota. PLoS ONE 2016, 11, e0153134. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green tea and its relation to human gut microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. JFST 2018, 55, 399–407. [Google Scholar] [CrossRef]
- Bustos, I.; Garcia-Cayuela, T.; Hernandez-Ledesma, B.; Pelaez, C.; Requena, T.; Martínez-Cuesta, M.C. Effect of flavan-3-ols on the adhesion of potential probiotic lactobacilli to intestinal cells. J. Agric. Food Chem. 2012, 60, 9082–9088. [Google Scholar] [CrossRef]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Z.; Qian, Y.; Li, X.; Wang, J.; Ma, J.; Guo, J.; Fu, F. Effects of different concentrations of ganpu tea on fecal microbiota and short chain fatty acids in mice. Nutrients 2021, 13, 3715. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zeng, X.; Chen, T.; Peng, W.; Su, W. Chemical Profile, Antioxidative, and Gut Microbiota Modulatory Properties of Ganpu Tea: A Derivative of Pu-erh Tea. Nutrients 2020, 12, 224. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, B.; Zheng, W.; Chen, X.; Zhang, J.; Yan, R.; Zhang, T.; Yu, L.; Dong, Y.; Ma, B. Liupao tea extract alleviates diabetes mellitus and modulates gut microbiota in rats induced by streptozotocin and high-fat, high-sugar diet. Biomed. Pharmacother. 2019, 118, 109262. [Google Scholar] [CrossRef] [PubMed]
- Dybkowska, E.; Sadowska, A.; Rakowska, R.; Debowska, M.; Swiderski, F.; Swiader, K. Assessing polyphenols content and antioxidant activity in coffee beans according to origin and the degree of roasting. Rocz. Panstw. Zakl. Hig. 2017, 68, 347–353. [Google Scholar] [PubMed]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhang, S.; Ma, W.; Wang, Q.; Li, Y.; Xia, C.; Hu, Y.; Zhang, T.; Yang, L.; Zhou, M. The impact of instant coffee and decaffeinated coffee on the gut microbiota and depression-like behaviors of sleep-deprived rats. Front. Microbiol. 2022, 13, 778512. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Mouatt, P.; Goncalves, P.; Thomas, T.; Brown, L.; Panchal, S.K. Modulation of gut microbiota by spent coffee grounds attenuates diet-induced metabolic syndrome in rats. FASEB J. 2020, 34, 4783–4797. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Brown, L.; Panchal, S.K. Coffee pulp, a by-product of coffee production, modulates gut microbiota and improves metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Pathogens 2021, 10, 1369. [Google Scholar] [CrossRef]
- González, S.; Salazar, N.; Ruiz-Saavedra, S.; Gómez-Martín, M.; de Los Reyes-Gavilán, C.G.; Gueimonde, M. Long-term coffee consumption is associated with fecal microbial composition in humans. Nutrients 2020, 12, 1287. [Google Scholar] [CrossRef]
- Molinari, R.; Merendino, N.; Costantini, L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: State-of-the-art. BioFactors 2022, 48, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic acid-induced gut microbiota improves metabolic endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lam, K.L.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Khymenets, O.; Urpí-Sardà, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Liorach, R.; Andres-Lacueva, C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa polyphenols and gut microbiota interplay: Bioavailability, prebiotic effect, and impact on human health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Laličić-Petronijević, J.; Komes, D.; Gorjanović, S.; Belščak-Cvitanović, A.; Pezo, L.; Pastor, F.; Ostojić, S.; Popov-Raljić, J.; Sužnjević, D. Content of total phenolics, flavan-3-ols and proanthocyanidins, oxidative stability and antioxidant capacity of chocolate during storage. Food Technol. Biotechnol. 2016, 54, 13. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- de Oliveira, V.P.S.; Dolinsky, M.; Barroso, S.G.; Pinto, M.B.S.; Uehara, S.K.; de Souza Rocha, G. Dark polyphenols-rich chocolate and gut microbiota: A literature review. DEMETRA 2017, 12, 399–409. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Pérez-Berezo, T.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F., Jr.; Davis, C.D.; Solano-Aguilar, G. Flavanol-enriched cocoa powder alters the intestinal microbiota, tissue and fluid metabolite profiles, and intestinal gene expression in pigs. J. Nutr. 2015, 146, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic review of phenolic compounds in apple fruits: Compositions, distribution, absorption, metabolism, and processing stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, L.; Martinez-Ferri, E.; Soria, C.; Ariza, M.T. Bioavailability of phenolic compounds in strawberry, raspberry and blueberry: Insights for breeding programs. Food Biosci. 2020, 37, 100680. [Google Scholar] [CrossRef]
- Wu, T.; Chu, X.; Cheng, Y.; Tang, S.; Zogona, D.; Pan, S.; Xu, X. Modulation of gut microbiota by lactobacillus casei fermented raspberry juice in vitro and in vivo. Foods 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.; Burns, G.; Sturgeon, N.; Mears, K.; Stote, K.; Blanton, C. The Effects of Berry Polyphenols on the Gut Microbiota and Blood Pressure: A Systematic Review of Randomized Clinical Trials in Humans. Nutrients 2022, 14, 2263. [Google Scholar] [CrossRef]
- Kan, J.; Chen, C.; Huo, T.; Xie, W.; Hui, Y.; Liu, J.; Jin, C. Polyphenolic-enriched peach peels extract regulates lipid metabolism and improves the gut microbiota composition in high fat diet-fed mice. J. Funct. Foods 2020, 72, 104082. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Huang, J.; Ding, Y.; Pan, Z.; Zhao, Y.; Zhang, R.; Hu, B.; Zeng, X. In vitro extraction and fermentation of polyphenols from grape seeds (Vitis vinifera) by human intestinal microbiota. Food Funct. 2016, 7, 1959–1967. [Google Scholar] [CrossRef]
- Cueva, C.; Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Bartolome, B.; Moreno-Arribas, M.V. An integrated view of the effects of wine polyphenols and their relevant metabolites on gut and host health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.O.; Carbonero, F. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2014, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Deng, R.; Zhang, Y.; Zheng, X. Dietary anthocyanin-rich extract of açai protects from diet-induced obesity, liver steatosis, and insulin resistance with modulation of gut microbiota in mice. Nutrition 2021, 86, 111176. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.P.; Young, W.; Armstrong, K.; Brewster, D.; Cooney, J.M.; Ellett, S.; Espley, R.V.; Laing, W.; Maclean, P.; McGhie, T.; et al. A polyphenol enriched variety of apple alters circulating immune cell gene expression and faecal microbiota composition in healthy adults: A randomized controlled trial. Nutrients 2021, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Butts, C.A.; Laing, W.A.; Martell, S.; Smith, H.; McGhie, T.K.; Zhang, J.; Paturi, G.; Hedderley, D.; Bovy, A.; et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J. Nutr. 2014, 144, 146–154. [Google Scholar] [CrossRef]
- Yu, C.; Guo, C.; Geng, X.; Yao, Y.; Guo, J.; Zhang, Y.; Zhang, J.; Mi, S. Effects of fruits and vegetables on gut microbiota in a mouse model of metabolic syndrome induced by high-fat diet. Food Sci. Nutr. 2023, 11, 794–805. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Holgado, F.; Campos-Monfort, G.; de las Heras, C.; Rupérez, P. Assessment of the prebiotic potential of globe artichoke by-product through in vitro fermentation by human faecal microbiota. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100328. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Villar, A.; Zangara, A.; Smidt, C.R.; Risco, E. In vitro evaluation of prebiotic properties of a commercial artichoke inflorescence extract revealed bifidogenic effects. Nutrients 2020, 12, 1552. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Costa, C.; Roupar, D.; Silva, S.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Modulation of the gut microbiota by tomato flours obtained after conventional and ohmic heating extraction and its prebiotic properties. Foods 2023, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Peng, Z.; Zheng, W.; Yang, S.; Wu, M.; Liu, K.; Xiao, M.; Huang, T.; Xie, M.; Xiong, T. Probiotic-fermented tomato alleviates high-fat diet-induced obesity in mice: Insights from microbiome and metabolomics. Food Chem. 2024, 436, 137719. [Google Scholar] [CrossRef] [PubMed]
- Goggans, M.L.; Bilbrey, E.A.; Quiroz-Moreno, C.D.; Francis, D.M.; Jacobi, S.K.; Kovac, J.; Cooperstone, J.L. Short-Term Tomato Consumption Alters the Pig Gut Microbiome toward a More Favorable Profile. Microbiol. Spectr. 2022, 10, e02506-22. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.; Swenson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, Q.; Huang, H.; Hou, K.; Dong, R.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. The effect of bound polyphenols on the fermentation and antioxidant properties of carrot dietary fiber in vivo and in vitro. Food Funct. 2020, 11, 748–758. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Xie, J.; Chen, Y.; Zheng, Y.; Zhang, X.; Zhang, X.; Zhao, E.; Wang, Z.; Xu, H.; et al. The recovery, catabolism and potential bioactivity of polyphenols from carrot subjected to in vitro simulated digestion and colonic fermentation. Food Res. Int. 2021, 143, 110263. [Google Scholar] [CrossRef]
- Bodai, B.I.; Nakata, T.E. Breast Cancer: Lifestyle, the Human Gut Microbiota/Microbiome, and Survivorship. Perm. J. 2020, 24, 129. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut microbiota and predicted metabolic pathways in a sample of Mexican women affected by obesity and obesity plus metabolic syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef]
- De La Cuesta-Zuluaga, J.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Body size phenotypes comprehensively assess cardiometabolic risk and refine the association between obesity and gut microbiota. Int. J. Obes. 2018, 42, 424–432. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Gut microbiota is associated with obesity and cardiometabolic disease in a population in the midst of Westernization. Sci. Rep. 2018, 8, 11356. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, M.; Xue, J.; Huang, J.; Zhuang, R.; Zhou, X.; Zhang, H.; Fu, Q.; Hao, Y. Body mass index differences in the gut microbiota are gender specific. Front. Microbiol. 2018, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Harakeh, S.; Angelakis, E.; Karamitros, T.; Bachar, D.; Bahijri, S.; Ajabnoor, G.; Alfadul, S.M.; Farraj, S.A.; Al Amri, T.; Al-Hejin, A.; et al. Impact of smoking cessation, coffee and bread consumption on the intestinal microbial composition among Saudis: A cross-sectional study. PLoS ONE 2020, 15, e0230895. [Google Scholar] [CrossRef] [PubMed]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; Herzig, K.H.; Caporaso, J.G.; Derkach, A.; Wan, Y.; Byrd, D.A.; Vogtmann, E.; Männikkö, M.; Karhunen, V.; Knight, R.; et al. Association of body mass index with fecal microbial diversity and metabolites in the northern Finland birth cohort. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2289–2299. [Google Scholar] [CrossRef]
- Oduaran, O.H.; Tamburini, F.B.; Sahibdeen, V.; Brewster, R.; Gómez-Olivé, F.X.; Kahn, K.; Norris, S.A.; Tollman, S.M.; Twine, R.; Wade, A.N.; et al. Gut microbiome profiling of a rural and urban South African cohort reveals biomarkers of a population in lifestyle transition. BMC Microbiol. 2020, 20, 330. [Google Scholar] [CrossRef]
- Ozato, N.; Saito, S.; Yamaguchi, T.; Katashima, M.; Tokuda, I.; Sawada, K.; Katsuragi, Y.; Kakuta, M.; Imoto, S.; Ihara, K.; et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes 2019, 5, 28. [Google Scholar] [CrossRef]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. 2014, 38, 1525–1531. [Google Scholar] [CrossRef]
- Yasir, M.; Angelakis, E.; Bibi, F.; Azhar, E.I.; Bachar, D.; Lagier, J.C.; Gaborit, B.; Hassan, A.M.; Jiman-Fatani, A.A.; Alshali, K.Z.; et al. Comparison of the gut microbiota of people in France and Saudi Arabia. Nutr. Diabetes 2015, 5, e153. [Google Scholar] [CrossRef]
- Duca, F.A.; Sakar, Y.; Lepage, P.; Devime, F.; Langelier, B.; Doré, J.; Covasa, M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes 2014, 63, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A high-fat diet increases gut microbiota biodiversity and energy expenditure due to nutrient difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Sreelatha, S.; Devang, N.; Alva, P.D.; Raveendran, A.V. The Microbiota–Gut–Brain Axis and Diabetic Cognitive Impairment: A Memorable Journey. Clin. Diabetol. 2023, 12, 261–271. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Craciun, C.I.; Neag, M.A.; Catinean, A.; Mitre, A.O.; Rusu, A.; Bala, C.; Roman, G.; Buzoianu, A.D.; Muntean, D.M.; Craciun, A.E. The relationships between gut microbiota and diabetes mellitus, and treatments for diabetes mellitus. Biomedicines 2022, 10, 308. [Google Scholar] [CrossRef]
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. 2017, 111, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Navab-Moghadam, F.; Sedighi, M.; Khamseh, M.E.; Alaei-Shahmiri, F.; Talebi, M.; Razavi, S.; Amirmozafari, N. The association of type II diabetes with gut microbiota composition. Microb. Pathog. 2017, 110, 630–636. [Google Scholar] [CrossRef]
- Tsai, H.J.; Tsai, W.C.; Hung, W.C.; Hung, W.W.; Chang, C.C.; Dai, C.Y.; Tsai, Y.C. Gut microbiota and subclinical cardiovascular disease in patients with type 2 diabetes mellitus. Nutrients 2021, 13, 2679. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef]
- Collins, B.; Hoffman, J.; Martinez, K.; Grace, M.; Lila, M.A.; Cockrell, C.; Anuradha Nadimpalli, A.; Chang, E.; Chuang, C.C.; Zhong, W.; et al. A polyphenol-rich fraction obtained from table grapes decreases adiposity, insulin resistance and markers of inflammation and impacts gut microbiota in high-fat-fed mice. J. Nutr. Biochem. 2016, 31, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Serino, M.; Blasco-Baque, V.; Azalbert, V.; Barton, R.H.; Cardellini, M.; Latorre, J.; Ortega, F.; Sabater-Masdeu, M.; Burcelin, R.; et al. Gut microbiota interacts with markers of adipose tissue browning, insulin action and plasma acetate in morbid obesity. Mol. Nutr. Food Res. 2018, 62, 1700721. [Google Scholar] [CrossRef] [PubMed]
- Bajinka, O.; Tan, Y.; Darboe, A.; Ighaede-Edwards, I.G.; Abdelhalim, K.A. The gut microbiota pathway mechanisms of diabetes. AMB Express 2023, 13, 16. [Google Scholar] [CrossRef]
- Sharma, B.R.; Jaiswal, S.; Ravindra, P.V. Modulation of gut microbiota by bioactive compounds for prevention and management of type 2 diabetes. Biomed. Pharmacother. 2022, 152, 113148. [Google Scholar] [CrossRef] [PubMed]
- Fryczkowski, M.; Hejmo, T.; Bułdak, M.; Stachowska, M.; Rokicka, J.; Żwirska-Korczala, K. Wpływ wybranych cytokin prozapalnych oraz stresu oksydacyjnego na kancerogenezę i progresję gruczolakoraków jelita i prostaty. Ann. Acad. Med. Siles. 2019, 73, 182–193. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Sezgin, B.; Pirinççi, F.; Camuzcuoğlu, A.; Erel, Ö.; Neşelioğlu, S.; Camuzcuoğlu, H. Assessment of thiol disulfide balance in early-stage endometrial cancer. J. Obstet. Gynaecol. Res. 2020, 46, 1140–1147. [Google Scholar] [CrossRef]
- Gifkins, D.; Olson, S.H.; Demissie, K.; Lu, S.E.; Kong, A.N.; Bandera, E.V. Total and individual antioxidant intake and endometrial cancer risk: Results from a population-based case-control study in New Jersey. Cancer Causes Control 2012, 23, 887–895. [Google Scholar] [CrossRef][Green Version]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—A review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.H.; Bouyahya, A. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Muhammad, T.; Park, J.; Kim, M.O. Caffeine modulates cadmium-induced oxidative stress, neuroin-flammation, an cognitive impairments by regulating Nrf-2/HO-1 in vivo and in vitro. J. Clin. Med. 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Krajewski, R.; Makuch, S.; Agrawal, S. Phytochemicals in Gynecological Cancer Prevention. Int. J. Mol. Sci. 2021, 22, 1219. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Currenti, W.; Godos, J. Plant-based polyphenol-rich foods and beverages influence metabolic health in a Mediterranean cohort. Eur. J. Public Health 2021, 31, 164–416. [Google Scholar] [CrossRef]
- Delgado, A.; Gonçalves, S.; Romano, A. Mediterranean Diet: The Role of Phenolic Compounds from Aromatic Plant Foods. Foods 2023, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed]
- Kapolou, A.; Karantonis, H.C.; Rigopoulos, N.; Koutelidakis, A.E. Association of Mean Daily Polyphenols Intake with Mediterranean Diet Adherence and Anthropometric Indices in Healthy Greek Adults: A Retrospective Study. Appl. Sci. 2021, 11, 4664. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Song, X.Y.; Pan, A.; Koh, W.P. Nutrition and Healthy Ageing in Asia: A Systematic Review. Nutrients 2023, 15, 3153. [Google Scholar] [CrossRef]
- Zhang, F.F.; Haslam, D.E.; Terry, M.B.; Knight, J.A.; Andrulis, I.L.; Daly, M.B.; Buys, S.S.; John, E.M. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer 2017, 123, 2070–2079. [Google Scholar] [CrossRef]
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef]
- Yoriki, K.; Mori, T.; Aoyama, K.; Tarumi, Y.; Kataoka, H.; Kokabu, T.; Kitawaki, J. Genistein induces long-term expression of progesterone receptor regardless of estrogen receptor status and improves the prognosis of endometrial cancer patients. Sci. Rep. 2022, 12, 10303. [Google Scholar] [CrossRef]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Topor-Mądry, R.; Szafraniec, K.; Pająk, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2014, 30, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Zloch, Z.; Sedlacek, P.; Langmajerová, J.; Mullerova, D. Intake and profile of plant polyphenols in the diet of the Czech population. Pol. J. Food Nutr. Sci. 2018, 68, 57–62. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Heuer, T.; Mulligan, A.A.; Lentjes, M.A.; Luben, R.N.; Kuhnle, G.G. Estimated dietary intakes and sources of flavanols in the German population (German National Nutrition Survey II). Eur. J. Nutr. 2014, 53, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols rich diets and risk of type 2 diabetes. Nutrients 2021, 13, 1445. [Google Scholar] [CrossRef]
| Animal Model | Time | Dosage (mg/kg b.w.) (Body Weight) | Effects | Ref. | |
|---|---|---|---|---|---|
| Apple polyphenol extract (49.27% epigallocatechin gallate, 10.09% epicatechin gallate, 15.49% epigallocatechin, and 7.18% epicatechin) | Male mice | 12 week | 125 and 500 mg/kg | Regulation of gut microbiota and its composition ↑ Akkermansia ↓ Lactobacillus | [53] |
| Grape extract | Obese mice | 11 weeks | 1% w/w extract dissolved in water | Restoration of gut microbiota dysbiosis ↑ Bifidobacteria, Akkermansia, Clostridiagenera and Firmicutes/Bacteroides | [54] |
| Green pea (Pisum sativum L.) hull extract (quercetin and its derivatives (2836.57 μg of quercetin/g of extracts), kaempferol trihexoside (1482.00 μg of quercetin/g of extracts), and catechin and its derivatives (1339.91 μg of quercetin/g of extracts)) | Mice | 7 days | 100 mg/kg | Regulation of gut microbiota ↑ Firmicutes/Bacteroidetes and promoting the growth of Lactobacilaceae and Lachnospiraceae | [55] |
| Blueberry polyphenols extract | Obese mice | 12 weeks | 200 mg/kg | Alteration of gut microbiota composition and modulation of gut microbiota composition ↑ Proteobacteria, Deferribacteres Bifidobacterium, Desulfovibrio, Adlercreutzia, Helicobacter, Flexispira ↓ Actinobacteria, Flexispira, Adlercreutzia, Prevotella | [56] |
| Blueberry anthocyanin extract | Obese mice | 12 weeks | 200 mg/kg | Modulation of gut microbiota, Improvement of insulin sensitivity | [57] |
| Honey polyphenols (caffeic acid, chlorogenic acid, rutin, etc.) | Adult male rats | 7 days | 10.5 mg/kg | Enhancement of resistance to oxidative stress through modulation of gut microbiota ↓ Bacteroides, Corynebacterium, Proteus | [58] |
| Aronia melanocarpa extract | Rats | 4 weeks | 200 mg/kg | Reinforcement of the gut barrier ↑ Lactobacillus, Enterobacteriaceae, Enterobacteriaceae, ↓ Lachnospiraceae, Phascolarctobacterium, Clostridiales | [59] |
| Grape seed proanthocyanidin | Male mice | 20 days | 250 mg/kg | Regulation of gut microbiota | [60] |
| Grape seed proanthocyanidin extract ((monomeric (21.3%), dimeric (17.4%), trimeric (16.3%), tetrameric (13.3%), and oligomeric (31.7%) proanthocyanidins) | Rats | 8 days | 500 mg/kg | Effectiveness in modifying microbiota ↑ Bacteroidetes, ↓ Firmicutes, Modifications in the microbiota led to changes in the profile of short-chain fatty acids, | [61] |
| Canarium album extract | Male mice | 4 weeks | 10 mg/kg | ↑ Firmicutes, Verrucomicrobia, ↓ Bacteroidetes | [62] |
| Arecanut (Areca catechu L.) seed polyphenols (28,284.5896 μg/g catechin, 5628.2237 μg/g procyanidin B1, 5486.037 μg/g quinic acid, 1150.3457 μg/g proanthocyanidin B2, etc.) | Female rats | 90 days. | 400 mg/kg. | ↓ Alistipes ↑ Phascolarctobacterium, Blautia, Faecalibaculum, Fusicatenibacter, Ruminococcaceae, Allobaculum, Negativibacillus, Turicibacter, Lactobacillus | [63] |
| Polyphenol extract and essential oil (TKO) from Amomum tsao-ko Crevost et Lemaire (tsao-ko) (50.00% epicatechin, 30.60% kaempferol, 7.10% protocatechuic acid, 2.58% vanillic acid, etc.) | Male hamsters | 6 weeks. | 1000 mg/kg | ↑ Bacteroidetes | [64] |
| Green tea extract (49.27% epi-gallocatechin gallate, 10.09% epicatechin gallate, 15.49% epigallocatechin, and 7.18% epicatechin) | Male mice | 28 days | 360 mg/kg | ↑ Lactobacillus, Akkermansia, Blautia, Roseburia, Eubacterium, Regulation of gut microbiota dysbiosis induced by antibiotics | [65] |
| Parsley (Petroselinum crispum) flavonoids extract (apigenin-7-O-glucuronide, diosmetin-7-O-glucoside, kaempferol-7-O-glucoside, and scutellarin) | Mice | 6 weeks | 50 mg/kg | Stimulation of probiotics and microbiota producing short-chain fatty acids | [66] |
| Lycium ruthenicum anthocyanins (petunidin (95.17%)) | Mice | 12 weeks | 50, 100, 200 mg/kg | Alleviation of colonic barrier dysfunction | [67] |
| Polyphenols | |||||
| Curcumin | Mice | 7 days | 50 or 150 mg kg | Strengthening the intestinal barrier, Modulating the abundance of bacteria: ↑ Odoribacter/Coprococcus, ↓ Turicibacter, Enterococcaceae | [68] |
| Baicalin | Male mice | 7 days | 100 mg/kg | Restoration of the normal microbiological composition of the intestines, ↑ Halomonas smyrnensis, Citromicrobium sp. WPS32, Eubacterium sp. CAG 86, ↓ Parabacteroides johnsonii, Bacteroides, | [69] |
| Caffeic acid | Male mice | 12 weeks | 50 mg/kg | Regulation of gut microbiota, ↑ Muribaculaceae ↓ Lachnospiraceae | [70] |
| Epigallocatechin- 3-gallate | Male mice | 2 weeks | 50 mg/kg | Significant improvement in gut microbiota dysbiosis caused by the methionine—choline-deficient diet, ↑ Bacteroidetes, Alloprevotella, Bifidobacteria, Lactobacillus ↓ Firmicutes | [71] |
| Ferulic acid | Rats | 8 weeks | 30 mg/kg | ↓ Lactobacillus, Ruminococcus, Oscillibacter, Desulfovibrio, ↑ Akkermansia, Phascolarctobacterium, Turicibacter | [72] |
| Genistein | Mice | 8 weeks | 40 mg/kg | ↓ Firmicutes/Bacteroidetes, ↓ Proteobacteria, Ruminococcus, Helicobacter ↑ Bacteroides, Prevotella | [73] |
| Kaempferol | Mice | 6 weeks | 100 mg/kg | ↑ Actinobacteria, Verrucomicrobia, Akkermansia, Alloprevotella, Bacteroides, Barnesiella, Gloebacter, Odoribacter, Parabacteroides ↓ Eubacterium | [74] |
| Luteolin | Rats | 12 weeks | 0.5% Luteolin | ↑ Parvibacter, Faecalitalea, Allobaculum sp., Bacteroides dorei | [75] |
| Myricetin | Mice | 12 weeks | 0.5% Myricetin | ↑ Allobaculum, Lactobacillus, Nocardiaceae Tyzzerella 4, Brachybacterium | [76] |
| Procyanidins | Obese male mice | 12 weeks | 100 mg/kg | ↑ Bacteroidetes ↓ Firmicutes/Bacteroidetes | [77] |
| Quercetin | Low-density lipoprotein receptor-null mice | 8 weeks | 100 μg/day | ↑ Actinobacteria, Bacteroidetes, Akkermansia, Bacteroides, Parabacteroides, Ruminococcus, ↓ Firmicutes, Lactobacillus | [78] |
| Resveratrol | Diabetic male rats | 28 days | 20 mg/kg | Increase in the richness indices of gut microbiota, Shaped composition of gut microbiota (e.g., increased beta diversity of the gut microbiota community), Impact on the metabolism of gut microbiota (amino acid and lipid metabolism) and defensive mechanisms | [79] |
| Rosmarinic acid | Diabetic rats | 8 weeks | 30 mg/kg | Prebiotic effect on gut microbiota | [80] |
| Vanillin | Obese mice | 12 weeks | 0.1% Vanillin | ↓ Firmicutes phylum, ↑ Bacteroidetes, Verrucomicrobiota phyla | [81] |
| Experimental Factor | Effects on Gut Microbiome | Ref. |
|---|---|---|
| Normal weight n = 10 Obese n = 10 | ↑ Firmicutes ↑ Fusobacteriums | [140] |
| Normal weight n = 25 Obese n = 17 Obese plus metabolic syndrome n = 25 | ↑ Firmicutes | [141] |
| Normal weight n = 138 Overweight n = 171 Obese n = 132 | ↓ Bakteroidetes ↓ Bacteroidetes/Firmicutes | [142,143] |
| Normal weight n = 261 Overweight n = 170 Obese n = 58 | ↑ Bacteroidetes ↑ Fusobacterium ↑ Proteobacteri | [144] |
| Normal weight n = 28 Overweight n = 24 | ↓ Fusobacterium | [145] |
| Normal weight n = 23 Obese n = 33 | ↓ Bacteroidetes ↑ Firmicutes/Bacteroidetes | [146] |
| Normal weight n = 217 Overweight n = 163 Obese n = 167 | ↑ Bacteroidetes | [147] |
| Normal weight = 30 Obese n = 106 | ↑ Bacteroidetes | [148] |
| Normal weight n = 209 Overweight n = 563 Obese n = 229 | ↓ Bacteroidetes ↑ Firmicutes | [149] |
| Normal weight n = 11 Obese and Overweight n = 11 | ↑ Firmicute ↑ Firmicutes/Bacteroidetes | [150] |
| France: Normal weight n = 16; Obese n = 12 Saudi Arabia: Normal weight n = 9; Obese n = 9 | France: ↑ Bacteroidetes, ↑ Proteobacteria Saudi Arabia: ↑ Firmicutes | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowska-Wójcik, E.; Winiarska-Mieczan, A.; Olcha, P.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Nowakowski, Ł.; Miturski, A.; Gałczyński, K. Polyphenols Influence the Development of Endometrial Cancer by Modulating the Gut Microbiota. Nutrients 2024, 16, 681. https://doi.org/10.3390/nu16050681
Baranowska-Wójcik E, Winiarska-Mieczan A, Olcha P, Kwiecień M, Jachimowicz-Rogowska K, Nowakowski Ł, Miturski A, Gałczyński K. Polyphenols Influence the Development of Endometrial Cancer by Modulating the Gut Microbiota. Nutrients. 2024; 16(5):681. https://doi.org/10.3390/nu16050681
Chicago/Turabian StyleBaranowska-Wójcik, Ewa, Anna Winiarska-Mieczan, Piotr Olcha, Małgorzata Kwiecień, Karolina Jachimowicz-Rogowska, Łukasz Nowakowski, Andrzej Miturski, and Krzysztof Gałczyński. 2024. "Polyphenols Influence the Development of Endometrial Cancer by Modulating the Gut Microbiota" Nutrients 16, no. 5: 681. https://doi.org/10.3390/nu16050681
APA StyleBaranowska-Wójcik, E., Winiarska-Mieczan, A., Olcha, P., Kwiecień, M., Jachimowicz-Rogowska, K., Nowakowski, Ł., Miturski, A., & Gałczyński, K. (2024). Polyphenols Influence the Development of Endometrial Cancer by Modulating the Gut Microbiota. Nutrients, 16(5), 681. https://doi.org/10.3390/nu16050681







