Epigenetic Genome Modifications during Pregnancy: The Impact of Essential Nutritional Supplements on DNA Methylation
Abstract
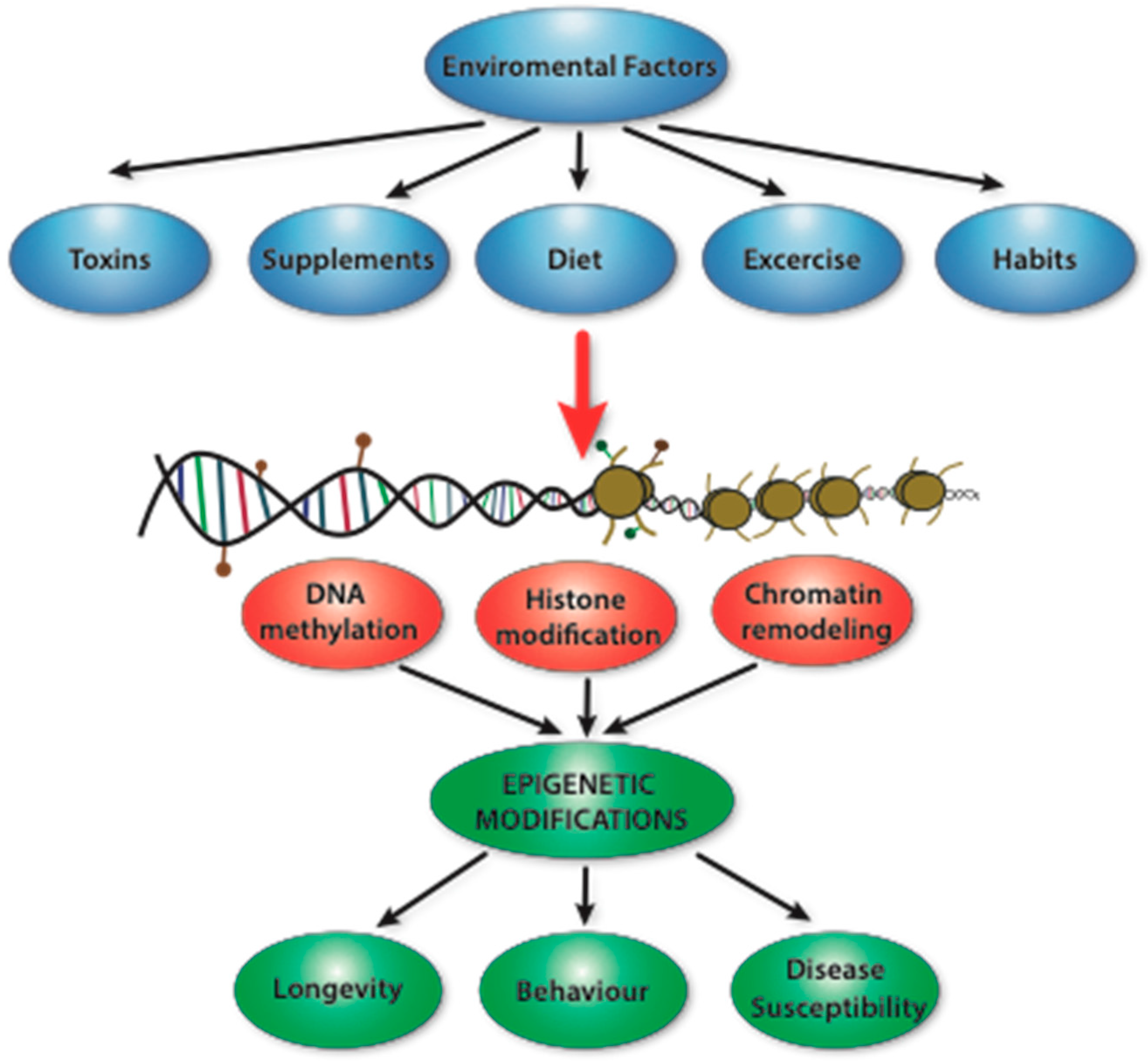
1. Introduction
2. Epigenetics
3. Choline
4. Folic Acid
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Zaidi, A.Z.; Moore, S.E.; Okala, S.G. Impact of Maternal Nutritional Supplementation during Pregnancy and Lactation on the Infant Gut or Breastmilk Microbiota: A Systematic Review. Nutrients 2021, 13, 1137. [Google Scholar] [CrossRef]
- Blondin, J.H.; LoGiudice, J.A. Pregnant women’s knowledge and awareness of nutrition. Appl. Nurs. Res. 2018, 39, 167–174. [Google Scholar] [CrossRef]
- Brown, B.; Wright, C. Safety and efficacy of supplements in pregnancy. Nutr. Rev. 2020, 78, 813–826. [Google Scholar] [CrossRef]
- Fallah, F.; Pourabbas, A.; Delpisheh, A.; Veisani, Y.; Shadnoush, M. Effects of nutrition education on levels of nutritional awareness of pregnant women in Western iran. Int. J. Endocrinol. Metab. 2013, 11, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Gahche, J.J.; Potischman, N.; Dwyer, J.T.; Guenther, P.M.; Sauder, K.A.; Bailey, R.L. Dietary Supplement Use and Its Micronutrient Contribution During Pregnancy and Lactation in the United States. Obstet. Gynecol. 2020, 135, 623–633. [Google Scholar] [CrossRef]
- Adams, J.B.; Kirby, J.K.; Sorensen, J.C.; Pollard, E.L.; Audhya, T. Evidence based recommendations for an optimal prenatal supplement for women in the US: Vitamins and related nutrients. Matern. Health Neonatol Perinatol 2022, 8, 4. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Zablotsky, B.; Black, L.I.; Blumberg, S.J. Estimated Prevalence of Children with Diagnosed Developmental Disabilities in the United States, 2014–2016. NCHS Data Brief. 2017, 291, 1–8. [Google Scholar]
- Vinyard, M.; Zimmer, M.; Herrick, K.A.; Story, M.; Juan, W.; Reedy, J. Healthy Eating Index-2015 Scores Vary by Types of Food Outlets in the United States. Nutrients 2021, 13, 2717. [Google Scholar] [CrossRef]
- Jaiswal, A.; Dewani, D.; Reddy, L.S.; Patel, A. Choline Supplementation in Pregnancy: Current Evidence and Implications. Cureus 2023, 15, e48538. [Google Scholar] [CrossRef]
- Adams, J.B.; Sorenson, J.C.; Pollard, E.L.; Kirby, J.K.; Audhya, T. Evidence-Based Recommendations for an Optimal Prenatal Supplement for Women in the U.S., Part Two: Minerals. Nutrients 2021, 13, 1849. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Feng, L.; Lou, J. DNA Methylation Analysis. Methods Mol. Biol. 2019, 1894, 181–227. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Camm, E.J. The Programming Power of the Placenta. Front. Physiol. 2016, 7, 33. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of well-being. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1359–1366. [Google Scholar] [CrossRef]
- Abdul, Q.A.; Yu, B.P.; Chung, H.Y.; Jung, H.A.; Choi, J.S. Epigenetic modifications of gene expression by lifestyle and environment. Arch. Pharm. Res. 2017, 40, 1219–1237. [Google Scholar] [CrossRef]
- Bollati, V.; Baccarelli, A. Environmental epigenetics. Heredity 2010, 105, 105–112. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.W. Epigenetics: The link between nature and nurture. Mol. Asp. Med. 2013, 34, 753–764. [Google Scholar] [CrossRef]
- Zeisel, S.H. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am. J. Clin. Nutr. 2009, 89, 1488S–1493S. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, F.; Li, X. Epigenetic Programming and Fetal Metabolic Programming. Front. Endocrinol. 2019, 10, 764. [Google Scholar] [CrossRef]
- Zeisel, S. Choline, Other Methyl-Donors and Epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef]
- Shenker, N.; Flanagan, J.M. Intragenic DNA methylation: Implications of this epigenetic mechanism for cancer research. Br. J. Cancer 2012, 106, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Bestor, T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 2000, 9, 2395–2402. [Google Scholar] [CrossRef]
- Mihai, D. Niculescu, Nutritional Epigenetics. ILAR J. 2012, 53, 270–278. [Google Scholar] [CrossRef]
- Dhar, G.A.; Saha, S.; Mitra, P.; Nag Chaudhuri, R. DNA methylation and regulation of gene expression: Guardian of our health. Nucleus 2021, 64, 259–270. [Google Scholar] [CrossRef]
- Long, H.K.; King, H.W.; Patient, R.K.; Odom, D.T.; Klose, R.J. Protection of CpG islands from DNA methylation is DNA-encoded and evolutionarily conserved. Nucleic Acids Res. 2016, 44, 6693–6706. [Google Scholar] [CrossRef]
- Verdone, L.; Caserta, M.; Di Mauro, E. Role of histone acetylation in the control of gene expression. Biochem. Cell Biol. 2005, 83, 344–353. [Google Scholar] [CrossRef]
- Margueron, R.; Reinberg, D. Chromatin structure and the inheritance of epigenetic information. Nat. Rev. Genet. 2010, 11, 285–296. [Google Scholar] [CrossRef]
- Martin, B.J.E.; Brind’Amour, J.; Kuzmin, A.; Jensen, K.N.; Liu, Z.C.; Lorincz, M.; Howe, L.J. Transcription shapes genome-wide histone acetylation patterns. Nat. Commun. 2021, 12, 210. [Google Scholar] [CrossRef]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Toraño, E.G.; García, M.G.; Fernández-Morera, J.L.; Niño-García, P.; Fernández, A.F. The Impact of External Factors on the Epigenome: In Utero and over Lifetime. Biomed. Res. Int. 2016, 2016, 2568635. [Google Scholar] [CrossRef]
- Jansson, T.; Powell, T.L. Role of the placenta in fetal programming: Underlying mechanisms and potential interventional approaches. Clin. Sci. 2007, 113, 1–13. [Google Scholar] [CrossRef]
- Cooney, C.A. Are somatic cells inherently deficient in methylation metabolism? A proposed mechanism for DNA methylation loss, senescence and aging. Growth Dev. Aging 1993, 57, 261–273. [Google Scholar]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Miller, K.M. Histone methylation and the DNA damage response. Mutat. Res. Rev. Mutat. Res. 2019, 780, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.A.; Marcum, R.D.; He, Y. Structure and Function of Chromatin Remodelers. J. Mol. Biol. 2021, 433, 166929. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Ramanathan, S.; Krishna, K. Factors, mechanisms and implications of chromatin condensation and chromosomal structural maintenance through the cell cycle. J. Cell Physiol. 2020, 235, 758–775. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, B.E.; Tieu, K.V.; Spagnol, S.T.; Vu, K.K.; Cabe, J.I.; Raisch, T.B.; Dahl, K.N.; Conway, D.E. Chromatin condensation regulates endothelial cell adaptation to shear stress. Mol. Biol. Cell. 2022, 33, ar101. [Google Scholar] [CrossRef]
- Alver, B.H.; Kim, K.H.; Lu, P.; Wang, X.; Manchester, H.E.; Wang, W.; Haswell, J.R.; Park, P.J.; Roberts, C.W. The SWI/SNF chromatin remodelling complex is required for maintenance of lineage specific enhancers. Nat. Commun. 2017, 8, 14648. [Google Scholar] [CrossRef]
- Blackledge, N.P.; Klose, R.J. The molecular principles of gene regulation by Polycomb repressive complexes. Nat. Rev. Mol. Cell Biol. 2021, 22, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Piunti, A.; Shilatifard, A. The roles of Polycomb repressive complexes in mammalian development and cancer. Nat. Rev. Mol. Cell Biol. 2021, 22, 326–345. [Google Scholar] [CrossRef]
- Khodadadi, E.; Fahmideh, L.; Khodadadi, E.; Dao, S.; Yousefi, M.; Taghizadeh, S.; Asgharzadeh, M.; Yousefi, B.; Kafil, H.S. Current Advances in DNA Methylation Analysis Methods. Biomed. Res. Int. 2021, 2021, 8827516. [Google Scholar] [CrossRef]
- Yi, P.; Melnyk, S.; Pogribna, M.; Pogribny, I.P.; Hine, R.J.; James, S.J. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J. Biol. Chem. 2000, 275, 29318–29323. [Google Scholar] [CrossRef]
- Mentch, S.J.; Locasale, J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef]
- Obeid, R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 2013, 5, 3481–3495. [Google Scholar] [CrossRef]
- Finkelstein, J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.; Li, X.; Liu, G.; Bin, P.; Yan, W.; Más, D.; Valdivié, M.; Hu, C.A.; Ren, W.; Yin, Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 2017, 49, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Mota-Martorell, N.; Jové, M.; Borrás, C.; Berdún, R.; Obis, È.; Sol, J.; Cabré, R.; Pradas, I.; Galo-Licona, J.D.; Puig, J.; et al.; et al. Methionine transsulfuration pathway is upregulated in long-lived humans. Free Radic. Biol. Med. 2021, 162, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Detich, N.; Hamm, S.; Just, G.; Knox, J.D.; Szyf, M. The methyl donor S-Adenosylmethionine inhibits active demethylation of DNA: A candidate novel mechanism for the pharmacological effects of S-Adenosylmethionine. J. Biol. Chem. 2003, 278, 20812–20820. [Google Scholar] [CrossRef] [PubMed]
- Zaric, B.L.; Obradovic, M.; Bajic, V.; Haidara, M.A.; Jovanovic, M.; Isenovic, E.R. Homocysteine and Hyperhomocysteinaemia. Curr. Med. Chem. 2019, 26, 2948–2961. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.W.; Glazier, J.D. Homocysteine Metabolism in Pregnancy and Developmental Impacts. Front. Cell Dev. Biol. 2022, 10, 802285. [Google Scholar] [CrossRef]
- Pérez-Miguelsanz, J.; Vallecillo, N.; Garrido, F.; Reytor, E.; Pérez-Sala, D.; Pajares, M.A. Betaine homocysteine S-methyltransferase emerges as a new player of the nuclear methionine cycle. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1165–1182. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic acid versus 5- methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Field, M.S.; Stover, P.J. Cell cycle regulation of folate-mediated one-carbon metabolism. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1426. [Google Scholar] [CrossRef] [PubMed]
- Lionaki, E.; Ploumi, C.; Tavernarakis, N. One-Carbon Metabolism: Pulling the Strings behind Aging and Neurodegeneration. Cells 2022, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Hajkova, P.; Erhardt, S.; Lane, N.; Haaf, T.; El-Maarri, O.; Reik, W.; Walter, J.; Surani, M.A. Epigenetic reprogramming in mouse primordial germ cells. Mech. Dev. 2002, 117, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Tiwari, V.K. Epigenetic reprogramming of cell identity: Lessons from development for regenerative medicine. Clin. Epigenetics 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.T.; Moreira, E.M.; Kiefte-de Jong, J.C.; Darweesh, S.K.; Visser, T.; Voortman, T.; Bautista, P.K.; Chowdhury, R.; Gorman, D.; Bramer, W.M.; et al. Effects of choline on health across the life course: A systematic review. Nutr. Rev. 2015, 73, 500–522. [Google Scholar] [CrossRef]
- Caudill, M.A. Pre- and postnatal health: Evidence of increased choline needs. J. Am. Diet. Assoc. 2010, 110, 1198–1206. [Google Scholar] [CrossRef]
- Jiang, X.; West, A.A.; Caudill, M.A. Maternal choline supplementation: A nutritional approach for improving offspring health? Trends Endocrinol. Metab. 2014, 25, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients 2019, 11, 2995. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.L.; Stead, L.M.; Devlin, C.; Tabas, I.; Brosnan, M.E.; Brosnan, J.T.; Vance, D.E. Physiological regulation of phospholipid methylation alters plasma homocysteine in mice. J. Biol. Chem. 2005, 280, 28299–28305. [Google Scholar] [CrossRef] [PubMed]
- Resseguie, M.; Song, J.; Niculescu, M.D.; da Costa, K.A.; Randall, T.A.; Zeisel, S.H. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007, 21, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, S.B.; Mayr, J.A. Choline-related-inherited metabolic diseases-A mini review. J. Inherit. Metab. Dis. 2019, 42, 237–242. [Google Scholar] [CrossRef]
- Tayebati, S.K. Phospholipid and Lipid Derivatives as Potential Neuroprotective Compounds. Molecules 2018, 23, 2257. [Google Scholar] [CrossRef]
- Skripuletz, T.; Manzel, A.; Gropengießer, K.; Schäfer, N.; Gudi, V.; Singh, V.; Salinas Tejedor, L.; Jörg, S.; Hammer, A.; Voss, E.; et al. Pivotal role of choline metabolites in remyelination. Brain 2015, 138 Pt 2, 398–413. [Google Scholar] [CrossRef]
- Mellott, T.J.; Huleatt, O.M.; Shade, B.N.; Pender, S.M.; Liu, Y.B.; Slack, B.E.; Blusztajn, J.K. Perinatal Choline Supplementation Reduces Amyloidosis and Increases Choline Acetyltransferase Expression in the Hippocampus of the APPswePS1dE9 Alzheimer’s Disease Model Mice. PLoS ONE 2017, 12, e0170450. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Zeisel, S.H.; Wurtman, R.J. Developmental changes in the activity of phosphatidylethanolamine N-methyltransferases in rat brain. Biochem. J. 1985, 232, 505–511. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef]
- Hayakawa, K.; Hirosawa, M.; Tabei, Y.; Arai, D.; Tanaka, S.; Murakami, N.; Yagi, S.; Shiota, K. Epigenetic switching by the metabolism-sensing factors in the generation of orexin neurons from mouse embryonic stem cells. J. Biol. Chem. 2013, 288, 17099–17110. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Yuan, Z.; Ramsubir, S.; Bakovic, M. Choline transport for phospholipid synthesis. Exp. Biol. Med. 2006, 231, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.Y.; Ikeda, Y. Regulation of membrane phospholipid biosynthesis in mammalian cells. Biochem. Pharmacol. 2022, 206, 115296. [Google Scholar] [CrossRef]
- Lykidis, A.; Jackowski, S. Regulation of mammalian cell membrane biosynthesis. Prog. Nucleic Acid Res. Mol. Biol. 2001, 65, 361–393. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M.; McCormack, J.M.; McNulty, H.; Walsh, P.M.; Robson, P.J.; Bonham, M.P.; Duffy, M.E.; Ward, M.; Molloy, A.M.; Scott, J.M.; et al. Choline supplementation and measures of choline and betaine status: A randomised, controlled trial in postmenopausal women. Br. J. Nutr. 2012, 108, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.K.; Paal, M.C.; Donohue TMJr Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Zeisel, S.H.; Jacques, P.; Selhub, J.; Dougherty, L.; Colditz, G.A.; Willett, W.C. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am. J. Clin. Nutr. 2006, 83, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Giovannucci, E.L.; Hankinson, S.E.; Zeisel, S.H.; Dougherty, L.W.; Willett, W.C.; Rimm, E.B. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am. J. Clin. Nutr. 2007, 86, 1073–1081. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Harris, B.J.; Kyle, W.E. Methionine metabolism in mammals: Kinetic study of betaine-homocysteine methyltransferase. Arch. Biochem. Biophys. 1972, 153, 320–324. [Google Scholar] [CrossRef]
- Meck, W.H.; Williams, C.L. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003, 27, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Meck, W.H.; Williams, C.L.; Cermak, J.M.; Blusztajn, J.K. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front. Integr. Neurosci. 2008, 1, 7. [Google Scholar] [CrossRef]
- Meck, W.H.; Williams, C.L. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport 1997, 8, 2831–2835. [Google Scholar] [CrossRef]
- Tomizawa, H.; Matsuzawa, D.; Ishii, D.; Matsuda, S.; Kawai, K.; Mashimo, Y.; Sutoh, C.; Shimizu, E. Methyl-donor deficiency in adolescence affects memory and epigenetic status in the mouse hippocampus. Genes Brain Behav. 2015, 14, 301–309. [Google Scholar] [CrossRef]
- Meck, W.H.; Williams, C.L. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res. Dev. Brain Res. 1999, 118, 51–59. [Google Scholar] [CrossRef]
- Albright, C.D.; Friedrich, C.B.; Brown, E.C.; Mar, M.H.; Zeisel, S.H. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res. Dev Brain Res. 1999, 115, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.M.; Gimbel, B.A.; de Water, E.; Eckerle, J.K.; Radke, J.P.; Georgieff, M.K.; Wozniak, J.R. Prenatal and Postnatal Choline Supplementation in Fetal Alcohol Spectrum Disorder. Nutrients 2022, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Guo-Ross, S.X.; Clark, S.; Montoya, D.A.; Jones, K.H.; Obernier, J.; Shetty, A.K.; White, A.M.; Blusztajn, J.K.; Wilson, W.A.; Swartzwelder, H.S. Prenatal choline supplementation protects against postnatal neurotoxicity. J. Neurosci. 2002, 22, RC195. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Cermak, J.M.; Tandon, P.; Sarkisian, M.R.; Stafstrom, C.E.; Neill, J.C.; Blusztajn, J.K.; Holmes, G.L. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. J. Neurosci. 2000, 20, RC109. [Google Scholar] [CrossRef]
- Boeke, C.E.; Gillman, M.W.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Oken, E. Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol. 2013, 177, 1338–1347. [Google Scholar] [CrossRef]
- Li, K.; Wahlqvist, M.L.; Li, D. Nutrition, One-Carbon Metabolism and Neural Tube Defects: A Review. Nutrients 2016, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Albright, C.D.; Tsai, A.Y.; Friedrich, C.B.; Mar, M.H.; Zeisel, S.H. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res. Dev. Brain Res. 1999, 113, 13–20. [Google Scholar] [CrossRef]
- Shaw, G.M.; Finnell, R.H.; Blom, H.J.; Carmichael, S.L.; Vollset, S.E.; Yang, W.; Ueland, P.M. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology 2009, 20, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Carmichael, S.L.; Yang, W.; Selvin, S.; Schaffer, D.M. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol. 2004, 160, 102–109. [Google Scholar] [CrossRef]
- Lee, H.S.; Barraza-Villarreal, A.; Biessy, C.; Duarte-Salles, T.; Sly, P.D.; Ramakrishnan, U.; Rivera, J.; Herceg, Z.; Romieu, I. Dietary supplementation with polyunsaturated fatty acid during pregnancy modulates DNA methylation at IGF2/H19 imprinted genes and growth of infants. Physiol. Genom. 2014, 46, 851–857. [Google Scholar] [CrossRef]
- Popova, S.; Charness, M.E.; Burd, L.; Crawford, A.; Hoyme, H.E.; Mukherjee, R.A.S.; Riley, E.P.; Elliott, E.J. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Primers. 2023, 9, 11. [Google Scholar] [CrossRef]
- Thomas, J.D.; Abou, E.J.; Dominguez, H.D. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol. Teratol. 2009, 31, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Fink, B.A.; Fuglestad, A.J.; Eckerle, J.K.; Boys, C.J.; Sandness, K.E.; Radke, J.P.; Miller, N.C.; Lindgren, C.; Brearley, A.M.; et al. Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J. Neurodev. Disord. 2020, 12, 9. [Google Scholar] [CrossRef]
- Jennings, L.; Basiri, R. Amino Acids, B Vitamins, and Choline May Independently and Collaboratively Influence the Incidence and Core Symptoms of Autism Spectrum Disorder. Nutrients 2022, 14, 2896. [Google Scholar] [CrossRef]
- Doyle-Thomas, K.A.; Card, D.; Soorya, L.V.; Wang, A.T.; Fan, J.; Anagnostou, E. Metabolic mapping of deep brain structures and associations with symptomatology in autism spectrum disorders. Res. Autism. Spectr. Disord. 2014, 8, 44–51. [Google Scholar] [CrossRef]
- Corrigan, N.M.; Shaw, D.W.; Estes, A.M.; Richards, T.L.; Munson, J.; Friedman, S.D.; Dawson, G.; Artru, A.A.; Dager, S.R. Atypical developmental patterns of brain chemistry in children with autism spectrum disorder. JAMA Psychiatry 2013, 70, 964–974. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, R.; Xu, Y.; Zhou, Z.; Guan, P.; Wu, Y.; Zhou, J.; Cheng, Z.; Zhang, L. Altered Metabolic Characteristics in Plasma of Young Boys with Autism Spectrum Disorder. J. Autism. Dev. Disord. 2022, 52, 4897–4907. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.G.; Hunter, S.K.; McCarthy, L.; Beuler, J.; Hutchison, A.K.; Wagner, B.D.; Leonard, S.; Stevens, K.E.; Freedman, R. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am. J. Psychiatry 2013, 170, 290–298. [Google Scholar] [CrossRef]
- Ross, R.G.; Hunter, S.K.; Hoffman, M.C.; McCarthy, L.; Chambers, B.M.; Law, A.J.; Leonard, S.; Zerbe, G.O.; Freedman, R. Perinatal Phosphatidylcholine Supplementation and Early Childhood Behavior Problems: Evidence for CHRNA7 Moderation. Am. J. Psychiatry 2016, 173, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, C.N.; Albright, C.D.; Mar, M.H.; Song, J.; Zeisel, S.H. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr. 2003, 133, 3614–3618. [Google Scholar] [CrossRef]
- Powers, B.E.; Velazquez, R.; Strawderman, M.S.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal Choline Supplementation as a Potential Therapy for Down Syndrome: Assessment of Effects Throughout the Lifespan. Front. Aging Neurosci. 2021, 13, 723046. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Chen, M.; Gandhy, S.U.; Strawderman, M.; Levitsky, D.A.; Maclean, K.N.; Strupp, B.J. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav. Neurosci. 2010, 124, 346–361. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Sukla, K.K.; Chauhan, A.; Lakhotia, A.R.; Kumar, A.; Rai, A.K. Choline metabolic pathway gene polymorphisms and risk for Down syndrome: An association study in a population with folate-homocysteine metabolic impairment. Eur. J. Clin. Nutr. 2017, 71, 45–50. [Google Scholar] [CrossRef]
- Scelfo, A.; Fachinetti, D. Keeping the Centromere under Control: A Promising Role for DNA Methylation. Cells 2019, 8, 912. [Google Scholar] [CrossRef]
- Jiang, X.; Bar, H.Y.; Yan, J.; Jones, S.; Brannon, P.M.; West, A.A.; Perry, C.A.; Ganti, A.; Pressman, E.; Devapatla, S.; et al. A higher maternal choline intake among third-trimester pregnant women lowers placental and circulating concentrations of the antiangiogenic factor fms-like tyrosine kinase-1 (sFLT1). FASEB J. 2013, 27, 1245–1253. [Google Scholar] [CrossRef]
- Jiang, X.; Jones, S.; Andrew, B.Y.; Ganti, A.; Malysheva, O.V.; Giallourou, N.; Brannon, P.M.; Roberson, M.S.; Caudill, M.A. Choline inadequacy impairs trophoblast function and vascularization in cultured human placental trophoblasts. J. Cell Physiol. 2014, 229, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Kwan, S.T.C.; King, J.H.; Yan, J.; Jiang, X.; Wei, E.; Fomin, V.G.; Roberson, M.S.; Caudill, M.A. Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta 2017, 53, 57–65. [Google Scholar] [CrossRef]
- King, J.H.; Kwan, S.T.C.; Yan, J.; Klatt, K.C.; Jiang, X.; Roberson, M.S.; Caudill, M.A. Maternal Choline Supplementation Alters Fetal Growth Patterns in a Mouse Model of Placental Insufficiency. Nutrients 2017, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Morasso, M.I.; Grinberg, A.; Robinson, G.; Sargent, T.D.; Mahon, K.A. Placental failure in mice lacking the homeobox gene Dlx3. Proc. Natl. Acad. Sci. USA 1999, 96, 162–167. [Google Scholar] [CrossRef]
- Napoli, I.; Blusztajn, J.K.; Mellott, T.J. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Res. 2008, 1237, 124–135. [Google Scholar] [CrossRef]
- Kovacheva, V.P.; Mellott, T.J.; Davison, J.M.; Wagner, N.; Lopez-Coviella, I.; Schnitzler, A.C.; Blusztajn, J.K. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J. Biol. Chem. 2007, 282, 31777–31788. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Zhang, L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front. Neuroendocrinol. 2013, 34, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Coussons-Read, M.E. Effects of prenatal stress on pregnancy and human development: Mechanisms and pathways. Obstet. Med. 2013, 6, 52–57. [Google Scholar] [CrossRef]
- Schatzberg, A.F.; Keller, J.; Tennakoon, L.; Lembke, A.; Williams, G.; Kraemer, F.B.; Sarginson, J.E.; Lazzeroni, L.C.; Murphy, G.M. HPA axis genetic variation, cortisol and psychosis in major depression. Mol. Psychiatry 2014, 19, 220–227. [Google Scholar] [CrossRef]
- Porter, R.J.; Gallagher, P. Abnormalities of the HPA axis in affective disorders: Clinical subtypes and potential treatments. Acta Neuropsychiatr. 2006, 18, 193–209. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Beedle, A.S. Early life events and their consequences for later disease: A life history and evolutionary perspective. Am. J. Hum. Biol. 2007, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yan, J.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Caudill, M.A. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J. 2012, 26, 3563–3574. [Google Scholar] [CrossRef] [PubMed]
- Thöny, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000, 347 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Caudill, M.A. Folate bioavailability: Implications for establishing dietary recommendations and optimizing status. Am. J. Clin. Nutr. 2010, 91, 1455S–1460S. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.; Bainbridge, S.; Fontaine-Bisson, B. A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes. Nutr. Rev. 2018, 76, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cantley, L.C. Toward a better understanding of folate metabolism in health and disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef]
- Obeid, R.; Fedosov, S.N.; Nexo, E. Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol. Nutr. Food Res. 2015, 59, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Castaño, E.; Piñuñuri, R.; Hirsch, S.; Ronco, A.M. Folatos y Embarazo, conceptos actuales: ¿Es necesaria una suplementación con Acido Fólico? [Folate and Pregnancy, current concepts: It is required folic acid supplementation?]. Rev. Chil. Pediatr. 2017, 88, 199–206. [Google Scholar] [CrossRef]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef]
- Annibal, A.; Tharyan, R.G.; Schonewolff, M.F.; Tam, H.; Latza, C.; Auler, M.M.K.; Grönke, S.; Partridge, L.; Antebi, A. Regulation of the one carbon folate cycle as a shared metabolic signature of longevity. Nat. Commun. 2021, 12, 3486. [Google Scholar] [CrossRef]
- Schaevitz, L.; Berger-Sweeney, J.; Ricceri, L. One-carbon metabolism in neurodevelopmental disorders: Using broad-based nutraceutics to treat cognitive deficits in complex spectrum disorders. Neurosci. Biobehav. Rev. 2014, 46 Pt 2, 270–284. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- van Gool, J.D.; Hirche, H.; Lax, H.; De Schaepdrijver, L. Folic acid and primary prevention of neural tube defects: A review. Reprod. Toxicol. 2018, 80, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bruinse, H.W.; van den Berg, H. Changes of some vitamin levels during and after normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 61, 31–37. [Google Scholar] [CrossRef]
- Fang, J.Y.; Xiao, S.D. Folic acid, polymorphism of methyl-group metabolism genes, and DNA methylation in relation to GI carcinogenesis. J. Gastroenterol. 2003, 38, 821–829. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Agodi, A. Dietary Folate Intake and Folic Acid Supplements among Pregnant Women from Southern Italy: Evidence from the “Mamma & Bambino” Cohort. Int. J. Environ. Res. Public Health 2020, 17, 638. [Google Scholar] [CrossRef]
- Wald, N.J. Folic acid and neural tube defects: Discovery, debate and the need for policy change. J. Med. Screen 2022, 29, 138–146. [Google Scholar] [CrossRef]
- Jonker, H.; Capelle, N.; Lanes, A.; Wen, S.W.; Walker, M.; Corsi, D.J. Maternal folic acid supplementation and infant birthweight in low- and middle-income countries: A systematic review. Matern. Child. Nutr. 2020, 16, e12895. [Google Scholar] [CrossRef]
- Willoughby, M.L.; Jewell, F.J. Investigation of folic acid requirements in pregnancy. Br. Med. J. 1966, 2, 1568–1571. [Google Scholar] [CrossRef]
- Engelhardt, D.M.; Martyr, C.A.; Niswander, L. Pathogenesis of neural tube defects: The regulation and disruption of cellular processes underlying neural tube closure. WIREs Mech. Dis. 2022, 14, e1559. [Google Scholar] [CrossRef]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- Dunlevy, L.P.; Burren, K.A.; Chitty, L.S.; Copp, A.J.; Greene, N.D. Excess methionine suppresses the methylation cycle and inhibits neural tube closure in mouse embryos. FEBS Lett. 2006, 580, 2803–2807. [Google Scholar] [CrossRef]
- Friso, S.; Choi, S.W.; Girelli, D.; Mason, J.B.; Dolnikowski, G.G.; Bagley, P.J.; Olivieri, O.; Jacques, P.F.; Rosenberg, I.H.; Corrocher, R.; et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 2002, 99, 5606–5611. [Google Scholar] [CrossRef]
- Cai, C.Q.; Fang, Y.L.; Shu, J.B.; Zhao, L.S.; Zhang, R.P.; Cao, L.R.; Wang, Y.Z.; Zhi, X.F.; Cui, H.L.; Shi, O.Y.; et al. Association of neural tube defects with maternal alterations and genetic polymorphisms in one-carbon metabolic pathway. Ital. J. Pediatr. 2019, 45, 37. [Google Scholar] [CrossRef]
- Ouyang, S.; Li, Y.; Liu, Z.; Chang, H.; Wu, J. Association between MTR A2756G and MTRR A66G polymorphisms and maternal risk for neural tube defects: A meta-analysis. Gene 2013, 515, 308–312. [Google Scholar] [CrossRef]
- Gaughan, D.J.; Kluijtmans, L.A.; Barbaux, S.; McMaster, D.; Young, I.S.; Yarnell, J.W.; Evans, A.; Whitehead, A.S. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis 2001, 157, 451–456. [Google Scholar] [CrossRef]
- Liew, S.C.; Gupta, E.D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: Epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 2015, 58, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Wang, B.; Ding, C.; Liu, H. Association between MTHFR C677T polymorphism and neural tube defect risks: A comprehensive evaluation in three groups of NTD patients, mothers, and fathers. Birth Defects Res. A Clin. Mol. Teratol. 2015, 103, 488–500. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Ji, W.; Qin, H.; Wu, H.; Xu, D.; Turtuohut, T.; Wang, Z. Variants in MTHFR gene and neural tube defects susceptibility in China. Metab. Brain Dis. 2015, 30, 1017–1026. [Google Scholar] [CrossRef]
- Wilson, R.D.; Genetics Committee; Wilson, R.D.; Audibert, F.; Brock, J.A.; Carroll, J.; Cartier, L.; Gagnon, A.; Johnson, J.A.; Langlois, S.; et al. Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies. J. Obstet. Gynaecol. Can. 2015, 37, 534–552. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obstet. Gynecol. 2015, 213 (Suppl. S4), S6.e1–S6.e4. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Raijmakers, M.T.; Burton, G.J.; Jauniaux, E.; Seed, P.T.; Peters, W.H.; Steegers, E.A.; Poston, L. Placental NAD(P)H oxidase mediated superoxide generation in early pregnancy. Placenta 2006, 27, 158–163. [Google Scholar] [CrossRef]
- Williams, P.J.; Bulmer, J.N.; Innes, B.A.; Broughton Pipkin, F. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biol. Reprod. 2011, 84, 1148–1153. [Google Scholar] [CrossRef]
- Pickell, L.; Li, D.; Brown, K.; Mikael, L.G.; Wang, X.L.; Wu, Q.; Luo, L.; Jerome-Majewska, L.; Rozen, R. Methylenetetrahydrofolate reductase deficiency and low dietary folate increase embryonic delay and placental abnormalities in mice. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.W.; Zhou, J.; Yang, Q.; Fraser, W.; Olatunbosun, O.; Walker, M. Maternal exposure to folic acid antagonists and placenta-mediated adverse pregnancy outcomes. CMAJ 2008, 179, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Mather, J.; Ramsay, M.M.; Kurlak, L.O.; Symonds, M.E.; Broughton Pipkin, F. Homocysteine and folate plasma concentrations in mother and baby at delivery after pre-eclamptic or normotensive pregnancy: Influence of parity. Pregnancy Hypertens. 2011, 1, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.W.; Chen, X.K.; Rodger, M.; White, R.R.; Yang, Q.; Smith, G.N.; Sigal, R.J.; Perkins, S.L.; Walker, M.C. Folic acid supplementation in early second trimester and the risk of preeclampsia. Am. J. Obstet. Gynecol. 2008, 198, 45.e1–45.e7. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, Y.H.; Dong, X.T.; Zhou, J.; Chen, X.; Wang, H.; Wu, S.X.; Xia, M.Z.; Zhang, C.; Xu, D.X. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PLoS ONE 2013, 8, e82713. [Google Scholar] [CrossRef]
- Balashova, O.A.; Visina, O.; Borodinsky, L.N. Folate action in nervous system development and disease. Dev. Neurobiol. 2018, 78, 391–402. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Sun, Q.; He, B.; Jia, Y.; Cai, D.; Zhao, R. Folate deprivation induces cell cycle arrest at G0/G1 phase and apoptosis in hippocampal neuron cells through down-regulation of IGF-1 signaling pathway. Int. J. Biochem. Cell Biol. 2016, 79, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Selhub, J.; Paul, L.; Ji, Y.; Wang, G.; Hong, X.; Zuckerman, B.; Fallin, M.D.; Wang, X. A prospective birth cohort study on cord blood folate subtypes and risk of autism spectrum disorder. Am. J. Clin. Nutr. 2020, 112, 1304–1317. [Google Scholar] [CrossRef]
- DeVilbiss, E.A.; Gardner, R.M.; Newschaffer, C.J.; Lee, B.K. Maternal folate status as a risk factor for autism spectrum disorders: A review of existing evidence. Br. J. Nutr. 2015, 114, 663–672. [Google Scholar] [CrossRef]
- Riahi, F.; Tashakori, A.; Vanani, G.S. Effects of Folic Acid on Appetite in Children with Attention Deficit Hyperactivity Disorder (ADHD) Treated with Methylphenidate: A Randomized Double-Blind Clinical Trial. Iran J. Med. Sci. 2018, 43, 9–17. [Google Scholar]
- Surén, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Magnus, P.; Reichborn-Kjennerud, T.; et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013, 309, 570–577. [Google Scholar] [CrossRef]
- Michels, K.B.; Binder, A.M. Impact of folic acid supplementation on the epigenetic profile in healthy unfortified individuals—A randomized intervention trial. Epigenetics 2024, 19, 2293410. [Google Scholar] [CrossRef]
- Zsigrai, S.; Kalmár, A.; Barták, B.K.; Nagy, Z.B.; Szigeti, K.A.; Valcz, G.; Kothalawala, W.; Dankó, T.; Sebestyén, A.; Barna, G.; et al. Folic Acid Treatment Directly Influences the Genetic and Epigenetic Regulation along with the Associated Cellular Maintenance Processes of HT-29 and SW480 Colorectal Cancer Cell Lines. Cancers 2022, 14, 1820. [Google Scholar] [CrossRef]
- Kruman, I.I.; Mouton, P.R.; Emokpae, R., Jr.; Cutler, R.G.; Mattson, M.P. Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport 2005, 16, 1055–1059. [Google Scholar] [CrossRef]
- Liu, H.Y.; Liu, S.M.; Zhang, Y.Z. Maternal Folic Acid Supplementation Mediates Offspring Health via DNA Methylation. Reprod. Sci. 2020, 27, 963–976. [Google Scholar] [CrossRef]
- Richmond, R.C.; Sharp, G.C.; Herbert, G.; Atkinson, C.; Taylor, C.; Bhattacharya, S.; Campbell, D.; Hall, M.; Kazmi, N.; Gaunt, T.; et al. The long-term impact of folic acid in pregnancy on offspring DNA methylation: Follow-up of the Aberdeen Folic Acid Supplementation Trial (AFAST). Int. J. Epidemiol. 2018, 47, 928–937. [Google Scholar] [CrossRef]
- Dutta, S.; Chatterjee, A.; Sinha, S.; Chattopadhyay, A.; Mukhopadhyay, K. Correlation between cystathionine beta synthase gene polymorphisms, plasma homocysteine and idiopathic mental retardation in Indian individuals from Kolkata. Neurosci. Lett. 2009, 453, 214–218. [Google Scholar] [CrossRef]
- Saha, T.; Chatterjee, M.; Sinha, S.; Rajamma, U.; Mukhopadhyay, K. Components of the folate metabolic pathway and ADHD core traits: An exploration in eastern Indian probands. J. Hum. Genet. 2017, 62, 687–695. [Google Scholar] [CrossRef]
- Ornoy, A.; Weinstein-Fudim, L.; Tfilin, M.; Ergaz, Z.; Yanai, J.; Szyf, M.; Turgeman, G. S-adenosyl methionine prevents ASD like behaviors triggered by early postnatal valproic acid exposure in very young mice. Neurotoxicol. Teratol. 2019, 71, 64–74. [Google Scholar] [CrossRef]
- Guo, B.Q.; Ding, S.B.; Li, H.B. Blood biomarker levels of methylation capacity in autism spectrum disorder: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2020, 141, 492–509. [Google Scholar] [CrossRef]
- Lintas, C. Linking genetics to epigenetics: The role of folate and folate-related pathways in neurodevelopmental disorders. Clin. Genet. 2019, 95, 241–252. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.T.; Luo, M.; Tang, Y.; Zhang, G.; Wu, D.; Yang, B.; Ruan, D.Y.; Wang, H.L. Multiple epigenetic factors predict the attention deficit/hyperactivity disorder among the Chinese Han children. J. Psychiatr. Res. 2015, 64, 40–50. [Google Scholar] [CrossRef]
- Skrypnik, D.; Moszak, M.; Wender-Ozegowska, E.; Bogdanski, P. Comparison of Polish and international guidelines on diet supplements in pregnancy—Review. Ginekol. Pol. 2021, 92, 322–330. [Google Scholar] [CrossRef]
- Wender-Ożegowska, E.; Bomba-Opoń, D.; Brązert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgoś, M. Standards of Polish Society of Gynecologists and Obstetricians in management of women with diabetes. Ginekol. Pol. 2018, 89, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Bomba-Opoń, D.; Hirnle, L.; Kalinka, J.; Seremak-Mrozikiewicz, A. Folate supplementation during the preconception period, pregnancy and puerperium. Polish Society of Gynecologists and Obstetricians Guidelines. Ginekol. Pol. 2017, 88, 633–636. [Google Scholar] [CrossRef] [PubMed]
- ACOG Committee Opinion. No. 495. Vitamin D: Screening and supplementation during pregnancy. Obstet. Gynecol. 2011, 118, 197–198. [Google Scholar] [CrossRef]
- Toriello, H.V.; Policy and Practice Guideline Committee of the American College of Medical Genetics. Policy statement on folic acid and neural tube defects. Genet. Med. 2011, 13, 593–596. [Google Scholar] [CrossRef]
- Antenatal Care for Uncomplicated Pregnancies; National Institute for Health and Care Excellence (NICE): London, UK, 2019.
- Ren, A.; Zhang, L.; Li, Z.; Hao, L.; Tian, Y.; Li, Z. Awareness and use of folic acid, and blood folate concentrations among pregnant women in northern China-an area with a high prevalence of neural tube defects. Reprod. Toxicol. 2006, 22, 431–436. [Google Scholar] [CrossRef]
- Hlushko, K.; Boyarchuk, O.; Kinash, M.; Burbela, E.; Rohalska, Y.; Dobrovolska, L. Awareness of folic acid use and its effects among medical students in Ukraine. Wiad. Lek. 2021, 74, 2033–2038. [Google Scholar] [CrossRef]
- Derbyshire, E.; Obeid, R.; Schön, C. Habitual Choline Intakes across the Childbearing Years: A Review. Nutrients 2021, 13, 4390. [Google Scholar] [CrossRef]
- Gómez, M.F.; Field, C.J.; Olstad, D.L.; Loehr, S.; Ramage, S.; McCargar, L.J.; APrON Study Team. Use of micronutrient supplements among pregnant women in Alberta: Results from the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort. Matern. Child. Nutr. 2015, 11, 497–510. [Google Scholar] [CrossRef]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; Casavale, K.O.; et al. Dietary patterns before and during pregnancy and birth outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109 (Suppl. S7), 729S–756S. [Google Scholar] [CrossRef]
- Obeid, R.; Holzgreve, W.; Pietrzik, K. Folate supplementation for prevention of congenital heart defects and low birth weight: An update. Cardiovasc. Diagn Ther. 2019, 9 (Suppl. S2), S424–S433. [Google Scholar] [CrossRef]
- Cheng, Z.; Gu, R.; Lian, Z.; Gu, H.F. Evaluation of the association between maternal folic acid supplementation and the risk of congenital heart disease: A systematic review and meta-analysis. Nutr. J. 2022, 21, 20. [Google Scholar] [CrossRef]
- Yan, M.X.; Zhao, Y.; Zhao, D.D.; Dang, S.N.; Zhang, R.; Duan, X.Y.; Rong, P.X.; Dang, Y.S.; Pei, L.L.; Qu, P.F. The Association of Folic Acid, Iron Nutrition during Pregnancy and Congenital Heart Disease in Northwestern China: A Matched Case-Control Study. Nutrients 2022, 14, 4541. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, J.; Feng, Q.L.; Gu, H.T.; Wan, G.; Zhang, H.M.; Xie, Y.J.; Li, X.S. Fetal echocardiography for congenital heart disease diagnosis: A meta-analysis, power analysis and missing data analysis. Eur. J. Prev. Cardiol. 2015, 22, 1531–1547. [Google Scholar] [CrossRef]
- Mozumdar, N.; Rowland, J.; Pan, S.; Rajagopal, H.; Geiger, M.K.; Srivastava, S.; Stern, K.W.D. Diagnostic Accuracy of Fetal Echocardiography in Congenital Heart Disease. J. Am. Soc. Echocardiogr. 2020, 33, 1384–1390. [Google Scholar] [CrossRef]
- Masih, S.P.; Plumptre, L.; Ly, A.; Berger, H.; Lausman, A.Y.; Croxford, R.; Kim, Y.I.; O’Connor, D.L. Pregnant Canadian Women Achieve Recommended Intakes of One-Carbon Nutrients through Prenatal Supplementation but the Supplement Composition, Including Choline, Requires Reconsideration. J. Nutr. 2015, 145, 1824–1834. [Google Scholar] [CrossRef]
- McGowan, E.C.; Hong, X.; Selhub, J.; Paul, L.; Wood, R.A.; Matsui, E.C.; Keet, C.A.; Wang, X. Association Between Folate Metabolites and the Development of Food Allergy in Children. J. Allergy Clin. Immunol. Pract. 2020, 8, 132–140.e5. [Google Scholar] [CrossRef]
- Whitrow, M.J.; Moore, V.M.; Rumbold, A.R.; Davies, M.J. Effect of supplemental folic acid in pregnancy on childhood asthma: A prospective birth cohort study. Am. J. Epidemiol. 2009, 170, 1486–1493. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, J.; Wang, Z.; Wang, L.; Tan, T.; Sun, L. Relationship between maternal folic acid supplementation during pregnancy and risk of childhood asthma: Systematic review and dose-response meta-analysis. Front. Pediatr. 2022, 10, 1000532. [Google Scholar] [CrossRef]
- Patel, K.R.; Sobczyńska-Malefora, A. The adverse effects of an excessive folic acid intake. Eur. J. Clin. Nutr. 2017, 71, 159–163. [Google Scholar] [CrossRef]
- Christensen, K.E.; Mikael, L.G.; Leung, K.Y.; Lévesque, N.; Deng, L.; Wu, Q.; Malysheva, O.V.; Best, A.; Caudill, M.A.; Greene, N.D.; et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015, 101, 646–658. [Google Scholar] [CrossRef]
- Fernàndez-Roig, S.; Cavallé-Busquets, P.; Fernandez-Ballart, J.D.; Ballesteros, M.; Berrocal-Zaragoza, M.I.; Salat-Batlle, J.; Ueland, P.M.; Murphy, M.M. Low folate status enhances pregnancy changes in plasma betaine and dimethylglycine concentrations and the association between betaine and homocysteine. Am. J. Clin. Nutr. 2013, 97, 1252–1259. [Google Scholar] [CrossRef]
- McGregor, D.O.; Dellow, W.J.; Robson, R.A.; Lever, M.; George, P.M.; Chambers, S.T. Betaine supplementation decreases post-methionine hyperhomocysteinemia in chronic renal failure. Kidney Int. 2002, 61, 1040–1046. [Google Scholar] [CrossRef]
- Allen, R.H.; Stabler, S.P.; Savage, D.G.; Lindenbaum, J. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J. 1993, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Sweiry, J.H.; Page, K.R.; Dacke, C.G.; Abramovich, D.R.; Yudilevich, D.L. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: Studies with calcium and choline. J. Dev. Physiol. 1986, 8, 435–445. [Google Scholar]
- Petersen, J.M.; Smith-Webb, R.S.; Shaw, G.M.; Carmichael, S.L.; Desrosiers, T.A.; Nestoridi, E.; Darling, A.M.; Parker, S.E.; Politis, M.D.; Yazdy, M.M.; et al. Periconceptional intakes of methyl donors and other micronutrients involved in one-carbon metabolism may further reduce the risk of neural tube defects in offspring: A United States population-based case-control study of women meeting the folic acid recommendations. Am. J. Clin. Nutr. 2023, 118, 720–728. [Google Scholar] [CrossRef]

| Dietary Supplement | American College of Obstetricians and Gynecologists | National Institute for Health and Care Excellence/Royal College of Obstetricians and Gynecologists (NICE/RJOG) | The Polish Society of Gynecologists and Obstetricians |
|---|---|---|---|
| Folic acid | 0.4 mg–0.6 mg/day | 0.4 mg | 0.4 mg–5 mg (depending on pregnancy complications) |
| Iodine | 200 μg/day | Not applicable | 150–200 μg/day |
| Vitamin D | 1000–2000 IU/day | 1000 IU/day | 1500–2000 IU/day |
| DHA | Not applicable | Not applicable | 600 mg/day |
| Choline | 450 mg/day | 400 mg/day | Not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socha, M.W.; Flis, W.; Wartęga, M. Epigenetic Genome Modifications during Pregnancy: The Impact of Essential Nutritional Supplements on DNA Methylation. Nutrients 2024, 16, 678. https://doi.org/10.3390/nu16050678
Socha MW, Flis W, Wartęga M. Epigenetic Genome Modifications during Pregnancy: The Impact of Essential Nutritional Supplements on DNA Methylation. Nutrients. 2024; 16(5):678. https://doi.org/10.3390/nu16050678
Chicago/Turabian StyleSocha, Maciej W., Wojciech Flis, and Mateusz Wartęga. 2024. "Epigenetic Genome Modifications during Pregnancy: The Impact of Essential Nutritional Supplements on DNA Methylation" Nutrients 16, no. 5: 678. https://doi.org/10.3390/nu16050678
APA StyleSocha, M. W., Flis, W., & Wartęga, M. (2024). Epigenetic Genome Modifications during Pregnancy: The Impact of Essential Nutritional Supplements on DNA Methylation. Nutrients, 16(5), 678. https://doi.org/10.3390/nu16050678








