Abstract
In recent decades, as a result of rising mortality rates due to cardiovascular diseases (CVDs), there has been a growing urgency to find alternative approaches to conventional pharmaceutical treatment to prevent the onset of chronic diseases. Arthrospira platensis, commonly known as Spirulina, is a blue-green cyanobacterium, classified as a “superfood”, used worldwide as a nutraceutical food supplement due to its remarkable nutritional value, lack of toxicity, and therapeutic effects. Several scientific studies have evaluated the cardioprotective role of Spirulina. This article presents a comprehensive review of the therapeutic benefits of Spirulina in improving cardio- and cerebrovascular health. It focuses on the latest experimental and clinical findings to evaluate its antihypertensive, antidiabetic, and antihyperlipidemic properties. The objective is to highlight its potential in preventing and managing risk factors associated with cardiovascular disease (CVD).
1. Introduction
Arthrospira platensis is a photosynthetic, microscopic filamentous blue-green microalga classified as a cyanobacterium belonging to the Microcoleaceae family [1]. A. maxima and A. platensis, commonly known as “Spirulina”, are the two most studied species for their considerable nutritional and therapeutic properties. Spirulina inhabits tropical regions, particularly alkaline lakes with a pH 11 and a high concentration of carbonate and bicarbonate salts. Additionally, these algae can survive in extreme environments, such as the frozen lakes of Antarctica [2,3]. These microorganisms were first discovered in Lake Texcoco in Mexico. The Aztecs were among the first to incorporate this microalga into their diet, particularly in the creation of a blue-green cake known as “tecuitlatl”, as unearthed by the Spanish army during their conquest of Mexico [4]. Since ancient times, Spirulina has been utilized for its beneficial characteristics. Today, Spirulina is still used in a wide range of applications. In recent decades, it has garnered the classification of a “superfood” because of its copious protein content (60–70% by dry weight) as well as its abundance of carbohydrates, essential fatty acids, vitamins, minerals, and pigments like carotenes, chlorophyll a, and phycocyanin [5]. Because of its impressive nutritional value, is widely utilized in both the food and pharmaceutical fields. In the food industry, Spirulina is used as a nutraceutical food supplement, added to foods such as baked goods, beverages, dairy products, sports supplements, and baby food [6]. On the other hand, the pharmaceutical sector has produced tablets, dehydrated powders or encapsulated forms, which are marketed as “nutraceuticals” [7].
As a consequence of the considerable market demand for Spirulina products, S. platensis has been classified as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA) [8]. Many research studies have demonstrated that Spirulina has therapeutic functions such as antioxidant, anti-inflammatory, hypolipidemic, antidiabetic, and brain-protective properties [9,10,11]. Remarkably, the abundant presence of natural pigments endows Spirulina with antioxidant potential, notably carotenoids and C-phycocyanin [12,13]. Several research investigations indicated that β-carotene, diadinoxanthin, diatoxanthin, and C-phycocyanin exhibited very high scavenging activity [14,15]. Thanks to its antioxidant properties, this microalga is considered beneficial in preventing cardiovascular diseases [11]. Today, CVDs are the main cause of death globally [16]. Therefore, drug therapies used today to prevent certain predisposing disorders such as diabetes, hypertension, and dyslipidemia display many benefits and, at the same time, some adverse effects. For this reason, the use of nutraceuticals, such as Spirulina, has been shown promising results as a support therapy for the maintenance of cardiovascular health and the reduction in cardiovascular risk [17,18]. This renewed focus on functional-food-based therapy is now seen as a new strategy to achieve a healthy generation. In the 21st century, it is essential to follow the ideologies established by Hippocrates (460–377 B.C.), who claimed “Let food be your medicine” [19].
Accordingly, this review aims to pick up the latest experimental and clinical findings on the potential therapeutic properties of Spirulina for the treatment of cardiovascular diseases (CVDs). The article is divided into three main sections, which discuss the antihypertensive, antidiabetic, and antihyperlipidemic effects of Spirulina, focusing on general characteristics, clinical trials, animal studies, and mechanisms of actions. Overall, the review concludes that Spirulina is a powerful therapeutic tool for the treatment of CVDs and can be beneficial for individuals with hypertension, diabetes, and hyperlipidemia.
Chemical Composition of Spirulina
Spirulina is mainly known for its valuable nutritional composition. Notwithstanding, significant differences in the main macromolecular composition were observed among the different phyla of this cyanobacterium [20]. In general, this microalga contains carbohydrates, lipids, vitamins, minerals, and a high protein content [21]. The high protein content on a dry weight basis is approximately 60% [22]. Specifically, the main proteins present in Spirulina consist of phycocyanin, allophycocyanin, and phycoerythrin [21]. Its low lipid composition is characterized by polyunsaturated essential fatty acids (PUFAs) such as omega-3 eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) [23], omega-6 arachidonic (AA), and γ linolenic acid (GLA) [24]. Moreover, Spirulina is also an excellent resource of minerals and vitamins. Researchers reported that the mineral composition includes potassium, calcium, magnesium, selenium, iron, zinc, and many others [25]. Spirulina is also valued for its content of vitamins, particularly B12 vitamin [26]. The vitamin C content is low due to exposure to light and heat during production, which can cause vitamin C degradation [25]. Finally, bioactive pigments including carotenoids, such as astaxanthin, zeaxanthin, and β-carotene [24] as well as chlorophylls [27], are present in Spirulina. Nevertheless, the macromolecular composition of different Spirulina strains can be strongly influenced by environmental and cultivation conditions, such as temperature, light, salinity, etc. [28]. For instance, a nitrogen and phosphorous limitation in S. platensis cultures can alter the biomass composition, leading to an increase in carbohydrate and lipid content [29]. Overall, its nutrient-rich composition had led to Spirulina being considered as a superfood that provides many health benefits.
2. Hypertension and Stroke
According to the World Health Organization (WHO), hypertension is considered one of the risk factors for CVDs and cerebrovascular diseases, such as cerebral hemorrhage and ischemic stroke, which are the leading cause of death worldwide [30,31]. The underlying causes of hypertension can be multiple and often unknown [32], resulting in increased resistance of peripheral vessels leading to increased blood pressure. As a result, systemic vascular damage occurs, and an adequate blood supply to the brain is not ensured [33,34]. The International Society of Hypertension Global Hypertension Practice Guidelines indicate that hypertension can be diagnosed as a systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg [35]. Despite advances in the understanding and treatment of hypertension, the ability to effectively regulate blood pressure is still insufficient for many patients. This is complicated by the fact that high blood pressure may show no symptoms even for many years, thus posing a significant challenge for its management [36]. Moreover, it has been demonstrated that monotherapy is often not sufficient to lower blood pressure in hypertensive patients [37]. Egan and colleagues reported that administering combination therapy, which involves the use of multiple antihypertensives, could be an alternative approach to managing hypertension because it induces better cardiovascular outcomes than monotherapy [38]. Therefore, considering the capability of Spirulina supplementation to reduce blood pressure levels, it can be used to complement common antihypertensive therapy without replacing it.
2.1. Clinical Studies
The impact of the intake of Spirulina on blood pressure has been evaluated in several randomized clinical trials. Recently, a randomized, triple-blind, placebo-controlled trial was conducted on 48 hypertensive patients to assess the effects of consuming a salad dressing enriched with 2 g/day of Spirulina powder over 8 weeks in patients with hypertension compared to the placebo group. The results showed that the oral consumption of Spirulina dressing had a significant impact on the reduction in systolic (p = 0.02) and diastolic (p = 0.01) blood pressure [39]. In a similar study conducted by Mickze et al. [40], the authors confirmed the hypotensive effect of Spirulina further. They concluded that after four capsules of 0.5 g Spirulina maxima each or placebo administration in 40 patients with hypertension, a noteworthy reduction in systolic blood pressure (p = 0.0023) and stiffness index (p < 0.001) was observed from the baseline to three months. Spirulina maxima contained 60–70% protein, gamma-linolenic acid (GLA), beta carotene, iron and phycocyanin (PC).
In line with these findings, Martínez-Sámano and colleagues [41] showed that the administration of 4.5 g/day of Spirulina maxima for 12 weeks in 16 patients with systemic arterial hypertension (SAH), treated with angiotensin-converting enzyme (ACE) inhibitors, resulted in a significant reduction in systolic blood pressure (p < 0.05) at the end of treatment, while significant differences in diastolic blood pressure were not observed [41]. Moreover, a reduction in blood pressure was also highlighted in 36 Mexican patients with pre-hypertension (120–139 mmHg for SBP and 80–89 mmHg for DBP), hypertension stage 1 (140–159 mmHg for SBP and 90–99 mmHg for DBP), and hypertension stage 2 (higher values than 160 mmHg for SBP and 100 mmHg DBP) after the consumption of three tablets of 0.5 g Spirulina each every 8 h for six weeks (p < 0.001). Following Spirulina supplementation, the authors observed that 36% of patients achieved normal blood pressure, while patients with 1 or 2 hypertension stages decreased their levels to pre-hypertension levels (50%). Notably, younger patients showed greater responsiveness to Spirulina-induced blood-pressure-lowering than the other groups [42]. In contrast, a clinical investigation did not observe any significant effects of dietary supplementation with Spirulina on the blood pressure of healthy subjects compared to the placebo group. Subjects enrolled in this study consumed four capsules of 4.8 g Spirulina or a placebo after breakfast, lunch, and dinner for 17 days. The researchers did not observe statistically significant differences because the subjects involved in the study were healthy. In this case, it might be worth considering increasing the duration of treatment [43]. The parameters at baseline and after Spirulina supplementation of the included studies are shown in Table 1.

Table 1.
Detailed characteristics of the included studies on hypertensive patients.
Unfortunately, there are a few clinical trials from the literature that evaluate the effect of Spirulina in hypertensive patients. Some of these studies lack a control group. Thus, further clinical trials with a larger number of patients treated with microalga and placebo are needed to evaluate its potential in reducing arterial blood pressure. In addition, not all studies reported specific details about the method of pressure measurement. It is not always discussed whether a manual or digital sphygmomanometer was used or if different measurements were taken. This lack of standardization makes it difficult to compare the results across studies.
2.2. Animal Studies
In the literature, studies have reported the significant antihypertensive effects of two tripeptides derived from the hydrolysis of blue algae Spirulina platensis powder, Ile-Gln-Pro (IQP) and Val-Glu-Pro (VEP), in vivo [44]. It was revealed that 8 weeks of oral treatment on spontaneously hypertensive rats (SHRs) led to a reduction in systolic and diastolic blood pressure, as well as a reduction in ventricular mass indices compared to the control group (p < 0.05). Compounds were administered 10 mg/kg every morning. Furthermore, the treatment had a significant impact on the expression levels of components of the renin–angiotensin system (RAS) in the myocardium [44].
Two other lyophilized peptides derived from Spirulina digestion were orally administered to SHR rats and have been shown to lower blood pressure for up to 8 h after supplementation compared to captopril, suggesting they have a longer-lasting ACE-inhibiting effect than captopril [45]. In another in vivo study conducted on SHR rats, tablets containing silicon-enriched Spirulina (15 mg Spirulina and 0.3 mg silicon) were administered orally for 3 months [46]. This supplementation induced a reduction in blood systolic (p < 0.001) and diastolic (p < 0.0001) pressure compared to SHR rats. In addition, they observed beneficial effects on vascular remodeling and improved vascular reactivity compared with Spirulina administration alone [46]. These findings suggest that the silicon enrichment contained in Spirulina may be able to improve arterial wall elasticity. Furthermore, Spirulina serves as a means by which elements can be assimilated in a form that the body can readily use. Consequently, it has been proven to be a promising dietary intervention for the treatment of hypertension in SHR rats.
Wang et al. showed that after ischemia/reperfusion, rats who were pre-treated with approximately 30 g of a Spirulina-enriched diet for four weeks showed reduced cerebral infarction and lower caspase-3 activity (p < 0.05) compared to the control group indicating a neuroprotective effect of blue-green alga [47]. The anti-apoptotic property was also demonstrated by Almeida and colleagues, who found that 30 days of Spirulina extract administration led to an increase in viable neurons in the perilesional fields of rats 24 h after an induced hemorrhage. All animals received 1.67 × 10−2 g of Spirulina extract diluted in 2 mL of distillated water via oral gavage [48]. Further studies are needed to investigate the potential of Spirulina as an agent to reduce injuries following cerebral hemorrhage and stroke.
2.3. Mechanisms of Action
The mechanisms of action of Spirulina in hypertension have been extensively studied. Several authors have proposed many mechanisms to demonstrate its efficiency in treating elevated blood pressure levels. According to Martínez-Sámano et al. [41], Spirulina supplementation induced a decrease in endothelial damage marker levels caused by an increase in blood pressure, such as sVCAM-1, sE-selectin, and endothelin-1, as well as the increase in glutathione peroxidase activity and oxidized glutathione levels, suggesting its potential to enhance endothelial function and its antioxidant properties that are beneficial in mitigating hypertension. Bioactive peptides derived from the hydrolysis of proteins contained in Spirulina have also been shown to possess antihypertensive properties. Only once released into the bloodstream can these peptides perform their function [49,50,51].
He et al. [52] demonstrated the total absorption of tripeptides through the intestinal epithelium using monolayers of human intestinal Caco-2 cells. They concluded that the main transport mechanism is the paracellular transport mechanism.
Pan et al. [44] assessed that two Spirulina-derived tripeptides can modulate the renin–angiotensin system (RAS), which has a central function in the regulation of blood pressure, specifically by activating the ACE2-Ang-(1-7)-Mas axis and inhibiting the ACE-Ang II-AT1 axis.
Recent work by our research group has demonstrated that a single decameric peptide called “SP6” (GIVAGDVTPI), derived from the gastrointestinal digestion (GID) of Spirulina, can lead to dose-dependent vasorelaxation in ex vivo mice vessels. In addition, this peptide can be effective as a blood-pressure-reducing agent by increasing PI3K/Akt/eNOS signaling, leading to the release of nitric oxide (NO), a well-known vasodilatation metabolite, whose bioavailability and signaling pathway are impaired in individuals with hypertension [53].
In line with our study, Majewski et al. [54] concluded that twelve peptides contained in Spirulina aqueous extract (SAE) can improve vascular function in the aorta of aged rats due to NO release and a decrease in superoxide production, associated with increased levels of p-eNOS and heme oxygenase-1 (HO-1), respectively [54].
Interestingly, another study proposed that a heptameric peptide (Thr-Met-Glu-Pro-Gly-Lys-Pro) can act as a mixed non-competitive inhibitor of ACE in human endothelial cells. Additionally, the expression of inducible NO synthase (iNOS) and endothelin-1 (ET-1) was reduced [55].
These results provide evidence to support the use of Spirulina as a preventive protection against vascular dysfunction and lay the foundation for its therapeutic use in hypotensive therapies in cardiovascular disease.
3. Diabetes
In the last decade, there has been a gradual increase in individuals afflicted with type 2 diabetes [56]. Diabetes mellitus (DM) represents a complex disease marked by elevated glucose levels and an increased basal metabolic rate because of a defect in insulin signaling.
In these conditions, hyperglycemia adversely affects the integrity of cellular membranes, leading to insulin resistance in both the liver and peripheral tissues and producing reactive oxygen species (ROS) [57].
Moreover, type 2 diabetes accounts for approximately 90% of all diagnosed cases of diabetes and is considered a risk factor for the development of CVDs, including myocardial infarction, peripheral vascular disease (PVD), heart failure, stroke, retinopathy, and neuropathy, as a result of microvascular and macrovascular complications due to hyperglycemia [58].
One of the proposed pharmacological approaches to treat hyperglycemia in diabetes is the utilization of metformin [59]. Generally, metformin has no impact on lipid profiles in subjects diagnosed with type 2 diabetes [60].
However, it is important to note that before considering the use of metformin, which can induce side effects of similar to those of digestive disorders, such as diarrhea and nausea [61], there are preventive measures that can be taken to avoid the development of diabetes. Pre-diabetes is a condition that occurs before the onset of diabetes, where blood sugar levels are higher than the normal range but not high enough to be considered type 2 diabetes.
Although it is often not possible to avoid the onset of the disease, exercise and food supplements can delay or improve the management of the disease because of improvements insulin sensitivity [62]. Furthermore, the bioactive ingredients contained in some food supplements, such as polyphenols, polysaccharides, and others, can affect the modulation of glucose metabolism [63].
Considerable research has focused on studying natural compounds with antihyperglycemic properties, such as Spirulina. Furthermore, unlike metformin, S. platensis not only reduces circulating glucose levels but can also influence lipoprotein metabolism, high levels of which are associated with diabetes. This would represent a possible cardiovascular benefit in diabetic patients [64].
Spirulina has gained attention as a functional food due to its potential to lower blood glucose levels, control cholesterol, and provide antioxidant benefits.
3.1. Clinical Studies
A recent randomized, double-blind, placebo-controlled study included 60 patients under usual treatment with metformin for type 2 diabetes [65]. The results suggest that 2 g of Spirulina platensis given as four capsules before meals, in addition to metformin therapy, markedly improved glycemic parameters, including glycosylated hemoglobin (HbA1c) (p < 0.001) and fasting blood glucose levels (FBS) (p < 0.001), compared to the placebo group under metformin treatment only. Hence, the supplementation of 2 g/day of S. platensis for 3 months is considered safe and free of side effects, making it an effective treatment option for the management of type 2 diabetes and its associated complications [65]. In line with this evidence, Alam et al. [66] evaluated that the administration of 7 g twice daily of Spirulina powder in patients with no pharmacological treatment can reduce FBS (p < 0.01) and postprandial glycemia (PPBS) similarly to the control group who received two capsules of 500 mg metformin before meals for 45 days. The authors observed no statistically significant differences in HbA1C levels induced by Spirulina treatment. As stated by the authors, this scenario could probably be due to the short duration of treatment and the small sample size. Further studies are needed to evaluate its pharmacological effect and its usefulness as a reliable alternative to classic antidiabetic agents [66]. Sowjanya and colleagues [67] divided diabetic patients into two groups. The first group (EG-1) was given 2 g of Spirulina contained in two snack bars (25 g each) in the mid-morning and evening. The second group (EG-2) was given two Spirulina capsules in the morning and evening, while the control group was given no supplementation. In both the EG-1 and EG-2 groups, their FBS, PPBS and HbA1c levels were significantly more reduced from the baseline to the endpoint both in males (p < 0.01) than in females (p < 0.05). In the EG-1 group, the reduction in FBS and PPBS levels was greater than in the EG-2 group, possibly due to the synergistic effects of other nutritional ingredients. In addition, the reduction in FBS and PPBS in females is lower than that in males [67]. Another clinical study showed that 2 g/day of Spirulina capsules, taken during lunch and dinner for 2 months, led to a reduction in FBS, PPBS, and HbA1c (p < 0.05) from the baseline to the endpoint [68]. A similar effect was induced by a dosage of 8 g of Spirulina administered daily for 3 months in tablet form to 50 patients with type 2 diabetes treated with their usual antidiabetic therapy, but the reduction in HbA1c levels was not significant. It is possible that Spirulina can lower serum glucose levels in the short term, but it may require a longer treatment period to affect hemoglobin A1C levels [69]. The reduction in the HbA1c level highlighted suggests an improvement in the regulation of long-term glucose management.
Moreover, in Cretan patients with non-alcoholic fatty liver disease (NAFLD), 6 g/day Spirulina (Greek production) supplementation for six months led to a significant reduction in the HOMA-IR index, a measure representing an enhancement in insulin sensitivity [70]. The main characteristics at the baseline and after Spirulina supplementation of the included studies are shown in Table 2.

Table 2.
Detailed characteristics of the included studies on diabetic patients.
Overall, Spirulina supplementation has been shown to be an effective agent for hyperglycemia; however, it is necessary to increase the number of studies and the duration of the treatment to confirm its effect on the regulation of HbA1c levels, indicative of long-term glucose management. These studies pave the way for future research on the use of nutraceuticals as an adjunct to basic therapy in managing diabetes mellitus.
3.2. Animal Studies
Similar research on the hypoglycemic property of Spirulina has been conducted in animal models. Spirulina oral supplementation in different concentrations (5, 10, and 15 mg/kg body weight) in streptozotocin-diabetic rats led to a decrease in FBS levels and, on the other hand, elevated plasma insulin and serum C-peptide concentrations (p < 0.05). Moreover, the researchers observed an increase in total hemoglobin levels in the Spirulina-treated group, which may be indirectly proportional to HbA1c formation, due to its ability to lower circulating glucose levels and its high iron content which is crucial for the metabolism of hemoglobin [71].
Further, the oral administration of Spirulina in albino diabetic rat models, at 10, 20, and 30 mg/kg body weight diluted in distillated water, resulted in a significant dose-dependent reduction in FBS compared to the diabetic control group (p < 0.01) [72].
Also, El-Sayed and colleagues [73] demonstrated that phenolic compounds and phycocyanin contained in Spirulina are responsible for the hypoglycemic effect. The four groups of diabetic rats treated for 30 days with oral Spirulina biomass suspension (50 mg/kg body weight), phycocyanin (50 mg/kg body weight), phycocyanobilin (982 µg/kg body weight), and phycopeptide (49 mg/kg body weight), respectively, showed a reduction in fasting blood glucose level and in the HOMA-IR score, which highlights insulin resistance, compared to the control and glibenclamide groups (p ≤ 0.05). In addition, a histopathological analysis revealed that diabetic rats treated with Spirulina, phycocyanin, and phycopeptide showed an improvement in their HOMA β-score which revealed an improvement in β cell function (p ≤ 0.05) [73].
3.3. Mechanism of Action
Although the mechanisms are not fully understood, Spirulina could be involved in pancreatic insulin secretion by islet β-cells or facilitate glucose transport from the blood to peripheral tissues [71].
The insulin-releasing impact of S. platensis occurs through a multitude of pathways, such as the adenylate cyclase/cAMP or phosphatidylinositol pathway or through direct influence on membrane depolarization [74].
Proteins extracted from Spirulina have been found to improve glucose entry into liver cells and promote glycogen synthesis by increasing the activities of hexokinase (HK) and pyruvate kinase (PK), which ultimately leads to lower blood glucose levels and improves insulin resistance.
Additionally, three peptides extracted from Spirulina platensis inhibit α-amylase, α-glucosidase, and dipeptidyl peptidase-4 (DPP-IV), which are critical enzymes involved in glycemic control. This makes them useful targets in treating type 2 diabetes [75].
The high fiber content of Spirulina may hinder glucose absorption, leading to a glucose-lowering effect [76].
According to Hozayen and colleagues [77], Spirulina could have a positive impact in diabetic rats enhancing serum adiponectin and decreasing TNF-α levels. High levels of adiponectin are commonly known to improve insulin sensitivity, while low levels of a pro-inflammatory cytokine TNF-α enhance glucose production in the liver and the ability of insulin to stimulate glucose uptake in peripheral tissues. Furthermore, the antioxidant capacity caused by Spirulina supplementation has been shown to increase GSH levels and SOD and GPx activity. As a result, it protects against oxidative-stress-induced cell damage associated with diabetes [77].
Some authors believe that the antioxidant capacity of Spirulina is attributed to phycocyanin. Selenium-bound phycocyanopeptide or/and phycocyanobilin are known for their antioxidant action in preventing diabetes-induced pancreatic cell damage, while chromium-bound phycocyanopeptide activates insulin receptors [73].
As a consequence of increased antioxidant enzymes, malondialdehyde (MDA) levels are decreased following Spirulina supplementation [78].
Lastly, Sadek et al. [79] provided evidence that Spirulina exerts its antidiabetic effect by attenuating the upregulation of gluconeogenic enzyme pyruvate carboxylase (PC) and pro-apoptotic Bax and caspase-3 (CASP-3) gene expression in diabetic rats, thereby resulting from its antioxidant activity. Furthermore, Spirulina has been shown to possess anti-apoptotic properties by mitigating the expression of pro-apoptotic MAPK pathways, consequently leading to the attenuation of apoptotic pathways induced by diabetes [79].
4. Hyperlipidemia
Hyperlipidemia refers to a condition characterized by elevated levels of lipids or lipoproteins in the blood that predisposes an individual to the development of atherosclerosis. Specifically, high blood levels of low-density lipoprotein cholesterol (LDL-C) and low blood levels of high-density lipoprotein (HDL) determine hypercholesterolemia. Blood cholesterol management guidelines state that LDL-C levels are one of the best predictors of CVDs [80]. In addition, the combination of other risk factors such as smoking and a sedentary lifestyle represents a significant correlation with atherosclerotic cardiovascular disorders [81]. Despite the high prevalence of hypercholesterolemia worldwide, the management of cardiovascular disease remains unsatisfactory. Typically, the conventional treatment of dyslipidemia involves the use of statins, which have been shown to reduce LDL-C levels and reduce CVD mortality. However, statin treatment is linked to adverse outcomes, including muscle weakness, muscle cramps, persistent myalgias, and elevations in creatine kinase levels [82]. Therefore, it is essential to enhance therapies by introducing adequate dietary therapy, including additional treatments, such as nutraceuticals, that can control plasma lipid and lipoprotein levels to improve outcomes for dyslipidemic individuals.
To this end, several studies have demonstrated Spirulina’s valuable effect in reducing plasma concentrations of LDL-C and triglycerides and increasing HDL-C levels following its supplementation, resulting in a reduced risk of developing cardiovascular diseases.
The effect of Spirulina on plasma lipid levels is independent of the dose administered and has no toxic effects [83]. Overall, Spirulina supplementation is shown to be effective in the treatment of hyperlipidemia and subsequent atherosclerosis to improve lipid profiles in patients.
4.1. Clinical Studies
Several recent clinical studies have been conducted to evaluate the lipid-lowering effect of Spirulina in obese subjects. Obesity is correlated with high blood lipid levels representing a risk factor linked to CVD.
Zeinalian and colleagues [84] conducted a study on obese individuals who were administrated 1 g (500 mg twice a day) of Spirulina pills for 3 months and observed a notable reduction in total cholesterol (TC) (p = 0.002). Furthermore, an increase in high-density lipoprotein-cholesterol (HDL-C) (p = 0.05) was observed with no significant change in triglycerides (TG) or low-density lipoprotein (LDL) between the baseline and after Spirulina treatment [84].
A further study in humans enrolled obese subjects, already undergoing antihypertensive treatment, who received four capsules of 0.5 g each of Spirulina maxima, taken daily in the morning, or a placebo for the same duration as the previous study. Spirulina supplementation resulted in a decrease in LDL-C (p < 0.001) and TC (p < 0.001) levels between the baseline and after administration, but it was not effective in reducing other lipid levels [85].
Although it is known that exercise can reduce CVD risk factors by improving the serum lipid profile, the synergistic action with Spirulina capsules has been evaluated in obese and sedentary individuals. A randomized double-blind study assessed the combined action of Spirulina supplementation (4.5 g/day for 6 weeks) in dyslipidemic subjects. Although synergetic action has proven advantageous, Spirulina supplementation alone has been shown to lower total cholesterol, LDL-C, and triglycerides, and to increase HDL-C in 6 weeks compared to control group [86].
In contrast, another clinical study evaluated the effect of Spirulina supplementation and high-intensity interval training on 20 overweight or obese women for 4 weeks. Administration was taken orally in pill form, once in the morning and again 48 h after the last exercise session. There were no significant differences observed in serum lipid levels, probably because of the low number of cohort participants involved in the study. Another limitation is the participation of only women [87].
In a study by Karizi et al., the efficacy of blue-green algae in reducing lipid levels in type 2 diabetes mellitus (T2DM) was evaluated. The authors studied the simultaneous action of metformin therapy and 2 g/day Spirulina in diabetic patients for 12 weeks. The supplementation of S. platensis before meals has been shown to have a hypolipidemic action and to be a valuable adjunct to metformin therapy. At the end of the intervention period, the researchers found a significant decrease in plasma values of atherogenic lipids and an increase in HDL levels compared to the baseline (p < 0.001) [65]. In a Cretan population, 3 months of Spirulina administration lowered all non-high-density lipoprotein cholesterol levels (p < 0.001), but the HDL-C levels remained unchanged [88].
Other studies have examined the potential role of Spirulina-fortified dressing or sauce as a functional food. Far and colleagues [39] emphasized a significant reduction in triglycerides (p < 0.01) in hypertensive patients when given salad dressing that contained 2 g of Spirulina powder [39], while the results from the study by Mazloomi et al. [89] confirmed the potential benefits in NAFLD patients. The investigation suggests that sauce prepared with 2 g of Spirulina may improve NAFLD by reducing the grade of fatty liver and liver enzymes (ALT and AST) as well as TG reduction (p = 0.03) and HDL-C (p = 0.02) levels increase between baseline and the end of the treatment. Additionally, the atherogenic index was significantly decreased (p = 0.007) [89]. The influence of a liquid extract of Arthrospira called “Spirulysat®” was examined in individuals with metabolic syndrome, demonstrating a reduction in triglycerides and an increase in HDL levels at the end of supplementation compared to placebo group. Spirulina water extract contained phycocyanin, polysaccharides and proteins, amino acids, enzymes, vitamins, and mineral salts [90]. On the other hand, the intake of Spirulina supplements for 17 days, with four capsules taken after each principal meal induced no effect either on plasma lipid levels or markers for synthesis and intestinal cholesterol absorption [43]. The basic characteristics of all included studies are summarized in Table 3.

Table 3.
Detailed characteristics of the included studies on hyperlipidemic patients.
However, most studies have evaluated the antihyperlipidemic effects of Spirulina in overweight and obese subjects. Therefore, the impact of this microalga on individuals with different conditions remains unclear. For this reason, further research is still required to fully understand and confirm Spirulina’s benefits in reducing serum lipid concentrations in a heterogeneous population sample.
4.2. Animal Studies
The impact of Spirulina on plasma lipid concentrations in animal models has also been assessed in the literature. The investigation by El-Sayed et al. [73] reported that 30 days of Spirulina feeding and its extracts, such as phycocyanin (PHY), phycocyanopeptide, and phycocyanobilin, resulted in a significant decrease in atherogenic serum lipid levels and an increase in HDL-cholesterol levels in diabetic rats. Specifically, it was emphasized that the main effect on HDL and LDL concentrations was mainly attributable to the antioxidant activity of phycocyanin and phycocyanopeptide [73].
In diabetic rats, similar results were presented by Nasirian et al. [91] who also observed a decrease in malondialdehyde (MDA) levels (p < 0.05), an index of lipid peroxidation, compared to the control group. Moreover, increasing concentrations of Spirulina extracts diluted in water were found to be correlated with high levels of antioxidant liver enzymes when orally consumed (p < 0.05) [91]. Furthermore, Spirulina concentrated (SPC), PHY, and PHY residues were added in rats’ high-cholesterol diet for 5 days. PHY has been shown to significantly reduce liver cholesterol levels compared with the SPC diet [92]. An improvement in lipid profiles was observed in two groups of hypercholesterolemic male rabbits treated orally for four weeks with different doses of algal alkaloid extract (33 and 66 mg/kg) (p ≤ 0.05) [93]. The researchers discovered that a Spirulina-enriched soy yogurt diet for administered for 4 weeks and oral treatment with Spirulina powder (500 mg/kg) for 8 weeks in hypercholesterolemic mice provided a protective effect against hepatic steatosis by reducing hepatic fat accumulation [94]. These findings are similar to those of Li et al. [95], who linked hepatic and plasma hypolipidemic activity to the ability to regulate the gut microbiota. Spirulina polysaccharides (150 mg/kg/day) were administered intragastrically for 8 weeks, thereby manifesting lipid-reducing effects in rats with hypercholesterolemia compared to the high-fat diet group (p < 0.01) [95]. These findings indicate that Spirulina may positively impact lipid profiles, which could lead to improved cardiovascular health.
4.3. Mechanism of Action
Several studies have shown that Spirulina supplementation can significantly lower levels of LDL-C, TC, and TG while increasing levels of HDL-C. These beneficial effects on blood lipid profiles are attributed to its nutritional content, but its mechanisms of action are not fully understood.
In the literature, it has been documented that S. platensis could impact lipid metabolism by down-regulating lipogenesis-related genes such as transcription factor-1c (SREBP-1c), acetyl CoA carboxylase (ACC), and peroxisome proliferator-activated receptor-g (PPARγ). Furthermore, researchers suggested an increase in peroxisome proliferator-activated receptor-a (PPARα) and adenosine 5′-monophosphate-activated protein kinase (AMPK) gene expression levels, which are involved in the regulation of fatty acid oxidation [95,96]. Related to the low levels of SREBP-1c, some authors have shown an increase in PGC-1α levels, a cofactor implicated both in the regulation of lipid oxidation gene expression and in hepatic mitochondrial biogenesis through the PGC-1α/Tfam/mtDNA pathway in the liver [97]. Additionally, Spirulina inhibits HMG COA reductase activity, a key enzyme in cholesterol synthesis, and increases the activity of Lecithin cholesterol acyltransferase (LCAT), which plays a central role in the reverse cholesterol transport process [98]. One of the first studies conducted on hypercholesterolemic Wistar rats revealed an improvement in lipoprotein lipase (LPL) and hepatic triglyceride lipase (H-TGL) activity [99]. Consequently, these alterations resulted in reduced levels of plasma cholesterol and triglycerides and elevated levels of HDL-C. Several investigations have proposed that Spirulina can positively impact dysbiosis by altering the composition of gut microbiota, which positively correlates with blood lipid levels [95,96].
According to Oriquat, Spirulina could alleviate non-alcoholic fatty liver disease (NAFLD) through the modulation of the hepatic expression of miR-122, miR-34a, and miR-21, as well as the SREBP-1c, SIRT1, and HPB1 genes [100].
Many researchers have attributed the mechanism underlying the hypolipidemic effect to C-phycocyanin, a blue-green pigment contained in Spirulina.
A study by Nagaoka et al. revealed, for the first time, that the consumption of C-phycocyanin (PHY) could suppress the intestinal absorption of cholesterol, as C-phycocyanin can bind to bile acids in the jejunum, resulting in an effect on the higher fecal excretion of cholesterol, which ultimately leads to a reduction in serum cholesterol levels [92]. Furthermore, according to Han et al., C-phycocyanin and glycolipid H-b2 inhibit pancreatic lipase activity in a dose-dependent manner [101]. A recent study also suggested that PHY could improve liver fat accumulation by regulating AMPK pathway [102]. PHY has antioxidant action, anti-inflammatory and free radical scavenging [13,15], thus exerting an inhibitory influence on lipid peroxidation. This confirmed the findings of Riss and colleagues which demonstrated that PHY can reduce the aortic fatty streak area by modulating NADPH oxidase and reducing superoxide anion accumulation, a marker of early atherosclerosis [103]. Phycocyanobilin (PCB), its tetrapyrrole chromophore, also showed an antioxidant effect, significantly increasing HMOX1 expression in aortic atherosclerotic plaques of ApoE-deficient mice, thus inducing atheroprotection [104]. Although several mechanisms by which Spirulina improves hyperlipidemia and consequently atherosclerosis have been suggested in the literature, further studies are needed to further investigate the mechanisms involved.
5. Conclusions
The present review was conducted to analyze the efficacy of Spirulina platensis supplementation on the current experimental and clinical findings in hypertension, diabetes, and dyslipidemic conditions.
According to the latest clinical research, consuming Spirulina has no health risks, as it has been classified as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA). Moreover, considering its “natural” origins, consumers prefer its use for human health promotion over pharmacological treatment. This classification has led researchers to investigate its possible beneficial role in different cardiovascular pathologies.
Several reports have demonstrated and highlighted the beneficial action of Spirulina in different cardio- and cerebrovascular diseases, acting by preventing or, at least, limiting cardiovascular risk factors such as high blood pressure, hyperglycemia, and hyperlipidemia (Figure 1). On the other hand, it is important to point out that its administration in healthy subjects did not evoke any modification of the physiological parameters, fully supporting the use of Spirulina as a potential preventive compound able to counteract the onset and progression of CVDs.
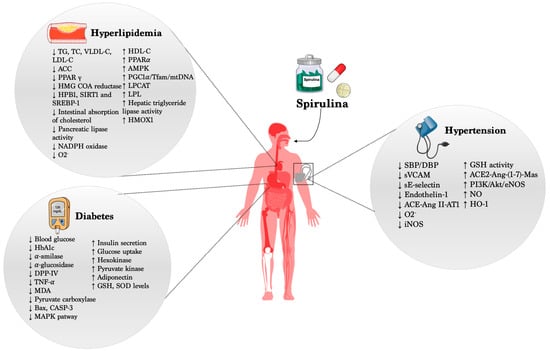
Figure 1.
Beneficial effects of Spirulina in CVDs.
Despite the scientific literature highlighting the potential beneficial role of Spirulina, it is important to emphasize that future research testing its therapeutic effects in a large heterogeneous population is needed, also taking into account the ethnicity, lifestyle, behavior, and gender-specific actions that can influence the physiological responses to nutraceutical treatment.
Therefore, the first goal to be achieved is to expand the number of studies evaluating the effects of Spirulina on populations from different regions of the world, so that the beneficial effects observed to date can be assessed unequivocally.
Environmental and growth conditions in which the algae are cultivated can affect the nutritional and pharmacological-like properties of the final Spirulina compound. Thus, the second milestone to achieve is to fully characterize the macronutrient composition of different strains type of Spirulina, aiming to establish a common denominator on macronutrients that can evoke the greatest beneficial effect on human health.
Finally, two big questions lie in the treatment dosage and timing to appreciate the impact of Spirulina treatment in specific vascular diseases. From the antihypertensive effect to the antidiabetic and antihyperlipidemic effects, the dosage ranges from 1 to 8 mg/die, and on the same line, the period of treatment changes from a minimum of 17 days to 12 weeks intra-pathology. Thus, until now, it has not been possible to establish a selective guideline on “how to administer” Spirulina for the prevention or treatment of specific cardiovascular diseases. Therefore, only by increasing the number of specific studies on CVDs will it be possible to achieve this final milestone in establishing the timing and dosages of Spirulina to be used, helping to define its preventive or adjuvant-drug-therapy use necessary to fight and contain the cardiovascular risk.
In conclusion, based on these data, more rigorous studies should be planned in the future aiming to address these critical questions, putting the foundations for developing a common guideline on “how and when” to use Spirulina.
Author Contributions
Conceptualization, V.P., C.V. and A.C.; original draft preparation, V.P., A.C.A., P.D.P. and M.D.L.; review and editing, C.V. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Volkmann, H.; Imianovsky, U.; Oliveira, J.L.; Sant’Anna, E.S. Cultivation of Arthrospira (Spirulina) platensis in desalinator wastewater and salinated synthetic medium: Protein content and amino-acid profile. Braz. J. Microbiol. 2008, 39, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Tamagnini, P.; Axelsson, R.; Lindberg, P.; Oxelfelt, F.; Wünschiers, R.B.; Lindblad, P. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A. Spirulina Platensis Arthrospira: Physiology, Cell-Biology and Biotechnology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Kulshreshtha, A.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.; Bisen, P. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef]
- Ahmad, A.M.R.; Intikhab, A.; Zafar, S.; Farooq, U.; Shah, H.B.U.; Akram, S.; Abid, J.; Parveen, Z.; Iqbal, S. Spirulina, an FDA-approved functional food: Worth the hype? Cell. Mol. Biol. 2023, 69, 137–144. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Morais, M.G.D.; Vaz, B.D.S.; Morais, E.G.D.; Costa, J.A.V. Biological effects of Spirulina (Arthrospira) biopolymers and biomass in the development of nanostructured scaffolds. BioMed Res. Int. 2014, 2014, 762705. [Google Scholar] [CrossRef]
- Karkos, P.; Leong, S.; Karkos, C.; Sivaji, N.; Assimakopoulos, D. Spirulina in clinical practice: Evidence-based human applications. Evid.-Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- Chei, S.; Oh, H.; Song, J. Spirulina maxima extract prevents activation of the NLRP3 in ammasome by inhibiting ERK signaling. Sci. Rep. 2020, 10, 2075. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Bhatt, A.N.; Nishad, D.K.; Purkayastha, J. C-Phycocyanin-a novel protein from Spirulina platensis-In vivo toxicity, antioxidant and immunomodulatory studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Conte, G.M.; Salviati, E.; Pepe, G.; Bertamino, A.; Ostacolo, C.; Sansone, F.; Prete, F.D.; Aquino, R.P.; Campiglia, P. Fast profiling of natural pigments in different spirulina (Arthrospira platensis) dietary supplements by DI-FT-ICR and evaluation of their antioxidant potential by pre-column DPPH-UHPLC assay. Molecules 2018, 23, 1152. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.B.; Madyastha, K. C-phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Gaidai, O.; Cao, Y.; Loginov, S. Global cardiovascular diseases death rate prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: A perspective on plant biotechnology application. Recent Pat. Biotechnol. 2007, 1, 75–97. [Google Scholar] [CrossRef] [PubMed]
- ElFar, O.A.; Billa, N.; Lim, H.R.; Chew, K.W.; Cheah, W.Y.; Munawaroh, H.S.H.; Balakrishnan, D.; Show, P.L. Advances in delivery methods of Arthrospira platensis (spirulina) for enhanced therapeutic outcomes. Bioengineered 2022, 13, 14681–14718. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.; Kumar, G.; Kalam, N.; Ansari, S.H. Current concepts and prospects of herbal nutraceutical: A review. J. Adv. Pharm. Technol. Res. 2013, 4, 4. [Google Scholar]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- da Rosa, G.M.; Moraes, L.; Cardias, B.B.; Costa, J.A.V. Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle. Bioresour. Technol. 2015, 192, 321–327. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical characterization of six microalgae with potential utility for food application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Ljubic, A.; Safafar, H.; Holdt, S.L.; Jacobsen, C. Biomass composition of Arthrospira platensis during cultivation on industrial process water and harvesting. J. Appl. Phycol. 2018, 30, 943–954. [Google Scholar] [CrossRef]
- Carcea, M.; Sorto, M.; Batello, C.; Narducci, V.; Aguzzi, A.; Azzini, E.; Fantauzzi, P.; Finotti, E.; Gabrielli, P.; Galli, V. Nutritional characterization of traditional and improved dihé, alimentary blue-green algae from the lake Chad region in Africa. LWT-Food Sci. Technol. 2015, 62, 753–763. [Google Scholar] [CrossRef]
- Watanabe, F.; Katsura, H.; Takenaka, S.; Fujita, T.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Pseudovitamin B12 is the predominant cobamide of an algal health food, spirulina tablets. J. Agric. Food Chem. 1999, 47, 4736–4741. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Rangel-Yagui, C.; Danesi, E.D.G.; De Carvalho, J.C.M.; Sato, S. Chlorophyll production from Spirulina platensis: Cultivation with urea addition by fed-batch process. Bioresour. Technol. 2004, 92, 133–141. [Google Scholar] [CrossRef]
- Lafarga, T. Cultured microalgae and compounds derived thereof for food applications: Strain selection and cultivation, drying, and processing strategies. Food Rev. Int. 2020, 36, 559–583. [Google Scholar] [CrossRef]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Carbohydrates production and bio-flocculation characteristics in cultures of Arthrospira (Spirulina) platensis: Improvements through phosphorus limitation process. BioEnergy Res. 2012, 5, 915–925. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.; Bakris, G.; Berlowitz, D.; Cifkova, R.; Dominiczak, A. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- Goodfriend, T.L.; Lyerly, K.M. Essential Hypertension. In Pathophysiology of Kidney Disease and Hypertension; Elsevier: Amsterdam, The Netherlands, 2009; pp. 179–195. [Google Scholar]
- Cifu, A.S.; Davis, A.M. Prevention, detection, evaluation, and management of high blood pressure in adults. JAMA 2017, 318, 2132–2134. [Google Scholar] [CrossRef]
- Iadecola, C.; Davisson, R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Wermelt, J.; Schunkert, H. Management of arterial hypertension. Herz 2017, 42, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, P.K.; Bahadur, S. Recent Developments in Drug Targets and Combination Therapy for the Clinical Management of Hypertension. Cardiovasc. Haematol. Disord.-Drug Targets (Former. Curr. Drug Targets-Cardiovasc. Hematol. Disord.) 2023, 23, 226–245. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.M.; Bandyopadhyay, D.; Shaftman, S.R.; Wagner, C.S.; Zhao, Y.; Yu-Isenberg, K.S. Initial monotherapy and combination therapy and hypertension control the first year. Hypertension 2012, 59, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Ghaem Far, Z.; Babajafari, S.; Kojuri, J.; Mohammadi, S.; Nouri, M.; Rostamizadeh, P.; Rahmani, M.H.; Azadian, M.; Ashrafi-Dehkordi, E.; Zareifard, A. Antihypertensive and antihyperlipemic of spirulina (Arthrospira platensis) sauce on patients with hypertension: A randomized triple-blind placebo-controlled clinical trial. Phytother. Res. 2021, 35, 6181–6190. [Google Scholar] [CrossRef]
- Miczke, A.; Szulińska, M.; Hansdorfer-Korzon, R.; Kręgielska-Narożna, M.; Suliburska, J.; Walkowiak, J.; Bogdański, P. Effects of spirulina consumption on body weight, blood pressure, and endothelial function in overweight hypertensive Caucasians: A doubleblind, placebo-controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 150–156. [Google Scholar] [PubMed]
- Martínez-Sámano, J.; Torres-Montes de Oca, A.; Luqueño-Bocardo, O.I.; Torres-Durán, P.V.; Juárez-Oropeza, M.A. Spirulina maxima Decreases Endothelial Damage and Oxidative Stress Indicators in Patients with Systemic Arterial Hypertension: Results from Exploratory Controlled Clinical Trial. Mar. Drugs 2018, 16, 496. [Google Scholar] [CrossRef]
- Torres-Duran, P.V.; Ferreira-Hermosillo, A.; Juarez-Oropeza, M.A. Antihyperlipemic and antihypertensive effects of Spirulina maxima in an open sample of Mexican population: A preliminary report. Lipids Health Dis. 2007, 6, 33. [Google Scholar] [CrossRef]
- van den Driessche, J.J.; Plat, J.; Konings, M.C.; Mensink, R.P. Effects of spirulina and wakame consumption on intestinal cholesterol absorption and serum lipid concentrations in non-hypercholesterolemic adult men and women. Eur. J. Nutr. 2020, 59, 2229–2236. [Google Scholar] [CrossRef]
- Pan, H.; She, X.; Wu, H.; Ma, J.; Ren, D.; Lu, J. Long-term regulation of the local renin–angiotensin system in the myocardium of spontaneously hypertensive rats by feeding bioactive peptides derived from Spirulina platensis. J. Agric. Food Chem. 2015, 63, 7765–7774. [Google Scholar] [CrossRef]
- Suo, Q.; Yue, Y.; Wang, J.; Wu, N.; Geng, L.; Zhang, Q. Isolation, identification and in vivo antihypertensive effect of novel angiotensin I-converting enzyme (ACE) inhibitory peptides from Spirulina protein hydrolysate. Food Funct. 2022, 13, 9108–9118. [Google Scholar] [CrossRef]
- Arthur-Ataam, J.; Bideaux, P.; Charrabi, A.; Sicard, P.; Fromy, B.; Liu, K.; Eddahibi, S.; Pasqualin, C.; Jouy, N.; Richard, S. Dietary supplementation with silicon-enriched spirulina improves arterial remodeling and function in hypertensive rats. Nutrients 2019, 11, 2574. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, C.-F.; Chou, J.; Chen, H.-L.; Deng, X.; Harvey, B.K.; Cadet, J.L.; Bickford, P.C. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp. Neurol. 2005, 193, 75–84. [Google Scholar] [CrossRef]
- Almeida, T.; Manfroi, G.; Silva, S.; Beggiora, P.; Schwingel, D.; Bertolin, T.E. Exploring the Neuroprotective Effects of Spirulina platensis: Insights Into Hemorrhagic Volume and Histological Outcomes. Cureus 2023, 15, e42078. [Google Scholar] [CrossRef]
- Lafarga, T.; Álvarez, C.; Hayes, M. Bioactive peptides derived from bovine and porcine co-products: A review. J. Food Biochem. 2017, 41, e12418. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Hao, Y.; Zhang, W.; Zhou, G. Antihypertensive Effects in Vitro and in Vivo of Novel Angiotensin-Converting Enzyme Inhibitory Peptides from Bovine Bone Gelatin Hydrolysate. J. Agric. Food Chem. 2020, 68, 759–768. [Google Scholar] [CrossRef]
- Giani, J.F.; Veiras, L.C.; Shen, J.Z.Y.; Bernstein, E.A.; Cao, D.; Okwan-Duodu, D.; Khan, Z.; Gonzalez-Villalobos, R.A.; Bernstein, K.E. Novel roles of the renal angiotensin-converting enzyme. Mol. Cell. Endocrinol. 2021, 529, 111257. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Li, T.T.; Chen, J.X.; She, X.X.; Ren, D.F.; Lu, J. Transport of ACE Inhibitory Peptides Ile-Gln-Pro and Val-Glu-Pro Derived from Spirulina platensis Across Caco-2 Monolayers. J. Food Sci. 2018, 83, 2586–2592. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Conte, G.M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala, M.C.; Aquino, R.P.; De Lucia, M.; Madonna, M.; et al. Novel Potent Decameric Peptide of Spirulina platensis Reduces Blood Pressure Levels Through a PI3K/AKT/eNOS-Dependent Mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Klett-Mingo, M.; Verdasco-Martin, C.M.; Otero, C.; Ferrer, M. Spirulina extract improves age-induced vascular dysfunction. Pharm. Biol. 2022, 60, 627–637. [Google Scholar] [CrossRef]
- Heo, S.Y.; Ko, S.C.; Kim, C.S.; Oh, G.W.; Ryu, B.; Qian, Z.J.; Kim, G.; Park, W.S.; Choi, I.W.; Phan, T.T.; et al. A heptameric peptide purified from Spirulina sp. gastrointestinal hydrolysate inhibits angiotensin I-converting enzyme- and angiotensin II-induced vascular dysfunction in human endothelial cells. Int. J. Mol. Med. 2017, 39, 1072–1082. [Google Scholar] [CrossRef]
- Haddad, J.A.; Haddad, A.N. The past decade in type 2 diabetes and future challenges. Hormones 2018, 17, 451–459. [Google Scholar] [CrossRef]
- Ha, H.; Kim, K.H. Pathogenesis of diabetic nephropathy: The role of oxidative stress and protein kinase C. Diabetes Res. Clin. Pract. 1999, 45, 147–151. [Google Scholar] [CrossRef]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Standards of Medical Care in Diabetes-2019 Abridged for Primary Care Providers. Clin. Diabetes 2019, 37, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Wulffele, M.G.; Kooy, A.; de Zeeuw, D.; Stehouwer, C.D.; Gansevoort, R.T. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: A systematic review. J. Intern. Med. 2004, 256, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saluja, M.; Pareek, K.K.; Swami, Y.K. Study of Diversity of Metformin Related Gastrointestinal Side Effects. J. Assoc. Physicians India 2020, 68, 36–38. [Google Scholar] [PubMed]
- Sivaraman, S.; Weickert, M.O. Nutrition and exercise in the treatment of type 2 diabetes mellitus. Hamdan Med. J. 2012, 5, 131–144. [Google Scholar] [CrossRef]
- Meng, X.; Li, Q.; Shi, R.; Chang, J.; Chang, H.; Li, M. Food supplements could be an effective improvement of diabetes mellitus: A review. J. Future Foods 2021, 1, 67–81. [Google Scholar] [CrossRef]
- Ray, K.K.; Seshasai, S.R.; Wijesuriya, S.; Sivakumaran, R.; Nethercott, S.; Preiss, D.; Erqou, S.; Sattar, N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials. Lancet 2009, 373, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Karizi, S.R.; Armanmehr, F.; Azadi, H.G.; Zahroodi, H.S.; Ghalibaf, A.M.; Bazzaz, B.S.F.; Abbaspour, M.; Boskabadi, J.; Eslami, S.; Taherzadeh, Z. A randomized, double-blind placebo-controlled add-on trial to assess the efficacy, safety, and anti-atherogenic effect of spirulina platensis in patients with inadequately controlled type 2 diabetes mellitus. Phytother. Res. 2023, 37, 1435–1448. [Google Scholar] [CrossRef]
- Alam, A.; Ma, S.; Quamri, A.; Fatima, S.; Roqaiya, M.; Ahmad, Z. Efficacy of Spirulina (Tahlab) in Patients of Type 2 Diabetes Mellitus (Ziabetus Shakri)—A Randomized Controlled Trial. J. Diabetes Metab. 2016, 7, 1–5. [Google Scholar] [CrossRef]
- Sowjanya, M.; Manjula, K. Effect of Food-based Approach with Spirulina on Blood Glucose Profile of Non-insulin Dependent Diabetics. Asian Pac. J. Health Sci. 2022. [Google Scholar] [CrossRef]
- Parikh, P.; Mani, U.V.; Iyer, U.M. Role of Spirulina in the Control of Glycemia and Lipidemia in Type 2 Diabetes Mellitus. J. Med. Food 2001, 4, 193–199. [Google Scholar] [CrossRef]
- Beihaghi, M.; Ghodrati Azadi, H.; Taherzadeh, Z.; Bahrami, H.R. The effects of oral administration of spirulina platensis (cultured iranian) on blood glucose and glycosylated hemoglobin blood in type ii diabetes mellitus patients. Iran. J. Diabetes Lipid Disord. 2017, 16, 183–190. [Google Scholar]
- Mazokopakis, E.E.; Papadomanolaki, M.G.; Fousteris, A.A.; Kotsiris, D.A.; Lampadakis, I.M.; Ganotakis, E.S. The hepatoprotective and hypolipidemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population with non-alcoholic fatty liver disease: A prospective pilot study. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2014, 27, 387–394. [Google Scholar]
- Layam, A.; Reddy, C.K. Antidiabetic property of spirulina. Diabetol. Croat. 2006, 35, 29–33. [Google Scholar]
- El-Moataaz, S.; Ismael, H.; Abo-Rhyem, S.M. Assessment of Chemical Composition of Spirulina Platensis and its Effect on Fasting Blood Glucose and Lipid Profile in Diabetic Rats. J. High Inst. Public Health 2019, 49, 199–211. [Google Scholar] [CrossRef]
- El-Sayed, E.-S.M.; Hikal, M.S.; Khair, B.E.A.E.; El-Ghobashy, R.E.; El-Assar, A.M. Hypoglycemic and hypolipidemic effects of spirulina platensis, phycocyanin, phycocyanopeptide and phycocyanobilin on male diabetic rats. Arab Univ. J. Agric. Sci. 2018, 26, 1121–1134. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Ansari, P.; Azam, S.; Flatt, P.R.; Abdel Wahab, Y.H.A. Effects of Spirulina platensis on insulin secretion, dipeptidyl peptidase IV activity and both carbohydrate digestion and absorption indicate potential as an adjunctive therapy for diabetes. Br. J. Nutr. 2020, 124, 1021–1034. [Google Scholar] [CrossRef]
- Hu, S.; Fan, X.; Qi, P.; Zhang, X. Identification of anti-diabetes peptides from Spirulina platensis. J. Funct. Foods 2019, 56, 333–341. [Google Scholar] [CrossRef]
- Yamina, M.; Oumelkheir, S.; Ismail, M. Effect of Adding the Spirulina (Arthrospira platensis), to Date Syrup on Glycemic Response and its Effectiveness to Reduce Post Prandial Blood Glucose. Int. J. Sci. Res. 2015, 4, 837–840. [Google Scholar]
- Hozayen, W.G.; Mahmoud, A.M.; Soliman, H.A.; Mostafa, S.R. Spirulina versicolor improves insulin sensitivity and attenuates hyperglycemia-mediated oxidative stress in fructose-fed rats. J. Intercult. Ethnopharmacol. 2016, 5, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.D.F.; Silva, A.S.; de Oliveira, C.V.C.; de Souza, A.A.; Ferreira, P.B.; de Souza, I.L.L.; da Cunha Araujo, L.C.; da Silva Félix, G.; de Souza Sampaio, R.; Tavares, R.L.; et al. Spirulina platensis prevents oxidative stress and inflammation promoted by strength training in rats: Dose-response relation study. Sci. Rep. 2020, 10, 6382. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; Lebda, M.A.; Nasr, S.M.; Shoukry, M. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed. Pharmacother. 2017, 92, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.T.; Birtcher, K.; Blumenthal, R.S.; Braun, L.; de Ferranti, S.D.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.M.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Clarkson, P.; Karas, R.H. Statin-associated myopathy. JAMA 2003, 289, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.-C.; Sahebkar, A.; Dragan, S.R.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef]
- Zeinalian, R.; Farhangi, M.A.; Shariat, A.; Saghafi-Asl, M. The effects of Spirulina Platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: A randomized double blinded placebo controlled trial. BMC Complement. Altern. Med. 2017, 17, 225. [Google Scholar] [CrossRef]
- Szulińska, M.; Gibas-Dorna, M.; Miller-Kasprzak, E.; Suliburska, J.; Miczke, A.; Walczak-Gałęzewska, M.; Stelmach-Mardas, M.; Walkowiak, J.; Bogdański, P. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2473–2481. [Google Scholar]
- Hernández-Lepe, M.A.; Wall-Medrano, A.; López-Díaz, J.A.; Juárez-Oropeza, M.A.; Hernández-Torres, R.P.; Ramos-Jiménez, A. Hypolipidemic Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program in Overweight and Obese Men: A Double-Blind, Randomized, and Crossover Controlled Trial. Mar. Drugs 2019, 17, 270. [Google Scholar] [CrossRef]
- Golestani, F.; Mogharnasi, M.; Erfani-Far, M.; Abtahi-Eivari, S.H. The effects of spirulina under high-intensity interval training on levels of nesfatin-1, omentin-1, and lipid profiles in overweight and obese females: A randomized, controlled, single-blind trial. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2021, 26, 10. [Google Scholar]
- Mazokopakis, E.E.; Starakis, I.K.; Papadomanolaki, M.G.; Mavroeidi, N.G.; Ganotakis, E.S. The hypolipidaemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population: A prospective study. J. Sci. Food Agric. 2014, 94, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, S.M.; Samadi, M.T.; Davarpanah, H.; Babajafari, S.; Clark, C.C.T.; Ghaemfar, Z.; Rezaiyan, M.; Mosallanezhad, A.; Shafiee, M.; Rostami, H. The effect of Spirulina sauce, as a functional food, on cardiometabolic risk factors, oxidative stress biomarkers, glycemic profile, and liver enzymes in nonalcoholic fatty liver disease patients: A randomized double-blinded clinical trial. Food Sci. Nutr. 2021, 10, 317–328. [Google Scholar] [CrossRef]
- Koite, N.; Sanogo, N.G.I.; Lépine, O.; Bard, J.M.; Ouguerram, K. Antioxidant Efficacy of a Spirulina Liquid Extract on Oxidative Stress Status and Metabolic Disturbances in Subjects with Metabolic Syndrome. Mar. Drugs 2022, 20, 441. [Google Scholar] [CrossRef]
- Nasirian, F.; Dadkhah, M.; Moradi-kor, N.; Obeidavi, Z. Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 375–380. [Google Scholar] [CrossRef]
- Nagaoka, S.; Shimizu, K.; Kaneko, H.; Shibayama, F.; Morikawa, K.; Kanamaru, Y.; Otsuka, A.; Hirahashi, T.; Kato, T. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J. Nutr. 2005, 135, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Kata, F.S.; Athbi, A.M.; Manwar, E.Q.; Al-Ashoor, A.; Abdel-Daim, M.M.; Aleya, L. Therapeutic effect of the alkaloid extract of the cyanobacterium Spirulina platensis on the lipid profile of hypercholesterolemic male rabbits. Environ. Sci. Pollut. Res. 2018, 25, 19635–19642. [Google Scholar] [CrossRef]
- Sengupta, S.; Koley, H.; Dutta, S.; Bhowal, J. Hypocholesterolemic effect of Spirulina platensis (SP) fortified functional soy yogurts on diet-induced hypercholesterolemia. J. Funct. Foods 2018, 48, 54–64. [Google Scholar] [CrossRef]
- Li, T.-T.; Huang, Z.-R.; Jia, R.-B.; Lv, X.-C.; Zhao, C.; Liu, B. Spirulina platensis polysaccharides attenuate lipid and carbohydrate metabolism disorder in high-sucrose and high-fat diet-fed rats in association with intestinal microbiota. Food Res. Int. 2021, 147, 110530. [Google Scholar] [CrossRef]
- Hua, P.; Yu, Z.; Xiong, Y.; Liu, B.; Zhao, L.-N. Regulatory Efficacy of Spirulina platensis Protease Hydrolyzate on Lipid Metabolism and Gut Microbiota in High-Fat Diet-Fed Rats. Int. J. Mol. Sci. 2018, 19, 4023. [Google Scholar] [CrossRef]
- Oriquat, G.A.; Ali, M.A.; Mahmoud, S.A.; Eid, R.S.M.; Hassan, R.; Kamel, M.A. Improving hepatic mitochondrial biogenesis as a postulated mechanism for the antidiabetic effect of Spirulina platensis in comparison with metformin. Appl. Physiol. Nutr. Metab. 2019, 44, 357–364. [Google Scholar] [CrossRef]
- Ama Moor, V.J.; Nya Biapa, P.C.; Nono Njinkio, B.L.; Moukette Moukette, B.; Sando, Z.; Kenfack, C.A.; Ateba, B.A.; Ngo Matip, M.E.; Pieme, C.A.; Ngogang, J.Y. Hypolipidemic effect and activation of Lecithin Cholesterol Acyl Transferase (LCAT) by aqueous extract of Spirulina platensis during toxicological investigation. BMC Nutr. 2017, 3, 25. [Google Scholar] [CrossRef]
- Iwata, K.; Inayama, T.; Kato, T. Effects of Spirulina platensis on plasma lipoprotein lipase activity in fructose-induced hyperlipidemic rats. J. Nutr. Sci. Vitaminol. 1990, 36, 165–171. [Google Scholar] [CrossRef]
- Oriquat, G.A. Therapeutic effects of Spirulina against experimentally-induced non-alcoholic fatty liver in rats may involve miR-21, -34a and -122. Meta Gene 2018, 18, 115–121. [Google Scholar] [CrossRef]
- Han, L.-K.; Li, D.-X.; Xiang, L.; Gong, X.J.; Kondo, Y.; Suzuki, I.; Okuda, H. Isolation of pancreatic lipase activity-inhibitory component of spirulina platensis and it reduce postprandial triacylglycerolemia. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2006, 126, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Huang, R.; Jiang, J.; Ding, Y.; Li, T.; Ou, Y. Potential use of C-phycocyanin in non-alcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2020, 526, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Riss, J.; Décordé, K.; Sutra, T.; Delage, M.; Baccou, J.C.; Jouy, N.; Brune, J.-P.; Oréal, H.; Cristol, J.-P.; Rouanet, J.M. Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J. Agric. Food Chem. 2007, 55, 7962–7967. [Google Scholar] [CrossRef] [PubMed]
- Strasky, Z.; Zemánková, L.; Němečková, I.; Rathouská, J.; Wong, R.J.; Muchová, L.; Subhanová, I.; Vaníková, J.; Vanova, K.H.; Vítek, L.; et al. Spirulina platensis and phycocyanobilin activate atheroprotective heme oxygenase-1: A possible implication for atherogenesis. Food Funct. 2013, 4, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).