Postprandial Micronutrient Variability and Bioavailability: An Interventional Meal Study in Young vs. Old Participants
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Intervention Meal
2.3. Determination of Anthropometric Parameters
2.4. Quantification of Trace Elements
2.5. Quantification of Free Zinc
2.6. Quantification of Carotenoids and Vitamins
2.7. Statistical Analyses
3. Results
3.1. Characteristics of the Study Groups
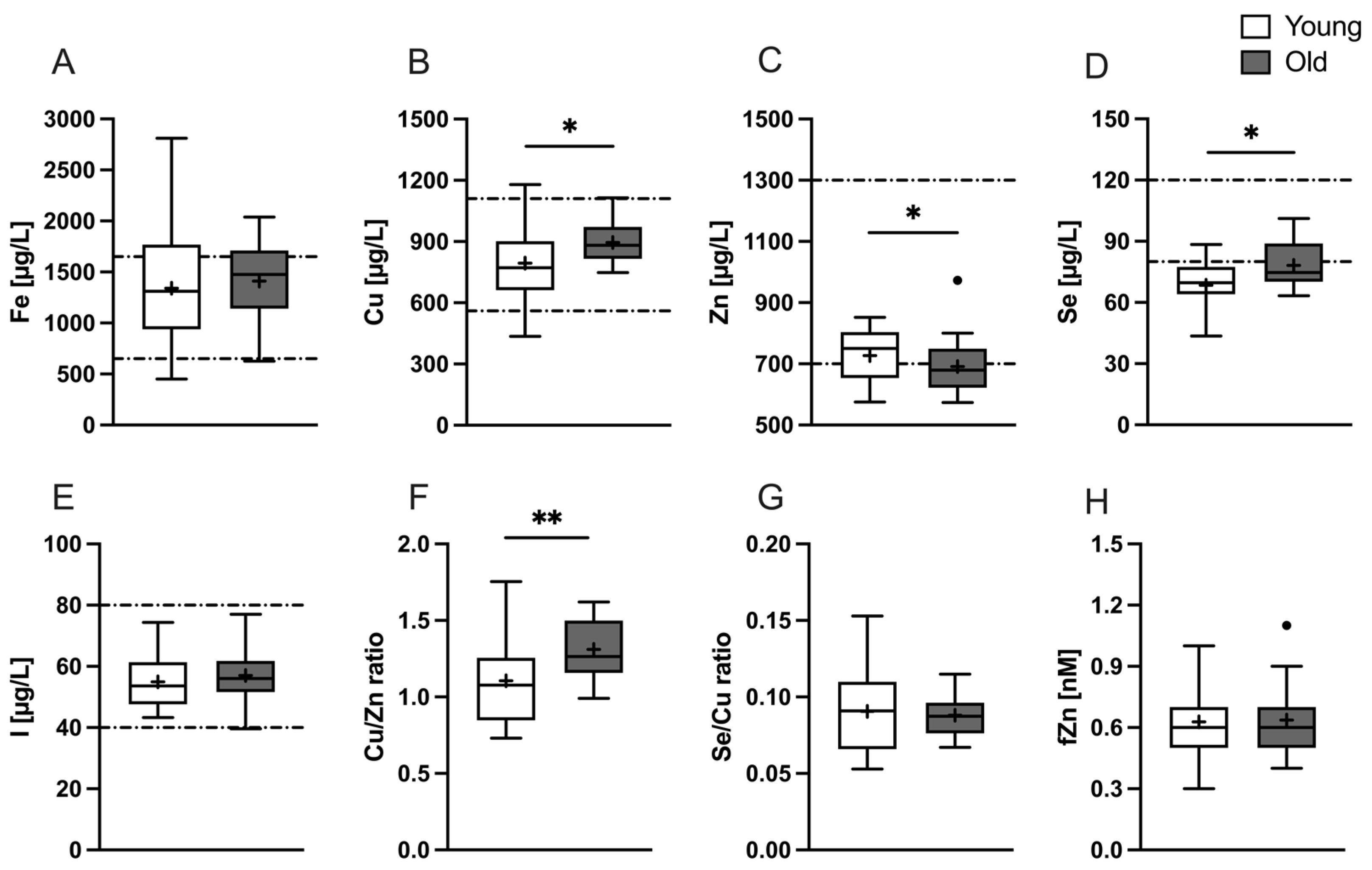
3.2. Baseline Status of Micronutrients in Young vs. Old Subjects
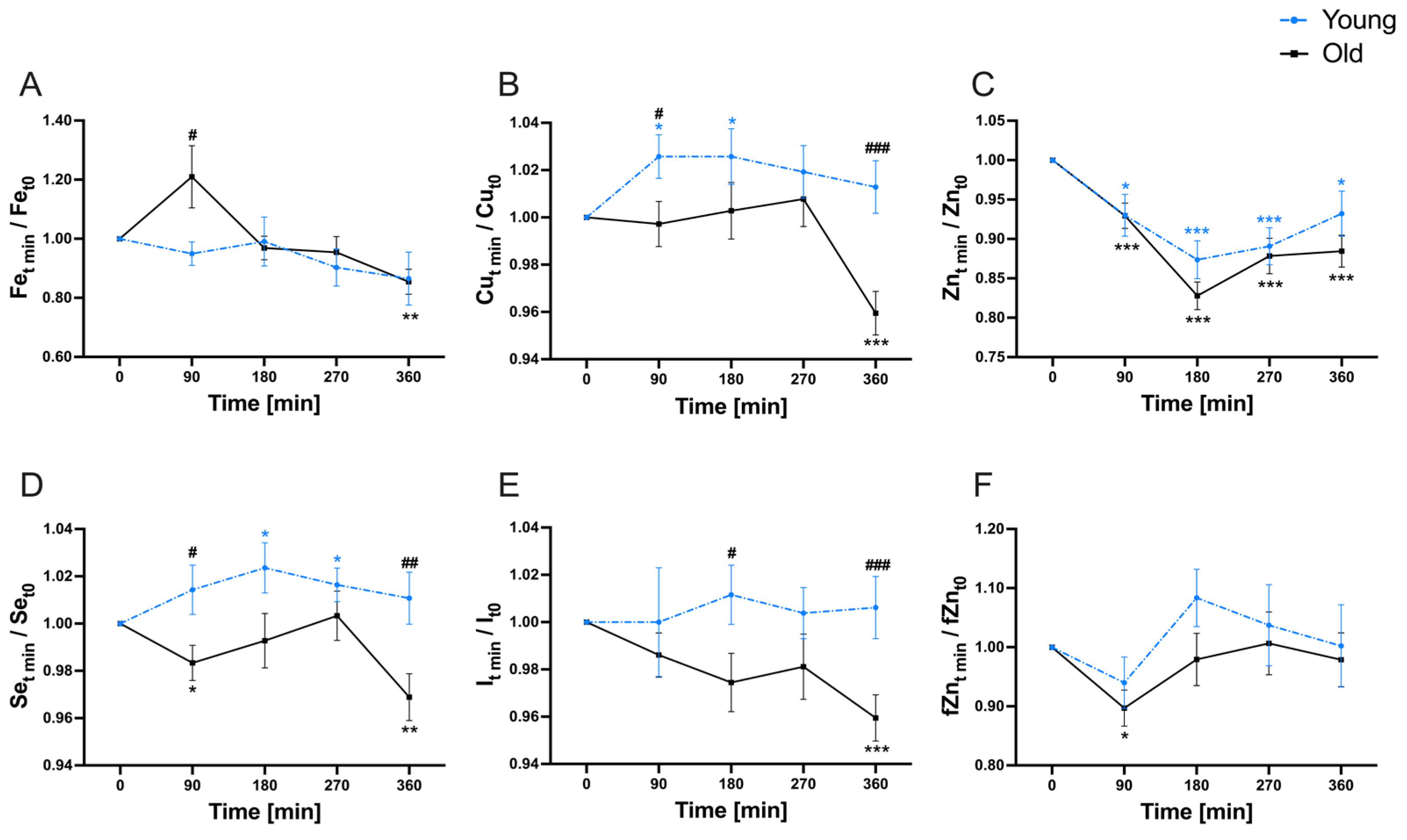
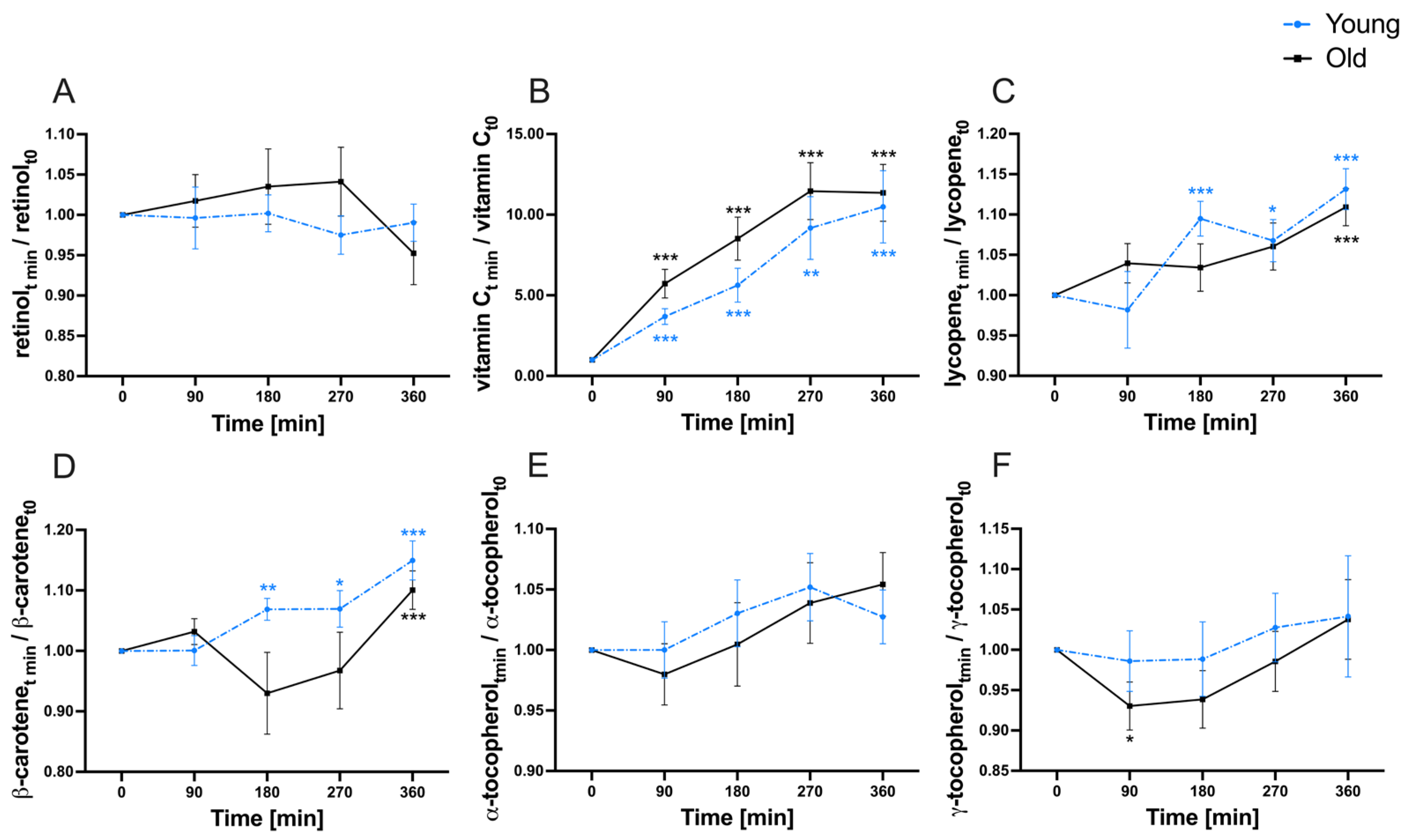
3.3. Postprandial Variability of Micronutrients
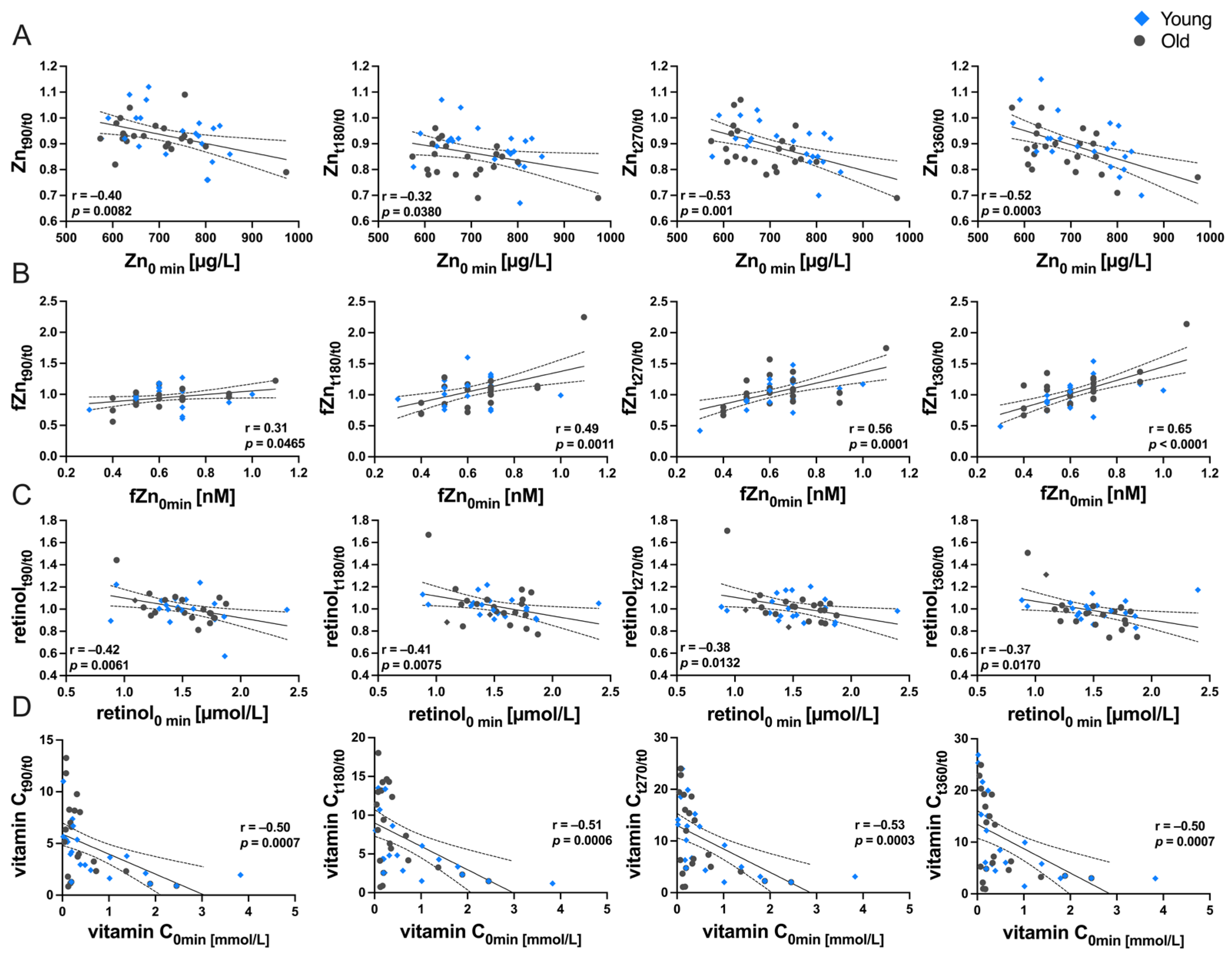
3.4. Relationship between Baseline Status and Postprandial Variability of Micronutrients in Serum/Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, R.M. Factors in Aging That Effect the Bioavailability of Nutrients. J. Nutr. 2001, 131, 1359S–1361S. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, J.; Kipp, A.P.; Haase, H.; Meyer, S.; Schwerdtle, T. The Crux of Inept Biomarkers for Risks and Benefits of Trace Elements. TrAC Trends Anal. Chem. 2018, 104, 183–190. [Google Scholar] [CrossRef]
- Baudry, J.; Kopp, J.F.; Boeing, H.; Kipp, A.P.; Schwerdtle, T.; Schulze, M.B. Changes of Trace Element Status during Aging: Results of the EPIC-Potsdam Cohort Study. Eur. J. Nutr. 2020, 59, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
- Trame, S.; Wessels, I.; Haase, H.; Rink, L. A Short 18 Items Food Frequency Questionnaire Biochemically Validated to Estimate Zinc Status in Humans. J. Trace Elem. Med. Biol. 2018, 49, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Ellinger, S.; Linseisen, J.; Neuhäuser-Berthold, M.; Richter, M. Revised D-A-CH-Reference Values for the Intake of Zinc. J. Trace Elem. Med. Biol. 2020, 61, 126536. [Google Scholar] [CrossRef]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)—Zinc Review. J. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Goodall, M.J.; Stall, C.; Pritts, J. Post-Prandial and Daily Changes in Plasma Zinc. J. Trace Elem. Electrolytes Health Dis. 1989, 3, 55–57. [Google Scholar]
- Maares, M.; Hackler, J.; Haupt, A.; Heller, R.A.; Bachmann, M.; Diegmann, J.; Moghaddam, A.; Schomburg, L.; Haase, H. Free Zinc as a Predictive Marker for COVID-19 Mortality Risk. Nutrients 2022, 14, 1407. [Google Scholar] [CrossRef]
- Kirby, J.K.; Lyons, G.H.; Karkkainen, M.P. Selenium Speciation and Bioavailability in Biofortified Products Using Species-Unspecific Isotope Dilution and Reverse Phase Ion Pairing−Inductively Coupled Plasma–Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 1772–1779. [Google Scholar] [CrossRef]
- Wastney, M.E.; Combs, G.F.; Canfield, W.K.; Taylor, P.R.; Patterson, K.Y.; Hill, A.D.; Moler, J.E.; Patterson, B.H. A Human Model of Selenium That Integrates Metabolism from Selenite and Selenomethionine. J. Nutr. 2011, 141, 708–717. [Google Scholar] [CrossRef]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and Mineral Status in a Vegan Diet. Dtsch. Arztebl. Int. 2020, 117, 575. [Google Scholar] [CrossRef]
- Klein, L.; Dawczynski, C.; Schwarz, M.; Maares, M.; Kipp, K.; Haase, H.; Kipp, A.P. Selenium, Zinc, and Copper Status of Vegetarians and Vegans in Comparison to Omnivores in the Nutritional Evaluation (NuEva) Study. Nutrients 2023, 15, 3538. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Stuetz, W.; Weber, D.; Dollé, M.; Jansen, E.; Grubeck-Loebenstein, B.; Fiegl, S.; Toussaint, O.; Bernhardt, J.; Gonos, E.; Franceschi, C.; et al. Plasma Carotenoids, Tocopherols, and Retinol in the Age-Stratified (35–74 Years) General Population: A Cross-Sectional Study in Six European Countries. Nutrients 2016, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Kochlik, B.; Demuth, I.; Steinhagen-Thiessen, E.; Grune, T.; Norman, K. Plasma Carotenoids, Tocopherols and Retinol—Association with Age in the Berlin Aging Study II. Redox Biol. 2020, 32, 101461. [Google Scholar] [CrossRef]
- Cardinault, N.; Tyssandier, V.; Grolier, P.; Winklhofer-Roob, B.M.; Ribalta, J.; Bouteloup-Demange, C.; Rock, E.; Borel, P. Comparison of the Postprandial Chylomicron Carotenoid Responses in Young and Older Subjects. Eur. J. Nutr. 2003, 42, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.F.; Müller, S.M.; Pohl, G.; Lossow, K.; Kipp, A.P.; Schwerdtle, T. A Quick and Simple Method for the Determination of Six Trace Elements in Mammalian Serum Samples Using ICP-MS/MS. J. Trace Elem. Med. Biol. 2019, 54, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Mocchegiani, E. Serum Copper to Zinc Ratio: Relationship with Aging and Health Status. Mech. Ageing Dev. 2015, 151, 93–100. [Google Scholar] [CrossRef]
- Mittag, J.; Schomburg, L. Der Selen/Kupfer Koeffizient—Ein Neuer Biomarker Für Schilddrüsenhormonresistenz? Perspect Sci 2015, 3, 44–45. [Google Scholar] [CrossRef]
- Cabral, M.; Kuxhaus, O.; Eichelmann, F.; Kopp, J.F.; Alker, W.; Hackler, J.; Kipp, A.P.; Schwerdtle, T.; Haase, H.; Schomburg, L.; et al. Trace Element Profile and Incidence of Type 2 Diabetes, Cardiovascular Disease and Colorectal Cancer: Results from the EPIC-Potsdam Cohort Study. Eur. J. Nutr. 2021, 60, 3267–3278. [Google Scholar] [CrossRef]
- Alker, W.; Schwerdtle, T.; Schomburg, L.; Haase, H. A Zinpyr-1-Based Fluorimetric Microassay for Free Zinc in Human Serum. Int. J. Mol. Sci. 2019, 20, 4006. [Google Scholar] [CrossRef]
- Henning, T.; Kochlik, B.; Ara, I.; González-Gross, M.; Fiorillo, E.; Marongiu, M.; Cucca, F.; Rodriguez-Artalejo, F.; Carnicero Carreño, J.A.; Rodriguez-Mañas, L.; et al. Patterns of Dietary Blood Markers Are Related to Frailty Status in the FRAILOMIC Validation Phase. Nutrients 2023, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Stuetz, W.; Toussaint, O.; Debacq-Chainiaux, F.; Dollé, M.E.T.; Jansen, E.; Gonos, E.S.; Franceschi, C.; Sikora, E.; Hervonen, A.; et al. Associations between Specific Redox Biomarkers and Age in a Large European Cohort: The MARK-AGE Project. Oxid. Med. Cell Longev. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Mocchegiani, E.; Rink, L. Correlation between Zinc Status and Immune Function in the Elderly. Biogerontology 2006, 7, 421–428. [Google Scholar] [CrossRef]
- King, J.C.; Michael Hambidge, K.; Westcott, J.L.; Kern, D.L.; Marshall, G. Daily Variation in Plasma Zinc Concentrations in Women Fed Meals at Six-Hour Intervals. J. Nutr. 1994, 124, 508–516. [Google Scholar] [CrossRef]
- Lossow, K.; Kopp, J.F.; Schwarz, M.; Finke, H.; Winkelbeiner, N.; Renko, K.; Meçi, X.; Ott, C.; Alker, W.; Hackler, J.; et al. Aging Affects Sex- and Organ-Specific Trace Element Profiles in Mice. Aging 2020, 12, 13762–13790. [Google Scholar] [CrossRef]
- Müller, S.M.; Dawczynski, C.; Wiest, J.; Lorkowski, S.; Kipp, A.P.; Schwerdtle, T. Functional Biomarkers for the Selenium Status in a Human Nutritional Intervention Study. Nutrients 2020, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Markova, M.; Pohl, G.; Marschall, T.A.; Pivovarova, O.; Pfeiffer, A.F.H.; Schwerdtle, T. Development, Validation and Application of an ICP-MS/MS Method to Quantify Minerals and (Ultra-)Trace Elements in Human Serum. J. Trace Elem. Med. Biol. 2018, 49, 157–163. [Google Scholar] [CrossRef]
- Holben, D.H.; Smith, A.M.; Ilich, J.Z.; Landoll, J.D.; Holcomb, J.P.; Matkovic, V. Selenium Intakes, Absorption, Retention, and Status in Adolescent Girls. J. Am. Diet. Assoc. 2002, 102, 1082–1087. [Google Scholar] [CrossRef]
- Babak, M.V.; Ahn, D. Modulation of Intracellular Copper Levels as the Mechanism of Action of Anticancer Copper Complexes: Clinical Relevance. Biomedicines 2021, 9, 852. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Methods to Assess Iron and Iodine Status. Br. J. Nutr. 2008, 99, S2–S9. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Buse, J.D.; Baskin, L.; Sadrzadeh, S.M.H.; Naugler, C. Influence of Diurnal Variation and Fasting on Serum Iron Concentrations in a Community-Based Population. Clin. Biochem. 2017, 50, 1237–1242. [Google Scholar] [CrossRef]
- Hutchinson, C.; Conway, R.E.; Bomford, A.; Hider, R.C.; Powell, J.J.; Geissler, C.A. Post-Prandial Iron Absorption in Humans: Comparison between HFE Genotypes and Iron Deficiency Anaemia. Clin. Nutr. 2008, 27, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. Aging and the Hypothalamic-Pituitary-Thyroid Axis. Vitam. Horm. 2021, 115, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K. Vitamine, Spurenelemente und Minerale; Thieme Medical Publishers: Leipzig, Germany, 2019. [Google Scholar] [CrossRef]
- Wagner, K.H.; Kamal-Eldin, A.; Elmadfa, I. Gamma-Tocopherol—An Underestimated Vitamin? Ann. Nutr. Metab. 2004, 48, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020, 41, 103. [Google Scholar] [CrossRef]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A Concentration-Function Approach Yields Pharmacology and Therapeutic Discoveries. Adv. Nutr. 2011, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Glube, N.; von Moos, L.; Duchateau, G. Capsule Shell Material Impacts the in Vitro Disintegration and Dissolution Behaviour of a Green Tea Extract. Results Pharma Sci. 2013, 3, 1–6. [Google Scholar] [CrossRef]

| Parameter | Young (n = 21) | Old (n = 22) | p |
|---|---|---|---|
| Age [Years] | 26.90 ± 3.25 | 66.77 ± 4.73 | <0.0001 a |
| sex, female [n (%)] | 12 (57) | 15 (68) | 0.4540 b |
| Vegan/vegetarian [n (%)] | 7 (33) | 4 (18) | 0.2550 b |
| BMI [kg/m2] | 24.02 ± 2.73 | 24.73 ± 3.21 | 0.4350 c |
| Grip strength [kg] | 41.79 ± 13.54 | 33.91 ± 10.80 | 0.0220 a |
| Grip normalized 1 | 1.73 ± 0.46 | 1.37 ± 0.37 | 0.0074 a |
| Absolute fat mass [kg] | 18.94 ± 4.62 | 23.33 ± 6.85 | 0.0184 c |
| Relative fat mass [%] | 25.57 ± 6.00 | 32.75 ± 6.90 | 0.0008 c |
| FMI | 5.90 ± 1.26 | 8.18 ± 2.23 | 0.0002 c |
| Lean body mass [kg] | 55.64 ± 10.69 | 48.00 ± 11.55 | 0.0074 a |
| LBMI | 17.85 ± 2.34 | 16.55 ± 2.18 | 0.0448 a |
| Bone mass [kg] | 26.50 ± 6.35 | 21.68 ± 6.69 | 0.0036 a |
| Total energy expenditure [kcal] | 2650 ± 412 | 2440 ± 472 | 0.0756 a |
| Resting energy expenditure [kcal] | 1628 ± 228 | 1433 ± 245 | 0.0028 a |
| Total plasma protein [mg/mL] | 74.98 ± 7.44 | 71.90 ± 6.45 | 0.1531 c |
| Skin Carotenoids | 386.6 ± 83.2 | 387.0 ± 92.4 | 0.9882 c |
| Micronutrient | % of Total Variation | p | ||||
|---|---|---|---|---|---|---|
| Time | Age | Subject | Time | Age | Subject | |
| Fe | 8.01 | 1.16 | 30.84 | 0.0055 | 0.2967 | 0.0010 |
| Cu | 6.51 | 7.60 | 31.41 | 0.0137 | 0.0115 | 0.0002 |
| Zn | 28.49 | 1.29 | 40.33 | <0.0001 | 0.3360 | <0.0001 |
| Se | 3.54 | 9.24 | 28.45 | 0.1395 | 0.0040 | 0.0028 |
| I | 1.29 | 6.20 | 31.77 | 0.5676 | 0.0242 | 0.0015 |
| fZn | 4.28 | 0.02 | 56.14 | 0.0341 | 0.9140 | <0.0001 |
| retinol | 1.49 | 0.40 | 61.64 | 0.2800 | 0.6619 | <0.0001 |
| vitamin C | 30.82 | 1.50 | 43.81 | <0.0001 | 0.3198 | <0.0001 |
| lycopene (normalized) | 15.16 | 0.08 | 39.00 | <0.0001 | 0.8014 | <0.0001 |
| β-carotene (normalized) | 8.33 | 0.11 | 35.51 | 0.0046 | 0.1577 | <0.0001 |
| α-tocopherol | 4.46 | 0.10 | 45.16 | 0.0424 | 0.8005 | <0.0001 |
| γ-tocopherol | 3.61 | 0.92 | 49.95 | 0.0824 | 0.4705 | <0.0001 |
| Micronutrient at Baseline | t90/t0 | t180/t0 | t270/t0 | t360/t0 | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Fe | −0.29 | 0.0977 | −0.32 | 0.0721 | −0.46 | 0.0074 | −0.34 | 0.0532 |
| Cu | −0.26 | 0.1514 | −0.23 | 0.1906 | −0.08 | 0.6525 | −0.14 | 0.4372 |
| Zn | −0.40 | 0.0082 | −0.32 | 0.0380 | −0.51 | 0.0005 | −0.52 | 0.0003 |
| Se | −0.15 | 0.4156 | −0.27 | 0.1317 | −0.50 | 0.0028 | −0.03 | 0.8803 |
| I | 0.03 | 0.8926 | 0.02 | 0.8985 | −0.03 | 0.8626 | −0.21 | 0.2455 |
| fZn | 0.31 | 0.0465 | 0.49 | 0.0011 | 0.56 | 0.0001 | 0.65 | <0.0001 |
| retinol | −0.42 | 0.0061 | −0.41 | 0.0075 | −0.38 | 0.0132 | −0.37 | 0.0170 |
| vitamin C | −0.50 | 0.0007 | −0.51 | 0.0006 | −0.53 | 0.0003 | −0.50 | 0.0007 |
| lycopene normalized | −0.26 | 0.1403 | 0.29 | 0.1084 | 0.06 | 0.7274 | −0.02 | 0.9257 |
| β-carotene normalized | −0.02 | 0.9064 | −0.14 | 0.4582 | −0.24 | 0.1710 | −0.47 | 0.0063 |
| α-tocopherol | −0.30 | 0.0900 | 0.15 | 0.4095 | −0.21 | 0.2340 | −0.18 | 0.3215 |
| γ-tocopherol | −0.12 | 0.5297 | −0.07 | 0.6958 | −0.19 | 0.2960 | −0.52 | 0.0021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellowski, D.; Kusch, P.; Henning, T.; Kochlik, B.; Maares, M.; Schmiedeskamp, A.; Pohl, G.; Schreiner, M.; Baldermann, S.; Haase, H.; et al. Postprandial Micronutrient Variability and Bioavailability: An Interventional Meal Study in Young vs. Old Participants. Nutrients 2024, 16, 625. https://doi.org/10.3390/nu16050625
Pellowski D, Kusch P, Henning T, Kochlik B, Maares M, Schmiedeskamp A, Pohl G, Schreiner M, Baldermann S, Haase H, et al. Postprandial Micronutrient Variability and Bioavailability: An Interventional Meal Study in Young vs. Old Participants. Nutrients. 2024; 16(5):625. https://doi.org/10.3390/nu16050625
Chicago/Turabian StylePellowski, Denny, Paula Kusch, Thorsten Henning, Bastian Kochlik, Maria Maares, Amy Schmiedeskamp, Gabriele Pohl, Monika Schreiner, Susanne Baldermann, Hajo Haase, and et al. 2024. "Postprandial Micronutrient Variability and Bioavailability: An Interventional Meal Study in Young vs. Old Participants" Nutrients 16, no. 5: 625. https://doi.org/10.3390/nu16050625
APA StylePellowski, D., Kusch, P., Henning, T., Kochlik, B., Maares, M., Schmiedeskamp, A., Pohl, G., Schreiner, M., Baldermann, S., Haase, H., Schwerdtle, T., Grune, T., & Weber, D. (2024). Postprandial Micronutrient Variability and Bioavailability: An Interventional Meal Study in Young vs. Old Participants. Nutrients, 16(5), 625. https://doi.org/10.3390/nu16050625








