Blood Selenium Concentrations Are Inversely Associated with the Risk of Undernutrition in Older Adults
Abstract
:1. Background
2. Methods
2.1. Study Population and Design
2.2. Study Variables
2.2.1. Whole Blood Selenium
2.2.2. Anthropometric Measures
2.2.3. Undernutrition
2.2.4. Dietary Intake
2.2.5. Other Potential Confounders
2.3. Statistical Analyses
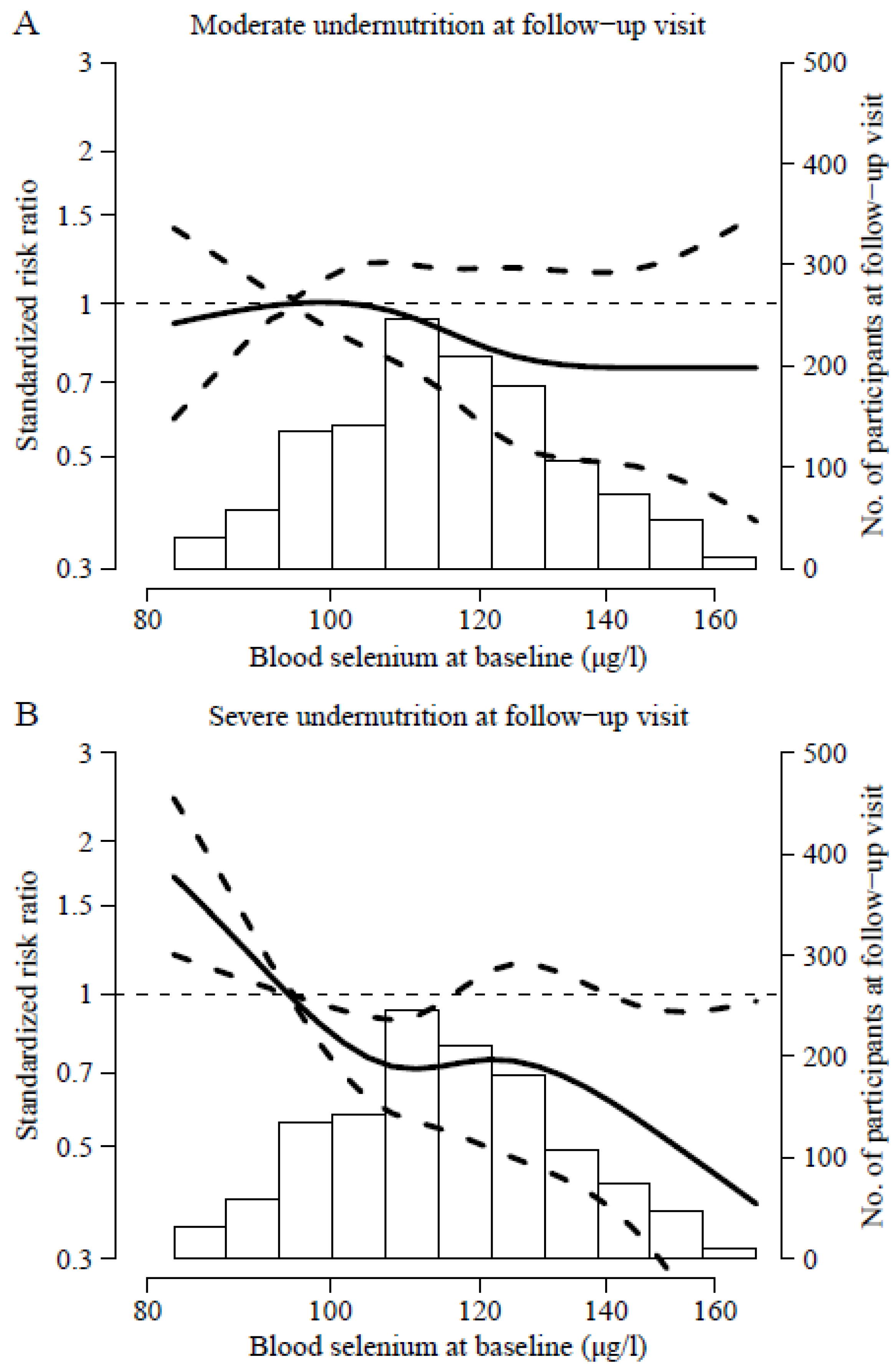
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leij-Halfwerk, S.; Verwijs, M.H.; van Houdt, S.; Borkent, J.W.; Guaitoli, P.R.; Pelgrim, T.; Heymans, M.W.; Power, L.; Visser, M.; Corish, C.A.; et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥ 65 years: A systematic review and meta-analysis. Maturitas 2019, 126, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Chan, R.S.M.; Kwok, T.; Lee, J.S.W.; Woo, J. Malnutrition According to GLIM Criteria and Adverse Outcomes in Community-Dwelling Chinese Older Adults: A Prospective Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 1953–1959.e4. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, D.; Locquet, M.; Reginster, J.-Y.; Cavalier, E.; Bruyère, O.; Beaudart, C. Mortality in malnourished older adults diagnosed by ESPEN and GLIM criteria in the SarcoPhAge study. J. Cachexia Sarcopenia Muscle 2020, 11, 1200–1211. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; Rodríguez-Sánchez, B.; Carnicero, J.A.; Rueda, R.; García-García, F.J.; Pereira, S.L.; Sulo, S. Impact of nutritional status according to GLIM criteria on the risk of incident frailty and mortality in community-dwelling older adults. Clin. Nutr. 2021, 40, 1192–1198. [Google Scholar] [CrossRef]
- Buys, D.R.; Roth, D.L.; Ritchie, C.S.; Sawyer, P.; Allman, R.M.; Funkhouser, E.M.; Hovater, M.; Locher, J.L. Nutritional Risk and Body Mass Index Predict Hospitalization, Nursing Home Admissions, and Mortality in Community-Dwelling Older Adults: Results from the UAB Study of Aging with 8.5 Years of Follow-Up. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1146–1153. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Carreras, O.; Nogales, F. The Role of Selenoprotein Tissue Homeostasis in MetS Programming: Energy Balance and Cardiometabolic Implications. Antioxidants 2022, 11, 394. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.P.; Filippini, T.; Zhou, J.G.; Gen Lei, X.; Gatiatulina, E.R.; Michalke, B.; Skalnaya, M.G.; Vinceti, M.; Aschner, M.; et al. Selenium and Selenoproteins in Adipose Tissue Physiology and Obesity. Biomolecules 2020, 10, 658. [Google Scholar] [CrossRef]
- Abo El-Magd, N.F.; Barbosa, P.O.; Nick, J.; Covalero, V.; Grignetti, G.; Bermano, G. Selenium, as selenite, prevents adipogenesis by modulating selenoproteins gene expression and oxidative stress–related genes. Nutrition 2022, 93, 111424. [Google Scholar] [CrossRef]
- Pitts, M.W.; Reeves, M.A.; Hashimoto, A.C.; Ogawa, A.; Kremer, P.; Seale, L.A.; Berry, M.J. Deletion of selenoprotein M leads to obesity without cognitive deficits. J. Biol. Chem. 2013, 288, 26121–26134. [Google Scholar] [CrossRef]
- Bunk, M.J.; Combs, G.F. Effect of selenium on appetite in the selenium-deficient chick. J. Nutr. 1980, 110, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Bortolatto, C.F.; Heck, S.O.; Zborowski, V.A.; Gai, B.M.; Neto, J.S.S.; Nogueira, C.W. Evidence for the contribution of multiple mechanisms in the feeding pattern of rats exposed to p-chloro-diphenyl diselenide-supplemented diets. Physiol. Behav. 2015, 151, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Nogales, F.; Carreras, O.; Pajuelo, E.; Gallego-López, M.C.; Romero-Herrera, I.; Begines, B.; Moreno-Fernández, J. Different Effects of Low Selenite and Selenium-Nanoparticle Supplementation on Adipose Tissue Function and Insulin Secretion in Adolescent Male Rats. Nutrients 2022, 14, 3571. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Carreras, O.; Díaz-Castro, J.; Murillo, M.L.; Nogales, F. High- and low- selenium diets affect endocrine energy balance during early programming. Toxicol. Appl. Pharmacol. 2019, 382, 114744. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Nogales, F.; Membrilla, A.; Carreras, O. Maternal selenium status is profoundly involved in metabolic fetal programming by modulating insulin resistance, oxidative balance and energy homeostasis. Eur. J. Nutr. 2019, 58, 3171–3181. [Google Scholar] [CrossRef]
- Molanouri Shamsi, M.; Chekachak, S.; Soudi, S.; Quinn, L.S.; Ranjbar, K.; Chenari, J.; Yazdi, M.H.; Mahdavi, M. Combined effect of aerobic interval training and selenium nanoparticles on expression of IL-15 and IL-10/TNF-α ratio in skeletal muscle of 4T1 breast cancer mice with cachexia. Cytokine 2017, 90, 100–108. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Kravtsova, M.A.; Lee, B.C.; Seravalli, J.; Peterson, D.A.; Walter, J.; Legge, R.; Benson, A.K.; Hatfiled, D.L.; Gladyshev, V.N. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011, 25, 2492–2499. [Google Scholar] [CrossRef]
- Song, X.; Qiao, L.; Chang, J.; Dou, X.; Zhang, X.; Pi, S.; Xu, C. Dietary supplementation with selenium nanoparticles-enriched Lactobacillus casei ATCC 393 alleviates intestinal barrier dysfunction of mice exposed to deoxynivalenol by regulating endoplasmic reticulum stress and gut microbiota. Ecotoxicol. Environ. Saf. 2022, 248, 114276. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, X.; Pi, S.; Chang, J.; Dou, X.; Yan, S.; Song, X.; Chen, Y.; Zeng, X.; Zhu, L.; et al. Dietary supplementation with biogenic selenium nanoparticles alleviate oxidative stress-induced intestinal barrier dysfunction. NPJ Sci. Food 2022, 6, 30. [Google Scholar] [CrossRef]
- Maggio, M.; Ceda, G.P.; Lauretani, F.; Bandinelli, S.; Dall’Aglio, E.; Guralnik, J.M.; Paolisso, G.; Semba, R.D.; Nouvenne, A.; Borghi, L.; et al. Association of plasma selenium concentrations with total IGF-1 among older community-dwelling adults: The InCHIANTI study. Clin. Nutr. 2010, 29, 674–677. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Yang, K.-C.; Chang, H.-H.; Lee, L.-T.; Lu, C.-W.; Huang, K.-C. Low serum selenium level is associated with low muscle mass in the community-dwelling elderly. J. Am. Med. Dir. Assoc. 2014, 15, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Ray, A.L.; Guralnik, J.M.; Ferrucci, L. Association of low plasma selenium concentrations with poor muscle strength in older community-dwelling adults: The InCHIANTI Study. Am. J. Clin. Nutr. 2007, 86, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Ray, A.L.; Ruggiero, C.; Cherubini, A.; Guralnik, J.M.; Ferrucci, L. Low plasma selenium concentrations and mortality among older community-dwelling adults: The InCHIANTI Study. Aging Clin. Exp. Res. 2008, 20, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Onder, G.; Lattanzio, F.; Bustacchini, S.; Di Stefano, G.; Moresi, R.; Russo, A.; Bernabei, R.; Landi, F. Selenium Concentrations and Mortality among Community-Dwelling Older Adults: Results from IlSIRENTE Study. J. Nutr. Health Aging 2018, 22, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Johansson, P.; Svensson, E.; Aaseth, J.; Alexander, J. Improved cardiovascular health by supplementation with selenium and coenzyme Q10: Applying structural equation modelling (SEM) to clinical outcomes and biomarkers to explore underlying mechanisms in a prospective randomized double-blind placebo-controlled intervention project in Sweden. Eur. J. Nutr. 2022, 61, 3135–3148. [Google Scholar] [PubMed]
- Dunning, B.J.; Bourgonje, A.R.; Bulthuis, M.L.C.; Alexander, J.; Aaseth, J.O.; Larsson, A.; van Goor, H.; Alehagen, U. Selenium and coenzyme Q10 improve the systemic redox status while reducing cardiovascular mortality in elderly population-based individuals. Free Radic. Biol. Med. 2023, 204, 207–214. [Google Scholar] [CrossRef]
- Alehagen, U.; Johansson, P.; Aaseth, J.; Alexander, J.; Brismar, K. Increase in insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 1 after supplementation with selenium and coenzyme Q10. A prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. PLoS ONE 2017, 12, e0178614. [Google Scholar] [CrossRef]
- García-Esquinas, E.; Carrasco-Rios, M.; Sotos-Prieto, M.; Pérez-Gómez, B.; Gutiérrez-González, E.; Banegas, J.; Queipo, R.; Olmedo, P.; Gil, F.; Tellez-Plaza, M.; et al. Selenium and impaired physical function in US and Spanish older adults. Redox Biol. 2020, 38, 101819. [Google Scholar] [CrossRef]
- Rodríguez-Artalejo, F.; Graciani, A.; Guallar-Castillón, P.; León-Muñoz, L.M.; Zuluaga, M.C.; López-García, E.; Gutiérrez-Fisac, J.L.; Taboada, J.M.; Aguilera, M.T.; Regidor, E.; et al. Rationale and methods of the study on nutrition and cardiovascular risk in Spain (ENRICA). Rev. Esp. Cardiol. 2011, 64, 876–882. [Google Scholar] [CrossRef]
- Rodríguez-Artalejo, F.; Graciani, A.; Guallar-Castillón, P.; León-Muñoz, L.M.; Zuluaga, M.C.; López-García, E.; Gutiérrez-Fisac, J.L.; Taboada, J.M.; Aguilera, M.T.; Regidor, E. Socioeconomic determinants of sarcopenic obesity and frail obesity in community-dwelling older adults: The Seniors-ENRICA Study. Sci. Rep. 2018, 8, 10760. [Google Scholar]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Fisac, J.L.; Guallar-Castillón, P.; León-Muñoz, L.M.; Graciani, A.; Banegas, J.R.; Rodríguez-Artalejo, F. Prevalence of general and abdominal obesity in the adult population of Spain, 2008–2010: The ENRICA study. Obes. Rev. 2012, 13, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Scafoglieri, A.; Clarys, J.P.; Bauer, J.M.; Verlaan, S.; Van Malderen, L.; Vantieghem, S.; Cederholm, T.; Sieber, C.C.; Mets, T.; Bautmans, I. Predicting appendicular lean and fat mass with bioelectrical impedance analysis in older adults with physical function decline—The PROVIDE study. Clin. Nutr. 2017, 36, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Aleman-Mateo, H.; Rush, E.; Esparza-Romero, J.; Ferriolli, E.; Ramirez-Zea, M.; Bour, A.; Yuchingtat, G.; Ndour, R.; Mokhtar, N.; Valencia, M.E.; et al. Prediction of fat-free mass by bioelectrical impedance analysis in older adults from developing countries: A cross-validation study using the deuterium dilution method. J. Nutr. Health Aging 2010, 14, 418–426. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clinical nutrition (Edinburgh, Scotland) 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Chen, L.Y.C.; Hoiland, R.L.; Stukas, S.; Wellington, C.L.; Sekhon, M.S. Confronting the controversy: Interleukin-6 and the COVID-19 cytokine storm syndrome. Eur. Respir. J. 2020, 56, 2003006. [Google Scholar] [CrossRef]
- Guallar-Castillón, P.; Sagardui-Villamor, J.; Balboa-Castillo, T.; Sala-Vila, A.; Ariza Astolfi, M.J.; Sarrión Pelous, M.D.; León-Muñoz, L.M.; Graciani, A.; Laclaustra, M.; Benito, C.; et al. Validity and reproducibility of a Spanish dietary history. PLoS ONE 2014, 9, e86074. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; Agricultural Research Service. USDA Food and Nutrient Database for Dietary Studies. Food Surveys Research Group Home Page. Available online: http://www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 13 September 2023).
- Ortega Anta, R.M.; López Sobaler, A.M.; Carvalajes, P.A. DIAL. 2017. Available online: http://alceingenieria.net/nutricion.htm (accessed on 28 January 2019).
- Moreiras, O.; Carvajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composicion de Alimentos, 11th ed.; Pirámide: Madrid, Spain, 2007. [Google Scholar]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Baum, J.I.; Kim, I.-Y.; Wolfe, R.R. Protein Consumption and the Elderly: What Is the Optimal Level of Intake? Nutrients 2008, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Pols, M.A.; Peeters, P.H.; Ocké, M.C.; Slimani, N.; Bueno-de-Mesquita, H.B.; Collette, H.J. Estimation of reproducibility and relative validity of the questions included in the EPIC Physical Activity Questionnaire. Int. J. Epidemiol. 1997, 26 (Suppl. S1), S181–S189. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Cao, M.; Zheng, S.; Zhang, W.; Hu, G. Progress in the study of nutritional status and selenium in dialysis patients. Ann. Med. 2023, 55, 2197296. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Li, S.; Xu, S.; Zhou, D.; Zhong, X.; Tan, R.; Liu, Y. Associations Between Blood Trace Element Levels and Nutritional Status in Maintenance Hemodialysis. J. Ren. Nutr. 2021, 31, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Sohrabi, Z.; Ekramzadeh, M.; Fallahzadeh, M.K.; Ayatollahi, M.; Geramizadeh, B.; Hassanzadeh, J.; Sagheb, M.M. Selenium supplementation improves the nutritional status of hemodialysis patients: A randomized, double-blind, placebo-controlled trial. Nephrol. Dial. Transplant. 2013, 28, 716–723. [Google Scholar] [CrossRef]
- Morley, J.E. Pathophysiology of anorexia. Clin. Geriatr. Med. 2002, 18, 661–673. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef]
- Cavedon, E.; Manso, J.; Negro, I.; Censi, S.; Serra, R.; Busetto, L.; Vettor, R.; Plebani, M.; Pezzani, R.; Nacamulli, D.; et al. Selenium Supplementation, Body Mass Composition, and Leptin Levels in Patients with Obesity on a Balanced Mildly Hypocaloric Diet: A Pilot Study. Int. J. Endocrinol. 2020, 2020, 4802739. [Google Scholar] [CrossRef]
- Guo, X.; Tang, P.; Hou, C.; Li, R. Mendelian randomization investigation highlights different roles of selenium status in mental disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 122, 110694. [Google Scholar] [CrossRef] [PubMed]
- Vajdi, M.; Hassanizadeh, S.; Gholami, Z.; Bagherniya, M. Selenium supplementation effect on glycemic control: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol. Res. 2023, 195, 106888. [Google Scholar] [CrossRef] [PubMed]
- Verlaan, S.; Aspray, T.J.; Bauer, J.M.; Cederholm, T.; Hemsworth, J.; Hill, T.R.; McPhee, J.S.; Piasecki, M.; Seal, C.; Sieber, C.C.; et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: A case-control study. Clin. Nutr. 2017, 36, 267–274. [Google Scholar] [CrossRef] [PubMed]
- ter Borg, S.; de Groot, L.C.P.G.M.; Mijnarends, D.M.; de Vries, J.H.M.; Verlaan, S.; Meijboom, S.; Luiking, Y.C.; Schols, J.M.G.A. Differences in Nutrient Intake and Biochemical Nutrient Status Between Sarcopenic and Nonsarcopenic Older Adults—Results from the Maastricht Sarcopenia Study. J. Am. Med. Dir. Assoc. 2016, 17, 393–401. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Aaseth, J.; Chirumbolo, S.; Pen, J.J. Cancer-associated Cachexia, Reactive Oxygen Species and Nutrition Therapy. Curr. Med. Chem. 2019, 26, 5728–5744. [Google Scholar] [CrossRef]
- Federico, A.; Iodice, P.; Federico, P.; Del Rio, A.; Mellone, M.C.; Catalano, G.; Federico, P. Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. Eur. J. Clin. Nutr. 2001, 55, 293–297. [Google Scholar] [CrossRef]
- Ghashut, R.A.; McMillan, D.C.; Kinsella, J.; Vasilaki, A.T.; Talwar, D.; Duncan, A. The effect of the systemic inflammatory response on plasma zinc and selenium adjusted for albumin. Clin. Nutr. 2016, 35, 381–387. [Google Scholar] [CrossRef]
- Carballo-Casla, A.; Sotos-Prieto, M.; García-Esquinas, E.; Struijk, E.A.; Caballero, F.F.; Calderón-Larrañaga, A.; Lopez-Garcia, E.; Rodríguez-Artalejo, F.; Ortolá, R. Animal and vegetable protein intake and malnutrition: A multicohort study. J. Nutr. Health Aging 2023. under review. [Google Scholar]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Peláez, C.; et al. Scientific opinion on the tolerable upper intake level for selenium. EFSA J. 2023, 21, e07704. [Google Scholar]
| Characteristics | Whole Blood Selenium (µg/L) | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| (<99) | (99–110) | (110–120) | (120–130) | (≥130) | |
| Number | 367 | 382 | 400 | 420 | 423 |
| Age (years), mean (SD) | 73.06 (4.63) | 71.89 (4.13) | 71.37 (4.23) | 71.18 (3.84) | 70.79 (3.60) |
| Sex, % | |||||
| Men | 47.68 | 53.93 | 52.75 | 50.48 | 47.99 |
| Women | 52.32 | 46.07 | 47.25 | 49.52 | 52.01 |
| Education, % | |||||
| <High School | 65.40 | 63.35 | 64.75 | 60.95 | 55.56 |
| High School | 20.16 | 18.32 | 18.00 | 21.67 | 19.15 |
| >High School | 14.44 | 18.32 | 17.25 | 17.38 | 25.30 |
| Living arrangement, % | |||||
| Alone | 74.66 | 77.49 | 78.25 | 80.24 | 75.89 |
| With family members | 23.16 | 21.73 | 20.00 | 18.33 | 23.17 |
| Other | 2.18 | 0.79 | 1.75 | 1.43 | 0.95 |
| MEDAS, mean (SD) | 6.74 (1.74) | 6.96 (1.74) | 7.13 (1.70) | 7.48 (1.71) | 7.39 (1.70) |
| Vitamins, above the RDA, % | |||||
| <5 | 45.78 | 41.10 | 40.50 | 36.67 | 29.31 |
| ≥5 | 54.22 | 58.90 | 59.50 | 63.33 | 70.69 |
| Daily protein intake (g/kg), mean (SD) | 1.21 (0.25) | 1.24 (0.26) | 1.24 (0.23) | 1.27 (0.24) | 1.30 (0.26) |
| Physical activity METs-hours/week, mean (SD) | 65.24 (36.76) | 68.50 (37.75) | 68.60 (32.71) | 68.97 (33.84) | 69.25 (37.34) |
| Watching TV h/week, mean (SD) | 24.06 (11.67) | 21.69 (11.34) | 22.17 (10.46) | 21.61 (10.41) | 21.08 (9.71) |
| Smoking, % | |||||
| Never | 48.50 | 50.26 | 47.50 | 52.86 | 50.12 |
| Former | 40.33 | 37.43 | 41.75 | 39.29 | 42.32 |
| Current | 11.17 | 12.30 | 10.75 | 7.86 | 7.57 |
| Alcohol consumption, % | |||||
| Never | 22.62 | 17.28 | 14.75 | 16.43 | 15.84 |
| Former | 66.76 | 73.82 | 75.00 | 72.86 | 71.39 |
| Moderate drinker | 5.72 | 4.19 | 4.25 | 6.43 | 6.86 |
| Heavy drinker | 4.90 | 4.71 | 6.00 | 4.29 | 5.91 |
| Hypertension, % | 69.48 | 70.68 | 67.75 | 67.14 | 66.43 |
| Cardiovascular disease, % | 4.36 | 2.09 | 2.50 | 1.67 | 2.60 |
| Diabetes mellitus, % | 24.25 | 21.99 | 20.00 | 20.71 | 14.18 |
| Cancer, % | 3.00 | 2.36 | 2.00 | 1.67 | 3.07 |
| Osteomuscular disease, % | 40.33 | 33.25 | 29.50 | 35.24 | 33.33 |
| Depression, % | 6.27 | 4.97 | 5.75 | 3.81 | 3.07 |
| Whole Blood Selenium ( ) | |||||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | p-trend | Per Interquartile Range | |
| (<99) | (99–110) | (110–120) | (120–130) | (≥130) | |||
| GLIM definition | |||||||
| Moderate undernutrition | 30 | 32 | 30 | 29 | 21 | 142 | |
| Model 1 | 1.00 | 1.06 (0.66–1.70) | 0.97 (0.60–1.57) | 0.91 (0.56–1.47) | 0.67 (0.39–1.14) | 0.06 | 0.86 (0.71–1.05) |
| Model 2 | 1.00 | 1.07 (0.67–1.71) | 1.00 (0.62–1.62) | 0.94 (0.57–1.53) | 0.70 (0.41–1.21) | 0.08 | 0.88 (0.72–1.07) |
| Model 3 | 1.00 | 1.07 (0.67–1.72) | 1.04 (0.64–1.67) | 0.98 (0.60–1.60) | 0.71 (0.41–1.23) | 0.10 | 0.90 (0.74–1.10) |
| Severe undernutrition | 33 | 22 | 15 | 21 | 22 | 113 | |
| Model 1 | 1.00 | 0.65 (0.39.1.09) | 0.43 (0.24–0.77) | 0.58 (0.34–0.98) | 0.60 (0.36–1.01) | 0.04 | 0.77 (0.61–0.96) |
| Model 2 | 1.00 | 0.62 (0.38–1.02) | 0.40 (0.22–0.70) | 0.53 (0.31–0.89) | 0.49 (0.29–0.82) | 0.01 | 0.70 (0.55–0.88) |
| Model 3 | 1.00 | 0.64 (0.39–1.06) | 0.41 (0.24–0.73) | 0.55 (0.33–0.91) | 0.51 (0.31–0.87) | 0.01 | 0.71 (0.57–0.89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Esquinas, E.; Carballo-Casla, A.; Ortolá, R.; Sotos-Prieto, M.; Olmedo, P.; Gil, F.; Plans-Beriso, E.; Fernández-Navarro, P.; Pastor-Barriuso, R.; Rodríguez-Artalejo, F. Blood Selenium Concentrations Are Inversely Associated with the Risk of Undernutrition in Older Adults. Nutrients 2023, 15, 4750. https://doi.org/10.3390/nu15224750
García-Esquinas E, Carballo-Casla A, Ortolá R, Sotos-Prieto M, Olmedo P, Gil F, Plans-Beriso E, Fernández-Navarro P, Pastor-Barriuso R, Rodríguez-Artalejo F. Blood Selenium Concentrations Are Inversely Associated with the Risk of Undernutrition in Older Adults. Nutrients. 2023; 15(22):4750. https://doi.org/10.3390/nu15224750
Chicago/Turabian StyleGarcía-Esquinas, Esther, Adrián Carballo-Casla, Rosario Ortolá, Mercedes Sotos-Prieto, Pablo Olmedo, Fernando Gil, Elena Plans-Beriso, Pablo Fernández-Navarro, Roberto Pastor-Barriuso, and Fernando Rodríguez-Artalejo. 2023. "Blood Selenium Concentrations Are Inversely Associated with the Risk of Undernutrition in Older Adults" Nutrients 15, no. 22: 4750. https://doi.org/10.3390/nu15224750
APA StyleGarcía-Esquinas, E., Carballo-Casla, A., Ortolá, R., Sotos-Prieto, M., Olmedo, P., Gil, F., Plans-Beriso, E., Fernández-Navarro, P., Pastor-Barriuso, R., & Rodríguez-Artalejo, F. (2023). Blood Selenium Concentrations Are Inversely Associated with the Risk of Undernutrition in Older Adults. Nutrients, 15(22), 4750. https://doi.org/10.3390/nu15224750






