Maternal Acylcarnitine Disruption as a Potential Predictor of Preterm Birth in Primigravida: A Preliminary Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Sample Collection
2.2. Sample Preparation and Measurement
2.3. Metabolite Identification and Statistical Analysis
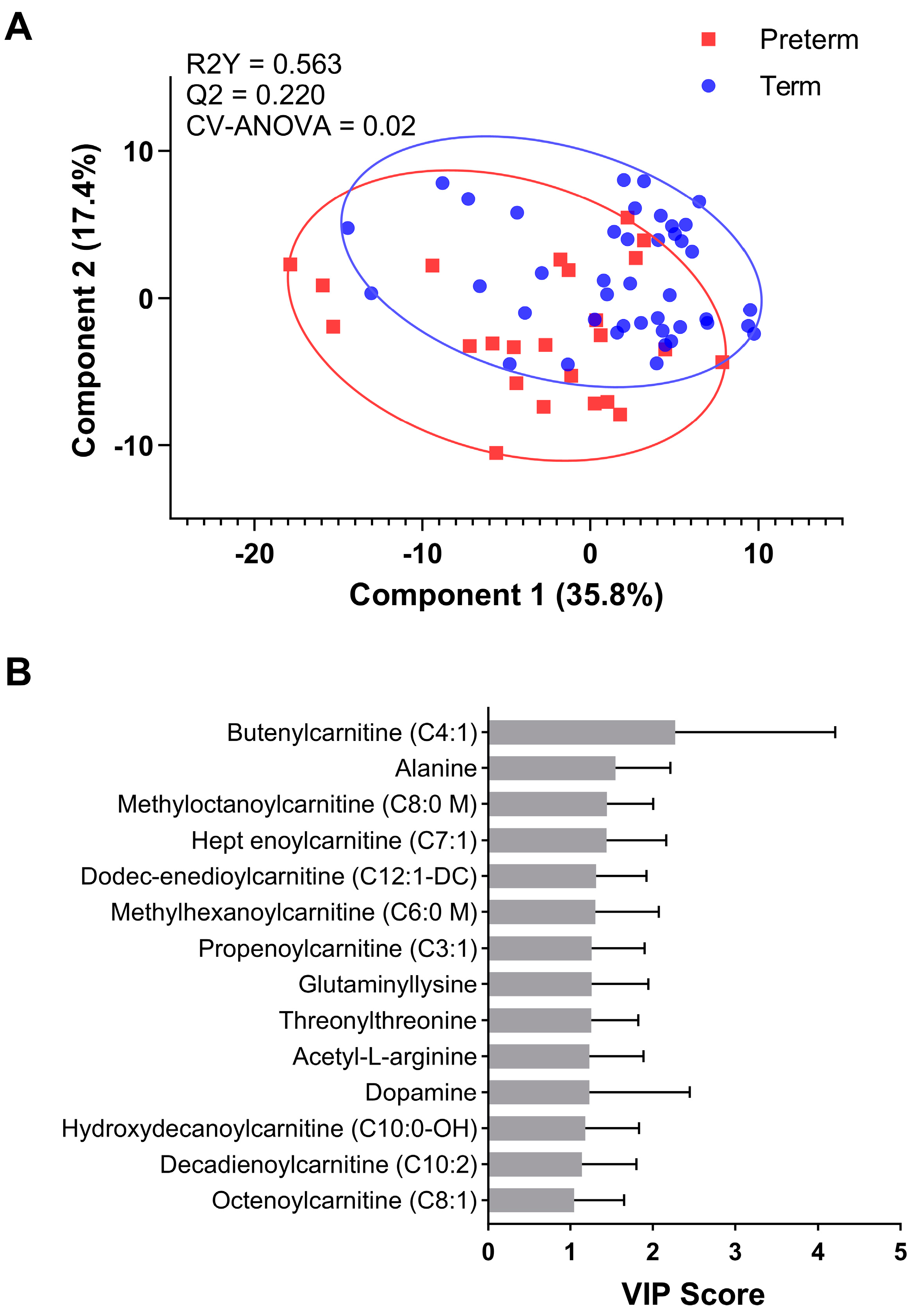
3. Results
3.1. Participant Characteristics
3.2. Metabolite Profiling
3.3. Predictive Modelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, M.S.; Goldenberg, R.L. Global Burden of Prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.A.; Klebanoff, M.A. The Epidemiology, Etiology, and Costs of Preterm Birth. Semin. Fetal Neonatal Med. 2016, 21, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Pravia, C.I.; Benny, M. Long-Term Consequences of Prematurity. Cleve Clin. J. Med. 2020, 87, 759–767. [Google Scholar] [CrossRef]
- Becerra-Mojica, C.H.; Parra-Saavedra, M.A.; Diaz-Martinez, L.A.; Martinez-Portilla, R.J.; Rincon Orozco, B. Cohort Profile: Colombian Cohort for the Early Prediction of Preterm Birth (COLPRET): Early Prediction of Preterm Birth Based on Personal Medical History, Clinical Characteristics, Vaginal Microbiome, Biophysical Characteristics of the Cervix and Maternal Serum Biochemical Markers. BMJ Open 2022, 12, e060556. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Klein, M.S.; Vogel, H.J.; Mohammad, S.; Bainbridge, S.; Adamo, K.B. Maternal and Cord Blood Metabolite Associations with Gestational Weight Gain and Pregnancy Health Outcomes. J. Proteome Res. 2021, 20, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Riano, C.; Santos, M.; Díaz, M.; García-Beltran, C.; Lerin, C.; Barbas, C.; Ibáñez, L.; Sánchez-Infantes, D. Birth Weight and Early Postnatal Outcomes: Association with the Cord Blood Lipidome. Nutrients 2022, 14, 3760. [Google Scholar] [CrossRef]
- da Silva, A.C.R.; Yadegari, A.; Tzaneva, V.; Vasanthan, T.; Laketic, K.; Shearer, J.; Bainbridge, S.A.; Harris, C.; Adamo, K.B. Metabolomics to Understand Alterations Induced by Physical Activity during Pregnancy. Metabolites 2023, 13, 1178. [Google Scholar] [CrossRef]
- Yao, M.; Xiao, Y.; Yang, Z.; Ge, W.; Liang, F.; Teng, H.; Gu, Y.; Yin, J. Identification of Biomarkers for Preeclampsia Based on Metabolomics. Clin. Epidemiol. 2022, 14, 337–360. [Google Scholar] [CrossRef]
- Aung, M.T.; Ashrap, P.; Watkins, D.J.; Mukherjee, B.; Rosario, Z.; Vélez-Vega, C.M.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Maternal Lipidomic Signatures in Relation to Spontaneous Preterm Birth and Large-for-Gestational Age Neonates. Sci. Rep. 2021, 11, 8115. [Google Scholar] [CrossRef]
- Lacey, L.; Daulton, E.; Wicaksono, A.; Covington, J.A.; Quenby, S. Volatile Organic Compound Analysis, a New Tool in the Quest for Preterm Birth Prediction—An Observational Cohort Study. Sci. Rep. 2020, 10, 12153. [Google Scholar] [CrossRef]
- Karahoda, R.; Robles, M.; Marushka, J.; Stranik, J.; Abad, C.; Horackova, H.; Tebbens, J.D.; Vaillancourt, C.; Kacerovsky, M.; Staud, F. Prenatal Inflammation as a Link between Placental Expression Signature of Tryptophan Metabolism and Preterm Birth. Hum. Mol. Genet. 2021, 30, 2053–2067. [Google Scholar] [CrossRef]
- Tough, S.C.; McDonald, S.W.; Collisson, B.A.; Graham, S.A.; Kehler, H.; Kingston, D.; Benzies, K. Cohort Profile: The All Our Babies Pregnancy Cohort (AOB). Int. J. Epidemiol. 2017, 46, 1389–1390k. [Google Scholar] [CrossRef]
- Breier, M.; Wahl, S.; Prehn, C.; Fugmann, M.; Ferrari, U.; Weise, M.; Banning, F.; Seissler, J.; Grallert, H.; Adamski, J.; et al. Targeted Metabolomics Identifies Reliable and Stable Metabolites in Human Serum and Plasma Samples. PLoS ONE 2014, 9, e89728. [Google Scholar] [CrossRef] [PubMed]
- Staneva, A.; Bogossian, F.; Pritchard, M.; Wittkowski, A. The Effects of Maternal Depression, Anxiety, and Perceived Stress during Pregnancy on Preterm Birth: A Systematic Review. Women Birth 2015, 28, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Laketic, K.; Lalonde-Bester, S.; Smyth, K.; Slater, D.M.; Tough, S.C.; Ishida, H.; Vogel, H.J.; Giesbrecht, G.F.; Mu, C.; Shearer, J. Maternal Metabolites Indicative of Mental Health Status during Pregnancy. Metabolites 2022, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Choudhary, A.; Mayengbam, S.; Barrett, K.T.; Rho, J.M.; Shearer, J.; Scantlebury, M.H. Seizure Modulation by the Gut Microbiota and Tryptophan-Kynurenine Metabolism in an Animal Model of Infantile Spasms. EBioMedicine 2022, 76, 103833. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Nikpoor, N.; Tompkins, T.A.; Choudhary, A.; Wang, M.; Marks, W.N.; Rho, J.M.; Scantlebury, M.H.; Shearer, J. Targeted Gut Microbiota Manipulation Attenuates Seizures in a Model of Infantile Spasms Syndrome. JCI Insight 2022, 7, e158521. [Google Scholar] [CrossRef]
- Cho, H.W.; Kim, S.B.; Jeong, M.K.; Park, Y.; Miller, N.G.; Ziegler, T.R.; Jones, D.P. Discovery of Metabolite Features for the Modelling and Analysis of High-Resolution NMR Spectra. Int. J. Data Min. Bioinform. 2008, 2, 176. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Brown, L.D.; Cai, T.T.; Das Gupta, A. Interval Estimation for a Binomial Proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- World Health Organization Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 2 January 2024).
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, Regional, and Global Estimates of Preterm Birth in 2020, with Trends from 2010: A Systematic Analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef]
- Oey, N.A.; van Vlies, N.; Wijburg, F.A.; Wanders, R.J.A.; Attie-Bitach, T.; Vaz, F.M. L-Carnitine Is Synthesized in the Human Fetal-Placental Unit: Potential Roles in Placental and Fetal Metabolism. Placenta 2006, 27, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Marchlewicz, E.H.; Dolinoy, D.C.; Tang, L.; Milewski, S.; Jones, T.R.; Goodrich, J.M.; Soni, T.; Domino, S.E.; Song, P.X.K.; Burant, C.F.; et al. Lipid Metabolism Is Associated with Developmental Epigenetic Programming. Sci. Rep. 2016, 6, 34857. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, B.; Sumner, S.J.; McRitchie, S.; Carlson, J.E.; Ananth, C.V.; Enquobahrie, D.A.; Qiu, C.; Sorensen, T.K.; Williams, M.A. Maternal Early Pregnancy Serum Metabolomics Profile and Abnormal Vaginal Bleeding as Predictors of Placental Abruption: A Prospective Study. PLoS ONE 2016, 11, e0156755. [Google Scholar] [CrossRef] [PubMed]
- Koster, M.P.H.; Vreeken, R.J.; Harms, A.C.; Dane, A.D.; Kuc, S.; Schielen, P.C.J.I.; Hankemeier, T.; Berger, R.; Visser, G.H.A.; Pennings, J.L.A. First-Trimester Serum Acylcarnitine Levels to Predict Preeclampsia: A Metabolomics Approach. Dis. Markers 2015, 2015, 857108. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, R.J.; Al-Akwaa, F.M.; Benny, P.A.; Gurary, A.; Xie, G.; Jia, W.; Chun, S.J.; Chern, I.; Garmire, L.X. Prepregnant Obesity of Mothers in a Multiethnic Cohort Is Associated with Cord Blood Metabolomic Changes in Offspring. J. Proteome Res. 2020, 19, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Batchuluun, B.; Al Rijjal, D.; Prentice, K.J.; Eversley, J.A.; Burdett, E.; Mohan, H.; Bhattacharjee, A.; Gunderson, E.P.; Liu, Y.; Wheeler, M.B. Elevated Medium-Chain Acylcarnitines Are Associated With Gestational Diabetes Mellitus and Early Progression to Type 2 Diabetes and Induce Pancreatic β-Cell Dysfunction. Diabetes 2018, 67, 885–897. [Google Scholar] [CrossRef]
- de la Barca, J.M.C.; Chabrun, F.; Lefebvre, T.; Roche, O.; Huetz, N.; Blanchet, O.; Legendre, G.; Simard, G.; Reynier, P.; Gascoin, G. A Metabolomic Profiling of Intra-Uterine Growth Restriction in Placenta and Cord Blood Points to an Impairment of Lipid and Energetic Metabolism. Biomedicines 2022, 10, 1411. [Google Scholar] [CrossRef]
- Elshenawy, S.; Pinney, S.E.; Stuart, T.; Doulias, P.T.; Zura, G.; Parry, S.; Elovitz, M.A.; Bennett, M.J.; Bansal, A.; Strauss, J.F.; et al. The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int. J. Mol. Sci. 2020, 21, 1043. [Google Scholar] [CrossRef]
- Menichini, D.; Feliciello, L.; Neri, I.; Facchinetti, F. L-Arginine Supplementation in Pregnancy: A Systematic Review of Maternal and Fetal Outcomes. J. Matern. Fetal Neonatal Med. 2023, 36, 2217465. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.; McDonald, C.R.; Urassa, W.S.; Kain, K.C.; Mwiru, R.S.; Fawzi, W.W. Maternal Dietary L-Arginine and Adverse Birth Outcomes in Dar Es Salaam, Tanzania. Am. J. Epidemiol. 2017, 186, 603–611. [Google Scholar] [CrossRef]
- Facchinetti, F.; Saade, G.R.; Neri, I.; Pizzi, C.; Longo, M.; Volpe, A. L-Arginine Supplementation in Patients with Gestational Hypertension: A Pilot Study. Hypertens. Pregnancy 2007, 26, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rytlewski, K.; Olszanecki, R.; Lauterbach, R.; Grzyb, A.; Kiec-Wilk, B.; Dembinska-Kiec, A.; Basta, A. Effects of Oral L-Arginine on the Pulsatility Indices of Umbilical Artery and Middle Cerebral Artery in Preterm Labor. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Goto, E. Effects of Prenatal Oral L-Arginine on Birth Outcomes: A Meta-Analysis. Sci. Rep. 2021, 11, 22748. [Google Scholar] [CrossRef]
- Contreras, M.T.; Gallardo, M.J.; Betancourt, L.R.; Rada, P.V.; Ceballos, G.A.; Hernandez, L.E.; Hernandez, L.F. Correlation between Plasma Levels of Arginine and Citrulline in Preterm and Full-term Neonates: Therapeutical Implications. J. Clin. Lab. Anal. 2017, 31, e22134. [Google Scholar] [CrossRef]



| Preterm (n = 24) | Term (n = 42) | p-Value | |
|---|---|---|---|
| Maternal | |||
| Pre-pregnancy BMI (kg/m2) | 24.0 ± 6.2 | 23.9 ± 5.1 | 0.993 |
| Maternal Age (y) | 30.8 ± 4.2 | 29.6 ± 4.4 | 0.310 |
| Gestation Weight Gain (kg) | 12.4 ± 5.6 | 13.1 ± 6.0 | 0.311 |
| Anxiety score 1 | 31.1 ± 6.7 | 30.8 ± 8.0 | 0.862 |
| Depression score 2 | 5.7 ± 3.8 | 5.6 ± 4.4 | 0.927 |
| Hypertensive 3 (n)(%) | 3 (12.5) | 2 (4.8) | 0.319 |
| Infant | |||
| Gestation (wk) | 34.3 ± 2.1 | 38.7 ± 1.1 | <0.001 |
| Fetal Birthweight (g) | 2383 ± 612 | 3137 ± 342 | <0.001 |
| Child Sex, female (%) | 10 (41.7) | 22 (52.4) | 0.461 |
| Delivery, n (%) | |||
| C-Section delivery | 10 (41.7) | 8 (18.2) | 0.066 |
| Spontaneous labor | 10 (41.7) | 25 (59.5) | 0.220 |
| Medically indicated (preterm) | 12 (50) | - | - |
| Induction (term) | - | 14 (33.3) | - |
| Heart rate abnormality 4 | 5 (20.8) | 9 (21.4) | - |
| Premature placental separation | 1 (4.2) | 0 (0) | - |
| Breech position | 3 (12.5) | 1 (2.4) | - |
| Uterine prolapse | 1 (4.2) | 0 (0) | - |
| Premature membrane rupture | 1 (4.2) | 0 (0) | - |
| Restricted fetal growth | 1 (4.2) | 0 (0) | - |
| Uterine inertia | 0 (0) | 2 (4.8) | - |
| Incomplete fetal head rotation | 0 (0) | 2 (4.8) | - |
| Acylcarnitine | AUC | p-Value | 95% CI |
|---|---|---|---|
| Decadienoylcarnitine (C10:2) | 0.74 | 0.0023 | 0.61 to 0.86 |
| Butenylcarnitine (C4:1) | 0.73 | 0.0018 | 0.60 to 0.86 |
| Propenoylcarnitine (C3:1) | 0.70 | 0.0080 | 0.56 to 0.83 |
| Dodec-enedioylcarnitine (C12:1-DC) | 0.70 | 0.0083 | 0.56 to 0.84 |
| Hydroxydecanoylcarnitine (C10:0-OH) | 0.70 | 0.0066 | 0.57 to 0.84 |
| Methylhexanoylcarnitine (C6:0 M) | 0.70 | 0.0109 | 0.56 to 0.84 |
| Hept enoylcarnitine (C7:1) | 0.69 | 0.0101 | 0.55 to 0.83 |
| Methyloctanoylcarnitine (C8:0 M) | 0.69 | 0.0113 | 0.55 to 0.83 |
| Octenoylcarnitine (C8:1) | 0.67 | 0.0204 | 0.53 to 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.-C.; Laketic, K.; Hornaday, K.K.; Slater, D.M.; Mu, C.; Tough, S.C.; Shearer, J. Maternal Acylcarnitine Disruption as a Potential Predictor of Preterm Birth in Primigravida: A Preliminary Investigation. Nutrients 2024, 16, 595. https://doi.org/10.3390/nu16050595
Han Y-C, Laketic K, Hornaday KK, Slater DM, Mu C, Tough SC, Shearer J. Maternal Acylcarnitine Disruption as a Potential Predictor of Preterm Birth in Primigravida: A Preliminary Investigation. Nutrients. 2024; 16(5):595. https://doi.org/10.3390/nu16050595
Chicago/Turabian StyleHan, Ying-Chieh, Katarina Laketic, Kylie K. Hornaday, Donna M. Slater, Chunlong Mu, Suzanne C. Tough, and Jane Shearer. 2024. "Maternal Acylcarnitine Disruption as a Potential Predictor of Preterm Birth in Primigravida: A Preliminary Investigation" Nutrients 16, no. 5: 595. https://doi.org/10.3390/nu16050595
APA StyleHan, Y.-C., Laketic, K., Hornaday, K. K., Slater, D. M., Mu, C., Tough, S. C., & Shearer, J. (2024). Maternal Acylcarnitine Disruption as a Potential Predictor of Preterm Birth in Primigravida: A Preliminary Investigation. Nutrients, 16(5), 595. https://doi.org/10.3390/nu16050595










