Protective Role of Lycopene in Subjects with Liver Disease: NUTRIHEP Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Assessments
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef]
- Tan, H.H.; Chang, J.P.E. Non-alcoholic Fatty Liver Disease. Proc. Singap. Healthc. 2010, 19, 1. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; McGreal, N.; Deutsch, R.; Finegold, M.J.; Lavine, J.E. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics 2005, 115, e561–e565. [Google Scholar] [CrossRef] [PubMed]
- Maurice, J.; Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. 2018, 18, 245–250. [Google Scholar] [CrossRef]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; De Minicis, S.; Yki-Järvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Tessari, P.; Coracina, A.; Cosma, A.; Tiengo, A. Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Thorup, A.C.; Kristensen, H.L.; Kidmose, U.; Lambert, M.N.T.; Christensen, L.P.; Fretté, X.; Clausen, M.R.; Hansen, S.M.; Jeppesen, P.B. Strong and Bitter Vegetables from Traditional Cultivars and Cropping Methods Improve the Health Status of Type 2 Diabetics: A Randomized Control Trial. Nutrients 2021, 13, 1813. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Zhang, D.; Lv, Y.; Wei, Y.; Wu, W.; Zhou, F.; Tang, M.; Mao, T.; Li, M.; et al. Inhibitory effect of blueberry polyphenolic compounds on oleic acid-induced hepatic steatosis in vitro. J. Agric. Food Chem. 2011, 59, 12254–12263. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Harrison, S.A.; Torgerson, S.; Hayashi, P.; Ward, J.; Schenker, S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2003, 98, 2485–2490. [Google Scholar] [CrossRef]
- Kugelmas, M.; Hill, D.B.; Vivian, B.; Marsano, L.; McClain, C.J. Cytokines and NASH: A pilot study of the effects of lifestyle modification and vitamin E. Hepatology 2003, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Thong-Ngam, D.; Samuhasaneeto, S.; Kulaputana, O.; Klaikeaw, N. N-acetylcysteine attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. World J. Gastroenterol. 2007, 13, 5127–5132. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, S.; Tokarczk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2002, 23, 1957. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sesso, H.D. Whole food versus supplement: Comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv. Nutr. 2014, 5, 457–485. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Maeda, H.; Fukaya, T.; Goto, M. Effects of Z-Isomerization on the Bioavailability and Functionality of Carotenoids: A Review. Prog. Carotenoid Res. 2018, 228, 139–159. [Google Scholar]
- Saini, R.K.; Bekhit, A.E.D.A.; Roohinejad, S.; Rengasamy, K.R.R.; Keum, Y.S. Chemical Stability of Lycopene in Processed Products: A Review of the Effects of Processing Methods and Modern Preservation Strategies. J. Agric. Food Chem. 2020, 68, 712–726. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Camargo, B.A.F.; de Thayanne, J.; de Araújo, J.T.C.; Chrorilli, M. Lycopene: From tomato to its nutraceutical use and its association with nanotechnology. Trends Food Sci. Technol. 2021, 118, 447–458. [Google Scholar] [CrossRef]
- Shi, J.; Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Biotechnol. 2000, 20, 293–334. [Google Scholar] [CrossRef]
- Colle, I.; Lemmens, L.; Van Buggenhout, S.; Loey, A.V.; Hendrick, M. Effect of thermal processing on the degradation, isomerization, and bioaccessibility of lycopene in tomato pulp. J. Food Sci. 2010, 75, C753–C759. [Google Scholar] [CrossRef]
- USDA National Nutrient Database for Standard Reference Legacy Release. FoodData Central. Available online: https://agdatacommons.nal.usda.gov/articles/dataset/USDA_National_Nutrient_Database_for_Standard_Reference_Legacy_Release/24661818 (accessed on 12 April 2019).
- Petyaev, I.M. Lycopene Deficiency in Ageing and Cardiovascular Disease. Oxidative Med. Cell. Longev. 2016, 2016, 321805. [Google Scholar] [CrossRef]
- Maiani, G.; Castó, M.J.P.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef]
- Puah, B.P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef]
- Arballo, J.; Amengual, J.; Erdman, J.J. Lycopene: A Critical Review of Digestion, Absorption, Metabolism, and Excretion. Antioxidans 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Z.; Shao, J.; Lin, L.; Jiang, M.; Wang, L.; Lu, X.; Zhang, H.; Chen, Y.; Zhang, R. Immune and Inflammation in Acute Coronary Syndrome: Molecular Mechanisms and Therapeutic Implications. J. Immunol. Res. 2020, 2020, 4904217. [Google Scholar] [CrossRef]
- Pereira, B.L.; Reis, P.P.; Severino, F.E.; Felix, T.F.; Braz, M.G.; Nogueira, F.R.; Silva, R.A.; Cardoso, A.C.; Lourenço, M.A.M.; Figueiredo, A.M.; et al. Tomato (Lycopersicon esculentum) or lycopene supplementation attenuates ventricular remodeling after myocardial infarction through different mechanistic pathways. J. Nutr. Biochem. 2017, 46, 117–124. [Google Scholar] [CrossRef]
- Cozzolongo, R.; Osella, A.R.; Elba, S.; Petruzzi, J.; Buongiorno, G.; Giannuzzi, V.; Leone, G.; Bonfiglio, C.; Lanzilotta, E.; Manghisi, O.G.; et al. Epidemiology of HCV infection in the general population: A survey in a southern Italian town. Am. J. Gastroenterol. 2009, 104, 2740–2746. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koening, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Sluijs, I.; Beulens, J.W.J.; Grobbee, D.E.; van der Schouw, Y.T. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J. Nutr. 2009, 139, 987–992. [Google Scholar] [CrossRef]
- Fenni, S.; Hammou, H.; Astier, J.; Bonnet, L.; Karkeni, E.; Couturier, C.; Tourniaire, F.; Landrier, J.F. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol. Nutr. Food Res. 2017, 61, 1601083. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Liu, C.; Fu, M.; Hu, K.Q.; Aizawa, K.; Takahashi, S.; Hiroyuki, S.; Cheng, J.; von Linting, J.; Wang, X.D. Tomato Powder Inhibits Hepatic Steatosis and Inflammation Potentially Through Restoring SIRT1 Activity and Adiponectin Function Independent of Carotenoid Cleavage Enzymes in Mice. Mol. Nutr. Food Res. 2018, 62, e1700738. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Khare, P.; Zhu, J.; Kondepudi, K.K.; Singh, J.; Baboota, R.K.; Boparai, R.K.; Khardori, R.; Chopra, K.; Bishnoi, M. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. Int. J. Obes. 2016, 40, 487–496. [Google Scholar] [CrossRef]
- Sesso, H.D.; Buring, J.E.; Norkus, E.P.; Gaziano, J.M. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am. J. Clin. Nutr. 2004, 79, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A. Effects of lycopene and tomato products on cholesterol metabolism and atherosclerosis. Pure Appl. Chem. 2002, 74, 1431–1439. [Google Scholar]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 15, 2005–2015. [Google Scholar]
- Abebavoli, L.; Procopio, A.C.; Paravati, M.R.; Costa, G.; Milić, N.; Alcaro, S.; Luzza, F. Mediterranean Diet: The Beneficial Effects of Lycopene in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2022, 11, 3477. [Google Scholar] [CrossRef]
- Stice, C.P.; Liu, C.; Aizawa, K.; Greenberg, A.S.; Ausman, L.M.; Wang, X.D. Dietary tomato powder inhibits alcohol-induced hepatic injury by suppressing cytochrome p450 2E1 induction in rodent models. Arch. Biochem. Biophys. 2015, 572, 81–88. [Google Scholar] [CrossRef] [PubMed]
| Parameters * | Cohorts | |||||
|---|---|---|---|---|---|---|
| NUTRIHEP 1 (2005–2006) (n = 969) | NUTRIHEP 2 (2014–2016) (n = 969) | |||||
| Absent (n = 748) | NAFLD (n = 114) | AFLD and FLD (n = 107) | Absent (n = 470) | NAFLD (n = 368) | AFLD and FLD (n = 131) | |
| Age (years) | 41.59 ± 13.88 | 46.67 ± 11.81 | 54.08 ± 10.23 | 48.11 ± 13.14 | 58.41 ± 13.39 | 61.51 ± 12.41 |
| Age Categories (%) | ||||||
| <50 | 424 (56.70) | 45 (39.50) | 13 (12.10) | 283 (60.20) | 96 (26.10) | 25 (19.10) |
| ≥50 | 324 (43.30) | 69 (60.50) | 94 (87.90) | 187 (39.80) | 272 (73.90) | 106 (80.90) |
| Gender (M) (%) | 324 (43.30) | 69 (60.50) | 94 (87.90) | 203 (43.20) | 179 (48.60) | 105 (80.20) |
| Smoker (%) | ||||||
| Never/Former | 636 (85.10) | 92 (81.40) | 87 (81.30) | 418 (88.90) | 322 (87.70) | 110 (84.60) |
| Current | 111 (14.90) | 21 (18.60) | 20 (18.70) | 52 (11.10) | 45 (12.30) | 20 (15.40) |
| Marital Status (%) | ||||||
| Single | 208 (28.00) | 15 (13.20) | 4 (3.70) | 91 (19.40) | 48 (13.00) | 7 (5.40) |
| Married/Coupled | 511 (68.70) | 94 (82.50) | 99 (92.50) | 358 (76.30) | 290 (78.80) | 113 (86.90) |
| Separated/Divorced | 15 (2.00) | 0 (0.00) | 1 (0.90) | 13 (2.80) | 7 (1.90) | 4 (3.10) |
| Widower | 10 (1.30) | 5 (4.40) | 3 (2.80) | 7 (1.50) | 23 (6.20) | 6 (4.60) |
| Hypertension (Yes) (%) | 72 (11.10) | 24 (26.40) | 29 (29.90) | 74 (16.90) | 155 (44.50) | 55 (44.40) |
| Diabetes (Yes) (%) | 9 (1.20) | 4 (3.50) | 10 (9.30) | 9 (2.10) | 34 (9.80) | 13 (10.50) |
| Weight (kg) | 66.07 ± 12.19 | 79.94 ± 17.14 | 83.93 ± 12.53 | 66.35 ± 12.06 | 78.63 ± 14.40 | 81.72 ± 15.50 |
| BMI (kg/m2) | 24.43 ± 3.80 | 29.02 ± 5.64 | 29.66 ± 3.58 | 24.88 ± 3.62 | 30.16 ± 4.81 | 29.72 ± 4.99 |
| Blood Parameters | ||||||
| Glucose (mmol/L) | 5.37 ± 0.87 | 5.84 ± 0.99 | 6.13 ± 1.17 | 4.98 ± 0.54 | 5.46 ± 1.11 | 5.79 ± 1.09 |
| Total Cholesterol (mmol/L) | 4.88 ± 0.97 | 5.19 ± 1.02 | 5.21 ± 0.99 | 4.85 ± 0.87 | 5.08 ± 0.95 | 4.84 ± 0.93 |
| Triglycerides (mmol/L) | 1.04 ± 0.69 | 1.64 ± 0.96 | 1.68 ± 1.13 | 0.89 ± 0.69 | 1.32 ± 0.85 | 1.41 ± 0.94 |
| HDL (mmol/L) | 1.40 ± 0.34 | 1.20 ± 0.30 | 1.17 ± 0.23 | 1.39 ± 0.33 | 1.27 ± 0.29 | 1.16 ± 0.28 |
| WBC (K/mcL) | 6.13 ± 1.57 | 6.81 ± 1.64 | 6.56 ± 1.63 | 5.63 ± 2.91 | 6.05 ± 1.62 | 6.36 ± 1.67 |
| GGT (µkat/L) | 0.19 ± 0.15 | 0.26 ± 0.12 | 0.27 ± 0.10 | 0.25 ± 0.14 | 0.32 ± 0.25 | 0.38 ± 0.26 |
| AST (µkat/L) | 0.18 ± 0.10 | 0.21 ± 0.08 | 0.21 ± 0.07 | 0.35 ± 0.12 | 0.38 ± 0.30 | 0.40 ± 0.10 |
| ALT (µkat/L) | 0.23 ± 0.21 | 0.35 ± 0.17 | 0.33 ± 0.16 | 0.33 ± 0.15 | 0.42 ± 0.44 | 0.43 ± 0.17 |
| Blood Lipids (Yes) (%) | 30 (4.60) | 4 (4.40) | 10 (10.30) | 54 (12.30) | 61 (17.60) | 25 (20.20) |
| rMed ^ (Yes) (%) | 8 (6–11) | 8 (5–11) | 8 (6–11) | 9 (7–11) | 9 (7–11) | 8 (6–10) |
| Kcal (die) | 2106.53 ± 770.46 | 2064.69 ± 838.32 | 2157.11 ± 761.54 | 2075.02 ± 707.08 | 1993.26 ± 775.25 | 2199.42 ± 775.94 |
| Cooked Tomato (gr/die) | 21.71 ± 24.84 | 22.10 ± 21.32 | 26.89 ± 24.09 | 11.98 ± 14.04 | 11.28 ± 14.03 | 16.14 ± 18.53 |
| Fresh Tomato (gr/die) | 45.36 ± 43.34 | 47.30 ± 66.78 | 51.23 ± 47.74 | 46.06 ± 46.66 | 49.27 ± 46.79 | 42.96 ± 34.48 |
| LYC Intake (mg/die) | 6.32 ± 5.43 | 6.78 ± 5.29 | 6.79 ± 5.31 | 4.82 ± 3.76 | 4.80 ± 3.74 | 5.48 ± 4.52 |
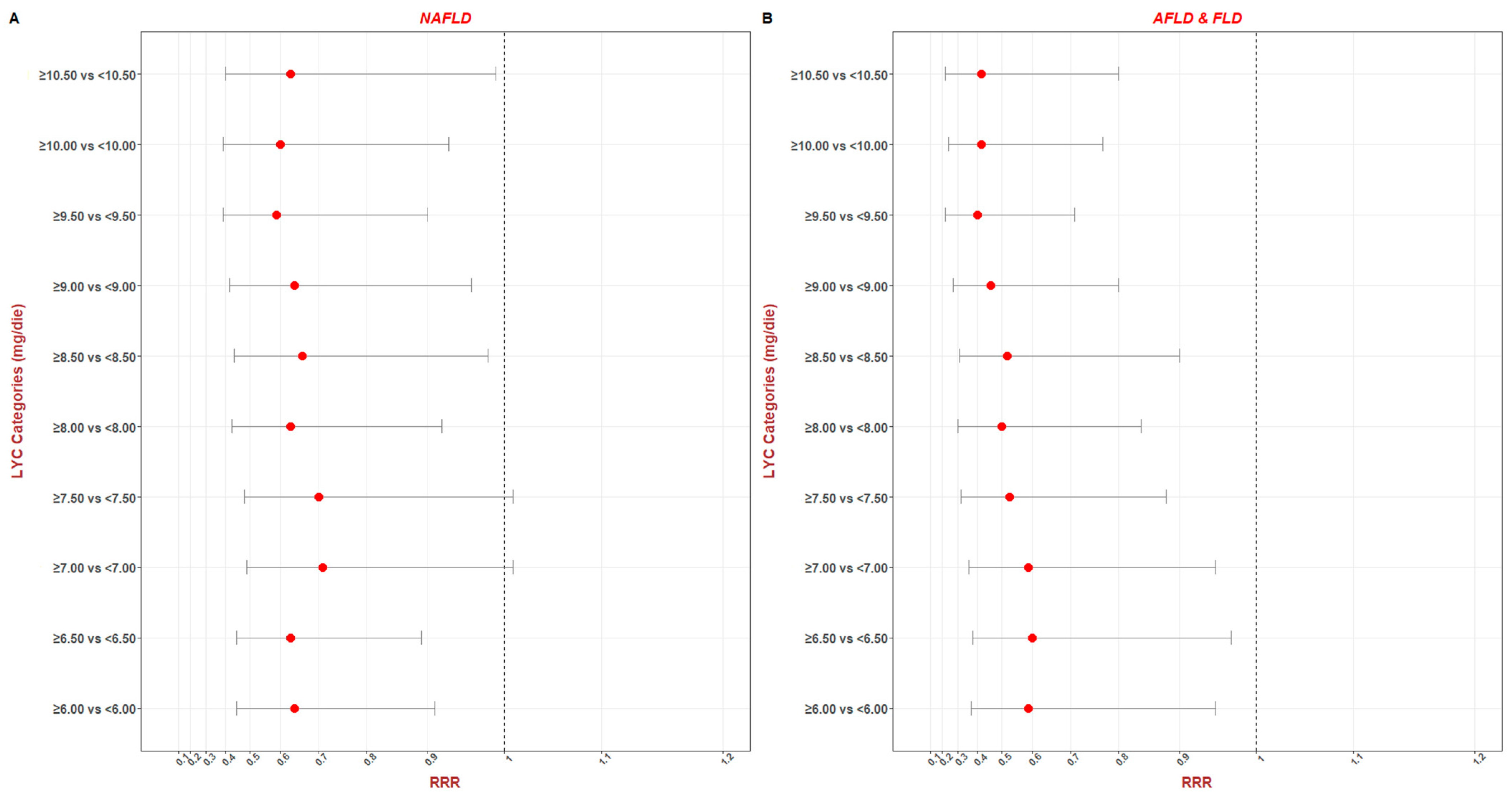
| Parameters | NAFLD ¥ | AFLD and FLD ¥ | ||||||
|---|---|---|---|---|---|---|---|---|
| RRR | se (RRR) | p | 95% C.I. | RRR | se (RRR) | p | 95% C.I. | |
| LYC (Continuous) | 0.96 | 0.02 | 0.008 | 0.93 to 0.99 | 0.94 | 0.02 | 0.006 | 0.90 to 0.98 |
| LYC (Categorical) | ||||||||
| ≥6.00 vs. <6.00 | 0.64 | 0.11 | 0.12 | 0.45 to 0.91 | 0.59 | 0.14 | 0.03 | 0.37 to 0.95 |
| ≥6.50 vs. <6.50 | 0.63 | 0.11 | 0.01 | 0.45 to 0.89 | 0.60 | 0.14 | 0.04 | 0.38 to 0.97 |
| ≥7.00 vs. <7.00 | 0.71 | 0.13 | 0.06 | 0.49 to 1.01 | 0.59 | 0.14 | 0.03 | 0.36 to 0.95 |
| ≥7.50 vs. <7.50 | 0.70 | 0.13 | 0.06 | 0.48 to 1.01 | 0.53 | 0.14 | 0.01 | 0.32 to 0.88 |
| ≥8.00 vs. <8.00 | 0.63 | 0.12 | 0.02 | 0.43 to 0.92 | 0.50 | 0.13 | 0.009 | 0.30 to 0.84 |
| ≥8.50 vs. <8.50 | 0.66 | 0.13 | 0.04 | 0.44 to 0.98 | 0.52 | 0.14 | 0.02 | 0.31 to 0.90 |
| ≥9.00 vs. <9.00 | 0.64 | 0.13 | 0.03 | 0.42 to 0.96 | 0.46 | 0.13 | 0.006 | 0.27 to 0.80 |
| ≥9.50 vs. <9.50 | 0.59 | 0.13 | 0.01 | 0.39 to 0.90 | 0.40 | 0.12 | 0.002 | 0.22 to 0.71 |
| ≥10.00 vs. <10.00 | 0.60 | 0.13 | 0.02 | 0.39 to 0.93 | 0.42 | 0.13 | 0.004 | 0.24 to 0.77 |
| ≥10.50 vs. <10.50 | 0.63 | 0.15 | 0.05 | 0.40 to 0.99 | 0.42 | 0.14 | 0.008 | 0.22 to 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donghia, R.; Campanella, A.; Bonfiglio, C.; Cuccaro, F.; Tatoli, R.; Giannelli, G. Protective Role of Lycopene in Subjects with Liver Disease: NUTRIHEP Study. Nutrients 2024, 16, 562. https://doi.org/10.3390/nu16040562
Donghia R, Campanella A, Bonfiglio C, Cuccaro F, Tatoli R, Giannelli G. Protective Role of Lycopene in Subjects with Liver Disease: NUTRIHEP Study. Nutrients. 2024; 16(4):562. https://doi.org/10.3390/nu16040562
Chicago/Turabian StyleDonghia, Rossella, Angelo Campanella, Caterina Bonfiglio, Francesco Cuccaro, Rossella Tatoli, and Gianluigi Giannelli. 2024. "Protective Role of Lycopene in Subjects with Liver Disease: NUTRIHEP Study" Nutrients 16, no. 4: 562. https://doi.org/10.3390/nu16040562
APA StyleDonghia, R., Campanella, A., Bonfiglio, C., Cuccaro, F., Tatoli, R., & Giannelli, G. (2024). Protective Role of Lycopene in Subjects with Liver Disease: NUTRIHEP Study. Nutrients, 16(4), 562. https://doi.org/10.3390/nu16040562






