Abstract
Several billion microorganisms reside in the gastrointestinal lumen, including viruses, bacteria, fungi, and yeast. Among them, probiotics were primarily used to cure digestive disorders such as intestinal infections and diarrhea; however, with a paradigm shift towards alleviating health through food, their importance is large. Moreover, recent studies have changed the perspective that probiotics prevent numerous ailments in the major organs. Probiotics primarily produce biologically active compounds targeting discommodious pathogens. This review demonstrates the implications of using probiotics from different genres to prevent and alleviate ailments in the primary human organs. The findings reveal that probiotics immediately activate anti-inflammatory mechanisms by producing anti-inflammatory cytokines such as interleukin (IL)-4, IL-10, IL-11, and IL-13, and hindering pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α by involving regulatory T cells (Tregs) and T helper cells (Th cells). Several strains of Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus reuteri, Bifidobacterium longum, and Bifidobacterium breve have been listed among the probiotics that are excellent in alleviating various simple to complex ailments. Therefore, the importance of probiotics necessitates robust research to unveil the implications of probiotics, including the potency of strains, the optimal dosages, the combination of probiotics, their habitat in the host, the host response, and other pertinent factors.
1. Introduction
The human body has an estimated count of 100 trillion microorganisms residing in the gastrointestinal lumen, more than the somatic cell count. The lumen anatomical region is the primary home for many microbial species, encompassing viruses, bacteria, fungi, and yeast [1]. The microflora residing in the gut is a significant repository of commensally existing bacteria that coexist harmoniously and perform beneficial metabolic and biological tasks for the host. Among several bacterial species, anaerobes, containing over three million different genes, as well as Firmicutes and Bacteroidetes (Gram-positive and Gram-negative, respectively) dominate the biosynthesis of short-chain fatty acids (SCFAs), i.e., butyrate, acetate, and propionate (Figure 1). Proteobacteria, Fusobacteria, Actinobacteria, and Verrucomicrobia are the other phyla that are listed as producers of SCFAs [2,3,4,5,6].
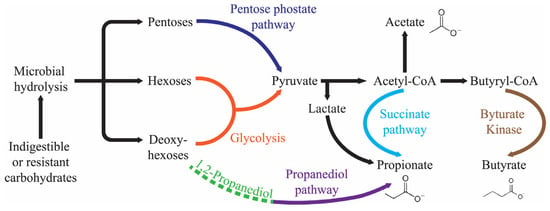
Figure 1.
Production pathway of the SCFAs in the human gut.
Probiotics initially treated gastrointestinal issues. They may prevent intestinal infections, aid constipation and diarrhea, improve lactose tolerance, and more [7]. The WHO and FAO define probiotics as affecting more than the intestines. Probiotics can prevent allergies, cancer, diabetes, and obesity and safeguard urogenital health (Figure 2) [8,9,10].
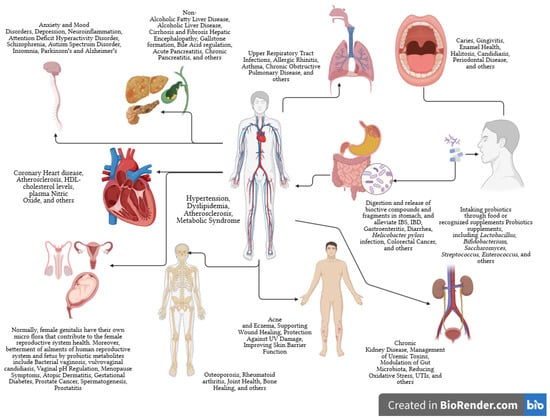
Figure 2.
The significant health effects probiotics have on the major organs of human body. The arrows represent the flow of beneficial compounds from probiotics.
So far, most of the probiotics studied by researchers are gut bacteria. Probiotics’ capacity to affect the immune system and ferment in the GI tract has opened up several medicinal uses. Probiotics inhibit pathogenic germs in several ways, such as by producing bacteriocins and bioactive peptides, competing for resources, changing pH, and creating an unfavorable environment for infections. Probiotics sticking to epithelial cells prevent pathogens from interacting with surface chemicals. They or their metabolites can also interact with several epithelial cell receptors. This connection activates pro- and anti-inflammatory signaling pathways, achieving homeostasis [11,12].
The gut microbiota and its metabolites affect the heart, brain, gut, vasculature, liver, kidneys, and host immunity; however, the research subjects in this discipline require further study. The gut microbiota is complex and inter-linked. Intestinal dysbiosis in various ailments reduces metabolic activity; hence, a “one-size-fits-all” approach to treatment is ineffective. Probiotic supplementation using bacterial strains that control metabolite synthesis may help manage several ailments. Characterizing interactions between various bacterial strains is essential for finding the best probiotic bacteria and metabolites for medicinal use [13].
Prebiotics are dietary fibers metabolized by the intestinal microbiota, resulting in the modulation of the microbiota and production of SCFAs. Metabolites are produced through prebiotic fermentation, exhibiting anti-inflammatory and immunomodulatory properties, indicating their potential for therapeutic applications in various pathological conditions. Galactooligosaccharide and short- and long-chain fructans such as fructooligosaccharides and inulin have been extensively used as prebiotics, although several other dietary compounds exhibiting similar characteristics are present [14].
Synbiotics refer to combinations of probiotics and prebiotics, which can synergistically work together. Synbiotics introduced into the gastrointestinal tract promote the growth and activate the metabolism of a natural intestinal microbiota, thereby positively impacting the host’s health. Synbiotics are products where a prebiotic component specifically benefits probiotic microorganisms to enhance their survival functioning in the GI. Hence, an appropriate amalgamation of both elements in a singular product can improve the outcome compared to the efficacy of the prebiotic or probiotic individually [15].
The probiotic strain, the targeted disease or condition, and the individual all need to be considered when analyzing a probiotic supplement’s efficiency and credible findings from carefully conducted human clinical studies. The palliating effects of probiotics on different organs are overviewed in Figure 2. The transnational agreement is that probiotic species help the host in many ways, such as contesting pathogenic microorganisms for adhesion sites and nutrition, improving the epithelial lining’s barrier function, modifying the immune system, and impacting other organs through neurotransmitter synthesis, and immune system modulation [16]. Probiotics increase the production of butyrate, a vital compound for eubiosis and human health. Beneficial microorganisms modulate an intestinal environment, optimizing nutrition absorption [17].
Previously, the alleviating effects of probiotics were elaborated on different organs separately, with efforts to demonstrate probiotics’ curative exponents on various organs simultaneously being rare. Therefore, this review seeks to establish the unquestionable advantages of probiotic therapies and their effects on critical bodily systems and organs and pursue the best-performing probiotics species involved in alleviating several ailments. Moreover, this review also aims to unveil the verity of the involvement of probiotics in soothing several organs simultaneously.
2. Probiotics Palliate Ailments in the Oral Cavity
Extensive studies have recently examined the deployment of probiotics to treat oral diseases and preserve oral health. The genera Lactobacillus, Streptococcus, Weissella, Bifidobacterium, as well as a few dispersed species like Saccharomyces cerevisiae and Bacillus subtilis, have been shown to have high concentrations of probiotics that benefit oral health. Many strains of the oral cavity-isolated microbes, Lactobacillus reuteri, Streptococcus salivarius, Lactobacillus brevis, and others, have been commercially created as probiotics that promote oral health [18,19]. The species regarded as probiotics have been shown to improve the symptoms of common oral disorders, including halitosis, dental caries, oral candida infection, and periodontal disease [20,21,22].
2.1. Periodontal Disorders and The Use of Probiotics to Alleviate Them
The most common periodontal disorders, gingivitis and periodontitis, are chronic inflammatory diseases that erode the gum- and teeth-supporting bone. Gingivitis, a moderate periodontal condition, can lead to periodontitis, which can lead to the loosening or loss of teeth. As of 2019, 1.1 billion people worldwide have severe periodontitis, the prevalence of which has increased by 8.44% since the early 1990s [23].
Probiotic usage limits bacteria development better than non-probiotic toothpaste, avoiding periodontal (Figure 3) and dental cavities. Toothpaste lingers on the tooth’s surface for longer, and the gingival sulcus can be easily reached using a toothbrush.
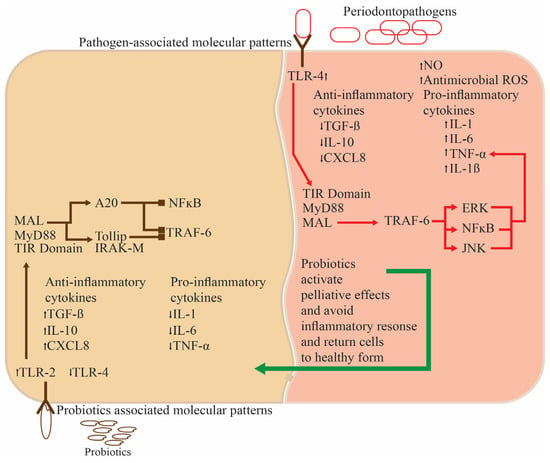
Figure 3.
The functioning of probiotics in the oral cavity for normal functioning and anti-inflammatory activity.
Many studies have examined probiotics’ immediate and long-term effects in periodontal non-surgical treatment. Ingesting yogurt containing Bifidobacterium animalis subsp. lactis DN-173010 and leaving the teeth unbrushed for five days can have soothing effects. Several parameters, including gingival crevicular fluid (GCF), the gingival index (GI), bleeding on probing (BOP), the plaque index (PI), periodontal pocket depth (PPD) volume, and GCF interleukin-1 (IL-1) concentration and quantity, showed significant betterment in the patients ingesting probiotics than the placebo, showing that probiotics improve gingival inflammatory parameters and plaque accumulation even after short-term use even during missing oral hygiene measures (Table 1) [24]. Intaking probiotics other than toothpaste is also beneficial for oral health. Chronic periodontitis patients receiving Lactobacillus reuteri lozenge had significantly better PI, GI, BOP, and PPD than those who received the placebo. Metalloproteinases-1 (TIMP-1) and matrix metalloproteinase-8 (MMP-8) levels in GCF also differed significantly [20].
2.2. Streptococcus and Lactobacillus spp. as Therapeutic Agents against Dental Caries
Dental caries is a bacterially caused, multifactorial illness that causes acid demineralization of tooth enamel [25]. According to the US Surgeon General, dental caries is a chronic illness that often affects children. Three main factors affect dental caries: carbohydrate consumption, especially sugar, Streptococcus mutans infection, and immune system responses [26,27].
Streptococcus thermophilus and Lactobacillus lactis spp. lactis, which are among the 23 major dairy sector strains, can form a biofilm on hydroxyapatite and inhibit the growth of cariogenic Streptococcus sobrinus. Weissella cibaria isolates can decrease S. mutans’ biofilm production in vitro and in vivo and hinder its proliferation [28,29]. Lactobacillus casei and one strain of Lactobacillus rhamnosus inhibit S. mutans and Streptococcus sobrinus growth in vitro. Moreover, Streptococcus thermophiles, L. reuteri, and Lactobacillus bulgaricus in yogurt hinder the prevalence of streptococci, especially S. mutans (Table 1). Hence, the regular use of probiotic-rich dairy products, including yogurt, milk, and cheese, reduces dental plaque and salivary cariogenic streptococci [30,31,32].
A statistically significant decrease in S. mutans was detected in a test group that received L. reuteri, and the impact persisted for at least 21 days. The effectiveness of mixed cultures against oral bacteria appears to be limited. The efficacy of the probiotic strain L. reuteri found in Indian curd in reducing the presence of salivary S. mutans is evident. Furthermore, this effect has been observed to persist for a certain duration following administration [33].
2.3. Bifidobacterium and Lactobacillus spp. Curing Gingivitis
Patients with mild to severe gingivitis receiving L. reuteri formulations at moderate doses exhibit a significantly decreased GI. L. reuteri also significantly reduces plaque and gingivitis in patients (Table 1) [34].
Several studies have observed the clinical effectiveness of several probiotics, such as Bifidobacterium animalis, Bacillus species, and L. reuteri, in treating gingivitis [35,36,37]. Several trials have shown that probiotics reduce gingival indices, bleeding, and plaque. A recent RCT found that a once-daily dose of B. animalis-enriched yogurt reduced plaque and bleeding scores compared to the people taking plain yogurt [24].
2.4. Halitosis and Its Establishment and Rectification by Probiotics
Volatile molecules arising from pathological or non-pathological oral or non-oral sources combine to generate halitosis. These volatile substances include short-chain fatty acids, amines, phenyl compounds or alcohols, ketones, aliphatic compounds, sulfur compounds, nitrogen-containing chemicals, and aromatic compounds [38,39]. Anaerobic bacteria cause halitosis by breaking down salivary and dietary proteins to produce amino acids, which are converted into volatile compounds such as methanethiol and hydrogen sulfide [40]. Different strains of W. cibaria can prevent Fusobacterium nucleatum from producing volatile sulfur compounds by synthesizing hydrogen peroxide and preventing F. nucleatum from proliferating (Table 1). Additionally, gargling with a W. cibaria-enriched solution causes a decrease in the generation of methanethiol and hydrogen sulfide, reducing foul breath [29].
2.4.1. Streptococcus salivarius: An Efficacious Element against Halitosis
Streptococcus salivarius is the most common commensal probiotic in the mouths of people without halitosis. S. salivarius produces bacteriocins which reduce the population of volatile sulfur compound-producing bacteria [41]. The administration of gum or lozenges containing S. salivarius K12 resulted in a decrease in volatile sulfur compounds in individuals with halitosis (Table 1) [42,43]. By establishing a healthy tongue microbial ecology, probiotics that protect periodontal health may reduce halitosis. In oral health, the dorsal posterior surface of the tongue near the circumvallate papillae is home to a large population of Gram-negative bacteria that cause bad breath. However, assessing and maintaining oral hygiene in these places is tricky [44]. The tongue is sometimes considered more prominent than the periodontal recesses in terms of the species that inhabit it. Specific adaptation to each recess is important, and probiotic strains meant to colonize periodontal recesses may not colonize the tongue and improve dental health [45,46].
2.4.2. Streptococcus salivarius’s Enmity against Streptococcus pyogenes—An Oral Pathogen
Streptococcus pyogenes, a major bacterial pathogen, predominantly affects humans and causes moderate localized infections to severe invasive infections with potentially deadly results. Acute rheumatic fever and poststreptococcal glomerulonephritis can result from ineffective S. pyogenes treatment. This pathogen also causes invasive infections, including necrotizing fasciitis and toxic shock syndrome, which cause high morbidity and mortality [47].
S. pyogenes causes non-bullous impetigo, a common childhood skin disease. The pruritic erythematous rash usually starts in the perioral or perinasal area and progresses to vesicular lesions. Blisters often burst and form a honey-colored crust. The face and lower extremities are particularly affected by highly localized lesions. Impetigo rarely causes systemic symptoms [47]. Two salivaricin-producing Streptococcus salivarius strains, 20P3 and 5, given to children via milk supplementation, were shown to exhibit higher colonization levels. After drinking milk enriched with S. salivarius, the children experienced a significant increase in SalA-like inhibitory activity in their indigenous streptococcal tongue populations. S. salivarius (SalA producer) made up fewer than 5% of the tongue bacteria. After drinking S. salivarius-supplemented milk, the children’s tongues contained more SalA synthesizers and had increased inhibitory activity. S. salivarius probiotics, which produce SalA, may persistently boost SalA-dependent protection against S. pyogenes infections. These outcomes signify that SalA strongly diminishes S. pyogenes, making this noteworthy [48].
2.5. Lactobacillus spp. as pH and Saliva Regulators in the Oral Cavity
Probiotic-treated dairy products elevate salivary pH significantly (Table 1). This notion is consistent with clinical trials showing that probiotic-containing yogurt and curd improve salivary pH. Probiotic consumption increases pH levels because probiotic bacteria compete with other microorganisms to reduce their numbers. Therefore, salivary pH rises when acidogenic bacteria decrease and acid production decreases. Due to the close relationship with pH imbalances, these fluctuations in pH affect the control of dental caries. In curd with probiotics, salivary pH increased compared to a curd lacking them, exhibiting that added probiotics cause salivary pH to rise (Table 1) [49,50].
Probiotics increase saliva production in edentulous patients, which helps xerostomia/hyposalivation. Patients receiving regular probiotics have significantly increased saliva volume and moderately changing saliva pH [51]. Probiotic strains change saliva’s immunoglobulins and mucins, according to animal research. Another finding of that experiment was the positive influence of probiotics on hyposalivation sufferers [52].
Probiotics, a type of commensal bacteria, effectively increase oral epithelial cell beta defensin-2 (BD-2) expression [53]. Lactobacillus strains that do not adhere to HT29 cells do not increase mucin gene expression. Mucin 3 (MUC3) mucin mRNA expression has a direct relationship with extracellular secretion. In coincubation investigations, the same Lactobacillus strains that increase MUC3 mucin synthesis inhibited E. coli E2348/69 adherence. Probiotics increase MUC3 mucin transcription, translation, extracellular secretion, and epithelial cell adhesion, which improves eukaryotic mucin effects [54], therefore influencing saliva production and their types [55]. After L. reuteri treatment, epithelial parotid gland BD-2 expression and levels increase. Studies on epithelial parotid glands have shown a strong connection between elevated BD-2 expression, and reduced S. mutans. L. reuteri supplementation significantly increases salivary BD-2 levels and glandular BD-2 expression. Probiotics may modify salivary gland epithelial cells such as the parotid gland to increase saliva production [56].
The regular usage of probiotics for Candida reduction without side effects is achievable. Probiotics vigorously improve oral health and reduce hyposalivation and dry mouth [55]. Probiotics and xylitol have been shown to reduce Streptococcus species in the saliva of orally healthy people. Therefore, probiotics and xylitol may complement each other to stabilize the salivary microbiota [57].

Table 1.
Alleviative effects of probiotics on the oral cavity, gastrointestinal tract, and liver established in placebo-controlled human trials.
Table 1.
Alleviative effects of probiotics on the oral cavity, gastrointestinal tract, and liver established in placebo-controlled human trials.
| Name of the Probiotic Strains | Age of the Participants | Dose of the Probiotics | Duration of Study | Outcomes of the Study | References |
|---|---|---|---|---|---|
| Probiotics Palliate Ailments in the Oral Cavity | |||||
| Lactobacillus. reuteri ATCC55730 and ATCC PTA5289 | ~38 years | 1 × 108 CFU/g each | 21 days | Gingival crevicular fluid (GCF) volume increased slightly, with a significantly increase in IL1-β and IL-18 and a significant decrease in IL-8 and MIP1-β also being found. | [58] |
| Lactobacillus reuteri PTA5289 | 31–46 years | 1 × 108 CFU/g | 14 days | A significant decrease in IL-17, TNF-α, and IL-1β, along with improved clinical indices, including clinical attachment level (CAL), periodontal probing depth (PPD), and sulcus bleeding index (SBI). | [59] |
| Lactobacillus curvatus EB10 DSM32307, Lactobacillus rhamnosus PB01 DSM14869 | 18–50 years | 1 × 108 CFU/g each | 28 days | Reduced bleeding on probing (BOP), amount of GCF, and decreased plaque levels. | [60] |
| Bifidobacterium animalis subsp. lactis DN 173010 | 16–26 years | 1 × 108 CFU/g | 28 days | Lowered gingivitis and plaque scores, lessened GCF volume and BOP, and lowered IL-1β concentration. | [24] |
| Bifidobacterium lactis BB-12, Lactobacillus rhamnosus GG | 13–15 years | 4.4 × 108 and 4.8 × 108 CFU/g | 28 days | Reduction in the gingival index (GI), plaque and Porphyromonas gingivalis in plaque, as well as a reduction in Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum in saliva. | [61] |
| Lactobacillus acidophilus, Enterococcus faecium Bifidobacterium infantis | 35–55 years | 1 × 107, 1 × 106, and 1 × 107 CFU/capsule | 30 days | A significant decrease in BOP after seven days and a reduction in the plaque index (PI), BOP, and periodontal pocket depth (PPD) after 30 days. | [62] |
| Bifidobacterium bifidum, Lactobacillus Acidophilus-HS101, Lactobacillus rhamnosus GG-HS111 | ≥60 years | 3.3 × 107 CFU/g | 60 days | Increased saliva in completely edentulous patients, which can be helpful in hyposalivation/xerostomia patients. | [51] |
| Lactobacillus rhamnosus GG, Bifidobacterium longum | 3–5 years | 7.5 × 105 and 4.5 × 105 CFU/mL of milk | 180 days | Significantly decreased Streptococcus mutans and pH, as well as the remineralization of 39.4% of caries. | [63] |
| Bifidobacterium lactis Bb-12, Lactobacillus acidophilus La-5 | 6–12 years | 1 × 106 CFU/g each | 30 days | Reduced Streptococcus mutans count after a week and also after 30 days. | [64] |
| Lactococcus reuteri ATCC PTA 5289 and DSM 17938 | 3–6 years | 1 × 108 CFU/g each | 28 days | Reduction in Mutans streptococci and lactobacilli and caries-associated bacterial counts. | [65] |
| Lactococcus rhamnosus GG, Lactobacillus helveticus, Lactococcus lactis, Lactococcus rhamnosus LC705, Propionibacterium freudenreichii ssp shermanii JS | 70–100 years | 1 × 107 CFU/g each | 120 days | Effectively controlled hyposalivation and oral Candida in the elderly. | [55] |
| Streptococcus salivarius M18 | 6–17 years | 1 × 109 CFU/ml | 90 days | Increased chances of avoiding the development of new dental caries in kids and reduced risk of tooth decay receptivity. | [66] |
| Lactobacillus salivarius WB21 | 22–67 years | 2.0 × 109 CFU/g | 14 days | Significantly decreased organoleptic test scores, the average probing pocket depth, the concentration of volatile sulfur compounds (VSCs), levels of Fusobacterium nucleatum and ubiquitous bacteria, which exhibited oral malodor and malodor-related factor control. | [67] |
| Lactobacillus salivarius Lactobacillus reuteri | 25–59 years | 2 × 109 CFU/g each | 90 days | Significantly reduced clinical and microbiological parameters and significantly improved bleeding index (BI), modified gingival index (MGI), and PI, leading to a significant decline in N-benzoyl-DL-arginine-naphthylamide and halitosis. | [68] |
| Lactobacillus reuteri ATCC PTA 5289 and DSM 17938 | 19–25 years | 1 × 108 CFU/g each | 28 days | Beneficial for oral malodor and malodourous compounds (other than VSCs) producing bacteria. | [69] |
| Streptococcus salivarius K12 | 23–44 years | 1 × 109 CFU/g | 30 days | Significantly decreased immediate organoleptic test (OLT) scores, tongue coating scores, and VSC levels in the absence of tongue coating. | [70] |
| Weissella cibaria CMU | 20–39 years | 1 × 108 CFU/g | 56 days | Significant decrease in OLT and VSC scores, along with bad breath improvement scores being reduced after eight weeks. | [71] |
| Probiotics Associated with the Small and Large Intestine | |||||
| Bifidobacterium longum BB536, Lactobacillus rhamnosus HN001 | 37–59 years | 4 × 108 and 1 × 108 CFU/g with 1.4 mg vitamin B6 | 60 days | Reduced abdominal pain, bloating, and disease severity; improved sucralose recovery (colonic permeability); increased relative abundance of hydrocarbons, butanoic, propanoic, and pentanoic acids; and decreased phenol. | [72] |
| Lactobacillus acidophilus subsp. helveticus LAFTI L10, Lactobacillus acidophilus NCFM | 30–60 years | 2.5 × 109 CFU/g each | 56 days | Significantly decreased flatus and composite scores. | [73] |
| Bifidobacterium longum, Lactobacillus paracasei | ≥18 years | 1 × 1010 CFU each | 84 days | Reduced symptoms in IBS and addition to the armamentarium of IBS management tools dependent on the IBS subtype. | [73] |
| Bifidobacterium bifidum HI-MIMBb75 | ≥18 years | 1 × 109 CFU/capsule (Non-viable) | 56 days | Substantially alleviated IBS and its symptoms and mediated specific beneficial effects independent of cell viability. | [74] |
| Bifidobacterium longum, Bifidobacterium breve, Lactobacillus paracasei HII01 | ~60 years | 2.0 × 1010, 2.0 × 1010, and 1.0 × 1010 CFU/g | 84 days | Improved intestinal barrier function (up to 48%), enhanced short-chain fatty acid levels, improved obesity-related anthropometric biomarkers, and significantly increased high-density lipoprotein–cholesterol. | [75] |
| Lactobacillus acidophilus W37, Lactococcus lactis W19 and W58, Lactobacillus brevis W63, Lactobacillus salivarius W24, Lactobacillus casei W56, Bifidobacterium lactis W52, Bifidobacterium bifidum W23, | 18–80 years | 2.5 × 109 CFU/g each | 180 days | Significantly increased production of reactive oxygen species by neutrophils and serum neopterin levels, maintaining or even improving liver functioning in sturdy cirrhosis with a slight impact on bacterial translocation and gut barrier function. | [76] |
| Clostridium butyricum | ≥18 years | 1 × 106 CFU/g | 15 days | Shortened duration of fever and constipation and significantly decreased bactericides, Escherichia coli, and Enterococcus. | [77] |
| Lactobacillus paracasei W20, Lactobacillus plantarum W1 and W62, Bifidobacterium bifidum W23, Lactobacillus acidophilus W37 and W55, Lactobacillus rhamnosus W71 Lactobacillus salivarius W24, Enterococcus faecium W54, Bifidobacterium lactis W51, | 45–65 years | 1.1 × 109 CFU/g each | 28 days | Increased probiotic strains in stool and improved microbiome composition and functional diversity, successfully modulating the microbiome and ultimately intervening in sepsis. | [78] |
| Bifidobacterium spp. | 27–55 years | - | 60 days | Decreased plasma levels of hs-CRP, TNF-α, plasma DA0, ET, D-lactic acid, IL-8, and IL-6 and increased CD4/CD8 ratio and CD4+ levels, enhancing the remedying impact in ulcerative colitis patients and regulating T cell frequency, in addition to reducing plasma inflammatory factors. | [79] |
| Bifidobacterium longum Enterococcus faecium, Lactobacillus acidophilus, Lactobacillus plantarum, Bifidobacterium lactis, Streptococcus thermophilus, | ≥18 years | 3 × 109 CFU/g each | 56 days | Decreased expression of serum C-reactive protein (CRP), significantly improved endoscopic and clinical activities, and positive impacts on the acute-phase reactants and endoscopic activity levels. | [80] |
| Lactobacillus rhamnosus NCIMB 30174, Enterococcus faecium NCIMB 30176, Lactobacillus acidophilus NCIMB 30175, Lactobacillus plantarum NCIMB 30173, | 18–70 years | 1 × 1010 CFU/g each | 28 days | Significantly reduced fecal calprotectin levels in ulcerative colitis patients and decreased intestinal inflammation. | [81] |
| Lactobacillus plantarum 299v | ≥18 years | 1 × 1010 CFU/g | 84 days | Reduced enteral nutrition-related gastrointestinal symptoms. Effectively improved the quality of life of cancer patients, nutritional status, and enteral nutrition tolerance. | [82] |
| Probiotics Tone Liver and Annihilate its Ailments | |||||
| Bifidobacterium spp., Lactobacillus spp., Enterococcus spp. | 18–59 years | - | 90 days | Significantly improved aspartate aminotransferase (AST), NAFLD activity score (NAS), total cholesterol (TC), alanine aminotransferase (ALT), glutamine transferase (GGT), triglyceride (TG) levels, and insulin resistance index (HOMA-IR). Improved liver functions, hepatic fatty deposition, and glucose and lipids metabolism in NAFLD patients, enhancing the therapeutic effects. | [83] |
| Pediococcus pentosaceus CBT SL4, Lactobacillus paracasei CBT LPC5, Lactobacillus rhamnosus CBT LR5, Lactobacillus acidophilus CBT LA1, Bifidobacterium breve CBT BR3, Bifidobacterium lactis CBT BL3, | 19–75 years | 1 × 109 CFU/1.4 g each | 84 days | Significantly decreased intrahepatic fat fraction after 12 weeks, along with significant triglyceride reduction. | [84] |
| Bifidobacterium animalis subsp. lactis BB-12 | ≥18 years | 1 × 108 CFU/g | 168 days | Significantly decreased alkaline phosphatase, aspartate aminotransferase, γ-glutamyltransferase, and alanine aminotransferase in serum and reduced NAFLD. | [85] |
| Acetobacter spp., Bifidobacterium spp., Propionibacterium spp., Lactobacillus spp., + Lactococcu spp., | 18–65 years | 6 × 1010, 1 × 1010, 3 × 1010, 1 × 106 CFU/g | 56 days | Significantly reduced the fatty liver index, serum GGT and AST values; diminished chronic systemic inflammatory state; and lowered IL-6 and TNF-α concentrations in NAFLD patients. | [86] |
| Lactobacillus lactis BCMC 12451, Lactobacillus casei BCMC 12313, Lactobacillus acidophilus BCMC 12130, Bifidobacterium longum BCMC 02120, Bifidobacterium infantis BCMC 02129, Bifidobacterium bifidum BCMC 02290 | 18 years and above | 3 × 109 CFU/g | 180 days | Stabilized mucosal immune function, protecting against risen intestinal permeability and playing a complementary role in ministering NAFLD. | [87] |
| Probiotics as Allayers of Gallbladder and Pancreatic Ailments | |||||
| Clostridium butyricum MIYAIRI | 35.5 ± 9.9 | 5 × 109 CFU/g | 180 days | Decreased incidence of gall bladder disease, adverse drug effects, and poor drug compliance rates, confirming the palliative effects of probiotics. | [88] |
| Lactobacillus acidophilus | 48.1 ± 13.8 | 5 × 106 CFU/g | 14 days | Significantly altered serum low-density lipoprotein cholesterol (LDL-C), total cholesterol, total bile acid (TBA), and triglyceride levels. Significantly differed glycoprotein, pH, and free Ca2+ of bile. Altered deoxycholic acid, chenodeoxycholic acid, and cholic acid levels, exhibiting the reverse development of bile composition in patients with cholecystolithiasis taking probiotics, thereby diminishing gallstones. | [89] |
| Enterococcus faecium Bacillus subtilis | 18–75 years | Manufacturer defined recipe | Significantly reduced length of stay (LOS) and shortened abdominal pain relief and oral feeding duration in patients with acute pancreatitis. | [90] | |
| Bifidobacterium infantalis, Bifidobacterium longus, Bifidobacterium bifidum, Lactobacillus acidophilus | 13–79 years | 2.5 × 109 CFU/g | 7 days | Significantly reduced immunoglobulins and C-reactive protein expression. | [91] |
| Acetobacter spp., Lactobacillus + Lactococcus spp., Propionibacterium spp., Bifidobacterium spp., | 18–75 years | 1 × 106, 6 × 1010, 3 × 1010, 1 × 1010 CFU/g | 56 days | Significantly improved β-cell function and reduced fasting glucose and hemoglobin A1C levels. Significantly affected chronic systemic inflammation by decreasing pro-inflammatory cytokines. | [92] |
| Bacillus mesentericus TO-A-, Clostridium butyricum TO-A, Lactobacillus Sporogenes, Streptococcus faecalis T-110 | 18–75 years | 1 × 108, 4 × 106, 2 × 106, 6 × 107 CFU/g | 15 days | Significantly lowered LOS, the duration of antibiotics therapy, and the incidence of postoperative infectious complications in patients with chronic pancreatitis. | [93] |
| Lactobacillus casei, Bifidobacterium bifidum Lactobacillus acidophilus, Lactobacillus rhamnosus | ≥18 years | 1 × 109 CFU/g | 90 days | Significantly reduced bowel frequency and total cholesterol levels. Significantly increased red blood cells, hematocrit, hemoglobin, albumin, serum magnesium, and total lymphocyte count. | [94] |
3. Probiotics Proven to Be Beneficial in Small and Large Intestine Disorders
Probiotics have gained attention for their ability to influence indicators of human health. Multiple meta-analyses have exhibited the beneficial effects of probiotics on the symptoms of different gastrointestinal (GI) ailments, including irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). Further meta-analyses have been conducted to evaluate the effectiveness of probiotic variations depending on the specific strain and the disease being targeted, with increasing compelling evidence portraying probiotics’ strain-specific effects in alleviating symptoms related to specific conditions or ailments (Table 1) [15,95,96,97].
The duodenum, jejunum, and ileum of the small intestine (SI) process and absorb macro- and micronutrients. The SI components’ luminal environments vary, affecting microbial abundance in each segment. Ileum bacteria concentrations rise to 108 bacteria/mL from 103 to 104 in the duodenum and jejunum. GI tract bacteria density grows along its length but stays low compared to colon bacteria concentration, which is 1011 bacteria per milliliter.
The GI tract mucosal epithelium protects the host from the environment. The intestinal barrier consists of junctional complexes (including adherens junctions, desmosomes, and tight junctions (TJs)), antimicrobial peptides (AMPs), the mucus layer, and the commensal gut microbiota. These adaptable parts maintain barrier haleness [98]. Damage to the epithelial mucosa or changes in dysbiosis, nutrition, or inflammation may increase barrier permeability [96].
3.1. Lactobacillus spp. as Small Intestinal Alleviators
The administration of three probiotic strains (L. reuteri G8-5, G22-2, and Lactobacillus salivarius G1-1), in comparison to an antibiotic control group, assisted in the expression of several pathogen defenses, the maintenance of cell structure integrity, and the maintenance of protein cell stability (Figure 4) [99]. Administrating Lactobacillus rhamnosus GG before challenging pigs with Salmonella infantis decreased S. Infantis-induced IL-7Rα production in the jejunum and T cells+ interferon-gamma (IFNγ)+ clusters of differentiation 4 (CD4) in Peyer’s patches. These facts establish the immunological benefits of L. rhamnosus GG as a probiotic and the complexity of its interactions [100]. Therefore, probiotics, especially LAB, protect the small intestine by increasing microbial diversity, homeostasis-related protein expression, and immune system integrity (Table 1).
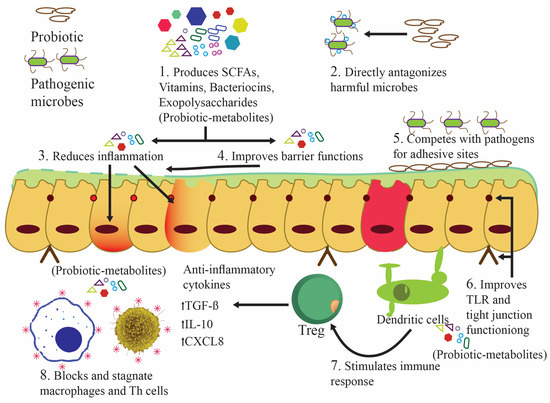
Figure 4.
The palliative effects of probiotics on intestinal integrity and the working of the anti-inflammatory mechanism in the gastrointestinal tract.
3.2. The Palliative Intestinal Permeability of Lactobacillus spp.
The selective permeability of the intestinal barrier lets water and nutrients flow while blocking germs and poisons. Tight junctions (TJ) mainly control paracellular permeability. By causing inflammation and limiting nutritional availability, prolonged gut barrier disruption may cause GI and autoimmune illnesses. Probiotics maintain intestinal barrier health throughout the intestines. If the microbiome is balanced, probiotics boost butyrate production, strengthen TJ proteins, and protect the mucosal lining. Thus, probiotics improve nutrient absorption [101].
A recent study examined L. reuteri LR1’s impact on the characteristics of the small intestine, particularly intestinal permeability. In this study, the weaned pigs, who received food fortified with L. reuteri LR1, exhibited higher mucosal TJ protein expression and villus height/crypt depth ratios in the jejunum and ileum than those fed antibiotics [102]. In another study, mice given lipopolysaccharide to hasten barrier dysfunction, followed by L. rhamnosus GG and L. reuteri ZJ617 as mitigators, showed unique attributes. Claudin-3 and occludin decreased after lipopolysaccharide consumption, and their functions were restored after intervention with probiotics. Therefore, the administrated probiotic strains effectively mitigated this dysfunction [103]. In another study, heavy kanamycin dosages compromised mice’s intestinal barriers. The LAB-fed mice exhibited higher ileal occludin and zonulin-1 expression than the control-fed mice. Participants’ Peyer’s patch cells had more elevated blood immunoglobulin A levels, showing that LAB mitigates kanamycin’s devastating effects [104]. Human research confirms animal studies’ claim that LAB can maintain barrier integrity (Figure 4). As a probiotic, Saccharomyces boulardii CNCM I-745 prevents and treats diarrhea caused by antibiotics, infections, and functional factors. S. boulardii CNCM I-745 improves intestinal microbiota and epithelial barrier abnormalities in diverse illnesses. The probiotic yeast S. boulardii CNCM I-745 helps maintain or repair the intestinal barrier in various ailments [105].
3.3. Probiotics as Lenitives against Impaired Nutrient Absorption and Chronic Diarrhea
Small intestinal bacterial overgrowth (SIBO) has been identified as a potential etiology for impaired nutrient absorption and chronic diarrhea. The quantitative characteristics of small intestinal bacterial cultures do not affect the functional gastrointestinal symptoms of SIBO. These symptoms do correlate with a microbial imbalance in the small intestine. Different levels of nutrient malabsorption cause weight loss and vitamin-deficient neuropathies [106].
Individuals with SIBO typically exhibit luminal content bacterial concentrations ranging from 105 to 106 bacteria per milliliter, which is approximately 2 to 3 log10/mL higher than those observed in healthy individuals. The bacteria found in the small intestine of patients with SIBO are typically the same as those found in the oropharynx and colon. Rifaximin is the most common SIBO treatment. However, it can disrupt good bacterial populations and induce antibiotic-associated diarrhea and Clostridium difficile infections. Thus, probiotics are being evaluated to treat bacterial giantism and restore small intestine commensal microorganisms (Figure 4 and Figure 5) [107].
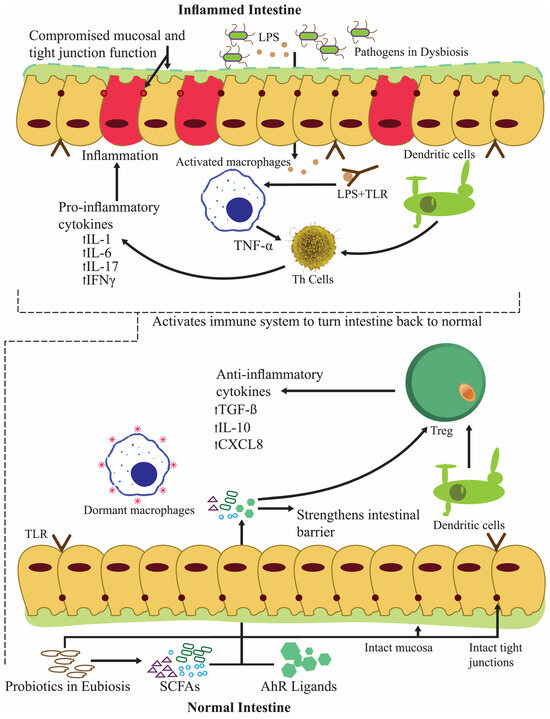
Figure 5.
Anti-inflammatory activity of probiotics during inflammatory bowel syndrome and reverting the intestine to normal functioning.
The complexity of irritable bowel syndrome (IBS) is compounded by various etiologies and symptomatic subtypes. Altered bowel habits, including diarrhea, constipation, or both, as well as stomach pain, characterize IBS [108]. SIBO can coexist with IBS. The evidence for small intestine dysbiosis in IBS is strong. However, a lack of knowledge about probiotic strains, dosages, and therapy duration hinders the potential of probiotic treatment for IBS [109]. Treatment with Bacillus spp. spores has been shown to improve IBS patient’s quality of life, likely due to alterations in the gut microbiota (Table 1) [110].
3.4. Probiotics Modulating Large Intestinal Microflora
Oral probiotic bacteria support and modify the metabolic processes and composition of the microflora of the large intestine. Fermentation by large intestine microorganisms aids digestion. Lowering the intestinal pH makes it more acidic, making it unsuitable for dangerous species. The microflora also guards against pathogenic microorganisms, preventing illnesses. In addition, they actively mature immune system components. Lactic acid bacteria play a crucial role in the gut microbiota, influencing the landscape for health advantages. Regular probiotic bacteria consumption maintains their health advantages.
3.5. The Alleviating Influence of Lactobacillus spp. on Colitis
In specific pathogen-free environments, mice lacking the interleukin (IL)-10 gene (IL-10-/-) develop colitis, while in sterile environments, they do not. Lactobacillus plantarum reduced colonic inflammation in SPF IL-10-/- mice by lowering mucosal IFN-gamma, immunoglobulin G2a, and IL-12 levels. Monoassociation with L. plantarum in gnotobiotic IL-10-/- mice causes considerable immune system activation but minimal colitis. In one specific study, probiotics administration in germ-free mice significantly lowered histologic colitis scores. These facts signify that L. plantarum reduces immune-mediated colitis and clinically treats inflammatory bowel diseases (Table 1) [111].
Another trial investigated whether exogenous Lactobacillus could help rats with acetic acid-induced colitis. Four days after acetic acid administration, uniform colitis, a three-fold increase in colonic tissue myeloperoxidase (MPO) activity (an indicator of neutrophil infiltration), and a six-fold increase in plasma exudation occurred. L. reuteri R2LC intracolonic injections after the administration of acetic acid alleviated colitis. Thus, Lactobacillus nearly normalized mucosal permeability, MPO activity, and morphologic score. The soothing impact of exogenous L. reuteri R2LC in preventing acetic acid-induced colitis in rats is evident [112].
Certain probiotics modulate allergic inflammation, reducing inflammation outside the gut (Table 1). The aggregate effects of these probiotic strains help neonates adjust during weaning, which begins with antigen sensitivity. Probiotics could help develop new allergy-fighting foods [113].
3.6. Bifidobacterium, Lactobacillus, and Other Probiotic spp. Modulating Gastrointestinal Cancers
Many gastrointestinal (GI) malignancies exist, including spontaneous and hereditary variants. Cancer can develop when genetic and environmental factors turn healthy tissue into a precursor or premalignant condition. Specific tissue and cell types have partially known genetic pathways of GI malignancies of various sources, and they share some similarities [114]. Probiotics are utilized as supplements, in line with the progress made to develop new diagnostic and therapeutic methods for GI cancers.
Different researchers have examined how probiotics help reduce symptoms and improve quality of life in colorectal cancer patients at various stages. According to one study, Lacidofil supplements reduced gastrointestinal discomfort and improved functional well-being in colorectal cancer patients [115]. Elevated serum levels of zonulin, a haptoglobin-2 precursor, have been linked to the presence of gastrointestinal cancers, inflammatory diseases, and autoimmunity [116]. Zonulin levels dropped significantly in colorectal cancer patients receiving B. longum-88, L. acidophilus-11, and L. plantarum. In addition, probiotics alleviate infection problems, decrease antibiotic use, and alleviate postoperative fever. Probiotics also inhibit the p38 mitogen-activated pathway, which controls cell differentiation, inflammation, growth, and death [117]. The use of E. faecalis, L. acidophilus, and B. longum shorten the time until first bowel movement, gas, and diarrhea (Yang et al., 2016). L. rhamnosus GG supplementation has been shown to significantly reduce diarrheal episodes in colorectal cancer patients using 5-Fluorouracil, a chemotherapy medication known to cause diarrhea [118]. Offering colorectal cancer patients a probiotic mixture of Bacillus mesentericus TO-A, Clostridium butyricum TO-A, and Enterococcus faecalis T110 reduced superficial incisional infection rates [119]. A combination of E. faecalis, L. acidophilus, and B. longum has been shown to change the gut flora of colorectal cancer patients. Moreover, those probiotics also reduce Fusobacterium, a cancer inducer, taxon secretion [120]. In colorectal cancer patients, a mixture of probiotics (L. plantarum, L. acidophilus, S. boulardii, and B. lactis) reduce pneumonia, mechanical ventilation, surgical site infections, and anastomosis leakage [121].
4. Probiotics Tone Liver and Annihilate Its Ailments
As the leading cause of chronic liver diseases, non-alcoholic fatty liver disease (NAFLD) is a global public health issue. The term “hepatic conditions” covers many liver-related issues. These conditions range from simple steatosis, which is the deposition of lipids on more than 5% of the liver without other causes, to severe forms like non-alcoholic steatohepatitis (NASH), hepatocellular carcinoma (HCC), cirrhosis, and fibrosis.
Probiotics Rectifying Non-Alcoholic Fatty Liver Disease (NAFLD) and Alcoholic Liver Disease (ALD)
Gut bacteria play a significant role in developing and progressing liver disease in metabolic syndrome and NAFLD, which affect children and adults. NAFLD risk increases due to dysbiosis, intestinal barrier dysregulation, and gut bacterial overgrowth. According to clinical guidelines, lifestyle changes and diet are the primary treatment avenues for NAFLD and related disorders. Patient non-adherence hinders these therapies’ efficacy, generating poor outcomes. To tailor NAFLD treatment, it is necessary to investigate various other therapies.
Numerous clinical research studies support probiotic supplements as a safe and effective treatment method. These findings highlight the untapped potential of restoring intestinal microbiota as a standard therapeutic therapy for NAFLD. Additionally, probiotics can be used alone or in combination with NAFLD treatments (Figure 6) [122].
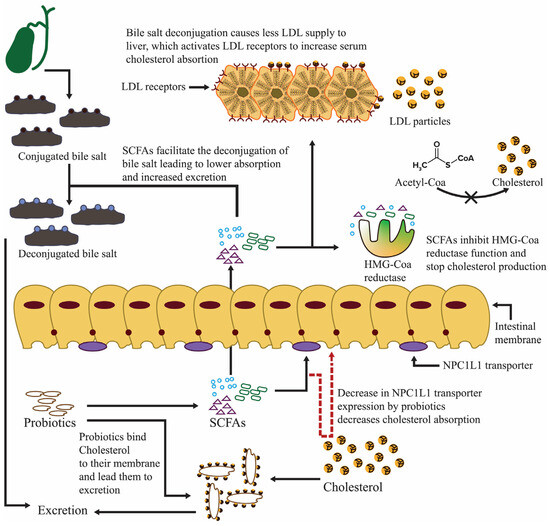
Figure 6.
The preventive mechanism of probiotics, which hinders NAFLD prevalence.
In one study, probiotic postadministration multivariate analysis demonstrated significantly lower alanine aminotransferase and antipeptidoglycan-polysaccharide antibodies despite changes in visceral fat and body mass index (BMI) z score. US light liver readings and TNF-α remained stable. L. rhamnosus GG, a potent probiotic, should be considered to treat hypertransaminasemia in hepatopathic obese youngsters with rebellious lifestyles [123].
Probiotics significantly decrease liver enzymes in non-alcoholic steatohepatitis, particularly alanine aminotransferase (ALT), and increase the expression of aspartate aminotransferase (AST). Dyspepsia symptoms also improve. The efficacy, safety, tolerability, affordability, long-term appropriateness, and potential multilevel downregulation of inflammatory mediators make probiotics a promising treatment [124].
Multistrain probiotics work better. Multistrain probiotics, L. rhamnosus DSMZ 21,690, L. acidophilus ATCC B3208, Bifidobacterium lactis DSMZ 32,269, and B. bifidum ATCC SD6576, intervention can reduce ALT levels, intrahepatic fat content, and sonographic lipid profiles. A “Symbiter” containing 14 live probiotic strains of Acetobacter, Propionibacterium, Bifidobacterium, and Lactobacillus + Lactococcus improved tumor necrosis factor (TNF)-α, IL6, aminotransferase activity, and hepatic steatosis in NAFLD patients [86]. Lepicol probiotics lower liver triglycerides and AST levels in NASH patients, as confirmed by histology [125]. Streptococcus, Bifidobacterium, and Lactobacillus probiotics have been shown to improve hepatic fat content, aminotransferase levels, total cholesterol, and a homeostatic model’s assessment of insulin resistance [126]. Probiotics improve insulin sensitivity and reduce TNF-α levels in NAFLD patients (Table 1, Figure 6). However, probiotics only improve dyslipidemia in Spanish and Italian people, suggesting that ethnicity has a connection with low-density lipoproteins (LDL), high-density lipoproteins (HDL), and triglyceride levels [127].
The most thoroughly studied probiotic with many strains, VSL#3, can protect the intestinal barrier. Furthermore, it reduces oxidative/nitrosative stress and endotoxemia, improving liver health in chronic liver disease patients [128] VSL#3’s potential to modify the gut microbiota with B. longum is intriguing because it produces conjugated linoleic acid, which changes liver fatty acid composition. These facts support the idea that gut–liver interaction is essential in designing NAFLD treatments [122,129]. The co-administration of B. longum and fructooligosaccharides (FOS) improved metabolic, inflammatory, and fibrosis scores in NASH patients [130].
5. Probiotics as Allayers of Gallbladder Ailments
The prevailing consensus in the scientific community is that dietary habits, particularly ingesting a high-fat diet over an extended period, constitute a substantial risk factor for developing cholesterol gallstones [131,132].
Probiotics significantly lower gall bladder disease prevalence when compared with digestive enzymes. This notion implies that probiotics are not inferior to other therapies. Probiotics also improve drug compliance compared to other treatments and lower pharmaceutical side effects. Therefore, probiotics are pretty effective in preventing gallbladder diseases (Table 1) [88].
5.1. Probiotics Repress Bile Acid Production and Diminish Gallstones
Probiotics prevent gallstones by lowering cholesterol. Additionally, probiotics may alter the profiles of serum bile acids by decreasing the proportion of deoxycholic acid in serum [133]. Oral Clostridium butyricum Miyairi therapy systematically reduces gallstone cholesterol content, incidence, and index in mice with cholesterol cholelithiasis. Probiotic treatments also dissolve gallstones well [134]. The intestinal prevalence of C. butyricum Miyairi No. 588 increases bile acid excretion and inhibits gallstone formation in mice [135].
Certain gut microorganisms produce cholesterol reductase, which converts cholesterol into insoluble coprostanol. The fecal excretion of coprostanol lowers exogenous cholesterol [136]. Probiotics, specifically L. acidophilus, B. lactis, VSL #3, and L. plantarum, noticeably reduce serum cholesterol levels [137]. Probiotics prevent and treat lipid-related diseases without medication. A BSH-positive Lactobacillus strain extensively diminished the cholesterol levels of hypercholesterolemia patients [138]. Farnesoid X receptor (FXR) agonists may reduce gallstones by adjusting bile salts and phospholipids [139]. Chenodeoxycholic acid (CDCA) and cholic acid (CA) help activate FXR. The gut bacteria metabolize these bile acids to produce secondary ones. Thus, metabolic processes affect FXR activity and signaling [140]. Probiotics and dietary changes modify the ‘gut microbiota–bile acid–host’ signaling connections. These treatments provide unique ways to treat bile acid metabolism problems [141].
It has been shown that L. acidophilus ATCC 43121 diet supplementation lowers blood low-density and total lipoprotein cholesterol by lowering 3-hydroxy-3-methylglutaryl coenzyme expression in Mice fed with a high-cholesterol and high-fat diet. Moreover, L. acidophilus ATCC 43121, along with L. fermentum MF27, lowers cholesterol and decreases the expression of gel-forming mucins like MUC5B and MUC5AB. Thus, the consistent dosing of these probiotics inhibits cholesterol gallstones. Both probiotic species also improve serum biochemical indices without affecting growth. Reduced liver HMG CoA R expression causes the serum to lower cholesterol, especially after ingesting L. acidophilus ATCC 43121. These traits may also reduce gallbladder gel-forming mucins like MUC5B and MUC5AC. Thus, consuming lactobacilli regularly helps prevent cholesterol gallstones in therapeutic circumstances (Table 1) [142].
5.2. Probiotics’ Connection with Bacterial Translocation and Acute and Chronic Pancreatitis
Acute pancreatitis (AP), a common gastrointestinal illness caused by gallstones and alcohol intake, can lead to hospitalization [143]. AP begins with acinar cells converting pancreatic enzymes from inactive to active, causing pancreatic tissue to autodigest. The release of proinflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α causes pancreatic inflammation [144,145].
Acute pancreatitis (AP) damages the pancreas. Gram-negative bacterial infections activate digestive enzymes pathologically, causing inflammation and cell signaling alterations. This increases intestinal permeability and lets microbes, endotoxins, and antigens into the pancreas, causing BT and acute illness. Direct transmural migration into the retroperitoneum or peritoneal cavity might lead to pancreatic, hematogenous, or lymphatic dispersion. It causes gut barrier breach, small bowel hypomotility, and systemic immunosuppression. Stellate cell and fibrotic tissue activation from recurrent pancreatitis induce chronic pancreatitis (CP). These findings suggest that acute, recurrent, and CP pancreatic cancer can metastasize [146,147,148].
Probiotics protect healthy gut ecology. The disruption of gut bacterial microflora homeostasis may increase bacterial translocation by altering barrier function. Bacterial translocation (BT) increases inflammation, leading to CP and pancreatic cancer [149,150]. Giving acute pancreatitis patients L. plantarum 299 reduced pancreatic sepsis and surgical procedures in [151]. Treating severe acute pancreatitis with synbiotics, including prebiotic fibers and LABs, reduces mortality rates. The probiotics S. boulardii and ciprofloxacin have been shown to reduce acute necrotizing pancreatitis histopathology scores. Moreover, the enteral feeding of probiotics is more beneficial than parenteral feeding. This method of probiotics feeding reduces the severity of pancreatic conditions like inflammation, edema, fibrosis, parenchymal necrosis, acinar cell loss, ductal damage, PMNL, MNL, vacuolization, and atypical reactive regeneration. These enteral administrations also prevent pancreatic cancer [152].
Probiotics decrease duodenal bacterial overgrowth and pancreatic translocation. Health scores and late-phase mortality have been shown to improve significantly. In acute pancreatitis, altering intestinal microbiota using probiotic species reduces BT, morbidity, and mortality [150].
The collapse of the intestinal barrier causes BT to enter into the circulation and necrotic tissues from the digestive tract, leading to pancreatic tissue infection. Typically, the pancreas has no well-defined microbiome. However, gastrointestinal tract dysbiosis commonly affects it. Pancreatic macrophages release TNF, IL-6, and IL-1 in necrotizing pancreatic tissues when bacterial antigens and endotoxins enter the portal circulation. These cytokines contribute to chronic pancreatitis and pancreatic tumors. Due to the lack of a screening modality and the poor outcomes of pancreatic cancer therapies, effective primary prevention techniques such as probiotic interventions are the best way to reduce morbidity and mortality [148].
In animals suffering from severe pancreatitis, Pseudomonas, Enterococcus faecalis, E. coli, and Proteus predominate. Animals with L. plantarum 299v ‘umbrella’ reduce mesenteric lymph node cultures. In animal pancreatic tissue cultures with E. faecalis or Escherichia coli, L. plantarum 299v effectively reduces microbiota translocation. Based on these facts, probiotic bacteria may replace antibiotics as a therapeutic strategy [153].
Probiotics significantly reduce pancreatic and oxidative damage. Probiotics strongly block AP-induced NF-kappaB activation, reduce AP-induced lipid peroxidation and glutathione depletion, and increase glutathione levels. Probiotics increase glutathione production, which may reduce inflammation and acinar cell injury. These actions likely mitigate oxidative stress and improve acute pancreatitis [154].
5.3. Probiotics Lower the Risks of Organ Failure and Systemic Inflammatory Response Syndrome
The administration of synbiotics has been shown to lower mortality, septic complications, and multiorgan failure (MOF) in pancreatic patients. Synbiotic treatment reduces systemic inflammatory response syndrome and MOF rates. Moreover, the early nasojejunal feeding of synbiotics can lead to an avoidance of organ failure in severe acute pancreatitis. Pancreatic necrosis infection may also influence early-stage organ failure [151].
Probiotics significantly reduced total leucocyte and neutrophil counts in patients of the same demographic and the same severity of pancreatitis. Hospitalization length (LOH) also shortened, significantly reducing non-septic morbidity and intensive care unit (ICU) stays. Synbiotics have also been shown to reduce septic complications in moderately severe and severe acute pancreatitis patients. Synbiotics significantly reduced LOH without reducing fatality rates or medical interventions (Table 1) in [155].
Pre/pro/synbiotics reduce hospital stays significantly, which proves their efficacy. Pre/pro/synbiotics also lower severe acute pancreatitis (SAP) patients’ risk of MOF and LOH. Pre/pro/synbiotics do not worsen SAP patients’ clinical outcomes. These individuals have a lower organ failure risk and shorter LOH [156].
High-temperature heating transforms heterocyclic aromatic amines (HCAs) in beef [157,158] into active derivatives, including pyrolyzates such as 3-amino-1-methyl-5H-pyrido-[4,3-b]indole [Trp-P-2], 3-amino-1,4-dimethyl-5H-pyrido-[4,3-b]indole [Trp-P-1] and compounds that promote tumorigenic mutations [159]. Commensal bacteria, especially LAB, retain or catabolize these mutagenic chemicals [160]. Probiotics bind or degrade HCAs, which then eliminate carcinogens from the body. Probiotics, especially LAB, adhere to carcinogenic HCAs formed during protein-rich food heating [161]. Zhang et al. observed a decrease in the genotoxicity of Trp-P-1, a nitroso compound, following its interaction with L. acidophilus and Bifidobacterium species [162]. The antimutagenic elements from L. plantarum KLAB2 affect N-methyl-N’-nitro-N-nitroso-guanidine’s mutagenesis effects in Salmonella enterica strain TA100 cells. The anti-mutagenic property is attributed to three glycoproteins located outside the bacterial cell wall [163].
Interestingly, probiotics reduce the perniciousness of toxic heavy metals and fungal mycotoxins, which contribute to pancreatic carcinogenesis [164]. Additionally, the influence of Propionibacteria, widely recognized dairy probiotics, in reducing cyanotoxins such as microcystin-LR, lead, and cadmium is well established [165,166]. Reducing carcinogenic chemical bioavailability reduces pancreatic cancer risk. L. rhamnosus GG’s cellular degradation of aflatoxin B1 is evident. L. acidophilus 24 and S. cerevisiae CECT 1891 remove fumonisin from the cells [167,168].
6. Probiotics Fortify the Respiratory Tract and Alleviate Rhinosinusitis and Rhinitis
Inflammatory illnesses affecting the upper respiratory tract (URT), including chronic rhinosinusitis (CRS), acute rhinosinusitis (ARS), and rhinitis, have a substantial impact on public wellness and significantly contribute to healthcare expenditures. Rhinitis, a URT disorder, is characterized by the symptomatic inflammation of the nasal lining caused by infectious agents, allergens, hormones, and medicines [169]. Allergic rhinitis (AR), a non-infectious form of rhinitis, is also common. Rhinosinusitis causes paranasal sinus and nose swelling. Experiencing more than two symptoms, including nasal blockage or drainage, is an indicator of rhinosinusitis. Illnesses lasting more than 12 weeks become CRS [170].
The indigenous microflora in the URT of children and healthy adults includes LAB members such as Lactococcus, Dolosigranulum, and Lacticaseibacillus species. Lactobacillus species in the nasopharynx and tonsillar crypts of adults and children from China, Canada, and Belgium have been found [171,172,173,174,175].
A decrease in specific LAB taxa such as Latilactobacillus sakei in CRS patients suggests sinus health benefits. In a study involving the use of a mouse model used to investigate sinus infection, the findings showed that L. sakei ATCC15521 protects sinus mucosa from C. tuberculostearicum pathogenesis after nasal inoculation [176].
Various LAB taxa are more common in the URT than Lactobacillaceae. Dolosigranulum pigrum, a neglected species, is being considered for the establishment of the URT’s next-generation probiotic. The main reason for this is its abundance, which can reach 50% in people with normal URTs. It is often more common in healthy people than sick people, suggesting a link to URT health [172,177,178,179].
6.1. Probiotics Have Yielded Encouraging Findings against Asthma
Asthma and other lower airway comorbidities must be considered when assessing the therapeutic effects of probiotics in chronic inflammatory diseases of the URT, such as CRS and allergic rhinitis [169]. The initial clinical trials have exhibited that Lactobacillus gasseri PM-A0005 has therapeutic effects in asthmatic children. L. gasseri PM-A0005 improves asthma, airway function, and, particularly, peak expiratory flow rates. Additionally, the probiotic strain significantly decreases the expression of pro-inflammatory cytokines such as IL-13, IL-12, IFN-γ, and TNF-α (Table 2) [180]. An oral mixture of B. bifidum, L. delbruecki ssp. bulgaricus, and L. acidophilus has been shown to improve pulmonary function and reduce asthma exacerbations [181]. Synbiotic therapy affects asthmatics and dust mite allergy sufferers. Fructo- and galactooligosaccharide and Bifidobacterium breve M-16V significantly improve serum IL-5 and initial lung function (Table 2) [182].

Table 2.
Alleviative effects of probiotics on the respiratory system, bone health, kidneys, cardiac health, and reproductive system, as established in placebo-controlled human studies.
6.2. Probiotics Counter Allergy Illnesses
High rates of allergic sickness are becoming a global health issue, especially in highly developed places like North America, Western Europe, and Australasia, where over 40% of the population may feel its effects [228]. Due to industrialization and Westernization, international patterns imply that environmental changes affect immune function regardless of genetics. However, growing evidence suggests that non-Caucasians may be more susceptible to allergic illnesses. This discovery is concerning, especially in densely populated, rapidly urbanizing locations [229,230].
Lactobacillus F19 shows a higher IFN-gamma/IL4 mRNA, implying that probiotics prevent early allergy diseases like eczema during weaning. This probiotic elevates the Type 2 T helper (Th2)–Type 1 T helper (Th1) ratio, suggesting that Lactobacillus F19 improves T cell-mediated immunity [231].
L. plantarum NumRes8 and B. breve M-16V inhibit several parameters, including methacholine responsiveness, bronchoalveolar lavage fluid eosinophil activity, and ovalbumin (OVA)-specific immunoglobulin E (IgE) and immunoglobulin G1 (IgG1) levels. B. breve M-16V also reduces IL 4, 5, or 10 and acute allergic skin reactions associated with OVA-induced asthma. Overall, B. breve M-16V is the most potent antiallergic strain [232].
B. breve-12 and L. rhamnosus GG reduce asthmatic symptoms such as pulmonary eosinophilia, antigen-specific immunoglobulin E production, and airway reactivity. Mesenteric lymph node cells produce fewer Th-2 cytokines (IL-4, IL-5, and IL-10), and spleen cells proliferate less in response to antigen-specific recall. The oral treatment of L. rhamnosus GG reduces allergen-induced proliferation. The mesenteric lymph nodes’ CD4+/CD3+ T cells, which release transforming growth factor-beta, increase with this suppression. Probiotics reduced allergic sensitization and airway disease in a mouse model of asthma. Promoting T regulatory cells, which increase TGF-beta production, achieves this effect [233].
Probiotics probably affect Th17, a niche subgroup of CD4+ T lymphocytes linked to allergic problems. Oral Enterococcus faecalis FK-23 (LFK) relieves inflammatory cell accumulation, bronchoalveolar lavage fluid (BALF), and airway resistance in lung tissue. In mice challenged with OVA, LFK also lowered the percentage of CD4+ cells expressing IL-17 in the lungs, spleen, and stomach. Oral leukotriene receptor antagonists reduce asthma symptoms and Th17 cell proliferation [234].
7. Probiotics Combat Osteoporosis and Build Up the Skeleton
The number of fractures resulting from osteoporosis has exceeded 2 million annually, and therapeutic approaches for osteoporosis prevention and treatment are manifold. Early intervention involves asking people to exercise, quit smoking, and take vitamin D and calcium supplements [235]. Patients with a high fracture risk are treated with medications and biologics. These treatments inhibit bone resorption or encourage development [236]. Given the momentous prevalence of bone decreases, it is crucial to identify additional osteoporosis treatment methods and targets [237].
L. paracasei DSM13434 or a mix of L. plantarum DSM 15313, DSM 15312, and L. paracasei DSM13434 (L. mix) have been shown to protect mice from bone resorption and OVX-induced cortical bone loss. L. mix and L. paracasei DSM13434 have been shown to elevate cortical bone mineral content in OVX mice. L. mix and L. paracasei DSM13434 lower the urine fractional excretion of calcium and resorption marker C-terminal telopeptides serum levels. Probiotics reduce IL-1β and TNFα levels and increase OPG expression. Probiotic treatment upholded regulatory T cell frequency in VEH-treated mice’s bone marrow. Overall, L. mix and L. paracasei DSM13434 reduce cortical bone loss, alter bone’s immune system, and reduce bone resorption in mice [238].
Fucooligosaccharides in yacon flour make it a prebiotic suitable for synbiotic food production. B. longum, along with yacon flour or diet, elevates tibia Mg, Ca, and P and enhances bone strength. Yacon flour assists in developing heavier anaerobes and cecums. Using B. longum in yacon flour or diet increases cecal propionate levels. Yacon flour and B. longum increase bone mineral levels, which helps prevent osteoporosis [239].
Insufficient estrogen accelerates osteoporosis, which causes bone resorption and inflammation. L. reuteri ATCC PTA 6475 releases immunomodulatory substances and protects mice from bone loss. L. reuteri reduces the receptor activator of nuclear factor kappa beta, Tartrate resistant acid phosphatase 5, and osteoclastogenesis. L. reuteri ATCC PTA 6475 inhibits OVX-induced bone marrow CD4+ T-lymphocytes, which increase osteoclastogenesis. L. reuteri ATCC PTA 6475 also suppresses osteoclastogenesis in vitro, affects the stomach microbial populations, and decreases bone resorption and loss in estrogen-deficient patients. L. reuteri ATCC PTA 6475 is a cost-effective way to reduce bone loss in postmenopausal women [240]. L. plantarum (NTU 102)-fermented soy milk or L. paracasei (NTU 101) increase bone trabecular number and volume fraction (BV/TV) [241].
7.1. Probiotics Meliorate Bone Health
Probiotics also contribute to improving bone health. L. reuteri, a probiotic recognized for its anti-inflammatory and bone health properties, protects mice from type 1 diabetes-induced bone loss and marrow obesity. L. reuteri prevents Wnt10b downregulation in type 1 diabetic bone because a lower bone-specific Wnt10b expression is linked to osteoporosis. L. reuteri substantially reduces the negative impact of TNF-α on Wnt10b expression and osteoblast formation. Probiotics protect bones from type 1 diabetes-induced degradation and elevate bone health [242].
Orally administrated L. reuteri ATCC 6475 elevates bone mineral density, content, number, thickness, and femoral and vertebral trabecular bone density [243]. During the onset of mild inflammation in mice, the administration of oral L. reuteri was shown to lead to improved bone health. Moreover, female mice ingesting probiotics with mild inflammation from a dorsal surgical incision (DSI) have higher bone density. Probiotic administration in DSI mice elevates femoral trabecular bone density, mineral apposition rates, and trabecular numbers [244]. Probiotic administration with no prior health issues yields a higher anti-inflammatory response. However, L. reuteri diminishes inflammation and improves bone production with enhanced inflammation in females with intact estrogen levels. Thus, L. reuteri improves bone density.
L. gasseri, L. reuteri, and L. casei-enriched yogurt increase calcium absorption in mice. This intervention increases bone mineral content (BMC), while L. rhamnosus (HN001) improves magnesium and calcium retention [245]. B. longum and other Lactobacillus strains also improve bone health. B. longum (ATCC 15707) increases tibia phosphorus, magnesium, and calcium levels. Probiotic supplementation also enhances bone strength [239]. Mice ingesting B. longum-fermented broccoli feeding on a cholesterol-rich diet demonstrated reduced tartrate-resistant acid phosphatase-positive osteoclasts in [246].
Lactobacilli reduces the adverse effects of estrogen deprivation on trabecular bone density. L. rhamnosus GG and VSL#3 administration positively impact femoral trabecular thickness, bone density, and number reduction. Genetic changes in probiotics cause a loss in their probiotic ability, such as L. rhamnosus GG pili mutant (ΔSpaC) and E. coli DH5alpha, which did not reduce bone loss in one specific study. The ingestion of VSL#3 and L. rhamnosus GG lowered blood collagen type I C-telopeptide levels, which indicates osteoclast-mediated bone resorption [247]. This implies that probiotics exert their impact on bone loss by reducing osteoclast activity.
B. longum affects bone mineral content, bone structure, bone density, bone remodeling, and the expression of genes associated with osteoclasts and osteoblasts by increasing bone density, trabecular number, and thickness. B. longum supplementation also increases femoral strength. B. longum therapy reduces serum C-terminal telopeptide and attenuates a decrease in osteoblast surface and an increase in osteoclast surface relative to the femur bone. These notions suggest that probiotics influence osteoclast activity and growth [248].
7.2. Probiotics Palliate Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a persistent disorder distinguished by inflammation, neuropathic uncomfortableness, rigidity, fractures, diminished functionality, and cartilage degeneration, which collectively lead to impaired physiological performance [249,250]. RA affects 1% of those aged 20–40 worldwide; however, it is more common in those 75 and older [251,252]. Psychological problems, asthma, cancer, heart illnesses, hypertension, diabetes, nephritis, and lung cancer/COPD are common comorbidities in RA patients. Comorbidities increase RA patients’ risk of death [253].
RA patients have higher amounts of Shigella, Escherichia, and Bacteroides bacteria in their guts but far lower levels of Lactobacillus spp. [254]. Well-balanced gut bacteria provide B vitamins like B6, B5, B3, B12, B7, K, tetrahydrofolate, and folate [255].
Inflammatory diseases like RA lower plasma folate. The long-term use of non-steroidal anti-inflammatory medicines, particularly cyclooxygenase blockers, inhibits vitamin B6 metabolism, lowering blood pyrophosphate [255]. Probiotics release several short-chain fatty acids and vitamins to help nourish the intestinal lumen and lower its pH [256]. L. casei ingestion improves rheumatoid arthritis pathology indicators. Lactobacillus spp. also hinders the functioning of high amounts of natriuretic and reactive oxygenated species (ROS) in degrading lipids and other macromolecular elements in the affected person’s matrix. L. casei reduces joint edema, joint problems, and inflammatory cytokines [257,258].
8. Probiotics Preserve Kidney Integrity and Combat Chronic Kidney Disease (CKD)
Chronic kidney disease (CKD) is anticipated to be the fifth major cause of death worldwide, especially in countries with long life expectancies; it could become the second most significant cause of death by the end of the century [259]. Recent studies have shown that finerenone and sodium–glucose co-transporter 2 inhibitors can slow CKD progression, offering promising future prospects. However, renal problems remain, especially in advanced CKD patients [260,261]. Reducing CKD progression and hastened aging requires more therapies. It is well known that various foods affect CKD and may cause acute kidney damage (AKI). Ingesting high levels of oxalate, phosphate, protein, and salt can accelerate CKD, while oxalate can cause acute renal damage [262,263,264].
L. casei Zhang protects renal function in mice models of AKI and CKD and in humans. Orally administrating L. acidophilus or L. casei Zhang to mice with ischemia-reperfusion injury (IRI) protected against AKI. Compared to L. acidophilus, L. casei Zhang enhances renal function, reduces fibrosis-related gene expression, and decreases kidney histological tubular injury. Prebiotics reduced kidney fibrosis in a subtotal nephrectomy model. L. casei Zhang lowers macrophage factor expression in the kidneys. L. casei Zhang’s beneficial effects remain unaffected by the gut microbiota, even during antibiotic disturbance in clinical settings, especially ICUs [265]
Lactobacillus casei Zhang improves renal ischemia/reperfusion (IRI) gut microbiota imbalances. Short-chain fatty acid (SCFA)-producing bacteria, especially Bacteroidetes, proliferate more after this intervention. After IRI induction, probiotics increase the expression of kidney and serum SCFAs such as propionate, butyrate, and acetate. SCFAs also reduce IRI and folic acid-induced nephropathy [266,267]. The intraperitoneal injection of butyrate, acetate, and propionate before ischemia and during reperfusion increases renal health associated with IRI. This improvement is because of the reduced histone deacetylase activity [266]. The oral delivery of SCFAs via drinking water reduces tubular injury caused by folic acid and alleviates interstitial fibrosis and chronic inflammation. Moreover, SCFAs activate receptors, namely Hydroxycarboxylic acid receptor 2 (GPR109A) and G-protein-coupled receptor 41 (GPR41), which protect the kidneys of mice lacking G-protein-coupled receptors in [267].
Moreover, CKD increases gastrointestinal tract urea and ammonium levels. As urea and ammonium levels rise, pH rises, promoting aerobic bacteria growth. Thus, aerobic microflora produce uremic toxins such as trimethylamine N-oxide, indoxyl sulfate, and phosphatidylcholine (PCS), which reduce beneficial anaerobic bacteria in the gut. These facts imply that butyrate-producing microbe reduction contributes to CKD inflammation and progression (Table 2) [268,269]. L. plantarum A7-supplemented soy milk significantly reduced oxidized glutathione buildup in people with diabetes with high proteinuria [270].
Probinul neutro® (CadiGroup, Rome, Italy) significantly decreases plasma p-cresol and moderately alleviates gastrointestinal symptoms [271]. Ingesting synbiotics decreases serum p-cresyl sulfate. Although the impact of synbiotics on indoxyl sulfate levels is minimal, the stool microbiota improves with the course of ingesting synbiotics [272]. Lactobacillales and Bifidobacteria markedly alter the gut microbiota and intestinal bacteria metabolism [200]. Probiotics alleviate fecal problems because they biosynthesize lactic acid, hinder the synthesis of toxic compounds by unwanted microbes, and protect kidneys (Table 2) [273].
B. bifidum (VDD088), B. longum subspecies infantis (BLI-02), and L. acidophilus significantly decrease blood urea nitrogen and creatinine with varying probiotic doses. High-dose probiotics protect the inflammatory characteristics of the glomerular corpuscles, renal cortex, and healthy renal pelvis from typical compact renal tubules [274]. In CKD, a protein-deficient diet with prebiotics and probiotics improves glomerular filtration rate in patients [275]. In end-stage kidney disease (ESKD) patients, lower levels of Faecalibacterium and Roseburia, which produce fewer SCFAs, prevail, causing intestinal dysbiosis. ESKD patients possess Fusobacterium, Shewanella, and Erwinia, which are usually absent in healthy individuals. The idea of using gut symbiosis as a CKD treatment is novel and could prove to be effective [276].
Probiotics Conciliate Kidney Ailments during Hemodialysis and Peritoneal Dialysis
Oral lactic acid bacteria lower bloodstream uremic toxins, particularly indican, in uremia patients. Fecal p-cresol levels decrease significantly in hemodialysis (HD) patients, although plasma p-cresol decreases slightly. Intestinal microbiota also repair suppressed bacterial formation. Probiotic strains reduce plasma indoxyl sulfate and slightly affect indoxyl glucuronide [277]. After ingesting oligofructose-enriched inulin, a great prebiotic, blood PCS levels decrease significantly, whereas IS levels moderately decrease [278]. In HD patients, resistant starch reduces blood indoxyl sulfate levels and also affects serum p-cresyl sulfate levels [279]. Given the information mentioned earlier, it is evident that adding prebiotics to one’s diet is an excellent way to avoid kidney-related diseases. These prebiotics provide nutrition directly to humans and act as a food source for probiotics. Therefore, caution is advised when selecting a probiotic therapy for individuals with HD.
In humans, probiotics reduce the expression of IL-6 and high-sensitivity C-reactive protein. Probiotics significantly improve triceps skinfold thickness, upper arm circumference, and blood albumin in peritoneal dialysis (PD) patients. Therefore, for PD patients, probiotic supplementation improves malnutrition and health [199].
Probiotics reduce proinflammatory cytokines such as tumor necrosis factor-a, IL-5, IL-6, and endotoxins. Probiotics also increase serum IL-10, an anti-inflammatory cytokine, preserving renal function in PD patients [280].
9. Probiotics Influence the Microflora of the Male and Female Reproductive Systems
Multiple studies have discussed the bacteria found in the female and male reproductive tracts. Semen contains most male reproductive microorganisms [281]. In contrast, females have microbiomes throughout their reproductive systems, with each tissue or organ colonized by a unique microbiota [282]. Pieces of evidence mark reproductive microbes as crucial for reproductive health and the development of associated illnesses. Commensal bacteria maintain ecological homeostasis in the reproductive tract, improving host fertility and fitness (Table 2) [283]. Dysbiosis in the reproductive microbiome can disturb normal reproductive physiology, causing several pregnancy complications [284]. Given the microbiome’s role in reproductive health and related diseases, probiotic therapies that target the microbiome as a therapeutic approach are rational. Due to the link between metabolic health and reproductive performance, probiotics may improve host reproductive function by modulating metabolism.
9.1. Probiotics Enhance Cell Membrane Integrity and Functioning
Probiotics and their bioactive components improve epithelial barrier function. Blastulation, placenta, chorion, and amnion development depend on cellular membrane integrity. Consequently, probiotic strains influence reproductive membrane architecture [285]. Numerous studies have shown probiotics’ immunomodulatory effects. Probiotic strains that alter the inflammatory cascade can yield benefits in terms of reproductive functions and alleviating illnesses related to the reproductive system [286]. Probiotics improve reproductive system performance, reduce illnesses related to the reproductive systems of males and females, and contribute to offspring well-being (Table 2).
9.2. Probiotics Conserve Male Reproductive Health
Seminal fluid contains a varied microbiota that protects male reproductive health. A link between serum bacteria and sperm quality exists. In semen, lactobacilli dominate, preserving sperm motility and viability [287]. Thus, semen with a majority of Lactobacillus bacteria is of superior quality than semen with other bacteria. Microorganisms can directly cling to sperm and affect spermatozoa. They also inhibit sperm motility indirectly through their metabolites (Table 2) [288].
9.2.1. Probiotics from the Vagina Protect Spermatozoa
Antioxidant enzymes, which protect somatic cells from free radicals, are rare in the cytoplasm of human spermatozoa. In the female reproductive system, spermatozoa may be more susceptible to reactive oxygen species (ROS), especially in infections. The absence of seminal plasma, which contains non-enzymatic antioxidants to protect against oxidative stress, causes susceptibility. Infertile spermatozoa produce excessively high ROS levels, which causes significant peroxidative damage. Sperm membranes with more polyunsaturated fatty acids (PUFAs) are more sensitive to lipid peroxidation [289,290]. A specific mix of three lactobacilli strains (L. plantarum FV9, L. salivarius FV2, and L. brevis CD2), usually used for treating bacterial vaginosis, prevent ferrous ion-induced sperm lipid peroxidation. Hence, this mix protects sperm viability and motility (Table 2). It appears that vaginal probiotic lactobacilli protect human spermatozoa from radical oxygen species during vaginal infections [289].
9.2.2. Probiotics Meliorate Reproductive Hormone Release
Probiotics increase follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone levels, which increases the percentage of progressively motile sperm and velocity characteristics (VCL, curvilinear velocity; VSL, straight-line velocity; VAP, average path velocity), while immotile sperm decreases. Probiotic administration increases the amplitude of lateral head displacement (ALH), linearity (LIN), straightness (STR), and beat cross frequency (BCF). L. rhamnosus PB01 is an effective weight loss and reproductive hormone agent, dramatically enhancing kinematic metrics and sperm motility [291]. Probiotics improve spermatogenesis, seminiferous tubule cross-sectional profiles, and testosterone levels, thereby increasing testicular function and semen quality. The systemic treatment of antibodies improves testicular mass and other age-related markers to youthful levels by suppressing the pro-inflammatory cytokine IL-17A. Probiotics mitigate low blood testosterone levels, which have several harmful effects. L. reuteri or other probiotic supplements prevent male hypogonadism naturally, avoiding conventional treatments’ controversies and side effects and offering practical solutions for aging-related diseases. Probiotic treatment improves public health by increasing hormonal and gonadal traits linked with reproductive fitness in younger and healthier people [292]. Therapy with B. longum CECT7347 and L. rhamnosus CECT8361 also increases sperm motility.
9.2.3. Probiotics Attenuate DNA Damage, Blood–Testis Barrier, and Spermatozoa Functionality
Probiotics also significantly reduce DNA fragmentation. Probiotics have been shown to reduce intracellular H2O2 by a considerable margin. Probiotics preserve cell viability. Moreover, probiotics may improve sperm motility, DNA fragmentation, and ROS in asthenozoospermic males [293]. Probiotics regulate testicular function and blood–testis barrier (BTB) permeability. Probiotics also improve rabbit semen quantity and quality. When mated with nitrate-supplemented bucks, these rabbits have larger litters and heavier babies. These findings imply that probiotics have anti-sterility and offspring-boosting effects [294,295]. Based on substantiated evidence, probiotics have the potential to open up fascinating treatment approaches for addressing infertility.
Research shows that Bacteroidetes longum CECT7347 and L. rhamnosus CECT8361 improve sperm motility, DNA fragmentation, and intracellular H2O2 levels. These probiotic strains improve sperm quality due to their antioxidant characteristics [293].
Lactobacillus casei significantly reduces P. aeruginosa-induced sperm motility and mitochondrial activity, implying that Lactobacillus improves semen quality by reducing harmful bacteria. Moreover, probiotic supplements also affect testicular function and spermatogenesis by altering the gut microbiota and its antioxidant characteristics [296,297]. Bacteroidetes, Deferribacteres, and Firmicutes are significantly correlated with diethylhexyl phthalate (DEHP)-induced testicular dysfunction. L. plantarum TW1-1 pretreatment reduces testicular damage by modulating microorganisms and reduces testicular injury in DEHP-exposed conditions. Therefore, probiotic strains enhance spermatozoa functionality by altering gut microbiota adaptability. It is also well known that oxidative stress, which probiotic interventions could alleviate, damages sperm DNA and impairs spermatozoa functionality [298].
9.2.4. Probiotics Attenuate Prostatitis and Modulate Lactic Acid Production
The effects of probiotics on the prostate have been narrowly studied in recent years. B. animalis Bb-12, L. casei-01, L. acidophilus La-05, and L. rhamnosus GG induce apoptosis in prostate cancer cells [299,300]. Probiotics can also prevent and treat Enterobacteriaceae-induced chronic bacterial prostatitis (Table 2) [301]. Furthermore, probiotics reduced Enterococcus faecalis and E. coli in prostatitis patients’ urine cultures in [302].
Lactic acid, a “postbiotic” metabolite with antibacterial and immunomodulatory properties, can solve probiotic strain colonization and regulation stability issues. Most eubiosis-associated vaginal bacteria produce more lactic acid [303]. Therefore, lactic acid may restore vaginal microbiome function without needing new and potentially under-evaluated probiotics.
The follicular fluid, which affects oocyte maturation, follicle growth, oviduct transit, steroidogenesis, and ovulation, possesses microorganisms found in the oral mucosa (Streptococcus spp.), skin (Staphylococcus spp.), gastrointestinal system (Bifidobacterium spp., enteric bacteria, Streptococcus agalactiae), and vagina (Lactobacillus spp.) [304]. Lactobacillus species dominate follicular fluids and impact embryo maturation and quality. Due to its antibacterial properties, Lactobacilli produce lactic acid to protect oocytes from harmful microorganisms during maturation [305]. The vaginal microbiota of reproductively active women contains L. gasseri, L. crispatus, L. jensenii, and L. iners, which produce lots of lactic acid, contributing to eubiosis [303,306,307]. Lactic acid content is inversely correlated with pH in women with Lactobacillus-dominated microbiota, suggesting its role in vaginal acidification [308].
9.3. Probiotics Alleviate Bacterial Vaginosis (BV)
The lower and upper reproductive tracts make up the female reproductive system. The reproductive organs—the uterus, fallopian tubes, and ovaries—are in the upper part, while the vagina and cervix are in the lower. Both the lower and upper reproductive tracts have microflora. There was formerly a belief that the upper system was microbial-free. Recent research has confirmed the presence of microflora in the placenta, fallopian tubes, follicles, and uterus [283,309,310]. Lower reproductive tract microbiomes are diverse and abundant. Each region owns a microbiome with a unique makeup and diversity. Age, physiological conditions, lifestyle, and environment affect reproductive tract microflora composition [284,288,311].
Little is known about the etiology and pathogenesis of bacterial vaginosis (BV), a common infection in reproductive-age women with harmful sexual and reproductive health effects [312].
The dominant attributes of probiotics in treating BV include synthesizing antimicrobial compounds such as bacteriocins and H2O2, adhering to vaginal epithelial cells, and having antimicrobial effects. Probiotics also acidify the vaginal environment by producing lactic acid, which has immunomodulatory effects. Moreover, probiotics outcompete unwanted bacteria, co-assemble, and resist antibiotics, excluding those used to treat BV [313,314,315,316]. Many Lactobacillus strains and species, including L. fermentum, L. reuteri RC-14, L. gasseri, L. brevis, L. plantarum, L. acidophilus, L. rhamnosus GR-1, and L. crispatus, are effective as vaginal probiotics for BV treatment or prevention (Table 2) [313,315,317].
Lactic acid alone, without bacteriocins, kills BV-associated bacteria in vaginal secretions in conditions that mimic ex vivo conditions. Lactal, a lactic acid gel used for BV recurrence prevention and therapy, results in a significant clearance rate for bacterial vaginosis without exhibiting deleterious effects. Treatment with Lactobacilli restores colonization in the lower female reproductive tract [318]. Lactic acid gels and other lactic acid sources re-colonize lactobacilli after a few days [319,320].
9.4. Products from Probiotics Hinder Sexually Transmitted Infections (STIs)
Lactic acid fights bacterial STIs better than hydrogen peroxide. L. gasseri and L. crispatus form lactic acid to inactivate E. coli, Neisseria gonorrhoeae, and Chlamydia trachomatis [321,322,323,324]. Lactic acid from L. crispatus inhibits bacterial growth in live tissue. A porcine vaginal mucosa model suppressed Gardnerella vaginalis and N. gonorrhoeae [325]. Lactic acid, directly or via a probiotic strain, helps to maintain eubiosis or restore dysbiosis in vaginal microorganisms, preventing bacterial STIs. Hydrogen peroxide-producing lactobacilli in women reduce dysbiotic microbiota. Lactobacillus spp., especially L. crispatus, exists in the vaginal microbiota and produces hydrogen peroxide in aerobic environments, establishing eubiosis [324].
9.5. Probiotics Countermine HIV and Herpes Simplex Virus 2 (HSV-2)
Women with a dominant lactobacillus vaginal microbiome are less likely to contract HIV from males. Furthermore, HIV-infected women with a lactobacillus-dominated microflora release fewer viral particles into the lower female reproductive tract [326,327]. This intervention protects males and vaginally delivered neonates from sexually transmitted HIV. Physiological concentrations of lactic acid inactivate HIV faster and more effectively than medium acidified to the same pH with acetic acid or HCl [328].
BV is also a good predictor of HSV-2. Women with a lactobacilli-dominated vaginal microbiota are less likely to contract HSV-2 [327]. Furthermore, vaginal lactobacilli and their products strongly inactivate STIs. Lactobacillus species inhibit HSV-2 in virucidal-independent and virucidal-dependent ways. Due to lactic acid or lactobacilli adhesion, the virucidal-independent processes suppress viral entry and reproduction. An acidic pH affects lactic acid’s HSV-2 inhibition. These facts suggest that protonated lactic acid mediates the effect [329,330,331].
9.6. Probiotics Improve Ovarian Function
Few studies have examined how probiotic strains affect follicular formation in women. Probiotics may delay ovarian function and estradiol decline in menopausal women to reduce menopause symptoms like dyslipidemia and obesity. Probiotics from healthy women raise estrogen levels in ovariectomized menopausal mice due to gut microflora’s versatile metabolites, suggesting that probiotic intervention regulates estradiol levels in ovarian failure. Perimenopausal women can preserve ovarian function by taking the probiotic Sanprobi Barrier, which raises FSH levels; a non-invasive intervention that regulates hormonal balance is possible [332,333]. Probiotic strains boost avian follicular growth. In Hy-Line layers, Bacillus improves egg bulk and production [334,335], and Enterococcus faecium, after being included in AA broiler breeders’, diets increased egg weight and shell thickness. Probiotics increase reproductive hormones like FSH, estradiol, and growth hormone and reduce adrenal cortical hormone levels, regulating follicle development [335,336]. Probiotics also improve fish follicle development. L. rhamnosus IMC 501 alters zebrafish oocyte constitution, facilitating maturation. Probiotics influence the endocrine system and peripheral tissues to upregulate leptin and Kiss2 and Kiss1 gene expression, modulating the constitution and maturation of oocytes [337].
9.7. Probiotics Maintain the Steady Provision of Crucial Elements through the Placenta
The transient placenta connects the mother’s uterus to the developing fetus during gestation. The placenta transfers essential nutrients and oxygen from the mother to the developing fetus, ensuring normal fetal growth [338]. Oral probiotics influence placental function. Probiotics such as E. faecium, especially its genetically modified strain, can migrate from the gastrointestinal tract to the developing fetus’s amniotic fluid via the placenta [339], suggesting that probiotic diets may influence the placental microbiome and placental functionality. Probiotics regulate placental genes associated with Toll-like receptors (TLRs) and autophagy-related proteins [340,341]. In infant–mother couples, placental tissue samples contain bacterial DNA. Microbial DNA in the placenta and amniotic fluid increases TLR-related gene expression in fetal intestinal tissue. Administering probiotics during pregnancy significantly regulated TLR-related genes in the gastrointestinal tract and placenta. During fetal development, microorganisms alter innate immunity gene expression in the intestines, implying that a maternal nutrition intervention with targeted probiotics may alter fetal and placental immune physiology [340]. L. rhamnosus GR-1 inhibits TNF-α generation in human placenta trophoblast cells induced by lipopolysaccharides (LPSs). Lipopolysaccharide administration increased IL-10, TNF-α, and prostaglandin-endoperoxide synthase 2 (PTGS2) expression, with a more significant prevalence in male placentae. The L. rhamnosus GR-1 supernatant hinders TNF-α production from LPS and promotes IL-10 production. The female placentae expressed more PGDH, while the male placentae had less LPS-stimulated PTGS2 and more TLR-4. These findings support lactobacilli as a treatment for premature labor [342]. Probiotics reduce placenta inflammation, reducing the risk of severe preeclampsia. First-time mothers who regularly consume milk-derived probiotics are less likely to develop preeclampsia [343]. Probiotics affect placental function by boosting immune response. Prebiotics and probiotics also increase sow serum triacylglycerol and decrease umbilical venous serum total cholesterol, suggesting that probiotics and prebiotics improve placental lipid metabolism [344]. These findings show that probiotic interventions improve placental function by changing microbiota composition, increasing immune response, and improving metabolic control during pregnancy.
Probiotic supplementation improves gut microflora and metabolism in pregnant women, elevating the well-being of pregnant women and the fetus [345,346]. In meconium composition, when ingested during pregnancy, B. lactis and L. rhamnosus probiotics affect TLR gene regulation in the developing gut of the fetus, suggesting that probiotic strains affect the fetus’ immune system [340]. Probiotics, as placental therapies, also have the potential to reduce preterm delivery and placental efficiency [347]. Probiotic supplements in the later stages of pregnancy significantly elevate birth weight and litter weight, regardless of the round of pregnancy. Probiotics affect fetal development by increasing feed intake and immunoglobulin levels and modulating gut flora [348,349,350]. In umbilical venous serum, Bacillus mixed with isomaltooligosaccharide, a prebiotic, increases placental antioxidant capacity and growth hormone levels, leading to enhanced fetal development [344].
10. Probiotics Preclude the Prevalence of Cardiovascular Disease (CVD)
Over the past few decades, cardiovascular disease (CVD) has caused the most premature death and disability in low- and middle-income countries. Most developed nations have over 50% of middle-aged fatalities and 33% of senior deaths due to CVD. Cardiovascular disease (CVD) encompasses many cardiovascular system abnormalities, including peripheral vascular disease, cerebrovascular disease, and coronary heart disease (CHD). These conditions affect blood flow to the heart, brain, and peripheral organs [351]. Lesions in coronary, cerebral, or peripheral arteries can cause CVDs, leading to atherosclerosis, thrombosis, and clots [352,353].
Immune responses play a significant role in the development of atherosclerosis. Lipid plaques characterize atherosclerosis in arterial walls that gradually grow. Cholesterol from bloodstream LDL particles makes up these plaques. Lipoproteins enter arterial walls’ subendothelial compartment, activating endothelial cells. Monocytes in the arterial wall differentiate into macrophages, internalizing lipoproteins and becoming foam cells, a hallmark of atherosclerotic plaque [354]. Lipid-driven atherosclerosis, a chronic disease causing inflammation, is a major risk factor for heart disease and stroke. Clotting is a common pathophysiological mechanism in CVD, which begins with an inactive precursor or zymogen and then involves a cascade of proteolytic processes [351].
10.1. Probiotics Regulate Plasma Glucose and Inulin Levels
Probiotic supplementation reduces fasting plasma glucose, insulin resistance, insulin, and serum high-sensitivity C-reactive protein and elevates glutathione and antioxidant capacity. Moreover, probiotics improve total-/HDL-cholesterol ratio, HDL-cholesterol, glycemic control, oxidative stress, and inflammation in diabetic CHD patients (Table 2) [355].
The homeostasis model shows that vitamin D and probiotics reduce serum insulin levels and insulin resistance. Additionally, serum 25-OH-vitamin D levels, HDL-cholesterol, and quantitative insulin sensitivity check index elevate. Probiotic interventions significantly influence the plasma total antioxidant capacity (TAC), plasma nitric oxide (NO), and serum high-sensitivity C-reactive protein (hs-CRP) levels. Vitamin D and probiotics co-administration in diabetics and CHD patients improves mental health, serum hs-CRP, plasma NO, TAC, glycemic management, and HDL cholesterol [356].
Probiotics and selenium reduce fasting plasma insulin resistance, serum insulin levels, glucose levels, and insulin sensitivity. Co-supplementation also significantly reduces hs-CRP (Table 2), very low-density lipoprotein (VLDL), total cholesterol, and triglycerides and increases serum NO, total glutathione, and total antioxidant capacity. Selenium and probiotic supplements improve metabolic health in diabetics and CHD patients [357].
Synbiotic capsules reduce fasting plasma glucose and serum insulin levels. The intervention decreases the homeostasis model of b-cell function, elevates the quantitative insulin sensitivity check index, and significantly changes HLDL-cholesterol changes. The administration of synbiotic supplements to diabetic and CHD patients improves insulin metabolism and HDL cholesterol [358].
10.2. Probiotics Engage as Comforters in Coronary Artery Disease (CAD)
Lactobacillus plantarum 299v, as a circulatory system comforter, significantly improves brachial flow dilation but moderately influences body mass index, fasting glucose, and plasma cholesterol. L. plantarum 299v supplementation also decreases circulation levels of leptin, IL-12, and IL-8; however, it minimally affects plasma trimethylamine oxide. Moreover, the intervention raises propionate levels in plasma while reducing acetate. In CAD patients, L. plantarum 299v plasma increases endothelium-dependent vasodilation, improves vascular endothelial function, and reduces systemic inflammation in male CAD patients regardless of trimethylamine oxide levels or risk factors [359].
Lactobacillus reuteri also reduces myocardial injury after ischemia/reperfusion (I/R). L. reuteri ingestion protects against heart damage regardless of cholesterol levels, demonstrating the anti-inflammatory effects of probiotics without cholesterol benefits. Daily L. reuteri administration to normal and hypercholesterolemic lipoprotein receptor deletion mice decreases myocardial damage following ischemia-reperfusion without lowering total serum cholesterol. L. reuteri ensures cardiac damage protection and reduces ischemic heart injury as a probiotic [360].
10.3. Probiotics Diminish Inflammation-Associated Ailments
In cardiac injury patients, probiotics lower peripheral inflammation and boost FoxP3+, CD25+, and CD4+ regulatory T cells (Tregs) [361,362]. Congestive heart failure (CHF) reduces Tregs. Tregs at a low level leads to a poor prognostic approach in CHF patients with vitiated cardiac functioning [363].
Bifidobacterium animalis subsp. lactis 420, a potent probiotic, reduces heart inflammation. Specifically, it reduces heart damage from ischemia/reperfusion and causes left coronary artery permanent closure. Probiotics lead to Treg cell activation and epigenetic changes. Probiotics possess various therapeutic benefits for human diseases; however, extending their findings to comprehensive clinical cardiovascular protection is tricky [364].
Probiotics reduce peripheral inflammation by converting gut and peripheral dendritic cells into Treg [361]. Gut metabolites from microflora target conserved non-coding sections of foxp3 genes to affect Tregs directly. This interaction can increase FoxP3 acetylation, improving Treg cell activity and expression [365,366]. B. animalis subsp. lactis 420, elevates Ac-H3, normalized compared to H3, and increases posttranslational and epigenetic remodeling [364]. L. rhamnosus GR-1 shows excellent potential as a therapy for reducing the severity of heart failure [367]
11. Probiotics Alleviate Neurodegenerative and Neurodevelopmental Disorders
Probiotics and vitamin D improve psychological measures, as they have been shown to prompt decreases in Beck Depression Inventory, General Health Questionnaire, and Beck Anxiety Inventory scores [356]. Moreover, probiotic and selenium co-administration significantly reduced the Beck Anxiety Inventory index and Beck Depression Inventory score (Table 2) in [357].
Psychobiotics are probiotics with mental health benefits which produce or induce anti-inflammatory cytokines, SCFAs, neurotransmitters, and enteroendocrine hormones. They reduce stress and mood and aid in treating neurodegenerative and neurodevelopmental problems. The most prevalent psychobiotics are Enterococci, Streptococci, Lactobacilli, Escherichia, and Bifidobacteria. These bacteria regulate the gut–brain connection. Gut bacteria biosynthesize substances that enteric nervous system neurons use to communicate with the CNS [368].
Psychobiotics can treat various neurological illnesses, from stress, anxiety, and mood swings to Parkinson’s and Alzheimer’s (Table 2). Chronic psychobiotic use normalizes anxiety and depressive behavior [369,370]. B. longum 1714 strain improves cognition, behavior, and physiological response. L. rhamnosus JB 1 reduces despair-induced corticosterone and raises plus maze anxiety [371,372,373].
11.1. Probiotics Combat Insomnia
Psychobiotics have tremendous potential to treat insomnia and can improve Non-Rapid Eye Movement sleep efficiency and reduce awakening episodes in insomniacs during the resting period [374,375].
11.2. Probiotics Assist in Accommodating for Autism Spectrum Disorder (ASD)
Psychobiotics fix the dysbiosis detected in ASD patients, as gut microbiota, including Clostridium, Prevotella, Firmicutes, and Bacteroidetes, change during ASD [376,377]. The composition of SCFAs in the stool samples of people with ASD differs, but the relevance of these variations concerning autistic symptoms has only been narrowly explored. Moreover, metabolic products from probiotics butyrate improve ASD symptoms [378]. Neuropsychiatric disorders, including schizophrenia, are associated with a specific neurotransmitter, dopamine, which is synthesized by microflora [379], suggesting that alterations in the gut microbiota have an association with the development of schizophrenia.
11.3. Probiotics Help in Coping with Attention Deficit Hyperactivity Disorder (ADHD)
Psychobiotics also aid those with ADHD and Tourette’s syndrome who experience involuntary vocalizations and movements called ‘tics.’ [379]. Pieces of evidence demonstrate that the CNS and gut microbiota are linked to this effect, since ADHD risk factors are directly linked to gut microflora changes [380].
11.4. Probiotics Temper Parkinson’s and Alzheimer’s Disease
Environmental factors such as the gut microbiota are major players in developing neurodegenerative disorders like Parkinson’s disease, as shown through the changes in bowel function that occur before the typical motor symptoms appear. Dysbiosis in such a situation creates abnormal levels of particular bacteria, including Proteobacteria of the genus Ralstonia, Faecalibacterium, Enterobacteriaceae, Prevotellaceae, and butyrate-producing bacteria with ‘anti-inflammatory’ properties [378]. Additionally, patients with Parkinson’s disease exhibit reduced levels of SCFAs, indicating their potential involvement in the progression of diseases [381].
Furthermore, manipulating the gut microflora can have a positive impact on microglial activation and neuronal function in Alzheimer’s disease (AD) (Table 2). The risks for AD, such as obesity and type 2 diabetes, have an influence on the constitution of the microflora [378]. Changes in the population of microorganisms result in increased intestinal permeability and systemic inflammation, which, in turn, can lead to diabetes mellitus and insulin resistance [382]. The pathophysiology of AD is characterized by the accumulation of misfolded amyloid proteins. These proteins undergo sequential cleavages by various proteases, resulting in the formation of the Aβ peptide. The gut microbiota regulates protease enzymatic activity, leading to inflammation. The gut microbiota is also a major element in the buildup of amyloid plaques [383]. These findings demonstrate psychobiotics’ potential as supplements to traditional drugs that can be employed to treat neuropsychiatric and neurodevelopmental diseases and for broader uses in the field.
12. Conclusions and Future Prospects
Probiotics exhibit considerable potential in promoting health and are frequently employed as agents that modulate the gastrointestinal tract to improve overall human health. The host possesses a significant defense mechanism known as the antioxidant system, as free radicals have been linked to many forms of cellular damage and consequent metabolic diseases or disorders. The therapeutic and prophylactic effects of probiotic microorganisms are attributed to their ability to produce numerous bioactive compounds and release them into the bloodstream through the digestive system or their area of prevalence, such as the oral and vaginal cavities. This is mainly achieved by forming short-chain fatty acids (SCFAs). These SCFAs serve as potent agents against several ailments and toxic conditions. Certain strains of probiotics can metabolize toxic chemicals, particularly amines and N-nitroso compounds. Short-chain fatty acids (SCFAs) and other bioactive compounds are produced in the colon through fermentation and delivered to the diseased areas through the bloodstream. These compounds elicit therapeutic effects on the host through a multitude of mechanisms, including alterations in the metabolic activity and composition of the gut microflora, maintaining intestinal health by strengthening the gut barrier and mucus layer, immunomodulation, the degradation and binding of toxic compounds, the modulation of the expression levels of genes in different organs, the altering of pathogen functioning, changes in host physiology, the inhibition of cell proliferation, anti-mutagenic effects, and the induction of apoptosis in cases involving cancer.
Moreover, it has been established that many probiotics, such as Lactobacillus rhamnosus GG, influence several organs simultaneously. For instance, L. rhamnosus GG palliates oral and gastrointestinal disorders, improves barrier dysfunction, reduces diarrheal episodes, treats hypertransaminasemia, reduces pancreatic cancer risk, reduces pathogenic Proteobacteria, exhibits beneficial effects on cardiac remodeling, and exerts healthful impacts on inflammatory biomarkers, anxiety, depression, etc. L. rhamnosus GG achieves this by promoting the production of anti-inflammatory cytokines such as interleukin (IL)-4 and IL-10, hindering pro-inflammatory cytokines, including IL-1, IL-6, and TNF-α. Several Lactobacillus spp. and Bifidobacterium spp. have the curative power needed to soothe several organs, requiring robust exploration. Furthermore, a sprucely designed cocktail of Lactobacillus and Bifidobacterium species can alleviate ailments throughout the human body, diminishing the need for multiple doses or medication.
The use of probiotics over an extended period has the potential to enhance and regulate the immune system by inflecting the related regulatory genes and releasing anti-inflammatory cytokines. Furthermore, incorporating food supplements containing a synergistic blend of appropriate probiotics has the potential to augment the functionality and viability of the host organism. Investigating the modification of the regulation of the immune system, gut microflora, and other pertinent pathways presents a captivating direction for further exploration. Furthermore, studying the influence of probiotics on receptors within human cells and their coordination with other organs is essential. These investigations could specifically aim to determine whether these effects are directly caused by probiotic interventions or whether alterations in the overall constitution of the microflora arbitrate them. Such research is crucial for comprehending the mechanisms elucidated in this review article.
Furthermore, the need for alternative therapeutics can be better understood by examining the escalating rate of antibiotic resistance in case of bacterial infections and the declining patient adherence to current treatment protocols. Therefore, while the antagonistic effects of probiotics on several pathogens are specific to certain strains, the potential for probiotics to be utilized as a future treatment modality, either alone or in conjunction with established therapeutic approaches such as adjuvant therapy, drug delivery systems, and immune system enhancement, may increase in light of the ongoing and anticipated rise in antibiotic resistance.
Hence, forthcoming investigations may need to contemplate conducting in vitro examinations on synthesizing probiotic bioactive compounds that exhibit gastrointestinal properties within a simulated gastrointestinal environment encompassing a combination of enzymes, acids, salts, mucus, and other relevant factors. To accurately determine the genes and bioactive chemicals that are activated and produced in eaten probiotics, it is imperative for such research to also take into account the impact of illness circumstances on the immune system, microbial competition, and gut host antimicrobial proteins.
It is imperative to conduct meticulously designed trials to enhance our knowledge and comprehension of the specific probiotic molecules that elicit particular effects. These trials should employ appropriate quantities of purified bioactive chemicals derived from probiotics or suitable numbers of probiotic mutants with targeted gene knock-outs or knock-ins. These challenges would necessitate the establishment of various crucial parameters, including the potency of the probiotic strain, its optimal dosage, the desired host response, its specific place within the host organism, and other pertinent factors.
Author Contributions
Conceptualization, M.S.V. and M.A.V.; methodology, M.S.V.; software, M.S.V. and M.A.V.; validation, M.S.V. and X.R.; formal analysis, M.S.V. and M.G.; investigation, M.S.V.; resources, M.S.V. and T.T.; data curation, M.S.V. and T.T.; writing—original draft preparation, M.S.V.; writing—review and editing, M.S.V., M.A.V., Y.H., M.G., A.Q., A.R. (Abdur Rehman), A.R. (Arif Rashid), J.-N.E., X.H., and J.W.; visualization, M.S.V. and X.R.; supervision, X.R.; project administration, X.R.; funding acquisition, X.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017, 32, 300–313. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.-R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Proctor, L.M. The Human Microbiome Project in 2011 and beyond. Cell Host Microbe 2011, 10, 287–291. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Lourens-Hattingh, A.; Viljoen, B.C. Yogurt as probiotic carrier food. Int. Dairy J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Kang, J.H.; Yun, S., II; Park, M.H.; Park, J.H.; Jeong, S.Y.; Park, H.O. Anti-Obesity Effect of Lactobacillus gasseri BNR17 in High-Sucrose Diet-Induced Obese Mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, I.K. In-Vitro Characterization of the Anti-Cancer Activity of the Probiotic Bacterium Lactobacillus fermentum NCIMB 5221 and Potential against Colorectal Cancer. J. Cancer Sci. Ther. 2015, 7, 224–235. [Google Scholar] [CrossRef]
- Grover, S.; Rashmi, H.M.; Srivastava, A.K.; Batish, V.K. Probiotics for human health -new innovations and emerging trends. Gut Pathog. 2012, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Im, S.-H. Probiotics as an Immune Modulator. J. Nutr. Sci. Vitaminol. 2015, 61 (Suppl. S61), S103–S105. [Google Scholar] [CrossRef]
- Britton, R.A.; Versalovic, J. Probiotics and Gastrointestinal Infections. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 290769. [Google Scholar] [CrossRef]
- Cookson, T.A. Bacterial-Induced Blood Pressure Reduction: Mechanisms for the Treatment of Hypertension via the Gut. Front. Cardiovasc. Med. 2021, 8, 721393. [Google Scholar] [CrossRef]
- Guarino, M.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Mahasneh, S.A.; Mahasneh, A.M. Probiotics: A promising role in dental health. Dent. J. 2017, 5, 26. [Google Scholar] [CrossRef]
- Allaker, R.P.; Stephen, A.S. Use of Probiotics and Oral Health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef] [PubMed]
- İnce, G.; Gürsoy, H.; İpçi, Ş.D.; Cakar, G.; Emekli-Alturfan, E.; Yılmaz, S. Clinical and Biochemical Evaluation of Lozenges Containing Lactobacillus reuteri as an Adjunct to Non-Surgical Periodontal Therapy in Chronic Periodontitis. J. Periodontol. 2015, 86, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Kojima, Y.; Seneviratne, C.J.; Maeda, N. Therapeutic application of synbiotics, a fusion of probiotics and prebiotics, and biogenics as a new concept for oral Candida infections: A mini review. Front. Microbiol. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A Review of the Role of Probiotic Supplementation in Dental Caries. Probiotics Antimicrob. Proteins 2020, 12, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Kuru, B.E.; Laleman, I.; Yalnızoğlu, T.; Kuru, L.; Teughels, W. The Influence of a Bifidobacterium animalis Probiotic on Gingival Health: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Jurevic, R.J.; Coulton, K.K.; Tsutsui, M.T.; Roberts, M.C.; Kimball, J.R.; Wells, N.; Berndt, J.; Dale, B.A. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob. Agents Chemother. 2005, 49, 3883–3888. [Google Scholar] [CrossRef] [PubMed]
- Shifa, S.; Muthu, M.S.; Amarlal, D.; Rathna Prabhu, V. Quantitative assessment of IgA levels in the unstimulated whole saliva of caries-free and caries-active children. J. Indian Soc. Pedod. Prev. Dent. 2008, 26, 158–161. [Google Scholar]
- Comelli, E.M.; Guggenheim, B.; Stingele, F.; Neeser, J.R. Selection of dairy bacterial strains as probiotics for oral health. Eur. J. Oral Sci. 2002, 110, 218–224. [Google Scholar] [CrossRef]
- Kang, M.S.; Chung, J.; Kim, S.M.; Yang, K.H.; Oh, J.S. Effect of Weissella cibaria isolates on the formation of Streptococcus mutans biofilm. Caries Res. 2006, 40, 418–425. [Google Scholar] [CrossRef]
- Nase, L.; Hatakka, K.; Savilahti, E.; Saxelin, M.; Pönkä, A.; Poussa, T.; Korpela, R.; Meurman, J.H. Effect of Long-Term Consumption of a Probiotic Bacterium, Lactobacillus rhamnosus GG, in Milk on Dental Caries and Caries Risk in Children. Caries Res. 2001, 35, 412–420. [Google Scholar] [CrossRef]
- Çaglar, E.; Kavaloglu, S.C.; Kuscu, O.O.; Sandalli, N.; Holgerson, P.L.; Twetman, S. Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin. Oral Investig. 2007, 11, 425–429. [Google Scholar] [CrossRef]
- Nikawa, H.; Makihira, S.; Fukushima, H.; Nishimura, H.; Ozaki, Y.; Ishida, K.; Darmawan, S.; Hamada, T.; Hara, K.; Matsumoto, A.; et al. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int. J. Food Microbiol. 2004, 95, 219–223. [Google Scholar] [CrossRef]
- Bolla, V.L.; Reddy, M.S.; Srinivas, N.; Reddy, C.S.; Koppolu, P. Investigation and comparison of the effects of two probiotic bacteria, and in reducing mutans streptococci levels in the saliva of children. Ann. Afr. Med. 2022, 21, 395–402. [Google Scholar] [CrossRef]
- Krasse, P.; Carlsson, B.; Dahl, C.; Paulsson, A.; Nilsson, A.; Sinkiewicz, G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed. Dent. J. 2006, 30, 55–60. [Google Scholar] [PubMed]
- Alkaya, B.; Laleman, I.; Keceli, S.; Ozcelik, O.; Cenk Haytac, M.; Teughels, W. Clinical effects of probiotics containing Bacillus species on gingivitis: A pilot randomized controlled trial. J. Periodontal Res. 2017, 52, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Toiviainen, A.; Jalasvuori, H.; Lahti, E.; Gursoy, U.; Salminen, S.; Fontana, M.; Flannagan, S.; Eckert, G.; Kokaras, A.; Paster, B.; et al. Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 on the number of salivary mutans streptococci, amount of plaque, gingival inflammation and the oral microbiome in healthy adults. Clin. Oral Investig. 2015, 19, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Lauritano, D.; Candotto, V.; Silvestre, F.J.; Nardi, G.M. Oral probiotics in the management of gingivitis in diabetic patients: A double blinded randomized controlled study. J. Biol. Regul. Homeost. Agents 2017, 31, 197–202. [Google Scholar] [PubMed]
- van den Velde, S.; Quirynen, M.; Van Hee, P.; van Steenberghe, D. Halitosis associated volatiles in breath of healthy subjects. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 853, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Musciotto, A.; Di Fede, O.; Di Marco, V.; Craxì, A. Halitosis: Could it be more than mere bad breath? Intern. Emerg. Med. 2011, 6, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Greenman, J. Halitosis (breath odor). Periodontol. 2000 2008, 48, 66–75. [Google Scholar] [CrossRef]
- Hyink, O.; Wescombe, P.A.; Upton, M.; Ragland, N.; Burton, J.P.; Tagg, J.R. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivanus K12. Appl. Environ. Microbiol. 2007, 73, 1107–1113. [Google Scholar] [CrossRef]
- Burton, J.P.; Chilcott, C.N.; Moore, C.J.; Speiser, G.; Tagg, J.R. A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J. Appl. Microbiol. 2006, 100, 754–764. [Google Scholar] [CrossRef]
- Burton, J.P.; Chilcott, C.N.; Tagg, J.R. The rationale and potential for the reduction of oral malodour using Streptococcus salivarius probiotics. Oral Dis. 2005, 11, 29–31. [Google Scholar] [CrossRef]
- Allaker, R.P.; Waite, R.D.; Hickling, J.; North, M.; McNab, R.; Bosma, M.P.; Hughes, F.J. Topographic distribution of bacteria associated with oral malodour on the tongue. Arch. Oral Biol. 2008, 53, S8–S12. [Google Scholar] [CrossRef]
- Eren, A.M.; Borisy, G.G.; Huse, S.M.; Mark Welch, J.L. Oligotyping analysis of the human oral microbiome. Proc. Natl. Acad. Sci. USA 2014, 111, E2875–E2884. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Vaitla, P. Streptococcus Pyogenes; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dierksen, K.P.; Moore, C.J.; Inglis, M.; Wescombe, P.A.; Tagg, J.R. The effect of ingestion of milk supplemented with salivaricin A-producing Streptococcus salivarius on the bacteriocin-like inhibitory activity of streptococcal populations on the tongue. FEMS Microbiol. Ecol. 2007, 59, 584–591. [Google Scholar] [CrossRef]
- Nadelman, P.; Magno, M.B.; Masterson, D.; da Cruz, A.G.; Maia, L.C. Are dairy products containing probiotics beneficial for oral health? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2763–2785. [Google Scholar] [CrossRef]
- Srivastava, S.; Saha, S.; Kumari, M.; Mohd, S. Effect of Probiotic Curd on Salivary pH and Streptococcus mutans: A Double Blind Parallel Randomized Controlled Trial. J. Clin. Diagn. Res. 2016, 10, ZC13–ZC16. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, U.; Chhabra, T.; Sethuraman, R. Effect of probiotics on the amount and pH of saliva in edentulous patients: A Prospective study. J. Indian Prosthodont. Soc. 2018, 18, 277–281. [Google Scholar] [PubMed]
- Pradeep, K.; Kuttappa, M.A.; Prasana, K.R. Probiotics and oral health: An update. SADJ 2014, 69, 20–24. [Google Scholar]
- Wallace, T.C.; Guarner, F.; Madsen, K.; Cabana, M.D.; Gibson, G.; Hentges, E.; Sanders, M.E. Human gut microbiota and its relationship to health and disease. Nutr. Rev. 2011, 69, 392–403. [Google Scholar] [CrossRef]
- Mack, D.R.; Ahrne, S.; Hyde, L.; Wei, S.; Hollingsworth, M.A. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 2003, 52, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Ahola, A.J.; Yli-Knuuttila, H.; Richardson, M.; Poussa, T.; Meurman, J.H.; Korpela, R. Probiotics Reduce the Prevalence of Oral Candida in the Elderly—A Randomized Controlled Trial. J. Dent. Res. 2007, 86, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kusumaningsih, T.; Subijanto, M.S.; Indrawati, R.; Devijanti, R.R. The level of beta defensin-2 in saliva and its expression in parotid gland epithelial cells after probiotic (Lactobacillus reuteri) induction to inhibit Streptococcus mutans in caries. Eur. J. Dent. 2016, 10, 556–560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lundtorp-Olsen, C.; Enevold, C.; Juel Jensen, C.A.; Stofberg, S.N.; Twetman, S.; Belstrøm, D. Impact of Probiotics on the Salivary Microbiota and Salivary Levels of Inflammation-Related Proteins during Short-Term Sugar Stress: A Randomized Controlled Trial. Pathogens 2021, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Hallström, H.; Lindgren, S.; Yucel-Lindberg, T.; Dahlén, G.; Renvert, S.; Twetman, S. Effect of probiotic lozenges on inflammatory reactions and oral biofilm during experimental gingivitis. Acta Odontol. Scand. 2013, 71, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Szkaradkiewicz, A.K.; Stopa, J.; Karpiński, T.M. Effect of Oral Administration Involving a Probiotic Strain of Lactobacillus reuteri on Pro-Inflammatory Cytokine Response in Patients with Chronic Periodontitis. Arch. Immunol. Ther. Exp. 2014, 62, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.K.; Brandsborg, E.; Holmstrøm, K.; Twetman, S. Effect of tablets containing probiotic candidate strains on gingival inflammation and composition of the salivary microbiome: A randomised controlled trial. Benef. Microbes 2018, 9, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Alanzi, A.; Honkala, S.; Honkala, E.; Varghese, A.; Tolvanen, M.; Söderling, E. Effect of Lactobacillus rhamnosus and Bifidobacterium lactis on gingival health, dental plaque, and periodontopathogens in adolescents: A randomised placebo-controlled clinical trial. Benef. Microbes 2018, 9, 593–602. [Google Scholar] [CrossRef]
- Minić, I.; Pejčić, A.; Bradić-Vasić, M. Effect of the local probiotics in the therapy of periodontitis A randomized prospective study. Int. J. Dent. Hyg. 2022, 20, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Angarita-Díaz, M.P.; Forero-Escobar, D.; Cerón-Bastidas, X.A.; Cisneros-Hidalgo, C.A.; Dávila-Narvaez, F.; Bedoya-Correa, C.M.; Freitas, S.C.; Cabrera-Arango, C.L.; Melo-Colina, R. Effects of a functional food supplemented with probiotics on biological factors related to dental caries in children: A pilot study. Eur. Arch. Paediatr. Dent. 2020, 21, 161–169. [Google Scholar] [CrossRef]
- Ashwin, D.; Ke, V.; Taranath, M.; Ramagoni, N.K.; Nara, A.; Sarpangala, M. Effect of Probiotic Containing Ice-cream on Salivary Mutans Streptococci (SMS) Levels in Children of 6-12 Years of Age: A Randomized Controlled Double Blind Study with Six-months Follow Up. J. Clin. Diagn. Res. 2015, 9, ZC06–ZC09. [Google Scholar] [CrossRef] [PubMed]
- Alamoudi, N.M.; Almabadi, E.S.; El Ashiry, E.A.; El Derwi, D.A. Effect of Probiotic Lactobacillus reuteri on Salivary Cariogenic Bacterial Counts among Groups of Preschool Children in Jeddah, Saudi Arabia: A Randomized Clinical Trial. J. Clin. Pediatr. Dent. 2018, 42, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Zanvit, A.; Nobili, P.; Risso, P.; Fornaini, C. Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: Results of a randomized, controlled study. Clin. Cosmet. Investig. Dent. 2015, 7, 107–113. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoneda, M.; Tanabe, K.; Fujimoto, A.; Iha, K.; Seno, K.; Yamada, K.; Iwamoto, T.; Masuo, Y.; Hirofuji, T. Lactobacillus salivarius WB21–containing tablets for the treatment of oral malodor: A double-blind, randomized, placebo-controlled crossover trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 462–470. [Google Scholar] [CrossRef]
- Penala, S.; Kalakonda, B.; Pathakota, K.R.; Jayakumar, A.; Koppolu, P.; Lakshmi, B.V.; Pandey, R.; Mishra, A. Efficacy of local use of probiotics as an adjunct to scaling and root planing in chronic periodontitis and halitosis: A randomized controlled trial. J. Res. Pharm. Pract. 2016, 5, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.K.; Bardow, A.; Jensdottir, T.; Lykkeaa, J.; Twetman, S. Effect of chewing gums containing the probiotic bacterium Lactobacillus reuteri on oral malodour. Acta Odontol. Scand. 2012, 70, 246–250. [Google Scholar] [CrossRef]
- He, L.; Yang, H.; Chen, Z.; Ouyang, X. The Effect of Streptococcus salivarius K12 on Halitosis: A Double-Blind, Randomized, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2020, 12, 1321–1329. [Google Scholar] [CrossRef]
- Lee, D.-S.; Lee, S.-A.; Kim, M.; Nam, S.-H.; Kang, M.-S. Reduction of Halitosis by a Tablet Containing Weissella cibaria CMU: A Randomized, Double-Blind, Placebo-Controlled Study. J. Med. Food 2020, 23, 649–657. [Google Scholar] [CrossRef]
- Bonfrate, L.; Di Palo, D.M.; Celano, G.; Albert, A.; Vitellio, P.; De Angelis, M.; Gobbetti, M.; Portincasa, P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020, 50, e13201. [Google Scholar] [CrossRef]
- Sadrin, S.; Sennoune, S.; Gout, B.; Marque, S.; Moreau, J.; Zinoune, K.; Grillasca, J.-P.; Pons, O.; Maixent, J.-M. A 2-strain mixture of Lactobacillus acidophilus in the treatment of irritable bowel syndrome: A placebo-controlled randomized clinical trial. Dig. Liver Dis. 2020, 52, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Andresen, V.; Gschossmann, J.; Layer, P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol. Hepatol. 2020, 5, 658–666. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Lailerd, N.; Sirilun, S.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Saelee, M.; Chaiyasut, K.; Kesika, P.; et al. Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial. Foods 2022, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Leber, B.; Schmerboeck, B.; Tawdrous, M.; Zettel, G.; Hartl, A.; Madl, T.; Stryeck, S.; Fuchs, D.; Lemesch, S.; et al. Randomised clinical trial: The effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 2016, 44, 926–935. [Google Scholar] [CrossRef]
- Wang, J.; Ke, H.; Liu, K.-X.; Qu, J.-M. Effects of exogenous probiotics on the gut microbiota and clinical outcomes in critically ill patients: A randomized controlled trial. Ann. Palliat. Med. 2021, 10, 1180–1190. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Horvath, A.; Komarova, I.; Schmerboeck, B.; Feldbacher, N.; Klymiuk, I.; Durdevic, M.; Rainer, F.; Blesl, A.; Stiegler, P.; et al. Dysbiosis in early sepsis can be modulated by a multispecies probiotic: A randomised controlled pilot trial. Benef. Microbes 2019, 10, 265–278. [Google Scholar] [CrossRef]
- Li, S.; Yin, Y.; Xiao, D.; Zou, Y. Supplemental bifid triple viable capsule treatment improves inflammatory response and T cell frequency in ulcerative colitis patients. BMC Gastroenterol. 2021, 21, 314. [Google Scholar] [CrossRef] [PubMed]
- Kamarlı Altun, H.; Akal Yıldız, E.; Akın, M. Effects of synbiotic therapy in mild-to-moderately active ulcerative colitis: A randomized placebo-controlled study. Turk. J. Gastroenterol. 2019, 30, 313–320. [Google Scholar] [CrossRef]
- Bjarnason, I.; Sission, G.; Hayee, B. A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology 2019, 27, 465–473. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Folwarski, M.; Skonieczna-Żydecka, K.; Ruszkowski, J.; Makarewicz, W. The use of Lactobacillus plantarum 299v (DSM 9843) in cancer patients receiving home enteral nutrition—Study protocol for a randomized, double-blind, and placebo-controlled trial. Nutr. J. 2020, 19, 98. [Google Scholar] [CrossRef]
- Cai, G.-S.; Su, H.; Zhang, J. Protective effect of probiotics in patients with non-alcoholic fatty liver disease. Medicine 2020, 99, e21464. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B.; Jun, D.W.; Kang, B.-K.; Lim, J.H.; Lim, S.; Chung, M.-J. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 5688. [Google Scholar] [CrossRef] [PubMed]
- Bakhshimoghaddam, F.; Shateri, K.; Sina, M.; Hashemian, M.; Alizadeh, M. Daily Consumption of Synbiotic Yogurt Decreases Liver Steatosis in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J. Nutr. 2018, 148, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointestin. Liver Dis. 2018, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Nor, M.H.; Ayob, N.; Mokhtar, N.M.; Raja Ali, R.A.; Tan, G.C.; Wong, Z.; Shafiee, N.H.; Wong, Y.P.; Mustangin, M.; Nawawi, K.N.M. The Effect of Probiotics (MCP® BCMC® Strains) on Hepatic Steatosis, Small Intestinal Mucosal Immune Function, and Intestinal Barrier in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 3192. [Google Scholar] [CrossRef]
- Han, M.-L.; Lee, M.-H.; Lee, W.-J.; Chen, S.-C.; Almalki, O.M.; Chen, J.-C.; Wu, C.-C. Probiotics for gallstone prevention in patients with bariatric surgery: A prospective randomized trial. Asian J. Surg. 2022, 45, 2664–2669. [Google Scholar] [CrossRef]
- Gao, F.; Guan, D.; Wang, G.; Zhang, L.; He, J.; Lv, W.; Zhang, X.; Tao, W.; Dai, Y.; Xu, S.; et al. Effects of oral tauroursodeoxycholic acid and/or intestinal probiotics on serum biochemical indexes and bile composition in patients with cholecystolithiasis. Front. Pharmacol. 2022, 13, 882764. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-D.; Zhu, R.-X.; Bian, Z.-Z.; Sun, T.-W. Effect of probiotics on length of hospitalization in mild acute pancreatitis: A randomized, double-blind, placebo-controlled trial. World J. Gastroenterol. 2021, 27, 224–232. [Google Scholar] [CrossRef]
- Sharma, B.; Srivastava, S.; Singh, N.; Sachdev, V.; Kapur, S.; Saraya, A. Role of Probiotics on Gut Permeability and Endotoxemia in Patients With Acute Pancreatitis. J. Clin. Gastroenterol. 2011, 45, 442–448. [Google Scholar] [CrossRef]
- Savytska, M.; Kyriienko, D.; Komisarenko, I.; Kovalchuk, O.; Falalyeyeva, T.; Kobyliak, N. Probiotic for Pancreatic β-Cell Function in Type 2 Diabetes: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Diabetes Ther. 2023, 14, 1915–1931. [Google Scholar] [CrossRef]
- Rammohan, A.; Sathyanesan, J.; Rajendran, K.; Pitchaimuthu, A.; Perumal, S.K.; Balaraman, K.; Ramasamy, R.; Palaniappan, R.; Govindan, M. Synbiotics in Surgery for Chronic Pancreatitis. Ann. Surg. 2015, 262, 31–37. [Google Scholar] [CrossRef]
- dos Santos, P.Q.; Guedes, J.C.; de Jesus, R.P.; dos Santos, R.R.; Fiaconne, R.L. Effects of using symbiotics in the clinical nutritional evolution of patients with chronic pancreatitis: Study prospective, randomized, controlled, double blind. Clin. Nutr. ESPEN 2017, 18, 9–15. [Google Scholar] [CrossRef]
- Braga, V.L.; Rocha, L.P.D.S.; Bernardo, D.D.; Cruz, C.d.O.; Riera, R. What do Cochrane systematic reviews say about probiotics as preventive interventions? Sao Paulo Med. J. 2017, 135, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J. Cell. Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, X.; Liu, M.; Guo, X. Effect of three lactobacilli with strain-specific activities on the growth performance, faecal microbiota and ileum mucosa proteomics of piglets. J. Anim. Sci. Biotechnol. 2017, 8, 52. [Google Scholar] [CrossRef]
- Yang, G.-Y.; Yu, J.; Su, J.-H.; Jiao, L.-G.; Liu, X.; Zhu, Y.-H. Oral Administration of Lactobacillus rhamnosus GG Ameliorates Salmonella Infantis-Induced Inflammation in a Pig Model via Activation of the IL-22BP/IL-22/STAT3 Pathway. Front. Cell. Infect. Microbiol. 2017, 7, 323. [Google Scholar] [CrossRef]
- Rao, R.K.; Samak, G. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr. Nutr. Food Sci. 2013, 9, 99–107. [Google Scholar]
- Yi, H.; Wang, L.; Xiong, Y.; Wen, X.; Wang, Z.; Yang, X.; Gao, K.; Jiang, Z. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J. Anim. Sci. 2018, 96, 2342–2351. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.; Dou, X.; Wang, C.; Zhang, W.; Gao, K.; Liu, J.; Wang, H. Lactobacillus reuteri ZJ617 maintains intestinal integrity via regulating tight junction, autophagy and apoptosis in mice challenged with lipopolysaccharide. Oncotarget 2017, 8, 77489–77499. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeung, W.; Choi, I.-D.; Jeong, J.-W.; Lee, D.E.; Huh, C.-S.; Kim, G.-B.; Hong, S.S.; Shim, J.-J.; Lee, J.L.; et al. Lactic Acid Bacteria Improves Peyer’s Patch Cell-Mediated Immunoglobulin A and Tight-Junction Expression in a Destructed Gut Microbial Environment. J. Microbiol. Biotechnol. 2016, 26, 1035–1045. [Google Scholar] [CrossRef]
- Terciolo, C.; Dapoigny, M.; Andre, F. Beneficial effects of Saccharomyces boulardii CNCM I-745 on clinical disorders associated with intestinal barrier disruption. Clin. Exp. Gastroenterol. 2019, 12, 67–82. [Google Scholar] [CrossRef]
- Dukowicz, A.C.; Lacy, B.E.; Levine, G.M. Small intestinal bacterial overgrowth: A comprehensive review. Gastroenterol. Hepatol. (N. Y). 2007, 3, 112–122. [Google Scholar]
- Grace, E.; Shaw, C.; Whelan, K.; Andreyev, H.J.N. Review article: Small intestinal bacterial overgrowth—Prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment. Pharmacol. Ther. 2013, 38, 674–688. [Google Scholar] [CrossRef]
- Stanghellini, V. Functional Dyspepsia and Irritable Bowel Syndrome: Beyond Rome IV. Dig. Dis. 2017, 35, 14–17. [Google Scholar] [CrossRef]
- Principi, N.; Cozzali, R.; Farinelli, E.; Brusaferro, A.; Esposito, S. Gut dysbiosis and irritable bowel syndrome: The potential role of probiotics. J. Infect. 2018, 76, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Catinean, A.; Neag, A.M.; Nita, A.; Buzea, M.; Buzoianu, A.D. Bacillus spp. Spores—A Promising Treatment Option for Patients with Irritable Bowel Syndrome. Nutrients 2019, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Veltkamp, C.; Dieleman, L.A.; Grenther, W.B.; Wyrick, P.B.; Tonkonogy, S.L.; Sartor, R.B. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm. Bowel Dis. 2002, 8, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Fabia, R.; Ar’Rajab, A.; Johansson, M.L.; Willén, R.; Andersson, R.; Molin, G.; Bengmark, S. The effect of exogenous administration of Lactobacillus reuteri R2LC and oat fiber on acetic acid-induced colitis in the rat. Scand. J. Gastroenterol. 1993, 28, 155–162. [Google Scholar] [CrossRef]
- Isolauri, E.; Arvola, T.; Sütas, Y.; Moilanen, E.; Salminen, S. Probiotics in the management of atopic eczema. Clin. Exp. Allergy 2000, 30, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Song, M.; Lu, X.; Zhu, X.; Deng, J. Gut microbes in gastrointestinal cancers. Semin. Cancer Biol. 2022, 86, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Chu, S.-H.; Jeon, J.Y.; Lee, M.-K.; Park, J.-H.; Lee, D.-C.; Lee, J.-W.; Kim, N.-K. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: A double-blind, randomized, placebo-controlled trial. Dig. Liver Dis. 2014, 46, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Mörkl, S.; Lackner, S.; Meinitzer, A.; Mangge, H.; Lehofer, M.; Halwachs, B.; Gorkiewicz, G.; Kashofer, K.; Painold, A.; Holl, A.K.; et al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur. J. Nutr. 2018, 57, 2985–2997. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Huang, M.-J.; Zhang, X.-W.; Wang, L.; Huang, N.-Q.; Peng, H.; Lan, P.; Peng, J.-S.; Yang, Z.; Xia, Y.; et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: A double-center and double-blind randomized clinical trial. Am. J. Clin. Nutr. 2013, 97, 117–126. [Google Scholar] [CrossRef]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef]
- Aisu, N.; Tanimura, S.; Yamashita, Y.; Yamashita, K.; Maki, K.; Yoshida, Y.; Sasaki, T.; Takeno, S.; Hoshino, S. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp. Ther. Med. 2015, 10, 966–972. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Wu, W.; Qin, H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol. Med. Rep. 2015, 12, 6119–6127. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients 2019, 11, 2642. [Google Scholar] [CrossRef]
- Vajro, P.; Mandato, C.; Licenziati, M.R.; Franzese, A.; Vitale, D.F.; Lenta, S.; Caropreso, M.; Vallone, G.; Meli, R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 740–743. [Google Scholar] [CrossRef]
- Abdel Monem, S.M. Probiotic Therapy in Patients with Nonalcoholic Steatohepatitis in Zagazig University Hospitals. Euroasian J. Hepato-Gastroenterol. 2017, 7, 101–106. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Wong, G.L.H.; Chim, A.M.L.; Chu, W.C.W.; Yeung, D.K.W.; Li, K.C.T.; Chan, H.L.Y. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann. Hepatol. 2013, 12, 256–262. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Li, L.; Yu, C.-H.; Shen, Z.; Chen, L.-H.; Li, Y.-M. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J. Gastroenterol. 2013, 19, 6911–6918. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, Y.; Wen, Y.; Liu, G.; Wan, C. Efficacy of probiotics in non-alcoholic fatty liver disease in adult and children: A meta-analysis of randomized controlled trials. Hepatol. Res. 2016, 46, 1226–1233. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. Alcohol or Gut Microbiota: Who Is the Guilty? Int. J. Mol. Sci. 2019, 20, 4568. [Google Scholar] [CrossRef]
- Loguercio, C.; Federico, A.; Tuccillo, C.; Terracciano, F.; D’Auria, M.V.; De Simone, C.; Blanco, C.D.V. Beneficial Effects of a Probiotic VSL#3 on Parameters of Liver Dysfunction in Chronic Liver Diseases. J. Clin. Gastroenterol. 2005, 39, 540–543. [Google Scholar] [PubMed]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium longum with Fructo-Oligosaccharides in Patients with Non Alcoholic Steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Acalovschi, M. Gallstones in patients with liver cirrhosis: Incidence, etiology, clinical and therapeutical aspects. World J. Gastroenterol. 2014, 20, 7277–7285. [Google Scholar] [CrossRef] [PubMed]
- Castro-Torres, I.G.; Cárdenas-Vázquez, R.d.J.; Velázquez-González, C.; Ventura-Martínez, R.; De la O-Arciniega, M.; Naranjo-Rodríguez, E.B.; Martínez-Vázquez, M. Future therapeutic targets for the treatment and prevention of cholesterol gallstones. Eur. J. Pharmacol. 2015, 765, 366–374. [Google Scholar] [CrossRef]
- Sato, S.; Nagai, H.; Igarashi, Y. Effect of Probiotics on Serum Bile Acids in Patients with Ulcerative Colitis. Hepatogastroenterology 2011, 59, 1804–1808. [Google Scholar]
- Takeda, Y.; Itoh, H.; Kobashi, K. Effect of Clostridium butyricum on the formation and dissolution of gallstones in experimental cholesterol cholelithiasis. Life Sci. 1983, 32, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, M.; Tanida, N.; Shimoyama, T. The role of intestinal bacteria in gallstone formation in animal model. A study on biliary lipid composition and bile acid profiles in bile, small intestinal contents and feces of Clostridium butyricum MIYAIRI No. 588 monocontaminated mice. Gastroenterol. Jpn. 1982, 17, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Horáčková, Š.; Plocková, M.; Demnerová, K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol. Adv. 2018, 36, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, M.-J.; Gao, Q.; Yang, J.-F.; Yang, L.; Pang, X.-L.; Jiang, X.-J. The effects of probiotics on total cholesterol. Medicine 2018, 97, e9679. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Parent, M.; Prakash, S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br. J. Nutr. 2012, 107, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, A.; Bookout, A.L.; Mangelsdorf, D.J. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat. Med. 2004, 10, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Kovatcheva-Datchary, P.; Ståhlman, M.; Bäckhed, F.; Marschall, H.-U. Crosstalk between Bile Acids and Gut Microbiota and Its Impact on Farnesoid X Receptor Signalling. Dig. Dis. 2017, 35, 246–250. [Google Scholar] [CrossRef]
- Devkota, S.; Chang, E.B. Interactions between Diet, Bile Acid Metabolism, Gut Microbiota, and Inflammatory Bowel Diseases. Dig. Dis. 2015, 33, 351–356. [Google Scholar] [CrossRef]
- Oh, J.K.; Kim, Y.R.; Lee, B.; Choi, Y.M.; Kim, S.H. Prevention of Cholesterol Gallstone Formation by Lactobacillus acidophilus ATCC 43121 and Lactobacillus fermentum MF27 in Lithogenic Diet-Induced Mice. Food Sci. Anim. Resour. 2021, 41, 343–352. [Google Scholar] [CrossRef]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef]
- Ko, J.-S.; Yang, H.-R.; Chang, J.-Y.; Seo, J.-K. Lactobacillus plantarum inhibits epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-alpha. World J. Gastroenterol. 2007, 13, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Gao, C.-F.; Wei, D.; Wang, C.; Ding, S.-Q. Acute pancreatitis: Etiology and common pathogenesis. World J. Gastroenterol. 2009, 15, 1427–1430. [Google Scholar] [CrossRef]
- Dervenis, C.; Hatzitheoklitos, E.; Smailis, D. Bacterial translocation and its prevention in acute pancreatitis. J. Hepatobiliary Pancreat. Surg. 2003, 10, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R.; et al. Burden of Gastrointestinal Disease in the United States: 2012 Update. Gastroenterology 2012, 143, 1179–1187.e3. [Google Scholar] [CrossRef]
- Singhal, B.; Mukherjee, A.; Srivastav, S. Role of Probiotics in Pancreatic Cancer Prevention: The Prospects and Challenges. Adv. Biosci. Biotechnol. 2016, 07, 468–500. [Google Scholar] [CrossRef]
- Muftuoglu, M.A.T.; Isikgor, S.; Tosun, S.; Saglam, A. Effects of probiotics on the severity of experimental acute pancreatitis. Eur. J. Clin. Nutr. 2006, 60, 464–468. [Google Scholar] [CrossRef]
- van Minnen, L.P.; Timmerman, H.M.; Lutgendorff, F.; Verheem, A.; Harmsen, W.; Konstantinov, S.R.; Smidt, H.; Visser, M.R.; Rijkers, G.T.; Gooszen, H.G.; et al. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery 2007, 141, 470–480. [Google Scholar] [CrossRef]
- Oláh, A.; Belágyi, T.; Pótó, L.; Romics, L.; Bengmark, S. Synbiotic control of inflammation and infection in severe acute pancreatitis: A prospective, randomized, double blind study. Hepatogastroenterology 2007, 54, 590–594. [Google Scholar]
- Oláh, A.; Romics, L., Jr. Early enteral nutrition in acute pancreatitis—Benefits and limitations. Langenbeck’s Arch. Surg. 2008, 393, 261–269. [Google Scholar] [CrossRef]
- Mangiante, G.; Colucci, G.; Canepari, P.; Bassi, C.; Nicoli, N.; Casaril, A.; Marinello, P.; Signoretto, C.; Bengmark, S. Lactobacillus plantarum reduces infection of pancreatic necrosis in experimental acute pancreatitis. Dig. Surg. 2001, 18, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorff, F.; Trulsson, L.M.; van Minnen, L.P.; Rijkers, G.T.; Timmerman, H.M.; Franzén, L.E.; Gooszen, H.G.; Akkermans, L.M.A.; Söderholm, J.D.; Sandström, P.A. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1111–G1121. [Google Scholar] [CrossRef]
- Rohith, G.; Sureshkumar, S.; Anandhi, A.; Kate, V.; Rajesh, B.S.; Abdulbasith, K.M.; Nanda, N.; Palanivel, C.; Vijayakumar, C. Effect of Synbiotics in Reducing the Systemic Inflammatory Response and Septic Complications in Moderately Severe and Severe Acute Pancreatitis: A Prospective Parallel-Arm Double-Blind Randomized Trial. Dig. Dis. Sci. 2023, 68, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, Y.; Yang, Q.; Lee, P.; Windsor, J.A.; Wu, D. An Updated Systematic Review With Meta-analysis: Efficacy of Prebiotic, Probiotic, and Synbiotic Treatment of Patients With Severe Acute Pancreatitis. Pancreas 2021, 50, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Felton, J.S.; Knize, M.G.; Wu, R.W.; Colvin, M.E.; Hatch, F.T.; Malfatti, M.A. Mutagenic potency of food-derived heterocyclic amines. Mutat. Res. Mol. Mech. Mutagen. 2007, 616, 90–94. [Google Scholar] [CrossRef]
- Turesky, R.J. Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol. Lett. 2007, 168, 219–227. [Google Scholar] [CrossRef]
- Sugimura, T.; Wakabayashi, K.; Nakagama, H.; Nagao, M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004, 95, 290–299. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, A.; Nagpal, R.; Mohania, D.; Behare, P.; Verma, V.; Kumar, P.; Poddar, D.; Aggarwal, P.K.; Henry, C.J.K.; et al. Cancer-preventing attributes of probiotics: An update. Int. J. Food Sci. Nutr. 2010, 61, 473–496. [Google Scholar] [CrossRef]
- Orrhage, K.M.; Annas, A.; Nord, C.E.; Brittebo, E.B.; Rafter, J.J. Effects of Lactic Acid Bacteria on the Uptake and Distribution of the Food Mutagen Trp-P-2 in Mice. Scand. J. Gastroenterol. 2002, 37, 215–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Zhang, J.; Li, Y.; He, Q.; Li, H.; Guo, X.; Guo, J.; Zhang, H. Probiotic Lactobacillus casei Zhang ameliorates high-fructose-induced impaired glucose tolerance in hyperinsulinemia rats. Eur. J. Nutr. 2014, 53, 221–232. [Google Scholar] [CrossRef]
- Rhee, C.-H.; Park, H.-D. Three Glycoproteins with Antimutagenic Activity Identified in Lactobacillus plantarum KLAB21. Appl. Environ. Microbiol. 2001, 67, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Niderkorn, V.; Boudra, H.; Morgavi, D.P. Binding of Fusarium mycotoxins by fermentative bacteria in vitro. J. Appl. Microbiol. 2006, 101, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Halttunen, T.; Tahvonen, R.; Salminen, S. Probiotic bacteria as potential detoxification tools: Assessing their heavy metal binding isotherms. Can. J. Microbiol. 2006, 52, 877–885. [Google Scholar] [CrossRef]
- Halttunen, T.; Collado, M.C.; El-Nezami, H.; Meriluoto, J.; Salminen, S. Combining strains of lactic acid bacteria may reduce their toxin and heavy metal removal efficiency from aqueous solution. Lett. Appl. Microbiol. 2007, 46, 160–165. [Google Scholar] [CrossRef]
- El-Nezami, H.S.; Chrevatidis, A.; Auriola, S.; Salminen, S.; Mykkänen, H. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit. Contam. 2002, 19, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J.; Haskard, C.A.; Ouwehand, A.C.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B 1 to cell wall components of Lactobacillus rhamnosus strain GG. Food Addit. Contam. 2004, 21, 158–164. [Google Scholar] [CrossRef]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. S8), 8–160. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- De Boeck, I.; van den Broek, M.F.L.; Allonsius, C.N.; Spacova, I.; Wittouck, S.; Martens, K.; Wuyts, S.; Cauwenberghs, E.; Jokicevic, K.; Vandenheuvel, D.; et al. Lactobacilli Have a Niche in the Human Nose. Cell Rep. 2020, 31, 107674. [Google Scholar] [CrossRef]
- Gan, W.; Yang, F.; Tang, Y.; Zhou, D.; Qing, D.; Hu, J.; Liu, S.; Liu, F.; Meng, J. The difference in nasal bacterial microbiome diversity between chronic rhinosinusitis patients with polyps and a control population. Int. Forum Allergy Rhinol. 2019, 9, 582–592. [Google Scholar] [CrossRef]
- Stearns, J.C.; Davidson, C.J.; McKeon, S.; Whelan, F.J.; Fontes, M.E.; Schryvers, A.B.; Bowdish, D.M.E.; Kellner, J.D.; Surette, M.G. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J. 2015, 9, 1246–1259. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Luo, Y.; Yuan, L.; Nelson, K.E.; Wang, Y.; Xiang, C.; Li, L. Pyrosequencing analysis of the human microbiota of healthy Chinese undergraduates. BMC Genom. 2013, 14, 390. [Google Scholar] [CrossRef]
- Jensen, A.; Fagö-Olsen, H.; Sørensen, C.H.; Kilian, M. Molecular mapping to species level of the tonsillar crypt microbiota associated with health and recurrent tonsillitis. PLoS ONE 2013, 8, e56418. [Google Scholar] [CrossRef]
- Abreu, N.A.; Nagalingam, N.A.; Song, Y.; Roediger, F.C.; Pletcher, S.D.; Goldberg, A.N.; Lynch, S. V Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci. Transl. Med. 2012, 4, 151ra124. [Google Scholar] [CrossRef]
- Hasegawa, K.; Linnemann, R.W.; Mansbach, J.M.; Ajami, N.J.; Espinola, J.A.; Petrosino, J.F.; Piedra, P.A.; Stevenson, M.D.; Sullivan, A.F.; Thompson, A.D.; et al. Nasal Airway Microbiota Profile and Severe Bronchiolitis in Infants: A Case-control Study. Pediatr. Infect. Dis. J. 2017, 36, 1044–1051. [Google Scholar] [CrossRef]
- Biesbroek, G.; Tsivtsivadze, E.; Sanders, E.A.M.; Montijn, R.; Veenhoven, R.H.; Keijser, B.J.F.; Bogaert, D. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am. J. Respir. Crit. Care Med. 2014, 190, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, I.; Wittouck, S.; Martens, K.; Claes, J.; Jorissen, M.; Steelant, B.; van den Broek, M.F.L.; Seys, S.F.; Hellings, P.W.; Vanderveken, O.M.; et al. Anterior Nares Diversity and Pathobionts Represent Sinus Microbiome in Chronic Rhinosinusitis. mSphere 2019, 4, e00532-19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Jan, R.-L.; Lin, Y.-L.; Chen, H.-H.; Wang, J.-Y. Randomized placebo-controlled trial of Lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010, 45, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Gutkowski, P.; Madaliński, K.; Grek, M.; Dmeńska, H.; Syczewska, M.; Michałkiewicz, J. Clinical immunology Effect of orally administered probiotic strains Lactobacillus and Bifidobacterium in children with atopic asthma. Cent. Eur. J. Immunol. 2010, 35, 233–238. [Google Scholar]
- van de Pol, M.A.; Lutter, R.; Smids, B.S.; Weersink, E.J.M.; van der Zee, J.S. Synbiotics reduce allergen-induced T-helper 2 response and improve peak expiratory flow in allergic asthmatics. Allergy 2011, 66, 39–47. [Google Scholar] [CrossRef]
- Dehnavi, S.; Azad, F.J.; Hoseini, R.F.; Moazzen, N.; Tavakkol-Afshari, J.; Nikpoor, A.R.; Salmani, A.A.; Ahanchian, H.; Mohammadi, M. A significant decrease in the gene expression of interleukin-17 following the administration of synbiotic in patients with allergic rhinitis who underwent immunotherapy: A placebo-controlled clinical trial. J. Res. Med. Sci. 2019, 24, 51. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; Indolfi, C.; Capasso, M.; Maiello, N.; Decimo, F.; Ciprandi, G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital. J. Pediatr. 2017, 43, 25. [Google Scholar] [CrossRef]
- Wardani, A.T.W.; Wiratno; Fatmawati, D. Potential Immuno-modulatory activity of Probiotics containing Lactobacillusacidophilus and Lacobacilluscasei to increase the ratio of IFN γ/IL-4 in patients with Allergic Rhinitis. Bangladesh J. Med. Sci. 2018, 18, 42–45. [Google Scholar] [CrossRef]
- Singh, A.; Hacini-Rachinel, F.; Gosoniu, M.L.; Bourdeau, T.; Holvoet, S.; Doucet-Ladeveze, R.; Beaumont, M.; Mercenier, A.; Nutten, S. Immune-modulatory effect of probiotic Bifidobacterium lactis NCC2818 in individuals suffering from seasonal allergic rhinitis to grass pollen: An exploratory, randomized, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2013, 67, 161–167. [Google Scholar] [CrossRef]
- Ahmed, M.; Billoo, A.G.; Iqbal, K. Efficacy of probiotic in perennial allergic rhinitis under five year children: A randomized controlled trial. Pak. J. Med. Sci. 2019, 35, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Cioffi, L.; Giuliano, M.; Pane, M.; Amoruso, A.; Schiavetti, I.; Reid, G.; Ciprandi, G.; PROPAM Study Group. The Probiotics in Pediatric Asthma Management (PROPAM) Study in the Primary Care Setting: A Randomized, Controlled, Double-Blind Trial with Ligilactobacillus salivarius LS01 (DSM 22775) and Bifidobacterium breve B632 (DSM 24706). J. Immunol. Res. 2022, 2022, 3837418. [Google Scholar] [CrossRef] [PubMed]
- Sadrifar, S.; Abbasi-Dokht, T.; Forouzandeh, S.; Malek, F.; Yousefi, B.; Salek Farrokhi, A.; Karami, J.; Baharlou, R. Immunomodulatory effects of probiotic supplementation in patients with asthma: A randomized, double-blind, placebo-controlled trial. Allergy Asthma Clin. Immunol. 2023, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Yang, Y.-H.; Chuang, S.-Y.; Huang, S.-Y.; Pan, W.-H. Reduced medication use and improved pulmonary function with supplements containing vegetable and fruit concentrate, fish oil and probiotics in asthmatic school children: A randomised controlled trial. Br. J. Nutr. 2013, 110, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Ahanchian, H.; Jafari, S.A.; Ansari, E.; Ganji, T.; Kiani, M.A.; Khalesi, M.; Momen, T.; Kianifar, H. A multi-strain Synbiotic may reduce viral respiratory infections in asthmatic children: A randomized controlled trial. Electron. Physician 2016, 8, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Hassanzad, M.; Maleki Mostashari, K.; Ghaffaripour, H.; Emami, H.; Rahimi Limouei, S.; Velayati, A.A. Synbiotics and Treatment of Asthma: A Double-Blinded, Randomized, Placebo-Controlled Clinical Trial. Galen Med. J. 2019, 8, e1350. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Djafarian, K.; Fazeli, M.R.; Yekaninejad, M.S.; Rostamian, A.; Keshavarz, S.A. Effects of a Multispecies Probiotic Supplement on Bone Health in Osteopenic Postmenopausal Women: A Randomized, Double-blind, Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.-A.; Curiac, D.; Lazou Ahrén, I.; Hansson, F.; Martinsson Niskanen, T.; Sjögren, K.; Ohlsson, C. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019, 1, e154–e162. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, É.M.R.; Meneses, G.C.; Carioca, A.A.F.; Martins, A.M.C.; Daher, E.D.F.; da Silva Junior, G.B. Use of probiotics in patients with chronic kidney disease on hemodialysis: A randomized clinical trial. Braz. J. Nephrol. 2023, 45, 152–161. [Google Scholar] [CrossRef]
- Lim, P.S.; Wang, H.F.; Lee, M.C.; Chiu, L.-S.; Wu, M.-Y.; Chang, W.-C.; Wu, T.K. The Efficacy of Lactobacillus-Containing Probiotic Supplementation in Hemodialysis Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Ren. Nutr. 2021, 31, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Íñiguez, J.S.; Ibarra-Estrada, M.; Gallardo-González, A.M.; Cisneros-Hernández, A.; Granado, R.C.-D.; Chávez-Alonso, G.; Hernández-Barajas, E.M.; Romero-Muñoz, A.C.; Ramos-Avellaneda, F.; Prieto-Magallanes, M.L.; et al. Probiotics in septic acute kidney injury, a double blind, randomized control trial. Ren. Fail. 2023, 45, 2260003. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, L.; Dai, B.; Lin, B.; Lin, S.; Lin, E. Effects of Probiotics on Malnutrition and Health-Related Quality of Life in Patients Undergoing Peritoneal Dialysis: A Randomized Controlled Trial. J. Ren. Nutr. 2021, 31, 199–205. [Google Scholar] [CrossRef]
- Simeoni, M.; Citraro, M.L.; Cerantonio, A.; Deodato, F.; Provenzano, M.; Cianfrone, P.; Capria, M.; Corrado, S.; Libri, E.; Comi, A.; et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur. J. Nutr. 2019, 58, 2145–2156. [Google Scholar] [CrossRef]
- Mitrović, M.; Stanković-Popović, V.; Tolinački, M.; Golić, N.; Soković Bajić, S.; Veljović, K.; Nastasijević, B.; Soldatović, I.; Svorcan, P.; Dimković, N. The Impact of Synbiotic Treatment on the Levels of Gut-Derived Uremic Toxins, Inflammation, and Gut Microbiome of Chronic Kidney Disease Patients—A Randomized Trial. J. Ren. Nutr. 2023, 33, 278–288. [Google Scholar] [CrossRef]
- Treewatchareekorn, S.; Tungsanga, S. WCN23-0326 effect of Lactobacillus rhamnosus GG on gut-derived uremic toxin and gut microbiome in non-dialysis chronic kidney disease patients: A randomized controlled trial. Kidney Int. Rep. 2023, 8, S211–S212. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Xu, D.; Wang, Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: A randomized clinical study. J. Clin. Lab. Anal. 2021, 35, e23650. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Mohammadshahi, M.; Shayanpour, S.; Haghighizadeh, M.H.; Rahmdel, S.; Rajaei, M. The Effect of Synbiotic and Probiotic Supplementation on Mental Health Parameters in Patients Undergoing Hemodialysis: A Double-blind, Randomized, Placebo-controlled Trial. Indian J. Nephrol. 2021, 31, 149–156. [Google Scholar]
- Liu, S.; Liu, H.; Chen, L.; Liang, S.-S.; Shi, K.; Meng, W.; Xue, J.; He, Q.; Jiang, H. Effect of probiotics on the intestinal microbiota of hemodialysis patients: A randomized trial. Eur. J. Nutr. 2020, 59, 3755–3766. [Google Scholar] [CrossRef]
- Haghighat, N.; Mohammadshahi, M.; Shayanpour, S.; Haghighizadeh, M.H. Effects of Synbiotics and Probiotics Supplementation on Serum Levels of Endotoxin, Heat Shock Protein 70 Antibodies and Inflammatory Markers in Hemodialysis Patients: A Randomized Double-Blinded Controlled Trial. Probiotics Antimicrob. Proteins 2020, 12, 144–151. [Google Scholar] [CrossRef]
- Moludi, J.; Saiedi, S.; Ebrahimi, B.; Alizadeh, M.; Khajebishak, Y.; Ghadimi, S.S. Probiotics Supplementation on Cardiac Remodeling Following Myocardial Infarction: A Single-Center Double-Blind Clinical Study. J. Cardiovasc. Transl. Res. 2021, 14, 299–307. [Google Scholar] [CrossRef]
- Pourrajab, B.; Naderi, N.; Janani, L.; Mofid, V.; Hajahmadi, M.; Dehnad, A.; Shidfar, F. Comparison of probiotic yogurt and ordinary yogurt consumption on serum Pentraxin3, NT-proBNP, oxLDL, and ApoB100 in patients with chronic heart failure: A randomized, triple-blind, controlled trial. Food Funct. 2020, 11, 10000–10010. [Google Scholar] [CrossRef]
- Moludi, J.; Alizadeh, M.; Behrooz, M.; Maleki, V.; Seyed Mohammadzad, M.H.; Golmohammadi, A. Interactive Effect of Probiotics Supplementation and Weight Loss Diet on Metabolic Syndrome Features in Patients With Coronary Artery Diseases: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Am. J. Lifestyle Med. 2021, 15, 653–663. [Google Scholar] [CrossRef]
- Shoaei Matin, S.; Shidfar, F.; Naderi, N.; Amin, A.; Hosseini-Baharanchi, F.S.; Dehnad, A. The Effect of Synbiotic Consumption on Serum NTproBNP, hsCRP and Blood Pressure in Patients with Chronic Heart Failure: A Randomized, Triple-Blind, Controlled Trial. Front. Nutr. 2022, 8, 822498. [Google Scholar] [CrossRef]
- Moludi, J.; Khedmatgozar, H.; Nachvak, S.M.; Abdollahzad, H.; Moradinazar, M.; Sadeghpour Tabaei, A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Romão da Silva, L.d.F.; de Oliveira, Y.; de Souza, E.L.; de Luna Freire, M.O.; Braga, V. de A.; Magnani, M.; de Brito Alves, J.L. Effects of probiotic therapy on cardio-metabolic parameters and autonomic modulation in hypertensive women: A randomized, triple-blind, placebo-controlled trial. Food Funct. 2020, 11, 7152–7163. [Google Scholar] [CrossRef]
- Moludi, J.; Kafil, H.S.; Qaisar, S.A.; Gholizadeh, P.; Alizadeh, M.; Vayghyan, H.J. Effect of probiotic supplementation along with calorie restriction on metabolic endotoxemia, and inflammation markers in coronary artery disease patients: A double blind placebo controlled randomized clinical trial. Nutr. J. 2021, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Gallelli, L.; Cione, E.; Perletti, G.; Ciarleglio, F.; Malossini, G.; De Pretis, G.; Palmieri, A.; Mirone, V.; Bartoletti, R.; et al. The use of Lactobacillus casei DG® prevents symptomatic episodes and reduces the antibiotic use in patients affected by chronic bacterial prostatitis: Results from a phase IV study. World J. Urol. 2021, 39, 3433–3440. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, C.; Calace, F.P.; Fusco, F.; Quattrone, C.; Giordano, D.; Crocetto, F.; Creta, M.; De Sio, M.; Arcaniolo, D. Escherichia coli Nissle 1917 as adjuvant therapy in patients with chronic bacterial prostatitis: A non-blinded, randomized, controlled trial. World J. Urol. 2021, 39, 4373–4379. [Google Scholar] [CrossRef]
- Abbasi, B.; Abbasi, H.; Niroumand, H. Synbiotic (FamiLact) administration in idiopathic male infertility enhances sperm quality, DNA integrity, and chromatin status: A triple-blinded randomized clinical trial. Int. J. Reprod. Biomed. 2021, 19, 235–244. [Google Scholar] [CrossRef]
- Asadi, M.; Gholipour, F.; Rahavian, A.; Javanbakht, M. Effects of probiotic supplementation on semen parameters after varicocelectomy: A randomized controlled trial. J. Res. Med. Sci. 2023, 28, 74. [Google Scholar]
- Park, S.-H.; Lee, E.S.; Park, S.T.; Jeong, S.Y.; Yun, Y.; Kim, Y.; Jeong, Y.; Kang, C.-H.; Choi, H.J. Efficacy and Safety of MED-01 Probiotics on Vaginal Health: A 12-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 331. [Google Scholar] [CrossRef]
- Mollazadeh-Narestan, Z.; Yavarikia, P.; Homayouni-Rad, A.; Samadi Kafil, H.; Mohammad-Alizadeh-Charandabi, S.; Gholizadeh, P.; Mirghafourvand, M. Comparing the Effect of Probiotic and Fluconazole on Treatment and Recurrence of Vulvovaginal Candidiasis: A Triple-Blinded Randomized Controlled Trial. Probiotics Antimicrob. Proteins 2023, 15, 1436–1446. [Google Scholar] [CrossRef]
- Ang, X.-Y.; Chung, F.-Y.-L.; Lee, B.-K.; Azhar, S.N.A.; Sany, S.; Roslan, N.S.; Ahmad, N.; Yusof, S.M.; Abdullah, N.; Nik Ab Rahman, N.N.; et al. Lactobacilli reduce recurrences of vaginal candidiasis in pregnant women: A randomized, double-blind, placebo-controlled study. J. Appl. Microbiol. 2022, 132, 3168–3180. [Google Scholar] [CrossRef] [PubMed]
- Mändar, R.; Sõerunurk, G.; Štšepetova, J.; Smidt, I.; Rööp, T.; Kõljalg, S.; Saare, M.; Ausmees, K.; Le, D.D.; Jaagura, M.; et al. Impact of Lactobacillus crispatus-containing oral and vaginal probiotics on vaginal health: A randomised double-blind placebo controlled clinical trial. Benef. Microbes 2023, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.A.; Choi, S.-B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Akhgarjand, C.; Vahabi, Z.; Shab-Bidar, S.; Etesam, F.; Djafarian, K. Effects of probiotic supplements on cognition, anxiety, and physical activity in subjects with mild and moderate Alzheimer’s disease: A randomized, double-blind, and placebo-controlled study. Front. Aging Neurosci. 2022, 14, 1032494. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E.; Luna, R.A.; Williams, K.; Chan, J.; Parker, R.A.; Wu, Q.; Hollway, J.A.; Jeffs, A.; Lu, F.; Coury, D.L.; et al. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J. Child Adolesc. Psychopharmacol. 2019, 29, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Papalini, S.; Michels, F.; Kohn, N.; Wegman, J.; van Hemert, S.; Roelofs, K.; Arias-Vasquez, A.; Aarts, E. Stress matters: Randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol. Stress 2019, 10, 100141. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Rajabi, S.; Mohammadshahi, M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: A randomized, double-blinded, clinical trial. Nutr. Neurosci. 2021, 24, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Salleh, R.M.; Kuan, G.; Aziz, M.N.A.; Rahim, M.R.A.; Rahayu, T.; Sulaiman, S.; Kusuma, D.W.Y.; Adikari, A.M.G.C.P.; Razam, M.S.M.; Radhakrishnan, A.K.; et al. Effects of Probiotics on Anxiety, Stress, Mood and Fitness of Badminton Players. Nutrients 2021, 13, 1783. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.W.; Strachan, D.P.; Weiland, S.K.; Williams, H.; ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Wood, R.A.; Stablein, D.; Lindblad, R.; Burks, A.W.; Liu, A.H.; Jones, S.M.; Fleischer, D.M.; Leung, D.Y.M.; Sampson, H.A. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J. Allergy Clin. Immunol. 2010, 126, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef]
- West, C.E.; Hammarström, M.-L.; Hernell, O. Probiotics during weaning reduce the incidence of eczema. Pediatr. Allergy Immunol. 2009, 20, 430–437. [Google Scholar] [CrossRef]
- Hougee, S.; Vriesema, A.J.M.; Wijering, S.C.; Knippels, L.M.J.; Folkerts, G.; Nijkamp, F.P.; Knol, J.; Garssen, J. Oral treatment with probiotics reduces allergic symptoms in ovalbumin-sensitized mice: A bacterial strain comparative study. Int. Arch. Allergy Immunol. 2010, 151, 107–117. [Google Scholar] [CrossRef]
- Feleszko, W.; Jaworska, J.; Rha, R.-D.; Steinhausen, S.; Avagyan, A.; Jaudszus, A.; Ahrens, B.; Groneberg, D.A.; Wahn, U.; Hamelmann, E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin. Exp. Allergy 2007, 37, 498–505. [Google Scholar] [CrossRef]
- Zhang, B.; An, J.; Shimada, T.; Liu, S.; Maeyama, K. Oral administration of Enterococcus faecalis FK-23 suppresses Th17 cell development and attenuates allergic airway responses in mice. Int. J. Mol. Med. 2012, 30, 248–254. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y.; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef]
- Collins, F.L.; Rios-Arce, N.D.; Schepper, J.D.; Parameswaran, N.; McCabe, L.R. The Potential of Probiotics as a Therapy for Osteoporosis. Microbiol. Spectr. 2017, 5, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Schepper, J.D.; Irwin, R.; Kang, J.; Dagenais, K.; Lemon, T.; Shinouskis, A.; Parameswaran, N.; McCabe, L.R. Probiotics in Gut-Bone Signaling. Adv. Exp. Med. Biol. 2017, 1033, 225–247. [Google Scholar] [PubMed]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.C.; Castro, A.S.B.; Rodrigues, V.C.; Fernandes, S.A.; Fontes, E.A.F.; de Oliveira, T.T.; Martino, H.S.D.; de Luces Fortes Ferreira, C.L. Yacon flour and Bifidobacterium longum modulate bone health in rats. J. Med. Food 2012, 15, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-S.; Pan, T.-M. Antiosteoporotic effects of Lactobacillus-fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J. Agric. Food Chem. 2011, 59, 7734–7742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Motyl, K.J.; Irwin, R.; MacDougald, O.A.; Britton, R.A.; McCabe, L.R. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology 2015, 156, 3169–3182. [Google Scholar] [CrossRef]
- McCabe, L.R.; Irwin, R.; Schaefer, L.; Britton, R.A. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J. Cell. Physiol. 2013, 228, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.L.; Irwin, R.; Bierhalter, H.; Schepper, J.; Britton, R.A.; Parameswaran, N.; McCabe, L.R. Lactobacillus reuteri 6475 Increases Bone Density in Intact Females Only under an Inflammatory Setting. PLoS ONE 2016, 11, e0153180. [Google Scholar] [CrossRef]
- Kruger, M.C.; Fear, A.; Chua, W.-H.; Plimmer, G.G.; Schollum, L.M. The effect of Lactobacillus rhamnosus HN001 on mineral absorption and bone health in growing male and ovariectomised female rats. Dairy Sci. Technol. 2009, 89, 219–231. [Google Scholar] [CrossRef]
- Tomofuji, T.; Ekuni, D.; Azuma, T.; Irie, K.; Endo, Y.; Yamamoto, T.; Ishikado, A.; Sato, T.; Harada, K.; Suido, H.; et al. Supplementation of broccoli or Bifidobacterium longum-fermented broccoli suppresses serum lipid peroxidation and osteoclast differentiation on alveolar bone surface in rats fed a high-cholesterol diet. Nutr. Res. 2012, 32, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, K.; Ebrahimi, M.; Sabran, M.R.; Karimi, G.; Hwei, A.N.M.; Abdul-Majeed, S.; Ahmad, Z.; Ibrahim, Z.; Jamaluddin, R. Probiotics (Bifidobacterium longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. BioMed Res. Int. 2015, 2015, 897639. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Lienesch, D.W. Rheumatoid Nodules. Dermatol. Clin. 2015, 33, 361–371. [Google Scholar] [CrossRef]
- Sepriano, A.; Kerschbaumer, A.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; McInnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; de Wit, M.; et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 760–770. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Dos Reis Neto, E.T.; Kakehasi, A.M.; de Medeiros Pinheiro, M.; Ferreira, G.A.; Marques, C.D.L.; da Mota, L.M.H.; Dos Santos Paiva, E.; Pileggi, G.C.S.; Sato, E.I.; Reis, A.P.M.G.; et al. Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv. Rheumatol. 2020, 60, 32. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.M.; Adebayo, A.S.; Bowyer, R.C.E.; Freidin, M.B.; Finckh, A.; Strowig, T.; Lesker, T.R.; Alpizar-Rodriguez, D.; Gilbert, B.; Kirkham, B.; et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: A cross-sectional study. Lancet Rheumatol. 2020, 2, e418–e427. [Google Scholar] [CrossRef] [PubMed]
- Pianta, A.; Arvikar, S.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2017, 69, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Luckey, D.; Taneja, V. Autoimmunity-Associated Gut Commensals Modulate Gut Permeability and Immunity in Humanized Mice. Mil. Med. 2019, 184, 529–536. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; Faria, A.V.d.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Chu, X.-J.; Cao, N.-W.; Zhou, H.-Y.; Meng, X.; Guo, B.; Zhang, H.-Y.; Li, B.-Z. The oral and gut microbiome in rheumatoid arthritis patients: A systematic review. Rheumatology 2021, 60, 1054–1066. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Ferro, C.J.; Balafa, O.; Burnier, M.; Ekart, R.; Halimi, J.-M.; Kreutz, R.; Mark, P.B.; Persu, A.; Rossignol, P.; et al. Mineralocorticoid receptor antagonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. Nephrol. Dial. Transplant. 2023, 38, 10–25. [Google Scholar] [CrossRef]
- Ortiz, A.; Fernandez-Fernandez, B. Atrasentan: The Difficult Task of Integrating Endothelin A Receptor Antagonists into Current Treatment Paradigm for Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2021, 16, 1775–1778. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 753–779. [Google Scholar] [CrossRef]
- Makkapati, S.; D’Agati, V.D.; Balsam, L. “Green Smoothie Cleanse” Causing Acute Oxalate Nephropathy. Am. J. Kidney Dis. 2018, 71, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Carriazo, S.; Perez-Gomez, M.V.; Cordido, A.; García-González, M.A.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Dietary Care for ADPKD Patients: Current Status and Future Directions. Nutrients 2019, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cao, C.; Wu, Z.; Zhang, H.; Sun, Z.; Wang, M.; Xu, H.; Zhao, Z.; Wang, Y.; Pei, G.; et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. 2021, 33, 1926–1942.e8. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.F.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.D.; et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.J.; Loh, Y.W.; Singer, J.; Zhu, W.; Macia, L.; Mackay, C.R.; Wang, W.; Chadban, S.J.; Wu, H. Fiber Derived Microbial Metabolites Prevent Acute Kidney Injury Through G-Protein Coupled Receptors and HDAC Inhibition. Front. Cell Dev. Biol. 2021, 9, 648639. [Google Scholar] [CrossRef]
- Miranda Alatriste, P.V.; Urbina Arronte, R.; Gómez Espinosa, C.O.; Espinosa Cuevas, M. de los Á. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr. Hosp. 2014, 29, 582–590. [Google Scholar]
- Jiang, S.; Xie, S.; Lv, D.; Zhang, Y.; Deng, J.; Zeng, L.; Chen, Y. A reduction in the butyrate producing species Roseburia spp. and Faecalibacterium prausnitzii is associated with chronic kidney disease progression. Antonie Van Leeuwenhoek 2016, 109, 1389–1396. [Google Scholar] [CrossRef]
- Miraghajani, M.; Zaghian, N.; Mirlohi, M.; Feizi, A.; Ghiasvand, R. The Impact of Probiotic Soy Milk Consumption on Oxidative Stress Among Type 2 Diabetic Kidney Disease Patients: A Randomized Controlled Clinical Trial. J. Ren. Nutr. 2017, 27, 317–324. [Google Scholar] [CrossRef]
- Guida, B.; Germanò, R.; Trio, R.; Russo, D.; Memoli, B.; Grumetto, L.; Barbato, F.; Cataldi, M. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Johnson, D.W.; Morrison, M.; Pascoe, E.M.; Coombes, J.S.; Forbes, J.M.; Szeto, C.-C.; McWhinney, B.C.; Ungerer, J.P.J.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Ranganathan, P.; Friedman, E.A.; Joseph, A.; Delano, B.; Goldfarb, D.S.; Tam, P.; Rao, A.V.; Anteyi, E.; Musso, C.G. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010, 27, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-K.; Yen, T.-H.; Hsieh, P.-S.; Ho, H.-H.; Kuo, Y.-W.; Huang, Y.-Y.; Kuo, Y.-L.; Li, C.-Y.; Lin, H.-C.; Wang, J.-Y. Effect of a Probiotic Combination in an Experimental Mouse Model and Clinical Patients With Chronic Kidney Disease: A Pilot Study. Front. Nutr. 2021, 8, 661794. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol. Nefrol. 2016, 68, 222–226. [Google Scholar]
- Yang, C.-Y.; Chen, T.-W.; Lu, W.-L.; Liang, S.-S.; Huang, H.-D.; Tseng, C.-P.; Tarng, D.-C. Synbiotics Alleviate the Gut Indole Load and Dysbiosis in Chronic Kidney Disease. Cells 2021, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Pechenyak, B.; Vyas, U.; Ranganathan, P.; Weinberg, A.; Liang, P.; Mallappallil, M.C.; Norin, A.J.; Friedman, E.A.; Saggi, S.J. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. BioMed Res. Int. 2014, 2014, 568571. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.I.; De Preter, V.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transplant. 2010, 25, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef]
- Wang, I.-K.; Wu, Y.-Y.; Yang, Y.-F.; Ting, I.-W.; Lin, C.-C.; Yen, T.-H.; Chen, J.-H.; Wang, C.-H.; Huang, C.-C.; Lin, H.-C. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 2015, 6, 423–430. [Google Scholar] [CrossRef]
- Lundy, S.D.; Sangwan, N.; Parekh, N.V.; Selvam, M.K.P.; Gupta, S.; McCaffrey, P.; Bessoff, K.; Vala, A.; Agarwal, A.; Sabanegh, E.S.; et al. Functional and Taxonomic Dysbiosis of the Gut, Urine, and Semen Microbiomes in Male Infertility. Eur. Urol. 2021, 79, 826–836. [Google Scholar] [CrossRef]
- Koedooder, R.; Mackens, S.; Budding, A.; Fares, D.; Blockeel, C.; Laven, J.; Schoenmakers, S. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum. Reprod. Update 2019, 25, 298–325. [Google Scholar] [CrossRef]
- Rowe, M.; Veerus, L.; Trosvik, P.; Buckling, A.; Pizzari, T. The Reproductive Microbiome: An Emerging Driver of Sexual Selection, Sexual Conflict, Mating Systems, and Reproductive Isolation. Trends Ecol. Evol. 2020, 35, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, S.; Steegers-Theunissen, R.; Faas, M. The matter of the reproductive microbiome. Obstet. Med. 2019, 12, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.N.S.; Bisanz, J.E.; Monachese, M.; Burton, J.P.; Reid, G. The Rationale for Probiotics Improving Reproductive Health and Pregnancy Outcome. Am. J. Reprod. Immunol. 2013, 69, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y. Gut microbiota and probiotics in maternal and infant health. Am. J. Clin. Nutr. 2011, 94, S2000–S2005. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-L.; Chiu, C.-M.; Lin, F.-M.; Huang, W.-C.; Liang, C.; Yang, T.; Yang, T.-L.; Liu, C.-Y.; Wu, W.-Y.; Chang, Y.-A.; et al. Bacterial Communities in Semen from Men of Infertile Couples: Metagenomic Sequencing Reveals Relationships of Seminal Microbiota to Semen Quality. PLoS ONE 2014, 9, e110152. [Google Scholar] [CrossRef]
- Tomaiuolo, R.; Veneruso, I.; Cariati, F.; D’Argenio, V. Microbiota and Human Reproduction: The Case of Male Infertility. High-Throughput 2020, 9, 10. [Google Scholar] [CrossRef]
- Barbonetti, A.; Cinque, B.; Vassallo, M.R.C.; Mineo, S.; Francavilla, S.; Cifone, M.G.; Francavilla, F. Effect of vaginal probiotic lactobacilli on in vitro–induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil. Steril. 2011, 95, 2485–2488. [Google Scholar] [CrossRef]
- Aitken, R.J.; Harkiss, D.; Buckingham, D.W. Analysis of lipid peroxidation mechanisms in human spermatozoa. Mol. Reprod. Dev. 1993, 35, 302–315. [Google Scholar] [CrossRef]
- Dardmeh, F.; Alipour, H.; Gazerani, P.; van der Horst, G.; Brandsborg, E.; Nielsen, H.I. Lactobacillus rhamnosus PB01 (DSM 14870) supplementation affects markers of sperm kinematic parameters in a diet-induced obesity mice model. PLoS ONE 2017, 12, e0185964. [Google Scholar] [CrossRef]
- Poutahidis, T.; Springer, A.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Probiotic Microbes Sustain Youthful Serum Testosterone Levels and Testicular Size in Aging Mice. PLoS ONE 2014, 9, e84877. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.G.; Genovés, S.; Riesco, M.F.; Martorell, P.; Herráez, M.P.; Ramón, D.; Robles, V. Probiotic administration improves sperm quality in asthenozoospermic human donors. Benef. Microbes 2017, 8, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Abd El Hamid, E.A.; Ismaiel, A.M.; El-Nagar, A. The detoxication of nitrate by two antioxidants or a probiotic, and the effects on blood and seminal plasma profiles and reproductive function of New Zealand White rabbit bucks. Animal 2013, 7, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmakh, M.; Stukenborg, J.-B.; Reda, A.; Anuar, F.; Strand, M.-L.; Hedin, L.; Pettersson, S.; Söder, O. The Gut Microbiota and Developmental Programming of the Testis in Mice. PLoS ONE 2014, 9, e103809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, H.; Yang, Q.; Li, P.; Wen, Y.; Han, X.; Li, B.; Jiang, H.; Li, X. Genomic Sequencing Reveals the Diversity of Seminal Bacteria and Relationships to Reproductive Potential in Boar Sperm. Front. Microbiol. 2020, 11, 1873. [Google Scholar] [CrossRef]
- Tian, X.; Yu, Z.; Feng, P.; Ye, Z.; Li, R.; Liu, J.; Hu, J.; Kakade, A.; Liu, P.; Li, X. Lactobacillus plantarum TW1-1 Alleviates Diethylhexylphthalate-Induced Testicular Damage in Mice by Modulating Gut Microbiota and Decreasing Inflammation. Front. Cell. Infect. Microbiol. 2019, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Balthazar, C.F.; Rocha, R.S.; Silva, H.L.A.; Esmerino, E.A.; Duarte, M.C.K.H.; Pimentel, T.C.; Freitas, M.Q.; et al. Antiproliferative and apoptotic effects of probiotic whey dairy beverages in human prostate cell lines. Food Res. Int. 2020, 137, 109450. [Google Scholar] [CrossRef]
- Celebioglu, H.U. Effects of potential synbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells. Arch. Microbiol. 2021, 203, 1221–1229. [Google Scholar] [CrossRef]
- Chiancone, F.; Carrino, M.; Meccariello, C.; Pucci, L.; Fedelini, M.; Fedelini, P. The Use of a Combination of Vaccinium Macracarpon, Lycium barbarum L. and Probiotics (Bifiprost®) for the Prevention of Chronic Bacterial Prostatitis: A Double-Blind Randomized Study. Urol. Int. 2019, 103, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, L.; Santacroce, L.; Dipalma, G.; Haxhirexha, K.; Topi, S.; Cantore, S.; Altini, V.; Pacifici, A.; De Vito, D.; Pettini, F. Gender medicine: The impact of probiotics on male patients. Clin. Ter. 2021, 172, 8–15. [Google Scholar]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Pelzer, E.S.; Allan, J.A.; Waterhouse, M.A.; Ross, T.; Beagley, K.W.; Knox, C.L. Microorganisms within Human Follicular Fluid: Effects on IVF. PLoS ONE 2013, 8, e59062. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. N. Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef]
- Verhelst, R.; Verstraelen, H.; Claeys, G.; Verschraegen, G.; Delanghe, J.; Van Simaey, L.; De Ganck, C.; Temmerman, M.; Vaneechoutte, M. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol. 2004, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, G.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation: A Potential Tool for Treatment of Human Female Reproductive Tract Diseases. Front. Immunol. 2019, 10, 2653. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine microbiota: Residents, tourists, or invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Heil, B.A.; Paccamonti, D.L.; Sones, J.L. Role for the mammalian female reproductive tract microbiome in pregnancy outcomes. Physiol. Genom. 2019, 51, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M. Vaginal microbiome and sexually transmitted infections: An epidemiologic perspective. J. Clin. Investig. 2011, 121, 4610–4617. [Google Scholar] [CrossRef]
- MacPhee, R.A.; Hummelen, R.; Bisanz, J.E.; Miller, W.L.; Reid, G. Probiotic strategies for the treatment and prevention of bacterial vaginosis. Expert Opin. Pharmacother. 2010, 11, 2985–2995. [Google Scholar] [CrossRef]
- Hütt, P.; Lapp, E.; Štšepetova, J.; Smidt, I.; Taelma, H.; Borovkova, N.; Oopkaup, H.; Ahelik, A.; Rööp, T.; Hoidmets, D.; et al. Characterisation of probiotic properties in human vaginal lactobacilli strains. Microb. Ecol. Health Dis. 2016, 27, 30484. [Google Scholar] [CrossRef]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef]
- Santos, C.M.A.; Pires, M.C.V.; Leão, T.L.; Hernández, Z.P.; Rodriguez, M.L.; Martins, A.K.S.; Miranda, L.S.; Martins, F.S.; Nicoli, J.R. Selection of Lactobacillus strains as potential probiotics for vaginitis treatment. Microbiology 2016, 162, 1195–1207. [Google Scholar] [CrossRef]
- Senok, A.C.; Verstraelen, H.; Temmerman, M.; Botta, G.A. Probiotics for the treatment of bacterial vaginosis. In Cochrane Database of Systematic Reviews; Senok, A.C., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Andersch, B.; Lindell, D.; Dahlén, I.; Brandberg, Å. Bacterial Vaginosis and the Effect of Intermittent Prophylactic Treatment with an Acid Lactate Gel. Gynecol. Obstet. Investig. 1990, 30, 114–119. [Google Scholar] [CrossRef]
- Decena, D.C.D.; Co, J.T.; Manalastas, R.M.; Palaypayon, E.P.; Padolina, C.S.; Sison, J.M.; Dancel, L.A.; Lelis, M.A. Metronidazole with Lactacyd vaginal gel in bacterial vaginosis. J. Obstet. Gynaecol. Res. 2006, 32, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Valeria Bahamondes, M.; Portugal, P.M.; Brolazo, E.M.; Simões, J.A.; Bahamondes, L. Use of a lactic acid plus lactoserum intimate liquid soap for external hygiene in the prevention of bacterial vaginosis recurrence after metronidazole oral treatment. Rev. Assoc. Med. Bras. 2011, 57, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Valore, E.V.; Park, C.H.; Igreti, S.L.; Ganz, T. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 2002, 187, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Graver, M.A.; Wade, J.J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Nardini, P.; Ñahui Palomino, R.A.; Parolin, C.; Laghi, L.; Foschi, C.; Cevenini, R.; Vitali, B.; Marangoni, A. Lactobacillus crispatus inhibits the infectivity of Chlamydia trachomatis elementary bodies, in vitro study. Sci. Rep. 2016, 6, 29024. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef] [PubMed]
- Breshears, L.M.; Edwards, V.L.; Ravel, J.; Peterson, M.L. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Balkus, J.E.; Fredricks, D.; Liu, C.; McKernan-Mullin, J.; Frenkel, L.M.; Mwachari, C.; Luque, A.; Cohn, S.E.; Cohen, C.R.; et al. Interaction Between Lactobacilli, Bacterial Vaginosis-Associated Bacteria, and HIV Type 1 RNA and DNA Genital Shedding in U.S. and Kenyan Women. AIDS Res. Hum. Retroviruses 2013, 29, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, H.; Tsivtsivadze, E.; Verhelst, R.; Marzorati, M.; Jurriaans, S.; Ndayisaba, G.F.; Schuren, F.H.; van de Wijgert, J.H. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014, 8, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Aldunate, M.; Tyssen, D.; Johnson, A.; Zakir, T.; Sonza, S.; Moench, T.; Cone, R.; Tachedjian, G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013, 68, 2015–2025. [Google Scholar] [CrossRef]
- Dimitonova, S.P.; Danova, S.T.; Serkedjieva, J.P.; Bakalov, B.V. Antimicrobial activity and protective properties of vaginal lactobacilli from healthy Bulgarian women. Anaerobe 2007, 13, 178–184. [Google Scholar] [CrossRef]
- Isaacs, C.E.; Xu, W. Theaflavin-3,3′-Digallate and Lactic Acid Combinations Reduce Herpes Simplex Virus Infectivity. Antimicrob. Agents Chemother. 2013, 57, 3806–3814. [Google Scholar] [CrossRef]
- Conti, C.; Malacrino, C.; Mastromarino, P. Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J. Physiol. Pharmacol. 2009, 60, 19–26. [Google Scholar]
- Chen, Q.; Wang, B.; Wang, S.; Qian, X.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice. Nutrients 2021, 13, 1793. [Google Scholar] [CrossRef]
- Szydłowska, I.; Marciniak, A.; Brodowska, A.; Loj, B.; Ciećwież, S.; Skonieczna-Żydecka, K.; Palma, J.; Łoniewski, I.; Stachowska, E. Effects of probiotics supplementation on the hormone and body mass index in perimenopausal and postmenopausal women using the standardized diet. A 5-week double-blind, placebo-controlled, and randomized clinical study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3859–3867. [Google Scholar] [PubMed]
- Lei, K.; Li, Y.L.; Yu, D.Y.; Rajput, I.R.; Li, W.F. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 2013, 92, 2389–2395. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Pang, Q.; Miao, Z. Bacillus amyloliquefaciens BLCC1-0238 Can Effectively Improve Laying Performance and Egg Quality Via Enhancing Immunity and Regulating Reproductive Hormones of Laying Hens. Probiotics Antimicrob. Proteins 2020, 12, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, K.; Ding, X.; Celi, P.; Yan, L.; Bai, S.; Zeng, Q.; Mao, X.; Xu, S.; Wang, J. The impact of dietary supplementation of different feed additives on performances of broiler breeders characterized by different egg-laying rate. Poult. Sci. 2019, 98, 6091–6099. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, G.; Maradonna, F.; Lombardo, F.; Bizzaro, D.; Olivotto, I.; Carnevali, O. Increase of fecundity by probiotic administration in zebrafish (Danio rerio). Reproduction 2010, 140, 953–959. [Google Scholar] [CrossRef]
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of Commensal Bacteria from Umbilical Cord Blood of Healthy Neonates Born by Cesarean Section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef]
- Rautava, S.; Collado, M.C.; Salminen, S.; Isolauri, E. Probiotics Modulate Host-Microbe Interaction in the Placenta and Fetal Gut: A Randomized, Double-Blind, Placebo-Controlled Trial. Neonatology 2012, 102, 178–184. [Google Scholar] [CrossRef]
- Yang, P.; Li, Z.; Tye, K.D.; Chen, Y.; Lu, T.; He, Z.; Zhou, J.; Xiao, X. Effects of an orally supplemented probiotic on the autophagy protein LC3 and Beclin1 in placentas undergoing spontaneous delivery during normal pregnancy. BMC Pregnancy Childbirth 2020, 20, 216. [Google Scholar] [CrossRef]
- Yeganegi, M.; Watson, C.S.; Martins, A.; Kim, S.O.; Reid, G.; Challis, J.R.G.; Bocking, A.D. Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: Implications for treatment of bacterial vaginosis and prevention of preterm. Am. J. Obstet. Gynecol. 2009, 200, e1–e532. [Google Scholar] [CrossRef]
- Brantsaeter, A.L.; Myhre, R.; Haugen, M.; Myking, S.; Sengpiel, V.; Magnus, P.; Jacobsson, B.; Meltzer, H.M. Intake of Probiotic Food and Risk of Preeclampsia in Primiparous Women: The Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2011, 174, 807–815. [Google Scholar] [CrossRef]
- Gu, X.L.; Li, H.; Song, Z.H.; Ding, Y.N.; He, X.; Fan, Z.Y. Effects of isomaltooligosaccharide and Bacillus supplementation on sow performance, serum metabolites, and serum and placental oxidative status. Anim. Reprod. Sci. 2019, 207, 52–60. [Google Scholar] [CrossRef]
- Jarrett, P.; Meczner, A.; Costeloe, K.; Fleming, P. Historical aspects of probiotic use to prevent necrotising enterocolitis in preterm babies. Early Hum. Dev. 2019, 135, 51–57. [Google Scholar] [CrossRef]
- Mirpuri, J.; Neu, J. Maternal microbial factors that affect the fetus and subsequent offspring. Semin. Perinatol. 2021, 45, 151449. [Google Scholar] [CrossRef]
- Rasool, A.; Alvarado-Flores, F.; O’Tierney-Ginn, P. Placental Impact of Dietary Supplements: More Than Micronutrients. Clin. Ther. 2021, 43, 226–245. [Google Scholar] [CrossRef]
- Böhmer, B.M.; Kramer, W.; Roth-Maier, D.A. Dietary probiotic supplementation and resulting effects on performance, health status, and microbial characteristics of primiparous sows. J. Anim. Physiol. Anim. Nutr. 2006, 90, 309–315. [Google Scholar] [CrossRef]
- Hayakawa, T.; Masuda, T.; Kurosawa, D.; Tsukahara, T. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim. Sci. J. 2016, 87, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Romano-Keeler, J.; Weitkamp, J.-H. Maternal influences on fetal microbial colonization and immune development. Pediatr. Res. 2015, 77, 189–195. [Google Scholar] [CrossRef]
- Saxelby, C. Cardiovascular Disease: Diet, Nutrition and Emerging Risk Factors. Nutr. Diet. 2006, 63, 189–190. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Asada, Y.; Yamashita, A.; Sato, Y.; Hatakeyama, K. Thrombus Formation and Propagation in the Onset of Cardiovascular Events. J. Atheroscler. Thromb. 2018, 25, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, F.; Mottola, G.; Fromonot, J.; Marlinge, M.; Deharo, P.; Guieu, R.; Ruf, J. Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link? Int. J. Mol. Sci. 2021, 22, 1690. [Google Scholar] [CrossRef] [PubMed]
- Raygan, F.; Rezavandi, Z.; Bahmani, F.; Ostadmohammadi, V.; Mansournia, M.A.; Tajabadi-Ebrahimi, M.; Borzabadi, S.; Asemi, Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 2018, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Raygan, F.; Ostadmohammadi, V.; Asemi, Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1594–1598. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi-Ebrahimi, M.; Sharifi, N.; Farrokhian, A.; Raygan, F.; Karamali, F.; Razzaghi, R.; Taheri, S.; Asemi, Z. A Randomized Controlled Clinical Trial Investigating the Effect of Synbiotic Administration on Markers of Insulin Metabolism and Lipid Profiles in Overweight Type 2 Diabetic Patients with Coronary Heart Disease. Exp. Clin. Endocrinol. Diabetes 2017, 125, 21–27. [Google Scholar] [CrossRef]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men With Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef]
- Koppinger, M.P.; Lopez-Pier, M.A.; Skaria, R.; Harris, P.R.; Konhilas, J.P. Lactobacillus reuteri attenuates cardiac injury without lowering cholesterol in low-density lipoprotein receptor-deficient mice fed standard chow. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H32–H41. [Google Scholar] [CrossRef]
- Mengheri, E. Health, probiotics, and inflammation. J. Clin. Gastroenterol. 2008, 42 (Suppl. S3), S177–S178. [Google Scholar] [CrossRef]
- Smelt, M.J.; de Haan, B.J.; Bron, P.A.; van Swam, I.; Meijerink, M.; Wells, J.M.; Faas, M.M.; de Vos, P. Probiotics can generate FoxP3 T-cell responses in the small intestine and simultaneously inducing CD4 and CD8 T cell activation in the large intestine. PLoS ONE 2013, 8, e68952. [Google Scholar] [CrossRef]
- Okamoto, N.; Noma, T.; Ishihara, Y.; Miyauchi, Y.; Takabatake, W.; Oomizu, S.; Yamaoka, G.; Ishizawa, M.; Namba, T.; Murakami, K.; et al. Prognostic value of circulating regulatory T cells for worsening heart failure in heart failure patients with reduced ejection fraction. Int. Heart J. 2014, 55, 271–277. [Google Scholar] [CrossRef]
- Danilo, C.A.; Constantopoulos, E.; McKee, L.A.; Chen, H.; Regan, J.A.; Lipovka, Y.; Lahtinen, S.; Stenman, L.K.; Nguyen, T.-V.V.; Doyle, K.P.; et al. Bifidobacterium animalis subsp. lactis 420 mitigates the pathological impact of myocardial infarction in the mouse. Benef. Microbes 2017, 8, 257–269. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Wang, L.; de Zoeten, E.F.; Greene, M.I.; Hancock, W.W. Immunomodulatory effects of deacetylase inhibitors: Therapeutic targeting of FOXP3+ regulatory T cells. Nat. Rev. Drug Discov. 2009, 8, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [PubMed]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Itoh, N.; Yamamoto, S.; Higo-Yamamoto, S.; Nakakita, Y.; Kaneda, H.; Shigyo, T.; Oishi, K. Dietary heat-killed Lactobacillus brevis SBC8803 promotes voluntary wheel-running and affects sleep rhythms in mice. Life Sci. 2014, 111, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Morishima, H.; Kumano-go, T.; Suganuma, N.; Matsumoto, H.; Adachi, H.; Sigedo, Y.; Mikami, A.; Kai, T.; Masuyama, A.; et al. The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. Eur. J. Clin. Nutr. 2009, 63, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; Labaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Borre, Y.E.; Cryan, J.F. Genomics of schizophrenia: Time to consider the gut microbiome? Mol. Psychiatry 2014, 19, 1252–1257. [Google Scholar] [CrossRef]
- Cenit, M.C.; Nuevo, I.C.; Codoñer-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Everard, A.; Cani, P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef]
- Harach, T.; Jammes, F.; Muller, C.; Duthilleul, N.; Cheatham, V.; Zufferey, V.; Cheatham, D.; Lukasheva, Y.A.; Lasser, T.; Bolmont, T. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2017, 51, 83–96. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).