Diabetes-Specific Complete Smoothie Formulas Improve Postprandial Glycemic Response in Obese Type 2 Diabetic Individuals: A Randomized Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of Modified Nutrition-Dense Smoothie Diets
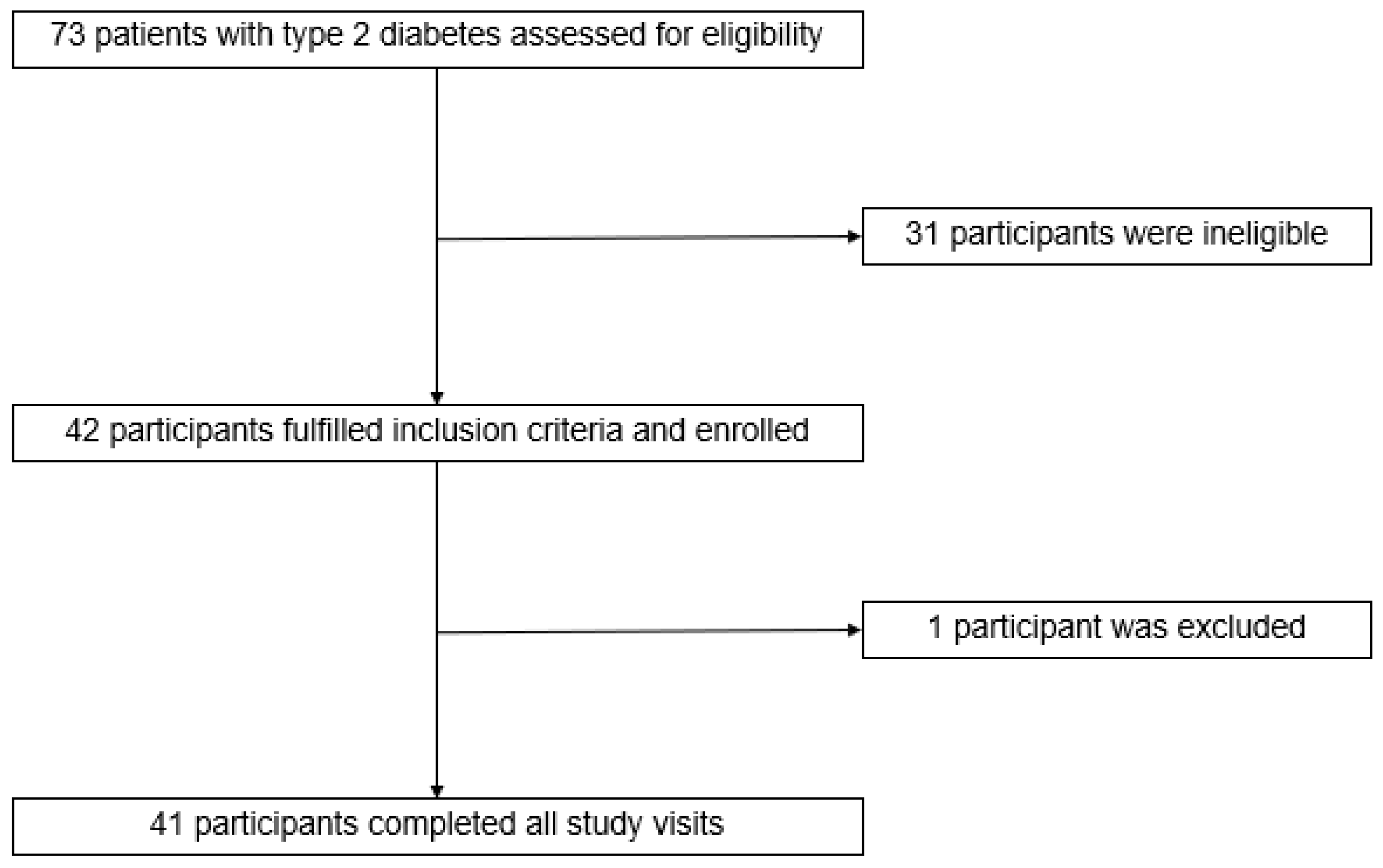
2.2. Study Design and Participants
2.3. Research Clinic Visits
2.4. Anthropometric Assessment
2.5. Laboratory Assessment
2.6. Statistical Analysis
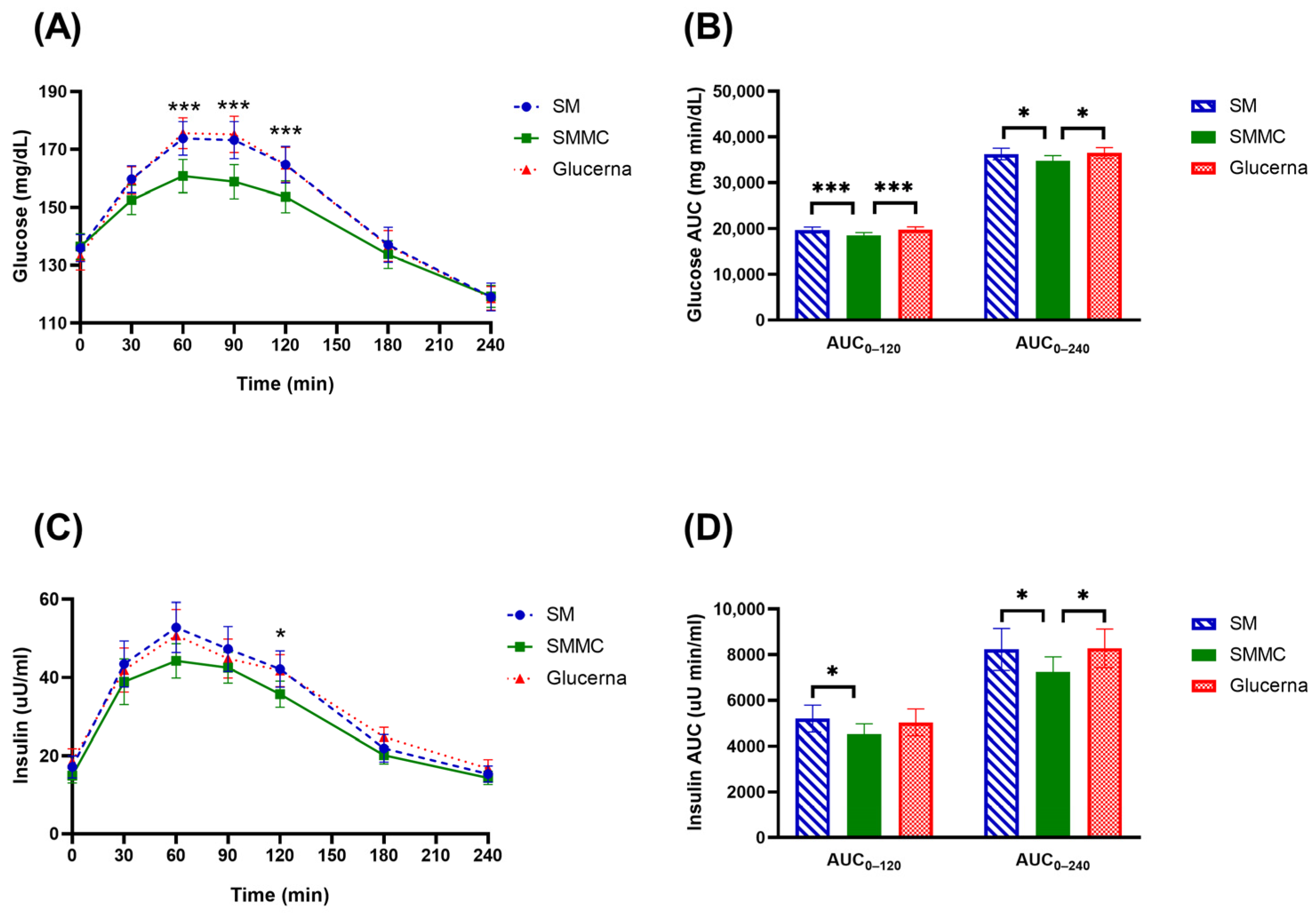
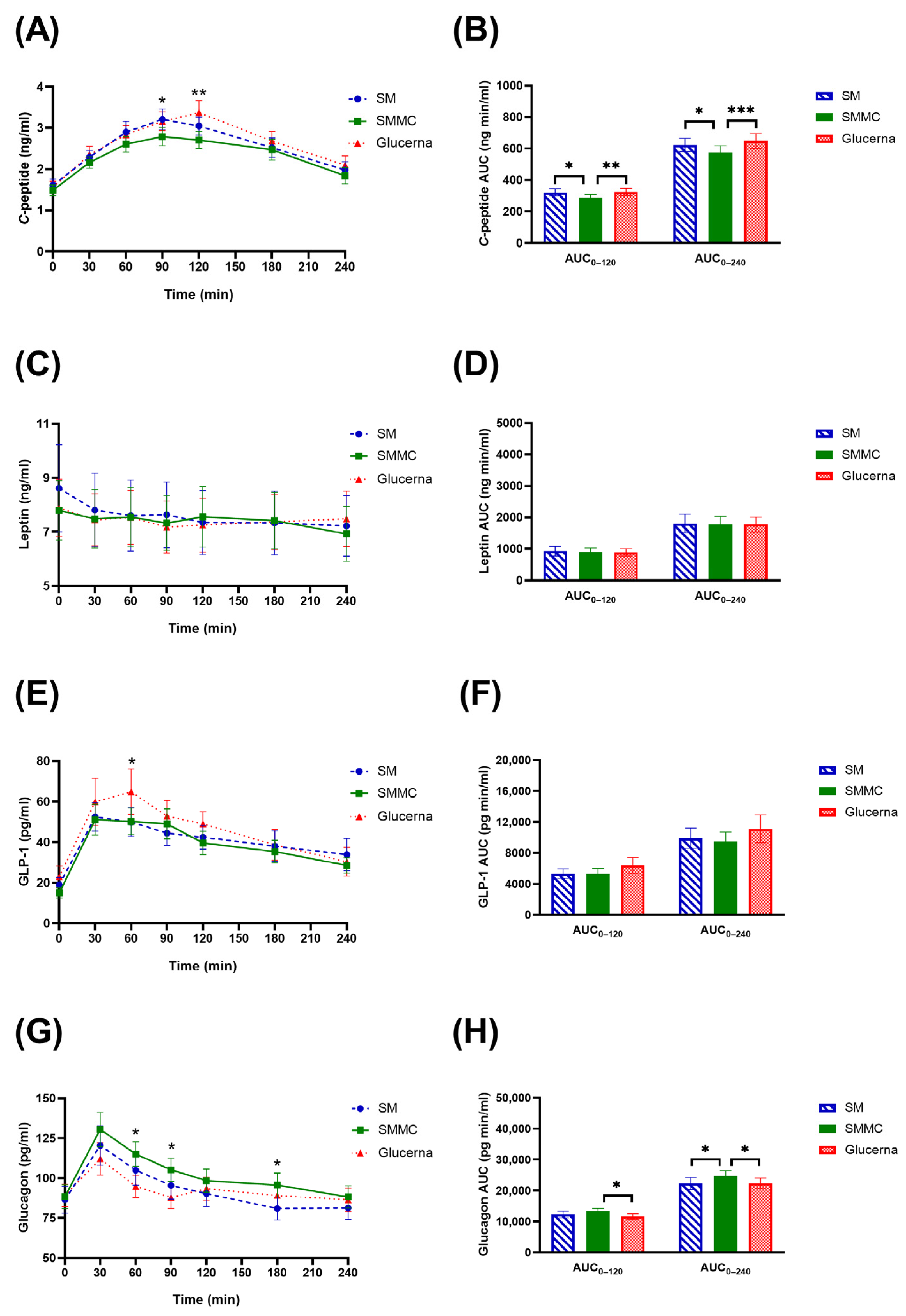
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Lifestyle management: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42, S46. [Google Scholar] [CrossRef] [PubMed]
- Mesejo, A.; Montejo-González, J.C.; Vaquerizo-Alonso, C.; Lobo-Tamer, G.; Zabarte-Martinez, M.; Herrero-Meseguer, J.I.; Acosta-Escribano, J.; Blesa-Malpica, A.; Martinez-Lozano, F. Diabetes-specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: A prospective, open-label, blind-randomized, multicenter study. Crit. Care 2015, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I. Metabolic mechanisms of stress hyperglycemia. J. Parenter. Enter. Nutr. 2006, 30, 157–163. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of care in diabetes—2023 abridged for primary care providers. Clin. Diabetes 2023, 41, 4–31. [Google Scholar] [CrossRef]
- Brown, B.; Roehl, K.; Betz, M. Enteral nutrition formula selection: Current evidence and implications for practice. Nutr. Clin. Pract. 2015, 30, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Mottalib, A.; Mohd-Yusof, B.N.; Shehabeldin, M.; Pober, D.M.; Mitri, J.; Hamdy, O. Impact of diabetes-specific nutritional formulas versus oatmeal on postprandial glucose, insulin, GLP-1 and postprandial lipidemia. Nutrients 2016, 8, 0443. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, G.; Yu, E.; Wang, B.; Wittenbecher, C.; Manson, J.E.; Rimm, E.B.; Liang, L.; Rexrode, K.; Willett, W.C. Low-carbohydrate diet scores and mortality among adults with incident type 2 diabetes. Diabetes Care 2023, 46, 874–884. [Google Scholar] [CrossRef]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: An open-label, non-randomized, controlled study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef]
- Barazzoni, R.; Deutz, N.; Biolo, G.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cuerda, C.; Delzenne, N.; Sanz, M.L.; Ljungqvist, O. Carbohydrates and insulin resistance in clinical nutrition: Recommendations from the ESPEN expert group. Clin. Nutr. 2017, 36, 355–363. [Google Scholar] [CrossRef]
- Sanz-Paris, A.; Hernández, J.Á.; Ballesteros-Pomar, M.D.; Botella-Romero, F.; León-Sanz, M.; Martín-Palmero, Á.; Olmos, M.Á.M.; Olveira, G. Evidence-based recommendations and expert consensus on enteral nutrition in the adult patient with diabetes mellitus or hyperglycemia. Nutrition 2017, 41, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Ceriello, A.; Laube, H.; Sinclair, A.J.; Engfer, M.; Stratton, R.J. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: A systematic review and meta-analysis. Diabetes Care 2005, 28, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Brooke, J. Evaluation of the role of enteral nutrition in managing patients with diabetes: A systematic review. Nutrients 2014, 6, 5142–5152. [Google Scholar] [CrossRef] [PubMed]
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.A.; Westerterp-Plantenga, M.S. Protein diets, body weight loss and weight maintenance. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 2004, 53, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, E.; Luscombe, N.D.; Noakes, M.; Wittert, G.; Argyiou, E.; Clifton, P.M. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am. J. Clin. Nutr. 2003, 78, 31–39. [Google Scholar] [CrossRef]
- Bowen, J.; Noakes, M.; Clifton, P.M. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J. Clin. Endocrinol. Metab. 2006, 91, 2913–2919. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Zhang, Y.-B.; Chi, M.-H. Soy protein supplementation reduces clinical indices in type 2 diabetes and metabolic syndrome. Yonsei Med. J. 2016, 57, 681–689. [Google Scholar] [CrossRef]
- Yost, T.J.; Erskine, J.M.; Gregg, T.S.; Podlecki, D.L.; Brass, E.P.; Eckel, R.H. Dietary substitution of medium chain triglycerides in subjects with non-insulin-dependent diabetes mellitus in an ambulatory setting: Impact on glycemic control and insulin-mediated glucose metabolism. J. Am. Coll. Nutr. 1994, 13, 615–622. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemistry (AOAC). Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- National Dysphagia Diet Task Force; American Dietetic Association. National Dysphagia Diet: Standardization for Optimal Care; American Dietetic Association: Chicago, IL, USA, 2002. [Google Scholar]
- Chirdkiatisak, M.; Sranacharoenpong, K.; Churak, P.; Praditsorn, P. Thai diabetes prevention education program: Development and validation of the Thai physical activity questionnaire for at-risk people. J. Public Health 2019, 27, 659–667. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, L.; Nsor-Atindana, J.; Goff, H.D.; Zhang, W.; Mao, J.; Zhong, F. The effect of viscous soluble dietary fiber on nutrient digestion and metabolic responses I: In vitro digestion process. Food Hydrocoll. 2020, 107, 105971. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L. Glycemic targets: Standards of care in diabetes—2023. Diabetes Care 2023, 46, S97–S110. [Google Scholar] [CrossRef] [PubMed]
- Huypens, P.; Ling, Z.; Pipeleers, D.; Schuit, F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 2000, 43, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Mayurasakorn, K.; Hasanah, N.; Homma, T.; Homma, M.; Rangel, I.K.; Garza, A.E.; Romero, J.R.; Adler, G.K.; Williams, G.H.; Pojoga, L.H. Caloric restriction improves glucose homeostasis, yet increases cardiometabolic risk in caveolin-1-deficient mice. Metabolism 2018, 83, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Pacini, G.; Ahrén, B.; D’Argenio, D.Z. Glucagon increases insulin levels by stimulating insulin secretion without effect on insulin clearance in mice. Peptides 2017, 88, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.; Arfuso, F.; Keane, K. Nutrient regulation of insulin secretion and action. J. Endocrinol. 2014, 221, R105–R120. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef]
- Ryan, A.T.; Luscombe-Marsh, N.D.; Saies, A.A.; Little, T.J.; Standfield, S.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 2013, 98, 300–311. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Wong, O.; Synnott, E.L.; Piyaratna, N.; Douglas, A.; Gribble, F.M.; Holst, J.J.; Chisholm, D.J.; Greenfield, J.R. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J. Nutr. 2011, 141, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Samkani, A.; Skytte, M.J.; Kandel, D.; Kjaer, S.; Astrup, A.; Deacon, C.F.; Holst, J.J.; Madsbad, S.; Rehfeld, J.F.; Haugaard, S.B.; et al. A carbohydrate-reduced high-protein diet acutely decreases postprandial and diurnal glucose excursions in type 2 diabetes patients. Br. J. Nutr. 2018, 119, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Wolstencroft, E.J.; Bateman, P.A.; Yaqoob, P.; Williams, C.M. Acute effects of meal fatty acids on postprandial NEFA, glucose and apo E response: Implications for insulin sensitivity and lipoprotein regulation? Br. J. Nutr. 2005, 93, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Barrows, B.R.; Parks, E.J. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J. Clin. Endocrinol. Metab. 2006, 91, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Asakura, L.; Lottenberg, A.M.P.; Neves, M.Q.T.S.; Nunes, V.S.; Rocha, J.C.; Passarelli, M.; Nakandakare, E.R.; Quintão, E.C.R. Dietary medium-chain triacylglycerol prevents the postprandial rise of plasma triacylglycerols but induces hypercholesterolemia in primary hypertriglyceridemic subjects. Am. J. Clin. Nutr. 2000, 71, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-chain triglycerides. Int. Dairy J. 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- Nielsen, S.; Guo, Z.; Johnson, C.M.; Hensrud, D.D.; Jensen, M.D. Splanchnic lipolysis in human obesity. J. Clin. Investig. 2004, 113, 1582–1588. [Google Scholar] [CrossRef]
- Samkani, A.; Skytte, M.J.; Anholm, C.; Astrup, A.; Deacon, C.F.; Holst, J.J.; Madsbad, S.; Boston, R.; Krarup, T.; Haugaard, S.B. The acute effects of dietary carbohydrate reduction on postprandial responses of non-esterified fatty acids and triglycerides: A randomized trial. Lipids Health Dis. 2018, 17, 295. [Google Scholar] [CrossRef]
- Wu, T.; Bound, M.J.; Standfield, S.D.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J. Clin. Endocrinol. Metab. 2013, 98, E718–E722. [Google Scholar] [CrossRef]
- Meessen, E.C.; Bakker, G.J.; Nieuwdorp, M.; Dallinga-Thie, G.M.; Kemper, E.M.; Damink, S.W.O.; Romijn, J.A.; Hartmann, B.; Holst, J.J.; Knop, F.K. Parenteral nutrition impairs plasma bile acid and gut hormone responses to mixed meal testing in lean healthy men. Clin. Nutr. 2021, 40, 1013–1021. [Google Scholar] [CrossRef]
- Horowitz, M.; Flint, A.; Jones, K.L.; Hindsberger, C.; Rasmussen, M.F.; Kapitza, C.; Doran, S.; Jax, T.; Zdravkovic, M.; Chapman, I.M. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res. Clin. Pract. 2012, 97, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.T.; Dirks, M.L.; Holwerda, A.M.; Kouw, I.W.; van Loon, L.J. Intermittent versus continuous enteral nutrition attenuates increases in insulin and leptin during short-term bed rest. Eur. J. Appl. Physiol. 2020, 120, 2083–2094. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Type 2 Diabetes. Available online: https://www.cdc.gov/diabetes/basics/type2.html (accessed on 25 January 2024).
- Voss, A.C.; Maki, K.C.; Garvey, W.T.; Hustead, D.S.; Alish, C.; Fix, B.; Mustad, V.A. Effect of two carbohydrate-modified tube-feeding formulas on metabolic responses in patients with type 2 diabetes. Nutrition 2008, 24, 990–997. [Google Scholar] [CrossRef]
- Ceriello, A.; Lansink, M.; Rouws, C.H.; van Laere, K.M.; Frost, G.S. Administration of a new diabetes-specific enteral formula results in an improved 24 h glucose profile in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2009, 84, 259–266. [Google Scholar] [CrossRef]


| Ingredients/100 g | SM | SMMC |
|---|---|---|
| Macronutrients | ||
| Soybean milk (g) | 35 | 35 |
| Mungbean milk (g) | 23 | 23 |
| Egg (g) | 2 | 10 |
| Soy isolated protein: Pea protein (ratio) | 2:2.5 | 2:2.5 |
| Apple juice (g) | 10 | 10 |
| Potato (g) | 5 | 2 |
| Maltodextrin (g) | 3 | 2 |
| Sugar (g) | 3 | 2 |
| Sesame (g) | 2 | 2 |
| SFA:MUFA:PUFA (ratio) | 2:1:0 | 3:2:1 |
| Vitamin Premix | ||
| Vitamin A (µg retinol) | 154 | 154 |
| Thiamin (mg) | 0.3 | 0.3 |
| Riboflavin (mg) | 0.4 | 0.4 |
| Niacin (mg) | 3.4 | 3.4 |
| Pantothenic acid (mg) | 1 | 1 |
| Vitamin B6 (mg) | 0.4 | 0.4 |
| Vitamin B12 (µg) | 0.4 | 0.4 |
| Vitamin C (mg) | 19 | 19 |
| Cholecalciferol (µg) | 1 | 1 |
| α-Tocopherol (mg) | 1.9 | 1.9 |
| Phytonadione (µg) | 18 | 18 |
| Biotin (µg) | 25 | 25 |
| Folic acid (µg) | 44 | 44 |
| SM 1 (285 g) | SMMC 1 (290 g) | Glucerna® (Control) 2 (300 g) | |
|---|---|---|---|
| Energy (kcal) | 300 | 300 | 300 |
| Carbohydrate (g, %) | 28.9, 39 | 20.7, 28 | 28.5, 38 |
| Protein (g, %) | 18.1, 24 | 21.3, 28 | 13.5, 18 |
| Fat (g, %) | 12.3, 37 | 14.8, 44 | 11.0, 33 |
| Saturated fat (g) | 5.5 | 6.1 | N/A 3 |
| Cholesterol (mg) | 16.4 | 46.2 | N/A 3 |
| Sugar (g) | 12.1 | 9.3 | N/A 3 |
| Sodium (mg) | 151.4 | 186.4 | 280.7 |
| Vitamin A (µg retinol) | 404.4 | 400.2 | 735.3 |
| Vitamin B1 (mg) | 0.9 | 0.8 | 0.5 |
| Vitamin B2 (mg) | 1.5 | 1.7 | 0.6 |
| Calcium (mg) | 68.6 | 72.8 | 223.8 |
| Iron (mg) | 2.3 | 2.3 | 1.8 |
| Measures (Mean ± Standard Error Mean) | n = 41 |
|---|---|
| Age (years) | 50.0 ± 1.39 |
| Male, n (%) | 17 (41.5) |
| Female, n (%) | 24 (58.5) |
| Underlying diseases 1 | |
| Diabetes Mellitus, n (%) | 41 (100.0) |
| Diabetes duration (years) | 6.2 ± 0.95 |
| Hypertension | 26 (63.4) |
| Dyslipidemia | 17 (41.5) |
| Body weight (kg) | 78.3 ± 2.30 |
| Height (cm) | 161.5 ± 1.28 |
| BMI (kg/m2) | 30.0 ± 0.80 |
| Waist circumference (cm) | 100.2 ± 1.71 |
| Systolic blood pressure (mmHg) | 132.9 ± 2.16 |
| Diastolic blood pressure (mmHg) | 85.4 ± 1.45 |
| HbA1c (%) | 7.2 ± 0.12 |
| Fasting blood glucose (mg/dL) | 139.2 ± 4.61 |
| Fasting insulin (μU/mL) | 18.4 ± 3.13 |
| Triglyceride (mg/dL) | 173.7 ± 17.89 |
| Cholesterol (mg/dL) | 176.8 ± 7.33 |
| HDL Cholesterol (mg/dL) | 50.4 ± 1.75 |
| LDL Cholesterol (mg/dL) | 114.0 ± 6.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongkolsucharitkul, P.; Pinsawas, B.; Surawit, A.; Pongkunakorn, T.; Manosan, T.; Ophakas, S.; Suta, S.; Pumeiam, S.; Mayurasakorn, K. Diabetes-Specific Complete Smoothie Formulas Improve Postprandial Glycemic Response in Obese Type 2 Diabetic Individuals: A Randomized Crossover Trial. Nutrients 2024, 16, 395. https://doi.org/10.3390/nu16030395
Mongkolsucharitkul P, Pinsawas B, Surawit A, Pongkunakorn T, Manosan T, Ophakas S, Suta S, Pumeiam S, Mayurasakorn K. Diabetes-Specific Complete Smoothie Formulas Improve Postprandial Glycemic Response in Obese Type 2 Diabetic Individuals: A Randomized Crossover Trial. Nutrients. 2024; 16(3):395. https://doi.org/10.3390/nu16030395
Chicago/Turabian StyleMongkolsucharitkul, Pichanun, Bonggochpass Pinsawas, Apinya Surawit, Tanyaporn Pongkunakorn, Thamonwan Manosan, Suphawan Ophakas, Sophida Suta, Sureeporn Pumeiam, and Korapat Mayurasakorn. 2024. "Diabetes-Specific Complete Smoothie Formulas Improve Postprandial Glycemic Response in Obese Type 2 Diabetic Individuals: A Randomized Crossover Trial" Nutrients 16, no. 3: 395. https://doi.org/10.3390/nu16030395
APA StyleMongkolsucharitkul, P., Pinsawas, B., Surawit, A., Pongkunakorn, T., Manosan, T., Ophakas, S., Suta, S., Pumeiam, S., & Mayurasakorn, K. (2024). Diabetes-Specific Complete Smoothie Formulas Improve Postprandial Glycemic Response in Obese Type 2 Diabetic Individuals: A Randomized Crossover Trial. Nutrients, 16(3), 395. https://doi.org/10.3390/nu16030395





