Sensor-Based Glucose Metrics during Different Diet Compositions in Type 1 Diabetes—A Randomized One-Week Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Study Design
2.2. Screening and Randomization Procedures
2.3. Pre- and Post-Intervention Visits
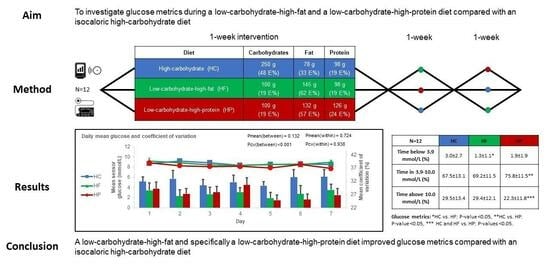
2.4. Composition of the Diets
2.5. Interventions
2.6. User Involvement
2.7. Statistical Analysis
3. Results
3.1. Participants
3.2. Sensor-Based Glucose Metrics
3.3. Insulin
3.4. Cardiovascular Risk Factors
3.5. Protocol Adherence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; Beaufort, C.D.; Donaghue, K.C.; Rai, P.; Ogle, G.D. Global Incidence, Prevalence, and Mortality of Type 1 Diabetes in 2021 with Projection to 2040: A Modelling Study. Lancet Diabetes Endocrinol. 2021, 10, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Dovc, K.; Battelino, T. Evolution of Diabetes Technology. Endocrinol. Metab. Clin. N. Am. 2020, 49, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; Dimeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, 81–90. [CrossRef] [PubMed]
- The Diabetes Control and Complication Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 6. Glycemic Targets: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S97–S110. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating Positive Health Behaviors and Well-Being to Improve Health Outcomes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S68–S96. [Google Scholar] [CrossRef]
- Seckold, R.; Fisher, E.; de Bock, M.; King, B.R.; Smart, C.E. The Ups and Downs of Low-Carbohydrate Diets in the Management of Type 1 Diabetes: A Review of Clinical Outcomes. Diabet. Med. 2019, 36, 326–334. [Google Scholar] [CrossRef]
- James, M.L.; Green, L.; Amiel, S.A.; Choudhary, P. Evaluation of the Effect of Carbohydrate Intake on Postprandial Glucose in Patients with Type 1 Diabetes Treated with Insulin Pumps. J. Diabetes Sci. Technol. 2016, 10, 1287–1293. [Google Scholar] [CrossRef]
- Turton, J.L.; Raab, R.; Rooney, K.B. Low-Carbohydrate Diets for Type 1 Diabetes Mellitus: A Systematic Review. PLoS ONE 2018, 13, e0194987. [Google Scholar] [CrossRef]
- Stamati, A.; Sotiriou, G.; Karagiannis, T.; Christoforidis, A. Efficacy and Safety of Carbohydrate Restriction in Patients with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 2023, 25, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Schmidt, S.; Damm-Frydenberg, C.; Holst, J.J.; Madsbad, S.; Nørgaard, K. Short-Term Effects of a Low Carbohydrate Diet on Glycaemic Variables and Cardiovascular Risk Markers in Patients with Type 1 Diabetes: A Randomized Open-Label Crossover Trial. Diabetes Obes. Metab. 2017, 19, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Christensen, M.B.; Serifovski, N.; Damm-Frydenberg, C.; Jensen, J.E.B.; Fløyel, T.; Størling, J.; Ranjan, A.; Nørgaard, K. Low versus High Carbohydrate Diet in Type 1 Diabetes: A 12-Week Randomized Open-Label Crossover Study. Diabetes Obes. Metab. 2019, 21, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Dimosthenopoulos, C.; Liatis, S.; Kourpas, E.; Athanasopoulou, E.; Driva, S.; Makrilakis, K.; Kokkinos, A. The Beneficial Short-Term Effects of a High-Protein/Low-Carbohydrate Diet on Glycaemic Control Assessed by Continuous Glucose Monitoring in Patients with Type 1 Diabetes. Diabetes Obes. Metab. 2021, 23, 1765–1774. [Google Scholar] [CrossRef]
- Scott, S.N.; Anderson, L.; Morton, J.P.; Wagenmakers, A.J.M.; Riddell, M.C. Carbohydrate Restriction in Type 1 Diabetes: A Realistic Therapy for Improved Glycaemic Control and Athletic Performance? Nutrients 2019, 11, 1022. [Google Scholar] [CrossRef]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of Fat, Protein, and Glycemic Index on Postprandial Glucose Control in Type 1 Diabetes: Implications for Intensive Diabetes Management in the Continuous Glucose Monitoring Era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef]
- Smart, C.E.; Ross, K.; Edge, J.A.; King, B.R.; McElduff, P.; Collins, C.E. Can Children with Type 1 Diabetes and Their Caregivers Estimate the Carbohydrate Content of Meals and Snacks? Diabet. Med. 2010, 27, 348–353. [Google Scholar] [CrossRef]
- Brazeau, A.S.; Mircescu, H.; Desjardins, K.; Leroux, C.; Strychar, I.; Ekoé, J.M.; Rabasa-Lhoret, R. Carbohydrate Counting Accuracy and Blood Glucose Variability in Adults with Type 1 Diabetes. Diabetes Res. Clin. Pract. 2013, 99, 19–23. [Google Scholar] [CrossRef]
- Smart, C.E.M.; Evans, M.; O’Connell, S.M.; McElduff, P.; Lopez, P.E.; Jones, T.W.; Davis, E.A.; King, B.R. Both Dietary Protein and Fat Increase Postprandial Glucose Excursions in Children with Type 1 Diabetes, and the Effect Is Additive. Diabetes Care 2013, 36, 3897–3902. [Google Scholar] [CrossRef]
- Paterson, M.; Bell, K.J.; O’Connell, S.M.; Smart, C.E.; Shafat, A.; King, B. The Role of Dietary Protein and Fat in Glycaemic Control in Type 1 Diabetes: Implications for Intensive Diabetes Management. Curr. Diabetes Rep. 2015, 15, 61. [Google Scholar] [CrossRef]
- Smart, C.E.M.; King, B.R.; Lopez, P.E. Insulin Dosing for Fat and Protein: Is It Time? Diabetes Care 2020, 43, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Barouti, A.A.; Björklund, A.; Catrina, S.B.; Brismar, K.; Rajamand Ekberg, N. Effect of Isocaloric Meals on Postprandial Glycemic and Metabolic Markers in Type 1 Diabetes—A Randomized Crossover Trial. Nutrients 2023, 15, 3092. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.B.; Ranjan, A.G.; Mccarthy, O.M.; Holst, J.J.; Bracken, R.M.; Nørgaard, K.; Schmidt, S. Effects of a Low-Carbohydrate-High-Protein Pre-Exercise Meal in Type 1 Diabetes—A Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2023, 109, 208–216. [Google Scholar] [CrossRef]
- Naude, C.E.; Schoonees, A.; Senekal, M.; Young, T.; Garner, P.; Volmink, J. Low Carbohydrate versus Isoenergetic Balanced Diets for Reducing Weight and Cardiovascular Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e0200284. [Google Scholar] [CrossRef]

| Diet Type | Carbohydrate | Fat | Protein |
|---|---|---|---|
| High-carbohydrate (HC) | 250 g | 78 g | 98 g |
| (48%) | (33%) | (19%) | |
| Low-carbohydrate-high-fat (HF) | 100 g | 145 g | 98 g |
| (19%) | (62%) | (19%) | |
| Low-carbohydrate-high-protein (HP) | 100 g | 132 g | 126 g |
| (19%) | (57%) | (24%) |
| n = 12 | HC | HF | HP | p-Value (Overall) | p-Value (HC vs. HF) | p-Value (HC vs. HP) | p-Value (HF vs. HP) |
|---|---|---|---|---|---|---|---|
| Mean sensor glucose (mmol/L) | 8.7 ± 1.0 | 8.7 ± 1.1 | 8.2 ± 1.0 | 0.08 | - | - | - |
| Standard deviation (mmol/L) | 3.0 ± 0.5 | 2.7 ± 0.7 | 2.5 ± 0.6 | 0.002 * | 0.042 * | 0.002 * | 0.371 |
| Coefficient of variation (%) | 34.5 ± 4.1 | 30.5 ± 6.2 | 30.0 ± 5.5 | 0.002 * | 0.009 * | 0.003 * | 0.906 |
| Time in 3.9–10.0 mmol/L (%) | 67.5 ± 13.1 | 69.2 ± 11.5 | 75.8 ± 11.5 | 0.041 * | 0.853 | 0.043 * | 0.126 |
| Time in 3.9–7.8 mmol/L (%) | 42.4 ± 12.4 | 41.7 ± 16.0 | 48.5 ± 16.1 | 0.157 | - | - | - |
| Time below 3.9 mmol/L (%) | 3.0 ± 2.7 | 1.3 ± 1.1 | 1.9 ± 1.9 | 0.022 * | 0.019 * | 0.140 | 0.601 |
| Time below 3.0 mmol/L (%) | 0.5 ± 0.7 | 0.1 ± 0.2 | 0.2 ± 0.5 | 0.148 | - | - | - |
| Time above 10.0 mmol/L (%) | 29.5 ± 13.4 | 29.4 ± 12.1 | 22.3 ± 11.8 | 0.019 * | 1.0 | 0.037 * | 0.037 * |
| Time above 13.9 mmol/L (%) | 6.9 ± 4.9 | 4.6 ± 5.4 | 3.1 ± 3.7 | 0.035 * | 0.230 | 0.007 * | 0.222 |
| Participants achieving glycemic targets † (number) | 3 | 3 | 7 | 0.033 * | 1.0 | 0.057 | 0.057 |
| Participants experiencing ≥1 hypoglycemia events (sensor glucose <3.9 mmol/L) during diet week (number) | 12 | 8 | 9 | - | - | - | - |
| Hypoglycemia events lasting ≥15 min per participant per week (number) | 5.4 | 3.1 | 2.6 | 0.015 * | 0.074 | 0.019 * | 0.873 |
| n = 12 | HC | HF | HP | p-Value (Overall) | p-Value (HC vs. HF) | p-Value (HC vs. HP) | p-Value (HF vs. HP) |
|---|---|---|---|---|---|---|---|
| Cardiovascular risk factors | |||||||
| ∆ Body weight (kg) | −0.2 (−0.8; 0.5) | −1.5 (−2.3; −0.7) | −1.0 (−1.4; −0.5) | 0.004 * | 0.003 * | 0.072 | 0.37 |
| ∆ Systolic blood pressure (mmHg) | −3 (−13; 2) | 2 (−2; 7) | −1 (−6; 3) | 0.37 | - | - | - |
| ∆ Diastolic blood pressure (mmHg) | 0 (−3; 3) | 2 (−2; 4) | 1 (−8; 4) | 0.88 | - | - | - |
| ∆ Heart rate (bpm) | 0 (−4; 3) | −4 (−9; −1) | −3 (−6; −1) | 0.10 | - | - | - |
| %-change UACR † | 6.2 (−32.0; 58.0) | −14.8 (−40.0; 50.7) | −16.5 (−42.3; 0.0) | 0.52 | - | - | - |
| ∆ eGFR † (mL/min/1.73 m2) | −2.6 (0.0; 1.5) | −1.0 (−1.0; 0.0) | −2.5 (−3.0; 0.0) | 0.48 NP | - | - | - |
| ∆ Total cholesterol (mmol/L) | −0.5 (−0.8; −0.3) | −0.2 (−0.3; −0.1) | −0.1 (−0.5; 0.3) | 0.11 | - | - | - |
| ∆ HDL (mmol/L) | −0.3 (−0.4; 0.0) | −0.1 (−0.5; 0.2) | −0.2 (−0.3; −0.1) | 0.22 | - | - | - |
| ∆ LDL (mmol/L) | −0.2 (−0.4; 0.0) | 0.0 (−0.2; 0.1) | 0.1 (−0.2; 0.5) | 0.11 | - | - | - |
| ∆ VLDL (mmol/L) | −0.0 (−0.1; 0.0) | −0.0 (−0.1; 0.0) | −0.2 (−0.1; 0.0) | 0.38 NP | - | - | - |
| ∆ Triglycerides (mmol/L) | −0.1 (−0.1; 0.1) | −0.1 (−0.4; 0.0) | −0.2 (−0.5; 0.3) | 0.75 NP | - | - | - |
| Insulin | |||||||
| Total daily basal insulin (U) | 20.6 (12.4; 22.4) | 20.7 (12.6; 22.0) | 20.7 (12.6; 22.8) | 0.91 | - | - | - |
| Total daily bolus insulin (U) | 29.1 (19.7; 32.3) | 14.5 (8.4; 17.3) | 13.5 (8.2; 14.8) | <0.001 * | <0.001 * | <0.001 * | 0.92 |
| Total daily insulin dose (U) | 49.8 (32.6; 52.9) | 35.2 (26.0; 34.6) | 34.2 (22.1; 35.6) | <0.001 * | <0.001 * | <0.001 * | 0.68 |
| Deviations from planned carbohydrate intake based on insulin pump entries | |||||||
| Deviation (g) | −4.8 (−25.3; 9.9) | 10.7 (−3.1; 28.4) | 5.7 (−1.2; 2.5) | 0.14 | - | - | - |
| Deviation (%) | 12.0 (2.2; 21.0) | 18.7 (3.7; 36.5) | 12.7 (0.8; 19.1) | 0.14 | - | - | - |
| Activity level | |||||||
| Energy expenditure per day (kcal) ‡ | 1481 (1220; 1752) | 1591 (1248; 1769) | 1563 (1184; 1855) | 0.70 | - | - | - |
| MET rate per day ‡ | 1.49 (1.40; 1.60) | 1.53 (1.39; 1.62) | 1.52 (1.40; 1.62) | 0.70 | - | - | - |
| Steps per day ‡ | 12,552 (9306; 15,374) | 12,815 (10,693; 16,258) | 12,908 (10,399; 15,597) | 0.90 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristensen, K.B.; Ranjan, A.G.; McCarthy, O.M.; Bracken, R.M.; Nørgaard, K.; Schmidt, S. Sensor-Based Glucose Metrics during Different Diet Compositions in Type 1 Diabetes—A Randomized One-Week Crossover Trial. Nutrients 2024, 16, 199. https://doi.org/10.3390/nu16020199
Kristensen KB, Ranjan AG, McCarthy OM, Bracken RM, Nørgaard K, Schmidt S. Sensor-Based Glucose Metrics during Different Diet Compositions in Type 1 Diabetes—A Randomized One-Week Crossover Trial. Nutrients. 2024; 16(2):199. https://doi.org/10.3390/nu16020199
Chicago/Turabian StyleKristensen, Kasper B., Ajenthen G. Ranjan, Olivia M. McCarthy, Richard M. Bracken, Kirsten Nørgaard, and Signe Schmidt. 2024. "Sensor-Based Glucose Metrics during Different Diet Compositions in Type 1 Diabetes—A Randomized One-Week Crossover Trial" Nutrients 16, no. 2: 199. https://doi.org/10.3390/nu16020199
APA StyleKristensen, K. B., Ranjan, A. G., McCarthy, O. M., Bracken, R. M., Nørgaard, K., & Schmidt, S. (2024). Sensor-Based Glucose Metrics during Different Diet Compositions in Type 1 Diabetes—A Randomized One-Week Crossover Trial. Nutrients, 16(2), 199. https://doi.org/10.3390/nu16020199