Vitamin D Supplementation: A Review of the Evidence Arguing for a Daily Dose of 2000 International Units (50 µg) of Vitamin D for Adults in the General Population
Abstract
1. Introduction
2. Current Vitamin D Guidelines
3. Safety of a Daily Vitamin D Supplementation with 2000 IU (50 µg)
4. Evidence Arguing for a Target Serum 25(OH)D Concentration of 75 nmol/L (30 ng/mL)
5. Practical and Pragmatic Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Martinaityte, I.; Kamycheva, E.; Didriksen, A.; Jakobsen, J.; Jorde, R. Vitamin D Stored in Fat Tissue During a 5-Year Intervention Affects Serum 25-Hydroxyvitamin D Levels the Following Year. J. Clin. Endocrinol. Metab. 2017, 102, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC-Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grubler, M.R.; Marz, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Ayoub, D.; Holick, M.F. Nutritional rickets and vitamin D deficiency: Consequences and strategies for treatment and prevention. Expert Rev. Endocrinol. Metab. 2022, 17, 351–364. [Google Scholar] [CrossRef]
- Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. Disassociation of Vitamin D’s Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 17685. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1357. [Google Scholar] [CrossRef]
- Kong, S.H.; Jang, H.N.; Kim, J.H.; Kim, S.W.; Shin, C.S. Effect of Vitamin D Supplementation on Risk of Fractures and Falls According to Dosage and Interval: A Meta-Analysis. Endocrinol. Metab. 2022, 37, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bouillon, R.; Binkley, N.; Sempos, C.; Adler, R.A.; Bollerslev, J.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Heijboer, A.; et al. Controversies in Vitamin D: A Statement From the Third International Conference. JBMR Plus 2020, 4, e10417. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Kiely, M. Contribution of nutrition science to the vitamin D field-Clarity or confusion? J. Steroid Biochem. Mol. Biol. 2019, 187, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Rosen, C.J. Vitamin D: 100 years of discoveries, yet controversy continues. Lancet Diabetes Endocrinol. 2023, 11, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Bennett, D.; Mafham, M.; Lin, X.; Chen, Z.; Armitage, J.; Clarke, R. Vitamin D and Calcium for the Prevention of Fracture: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1917789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between vitamin D supplementation and mortality: Systematic review and meta-analysis. BMJ 2019, 366, l4673. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Mathyssen, C.; Rafiq, R.; de Jongh, R.T.; Camargo, C.A.; Griffiths, C.J.; Janssens, W.; Martineau, A.R. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax 2019, 74, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Zhao, Y.; Yang, P.; Zhang, X.; Giovannucci, E.L. Vitamin D and human health: Evidence from Mendelian randomization studies. Eur. J. Epidemiol. 2024, 1–24. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Wetterslev, J.; Simonetti, R.G.; Bjelakovic, M.; Gluud, C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 2014, CD007470. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; Pallares-Carratala, V.; Turegano-Yedro, M.; Torres, F.; Sapena, V.; Martin-Gorgojo, A.; Martin-Moreno, J.M. Vitamin D Supplementation and Its Impact on Mortality and Cardiovascular Outcomes: Systematic Review and Meta-Analysis of 80 Randomized Clinical Trials. Nutrients 2023, 15, 1810. [Google Scholar] [CrossRef]
- Kuznia, S.; Zhu, A.; Akutsu, T.; Buring, J.E.; Camargo, C.A., Jr.; Cook, N.R.; Chen, L.J.; Cheng, T.D.; Hantunen, S.; Lee, I.M.; et al. Efficacy of vitamin D(3) supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res. Rev. 2023, 87, 101923. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A., Jr.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Williamson, A.; Martineau, A.R.; Sheikh, A.; Jolliffe, D.; Griffiths, C.J. Vitamin D for the management of asthma. Cochrane Database Syst. Rev. 2023, 2, CD011511. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Chen, L.; Zhang, H.; Yu, L.; Chi, Y.; Chen, M.; Cai, Y. Efficacy of vitamin D supplementation on COPD and asthma control: A systematic review and meta-analysis. J. Glob. Health 2022, 12, 04100. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Jiang, T.; Feng, J.; Zou, M. Negligible effect of vitamin D supplementation on exacerbation in patients with chronic obstructive pulmonary disease: Meta-analysis. Biochem. Med. 2023, 33, 030703. [Google Scholar] [CrossRef]
- Mattumpuram, J.; Maniya, M.T.; Faruqui, S.K.; Ahmed, A.; Jaiswal, V.; Harshakumar, S.P. Cardiovascular and Cerebrovascular Outcomes With Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2024, 49, 102119. [Google Scholar] [CrossRef]
- Barbarawi, M.; Kheiri, B.; Zayed, Y.; Barbarawi, O.; Dhillon, H.; Swaid, B.; Yelangi, A.; Sundus, S.; Bachuwa, G.; Alkotob, M.L.; et al. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol. 2019, 4, 765–776. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Pena-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef] [PubMed]
- Ganmaa, D.; Enkhmaa, D.; Nasantogtokh, E.; Sukhbaatar, S.; Tumur-Ochir, K.E.; Manson, J.E. Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials. J. Intern Med. 2021, 291, 141–164. [Google Scholar] [CrossRef]
- Scragg, R.; Sluyter, J.D. Is There Proof of Extraskeletal Benefits From Vitamin D Supplementation From Recent Mega Trials of Vitamin D? JBMR Plus 2021, 5, e10459. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Pilz, S.; Trummer, C.; Pandis, M.; Schwetz, V.; Aberer, F.; Grubler, M.; Verheyen, N.; Tomaschitz, A.; Marz, W. Vitamin D: Current Guidelines and Future Outlook. Anticancer Res. 2018, 38, 1145–1151. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative analysis of nutritional guidelines for vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Vitamin D Requirements for the Future-Lessons Learned and Charting a Path Forward. Nutrients 2018, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef]
- Pludowski, P.; Kos-Kudla, B.; Walczak, M.; Fal, A.; Zozulinska-Ziolkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewinski, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Buttriss, J.L.; Lanham-New, S.A.; Steenson, S.; Levy, L.; Swan, G.E.; Darling, A.L.; Cashman, K.D.; Allen, R.E.; Durrant, L.R.; Smith, C.P.; et al. Implementation strategies for improving vitamin D status and increasing vitamin D intake in the UK: Current controversies and future perspectives: Proceedings of the 2nd Rank Prize Funds Forum on vitamin D. Br. J. Nutr. 2022, 127, 1567–1587. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Ritz, C.; Kiely, M.; Odin, C. Improved Dietary Guidelines for Vitamin D: Application of Individual Participant Data (IPD)-Level Meta-Regression Analyses. Nutrients 2017, 9, 469. [Google Scholar] [CrossRef]
- Cashman, K.D.; Ritz, C.; Adebayo, F.A.; Dowling, K.G.; Itkonen, S.T.; Ohman, T.; Skaffari, E.; Saarnio, E.M.; Kiely, M.; Lamberg-Allardt, C. Differences in the dietary requirement for vitamin D among Caucasian and East African women at Northern latitude. Eur. J. Nutr. 2019, 58, 2281–2291. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kiely, M.E.; Andersen, R.; Gronborg, I.M.; Tetens, I.; Tripkovic, L.; Lanham-New, S.A.; Lamberg-Allardt, C.; Adebayo, F.A.; Gallagher, J.C.; et al. Individual participant data (IPD)-level meta-analysis of randomised controlled trials to estimate the vitamin D dietary requirements in dark-skinned individuals resident at high latitude. Eur. J. Nutr. 2022, 61, 1015–1034. [Google Scholar] [CrossRef]
- Mo, M.; Wang, S.; Chen, Z.; Muyiduli, X.; Wang, S.; Shen, Y.; Shao, B.; Li, M.; Chen, D.; Chen, Z.; et al. A systematic review and meta-analysis of the response of serum 25-hydroxyvitamin D concentration to vitamin D supplementation from RCTs from around the globe. Eur. J. Clin. Nutr. 2019, 73, 816–834. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Luttmann-Gibson, H.; Mora, S.; Camargo, C.A.; Cook, N.R.; Demler, O.V.; Ghoshal, A.; Wohlgemuth, J.; Kulkarni, K.; Larsen, J.; Prentice, J.; et al. Serum 25-hydroxyvitamin D in the VITamin D and OmegA-3 TriaL (VITAL): Clinical and demographic characteristics associated with baseline and change with randomized vitamin D treatment. Contemp. Clin. Trials 2019, 87, 105854. [Google Scholar] [CrossRef]
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef]
- Pilz, S.; Marz, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, F.A.; Itkonen, S.T.; Ohman, T.; Kiely, M.; Cashman, K.D.; Lamberg-Allardt, C.; On Behalf Of The Odin, C. Safety of Vitamin D Food Fortification and Supplementation: Evidence from Randomized Controlled Trials and Observational Studies. Foods 2021, 10, 3065. [Google Scholar] [CrossRef]
- Malihi, Z.; Wu, Z.; Lawes, C.M.M.; Scragg, R. Adverse events from large dose vitamin D supplementation taken for one year or longer. J. Steroid Biochem. Mol. Biol. 2019, 188, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Pilz, S. Long-term supplementation with 3200 to 4000 IU of vitamin D daily and adverse events: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2023, 62, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.G.; Palmer, S.C.; Strippoli, G.F.M.; Talbot, B.; Shah, N.; Hawley, C.M.; Toussaint, N.D.; Badve, S.V. Vitamin D Therapy in Adults With CKD: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2023, 82, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Tehrani, F.R.; Simbar, M.; Bidhendi Yarandi, R.; Minooee, S.; Hollis, B.W.; Hosseinpanah, F. Effectiveness of Prenatal Vitamin D Deficiency Screening and Treatment Program: A Stratified Randomized Field Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2936–2948. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Nino, J.F.; et al. Maternal 25(OH)D concentrations ≥40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef]
- Roth, D.E.; Morris, S.K.; Zlotkin, S.; Gernand, A.D.; Ahmed, T.; Shanta, S.S.; Papp, E.; Korsiak, J.; Shi, J.; Islam, M.M.; et al. Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. N. Engl. J. Med. 2018, 379, 535–546. [Google Scholar] [CrossRef]
- O’Callaghan, K.M.; Hennessy, A.; Hull, G.L.J.; Healy, K.; Ritz, C.; Kenny, L.C.; Cashman, K.D.; Kiely, M.E. Estimation of the maternal vitamin D intake that maintains circulating 25-hydroxyvitamin D in late gestation at a concentration sufficient to keep umbilical cord sera ≥25–30 nmol/L: A dose-response, double-blind, randomized placebo-controlled trial in pregnant women at northern latitude. Am. J. Clin. Nutr. 2018, 108, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska, E.; Kupisz-Urbanska, M.; Lukaszkiewicz, J.; Pludowski, P.; Jones, G. Vitamin D Toxicity-A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]
- Billington, E.O.; Burt, L.A.; Rose, M.S.; Davison, E.M.; Gaudet, S.; Kan, M.; Boyd, S.K.; Hanley, D.A. Safety of High-Dose Vitamin D Supplementation: Secondary Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2020, 105, 1261–1273. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Berthold, H.K.; Gouni-Berthold, I.; Gummert, J.F.; Pilz, S. A 3 year post-intervention follow-up on mortality in advanced heart failure (EVITA vitamin D supplementation trial). ESC Heart Fail. 2020, 7, 3754–3761. [Google Scholar] [CrossRef] [PubMed]
- Cappellani, D.; Brancatella, A.; Morganti, R.; Borsari, S.; Baldinotti, F.; Caligo, M.A.; Kaufmann, M.; Jones, G.; Marcocci, C.; Cetani, F. Hypercalcemia due to CYP24A1 mutations: A systematic descriptive review. Eur. J. Endocrinol. 2021, 186, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Mazess, R.B.; Bischoff-Ferrari, H.A.; Dawson-Hughes, B. Vitamin D: Bolus Is Bogus-A Narrative Review. JBMR Plus 2021, 5, e10567. [Google Scholar] [CrossRef]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grubler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; Marz, W. Critical Appraisal of Large Vitamin D Randomized Controlled Trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Orav, E.J.; Staehelin, H.B.; Meyer, O.W.; Theiler, R.; Dick, W.; Willett, W.C.; Egli, A. Monthly High-Dose Vitamin D Treatment for the Prevention of Functional Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 175–183. [Google Scholar] [CrossRef]
- Hanel, A.; Carlberg, C. Vitamin D and evolution: Pharmacologic implications. Biochem. Pharmacol. 2020, 173, 113595. [Google Scholar] [CrossRef] [PubMed]
- Gaksch, M.; Jorde, R.; Grimnes, G.; Joakimsen, R.; Schirmer, H.; Wilsgaard, T.; Mathiesen, E.B.; Njolstad, I.; Lochen, M.L.; Marz, W.; et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS ONE 2017, 12, e0170791. [Google Scholar] [CrossRef]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef]
- Durazo-Arvizu, R.A.; Dawson-Hughes, B.; Kramer, H.; Cao, G.; Merkel, J.; Coates, P.M.; Sempos, C.T. The Reverse J-Shaped Association Between Serum Total 25-Hydroxyvitamin D Concentration and All-Cause Mortality: The Impact of Assay Standardization. Am. J. Epidemiol. 2017, 185, 720–726. [Google Scholar] [CrossRef]
- Acharya, P.; Dalia, T.; Ranka, S.; Sethi, P.; Oni, O.A.; Safarova, M.S.; Parashara, D.; Gupta, K.; Barua, R.S. The Effects of Vitamin D Supplementation and 25-Hydroxyvitamin D Levels on the Risk of Myocardial Infarction and Mortality. J. Endocr. Soc. 2021, 5, bvab124. [Google Scholar] [CrossRef]
- Gibbons, J.B.; Norton, E.C.; McCullough, J.S.; Meltzer, D.O.; Lavigne, J.; Fiedler, V.C.; Gibbons, R.D. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci. Rep. 2022, 12, 19397. [Google Scholar] [CrossRef]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Dominguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv. Exp. Med. Biol. 2014, 810, 500–525. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G.; Group, D.d.R. Intratrial Exposure to Vitamin D and New-Onset Diabetes Among Adults With Prediabetes: A Secondary Analysis From the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care 2020, 43, 2916–2922. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥ 60 vs <20 ng/ml (150 vs 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Buring, J.E.; Group, V.R. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J. Steroid Biochem. Mol. Biol. 2020, 198, 105522. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Chen, L.; Huang, Y.; Christen, W.; Cook, N.R.; Copeland, T.; Mora, S.; Buring, J.E.; Lee, I.M.; et al. Effects of Vitamin D(3) and Marine Omega-3 Fatty Acids Supplementation on Biomarkers of Systemic Inflammation: 4-Year Findings from the VITAL Randomized Trial. Nutrients 2022, 14, 5307. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Jorde, R.; Kawahara, T.; Dawson-Hughes, B. Vitamin D Supplementation for Prevention of Type 2 Diabetes Mellitus: To D or Not to D? J. Clin. Endocrinol. Metab. 2020, 105, 3721–3733. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Avenell, A. Assessment of research waste part 2: Wrong study populations- an exemplar of baseline vitamin D status of participants in trials of vitamin D supplementation. BMC Med. Res. Methodol. 2018, 18, 101. [Google Scholar] [CrossRef]
- Carlberg, C.; Munoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef]
- Kanno, K.; Akutsu, T.; Ohdaira, H.; Suzuki, Y.; Urashima, M. Effect of Vitamin D Supplements on Relapse or Death in a p53-Immunoreactive Subgroup With Digestive Tract Cancer: Post Hoc Analysis of the AMATERASU Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2328886. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.C.; Wetterslev, J.; Winkel, P.; Lange, T.; Gluud, C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med. Res. Methodol. 2014, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Dawson-Hughes, B.; Durazo-Arvizu, R.; Thamm, M.; Tian, L.; Merkel, J.M.; Jones, J.C.; Carter, G.D.; Sempos, C.T. Vitamin D measurement standardization: The way out of the chaos. J. Steroid Biochem. Mol. Biol. 2017, 173, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.A.; Camara, J.E.; Sempos, C.T.; Lukas, P.; Le Goff, C.; Peeters, S.; Burdette, C.Q.; Nalin, F.; Hahm, G.; Durazo-Arvizu, R.A.; et al. Vitamin D Standardization Program (VDSP) intralaboratory study for the assessment of 25-hydroxyvitamin D assay variability and bias. J. Steroid Biochem. Mol. Biol. 2021, 212, 105917. [Google Scholar] [CrossRef] [PubMed]
- Saksa, N.; Neme, A.; Ryynanen, J.; Uusitupa, M.; de Mello, V.D.; Voutilainen, S.; Nurmi, T.; Virtanen, J.K.; Tuomainen, T.P.; Carlberg, C. Dissecting high from low responders in a vitamin D3 intervention study. J. Steroid Biochem. Mol. Biol. 2015, 148, 275–282. [Google Scholar] [CrossRef]
- Seuter, S.; Virtanen, J.K.; Nurmi, T.; Pihlajamaki, J.; Mursu, J.; Voutilainen, S.; Tuomainen, T.P.; Neme, A.; Carlberg, C. Molecular evaluation of vitamin D responsiveness of healthy young adults. J. Steroid Biochem. Mol. Biol. 2017, 174, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Vukic, M.; Neme, A.; Seuter, S.; Saksa, N.; de Mello, V.D.; Nurmi, T.; Uusitupa, M.; Tuomainen, T.P.; Virtanen, J.K.; Carlberg, C. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS ONE 2015, 10, e0124339. [Google Scholar] [CrossRef] [PubMed]
- Gospodarska, E.; Ghosh Dastidar, R.; Carlberg, C. Intervention Approaches in Studying the Response to Vitamin D(3) Supplementation. Nutrients 2023, 15, 3382. [Google Scholar] [CrossRef]
- Nikolova, M.G.; Boyanov, M.A.; Tsakova, A.D. Correlations of Serum Vitamin D with Metabolic Parameters in Adult Outpatients with Different Degrees of Overweight / Obesity Coming from an Urban Community. Acta Endocrinol. 2018, 14, 375–383. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Formenti, A.M.; Adler, R.A.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.M.; Buring, J.E.; et al. Association of Body Weight With Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Gummert, J.F.; Borgermann, J. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: A systematic review. Eur. J. Nutr. 2014, 53, 367–374. [Google Scholar] [CrossRef]
- Burrelli Scotti, G.; Afferri, M.T.; De Carolis, A.; Vaiarello, V.; Fassino, V.; Ferrone, F.; Minisola, S.; Nieddu, L.; Vernia, P. Factors affecting vitamin D deficiency in active inflammatory bowel diseases. Dig. Liver Dis. 2019, 51, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.H.; Hansen, T.I.; Gubatan, J.M.; Jensen, K.B.; Rejnmark, L. Managing vitamin D deficiency in inflammatory bowel disease. Frontline Gastroenterol. 2019, 10, 394–400. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn, J.R.; Bossuyt, P.; Ferrante, M.; West, R.L.; Dijkstra, G.; Witteman, B.J.; Wildenberg, M.; Hoentjen, F.; Franchimont, D.; Clasquin, E.; et al. High-Dose Vitamin D Does Not Prevent Postoperative Recurrence of Crohn’s Disease in a Randomized Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2021, 19, 1573–1582. [Google Scholar] [CrossRef]
- LoPinto-Khoury, C.; Brennan, L.; Mintzer, S. Impact of carbamazepine on vitamin D levels: A meta-analysis. Epilepsy Res. 2021, 178, 106829. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Gomez, J.M.; Bouillon, R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Wylon, K.; Drozdenko, G.; Krannich, A.; Heine, G.; Dolle, S.; Worm, M. Pharmacokinetic Evaluation of a Single Intramuscular High Dose versus an Oral Long-Term Supplementation of Cholecalciferol. PLoS ONE 2017, 12, e0169620. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hypponen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, E.G.; Lips, P.; Schoonmade, L.J.; Lanham-New, S.A.; van Schoor, N.M. Comparison of the Effect of Daily Vitamin D2 and Vitamin D3 Supplementation on Serum 25-Hydroxyvitamin D Concentration (Total 25(OH)D, 25(OH)D2, and 25(OH)D3) and Importance of Body Mass Index: A Systematic Review and Meta-Analysis. Adv. Nutr. 2023, 15, 100133. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Drincic, A.; Fuller, E.; Heaney, R.P.; Armas, L.A. 25-Hydroxyvitamin D response to graded vitamin D(3) supplementation among obese adults. J. Clin. Endocrinol. Meta. 2013, 98, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.R.; Harnack, L.; Michos, E.D.; Ogilvie, R.P.; Sempos, C.T.; Lutsey, P.L. Trends in Use of High-Dose Vitamin D Supplements Exceeding 1000 or 4000 International Units Daily, 1999–2014. JAMA 2017, 317, 2448–2450. [Google Scholar] [CrossRef]
- Rybchyn, M.S.; Abboud, M.; Puglisi, D.A.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Mason, R.S.; Fraser, D.R. Skeletal Muscle and the Maintenance of Vitamin D Status. Nutrients 2020, 12, 3270. [Google Scholar] [CrossRef]
- Kroll, M.H.; Bi, C.; Garber, C.C.; Kaufman, H.W.; Liu, D.; Caston-Balderrama, A.; Zhang, K.; Clarke, N.; Xie, M.; Reitz, R.E.; et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE 2015, 10, e0118108. [Google Scholar] [CrossRef]
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr. Dev. Nutr. 2019, 3, nzz087. [Google Scholar] [CrossRef]
- Levis, S.; Gomez, A.; Jimenez, C.; Veras, L.; Ma, F.; Lai, S.; Hollis, B.; Roos, B.A. Vitamin d deficiency and seasonal variation in an adult South Florida population. J. Clin. Endocrinol. Metab. 2005, 90, 1557–1562. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Taniguchi, H.; Kubo, T.; Higuchi, M. Effects of chronic endurance exercise training on serum 25(OH)D concentrations in elderly Japanese men. Endocrine 2018, 59, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.B.; Taniguchi, H.; Tanisawa, K.; Higuchi, M. Effect of an Acute Bout of Endurance Exercise on Serum 25(OH)D Concentrations in Young Adults. J. Clin. Endocrinol. Metab. 2017, 102, 3937–3944. [Google Scholar] [CrossRef]
- Dzik, K.P.; Grzywacz, T.; Luszczyk, M.; Kujach, S.; Flis, D.J.; Kaczor, J.J. Single bout of exercise triggers the increase of vitamin D blood concentration in adolescent trained boys: A pilot study. Sci. Rep. 2022, 12, 1825. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Global differences in vitamin D status and dietary intake: A review of the data. Endocr. Connect. 2021, 11, e210282. [Google Scholar] [CrossRef]
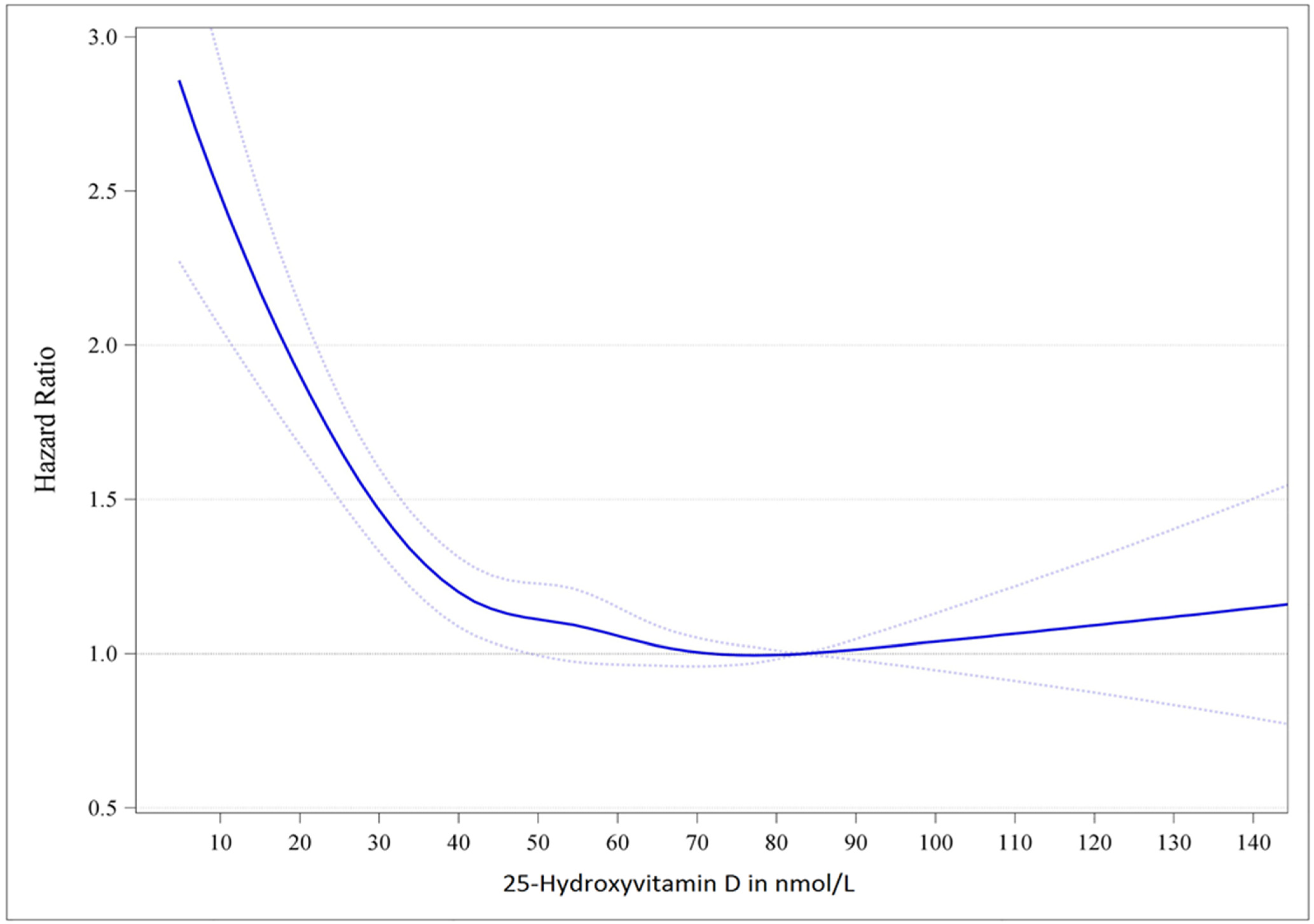
| Previous publications and guidelines may have partially underestimated the vitamin D requirements to achieve certain target serum 25(OH)D concentrations. |
| The high safety of a daily vitamin D supplementation dose of 2000 IU (50 µg) has been well established by recent RCT data documenting this over several years of treatment. |
| Clinical studies support a serum 25(OH)D concentration of 75 nmol/L (30 ng/mL) and higher as the optimal level. |
| Some RCT data support clinical extraskeletal benefits of vitamin D supplementation with 2000 (IU) (50 µg) per day. |
| IU, international units; 25(OH)D, 25-hydroxyvitamin D; RCT, randomized controlled trial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pludowski, P.; Grant, W.B.; Karras, S.N.; Zittermann, A.; Pilz, S. Vitamin D Supplementation: A Review of the Evidence Arguing for a Daily Dose of 2000 International Units (50 µg) of Vitamin D for Adults in the General Population. Nutrients 2024, 16, 391. https://doi.org/10.3390/nu16030391
Pludowski P, Grant WB, Karras SN, Zittermann A, Pilz S. Vitamin D Supplementation: A Review of the Evidence Arguing for a Daily Dose of 2000 International Units (50 µg) of Vitamin D for Adults in the General Population. Nutrients. 2024; 16(3):391. https://doi.org/10.3390/nu16030391
Chicago/Turabian StylePludowski, Pawel, William B. Grant, Spyridon N. Karras, Armin Zittermann, and Stefan Pilz. 2024. "Vitamin D Supplementation: A Review of the Evidence Arguing for a Daily Dose of 2000 International Units (50 µg) of Vitamin D for Adults in the General Population" Nutrients 16, no. 3: 391. https://doi.org/10.3390/nu16030391
APA StylePludowski, P., Grant, W. B., Karras, S. N., Zittermann, A., & Pilz, S. (2024). Vitamin D Supplementation: A Review of the Evidence Arguing for a Daily Dose of 2000 International Units (50 µg) of Vitamin D for Adults in the General Population. Nutrients, 16(3), 391. https://doi.org/10.3390/nu16030391










