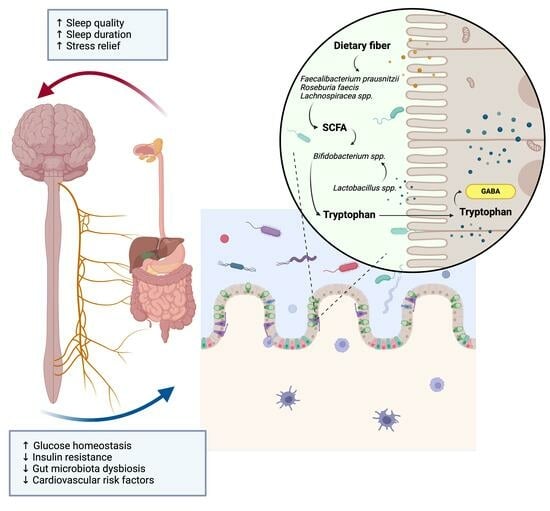
The Microbiota–Gut–Brain Axis in Metabolic Syndrome and Sleep Disorders: A Systematic Review
Abstract
1. Introduction
2. Sleep Disorders
3. Exogenous and Endogenous Factors Influencing Sleep
4. The Role of Host–Microbial Mechanisms in the Sleep Clock
5. The Neural System of the Gut–Brain Axis
6. The Role of the Immune System in the Gut–Brain Axis
7. The Role of Gut Microbiome in Metabolic Health
8. Biological Mechanisms Involved in Metabolic Syndrome
9. The Role of Gut Microbial Metabolism in Metabolic Syndrome
10. Dietary Modulation of Gut Microbiota in Metabolic Syndrome
| N | Authors | Title | Sample Size (n) | Disease Status | Type of Exposure | Methods | Outcome | Microbiota-Related Outcome(s) | Other Outcome(s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Wang et al., 2021 [72] | Dietary Supplementation with Inulin Modulates the Gut Microbiota and Improves Insulin Sensitivity in Prediabetes | 49 | Pre-diabetes | Inulin | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Bifidobacteriales, Bifidobacteriaceae, Bifidobacterium, Lactobacillae, and Lactobacillus increased after inulin supplementation and improved lipid levels. Eubacterium rectale, Butyricimonas, and Odoribacter positively correlated with insulin and triglyceride levels. | Improvement in lipid parameters. |
| 2 | Ismael et al., 2021 [85] | A Pilot Study on the Metabolic Impact of Mediterranean Diet in Type 2 Diabetes: Is Gut Microbiota the Key? | 9 | T2D | MedDiet | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Increase in bacterial richness negatively correlated with glucose and insulin levels. | |
| 3 | Guo et al., 2021 [86] | Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients | 39 | Metabolic Syndrome | IF | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Acidobacteria bacterium, Eubacterium sp. 1_3, and Roseburia faecis associated with improvements in cardiometabolic markers. | Improvement in cardiometabolic risk factors. |
| 4 | Marungruang et al., 2018 [87] | Improvement in cardiometabolic risk markers following a multifunctional diet is associated with gut microbial taxa in healthy overweight and obese subjects | 47 | Overweight | MFD | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Increased abundance of Prevotella copri in the MFD group as compared to the control group. Treponema correlated positively with blood pressure. In contrast, Faecalibacterium showed a negative association with blood pressure, while Bilophila appeared to associate with a negative blood lipid profile. | Improved blood pressure and lipid profile. |
| 5 | Haro et al., 2017 [88] | Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction | 106 | Obese | Healthy Dietary patterns | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Restoration of the gut microbiome dysbiosis. | |
| 6 | Wastyk et al., 2023 [81] | Randomized controlled trial demonstrates response to a probiotic intervention for metabolic syndrome that may correspond to diet | 39 | Metabolic Syndrome | Probiotic | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Metabolic shifts associated with a distinct microbiome profile. | Improvements in triglycerides and DBP in a subset of participants. |
| 7 | Gilijamse et al., 2020 [83] | Treatment with Anaerobutyricum soehngenii: a pilot study of safety and dose-response effects on glucose metabolism in human subjects with metabolic syndrome | 24 | Metabolic Syndrome | Anaerobutyricum soehngenii | Shotgun metagenomics | Metabolic Syndrome/Gut Microbiota | Gut microbiota alteration and change in bile acid metabolism. Levels of endogenous Anaerobutyricum spp. were not significantly different in fecal baseline samples when comparing the three dose groups (Kruskal–Wallis, p = 0.10). | Improvement in insulin sensitivity. |
| 8 | Sangouni et al., 2023 [89] | Garlic supplementation improves intestinal transit time, lipid accumulation product and cardiometabolic indices in subjects with metabolic syndrome: A randomized controlled trial | 90 | Metabolic Syndrome | Garlic Powder | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Garlic powder improved intestinal transit time. | Improvements in cardiometabolic indexes. |
| 9 | Eriksen et al., 2020 [74] | Effects of whole-grain wheat, rye, and lignan supplementation on cardiometabolic risk factors in men with metabolic syndrome: a randomized crossover trial | 40 | Metabolic Syndrome | Whole-grain rich diet | 16S sequencing | Metabolic Syndrome/Gut Microbiota | WG rye is associated with higher Bifidobaterium and lower Clostridium. The effect of WG diets appeared to differ according to baseline microbial enterotype. WG rye resulted in higher abundance of Bifidobacterium lower abundance of Clostridium genus compared with WG wheat (FC = 2.58, p < 0.001) and baseline (FC = 0.54, p = 0.02). | WG rye, alone or with SDG supplementation, compared with WG wheat did not affect glucose metabolism but caused transient LDL-cholesterol reduction. |
| 10 | Rabiei et al., 2018 [82] | The Effects of Synbiotic Supplementation on Body Mass Index, Metabolic and Inflammatory Biomarkers, and Appetite in Patients with Metabolic Syndrome: A Triple-Blind Randomized Controlled Trial | 46 | Metabolic Syndrome | Synbiotic | 16S sequencing | Metabolic Syndrome | Symbiotic treatment improved the status of BMI, FBS, insulin resistance, HOMA-IR, GLP-1, and PYY in patients with metabolic syndrome. | |
| 11 | Velikonja et al., 2019 [75] | Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development | 43 | Healthy | Barley Bread | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Decrease in microbial diversity and richness in the test group. The pre-intervention gut microbiota composition showed higher abundance of health associated Bifidobacterium spp. and Akkermansia municiphila within cholesterol-responsive group. | |
| 12 | Xiao et al., 2014 [90] | A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome | 93 | Obese | WTP diet | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Improved lipid profile correlated to decrease in opportunistic pathogens of Enterobacteriaceae and Desulfovibrionaceae. | Improvement in insulin sensitivity and lipid profile. |
| 13 | Kjølbæk et al., 2020 [73] | Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial | 27 | Overweight | AXOS | 16S sequencing | Metabolic Syndrome/Gut Microbiota | AXOS intake has a bifidogenic effect and also increases butyrate producers in the gut microbiota. | No modulation of lipid or glucose metabolic parameters related to metabolic syndrome. |
| 14 | Wang et al., 2021 [80] | Gut Microbiota Composition is Associated with Responses to Peanut Intervention in Multiple Parameters Among Adults with Metabolic Syndrome Risk | 209 | Obese | Peanuts | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Minor modification of gut microbiome, except Bilophila, Coprococcus, and Dorea, appeared to be decreased after peanut intervention. | Decreased body weight, waist circumference and fasting blood glucose. |
| 15 | Akamine et al., 2022 [76] | Fermented brown rice beverage distinctively modulates the gut microbiota in Okinawans with metabolic syndrome: A randomized controlled trial | 40 | Metabolic Syndrome | Brown rice | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Increase in the number of beneficial species belonging to the Clostridia class, associated with reduced inflammation and increased SCFA production. Interestingly, ingestion of BA in contrast to WA resulted in a unique elevation in the abundance of number of beneficial species belonging to the Clostridia class, associated with reduced inflammation, and increased short-chain fatty acid production: Lactobacillales bacterium DJF B280 (p = 0.005), Butyrate-producing bacterium A2 207 (p = 0.012), and Firmicutes bacterium DJF VP44 (p = 0.038). | Reduction in inflammation. |
| 16 | Tian et al., 2022 [53] | Overall Structural Alteration of Gut Microbiota and Relationships with Risk Factors in Patients with Metabolic Syndrome Treated with Inulin Alone and with Other Agents: An Open-Label Pilot Study | 60 | Metabolic Syndrome | Inulin | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Inulin alone or combined with metformin or TCM altered specific gut microbiota taxa, like a decrease in Bacteroides, but not the general microbial diversity. | |
| 17 | Munch et al., 2019 [77] | Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial | 60 | Healthy | Refined Grain | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Compared with refined grain, whole grain did not significantly alter glucose homeostasis and did not induce major changes in the fecal microbiome. | Whole-grain diet did not alter insulin sensitivity and gut microbiome but reduced body weight and systemic low-grade inflammation. |
| 18 | Hibberd et al., 2018 [91] | Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults | 134 | Overweight | Synbiotic (B420+LU) or Prebiotic (LU) | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Christensenellaceae was consistently increased in the LU and LU+B420 groups across the intervention time points. | LU+B420 correlated negatively to waist/hip ratio and energy intake at baseline, and waist-area body fat mass. |
| 19 | Bellikci-Koyu et al., 2019 [84] | Effects of Regular Kefir Consumption on Gut Microbiota in Patients with Metabolic Syndrome: A Parallel-Group, Randomized, Controlled Study | 22 | Metabolic Syndrome | Kefir | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Only Actinobacteria was significantly increased after kefir intervention. | Fasting insulin, HOMA-IR, TNF-α, IFN-γ, and systolic and diastolic blood pressure decreased after kefir intervention, not differently as with the control. |
| 20 | Thomas et al., 2022 [92] | Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome | 23 | Metabolic Syndrome | Egg Consumption | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Diet intervention had no effects on microbiota diversity measures or relative taxa abundances. | Bacterial biodiversity correlated with HDL. |
| 21 | Guevara-Cruz et al., 2019 [93] | Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects With Metabolic Syndrome | 21 | Metabolic Syndrome | Lifestyle Intervention | 16S sequencing | Metabolic Syndrome/Gut Microbiota | A decrease in the dysbiosis of the gut microbiota associated with a reduction in the Prevotella/Bacteroides ratio and an increase in the abundance of Akkermansia muciniphila and Faecalibacterium prausnitzii after intervention. | Twenty-four percent reduction in serum triglycerides and a 44.8% reduction in MetS after a 75-day lifestyle intervention. |
| 22 | Galié et al., 2021 [62] | Effects of Mediterranean Diet on plasma metabolites and their relationship with insulin resistance and gut microbiota composition in a crossover randomized clinical trial | 44 | Metabolic Syndrome | Mediterranean Diet and Nuts | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Different microbial clusters that correlate with plasma metabolomics modules associated with improvements in cardiometabolic risk factors. | |
| 23 | Galié et al., 2021 [78] | Composition and Fecal Metabolites and their Relationship with Cardiometabolic Risk Factors | 50 | Metabolic Syndrome | MedDiet Nuts | 16S sequencing | Metabolic Syndrome/Gut Microbiota | MedDiet induced higher abundances of Lachnospiraceae NK4A136, which correlated with insulin homeostasis. | Improvement in insulin resistance and glucose levels after MedDiet versus nut supplementation. |
| 24 | Tagliamonte et al., 2021 [61] | Mediterranean diet consumption affects the endocannabinoid system in overweight and obese subjects: possible links with gut microbiome, insulin resistance and inflammation. | 82 | Obese/Overweight | MedDiet | 16S sequencing | Metabolic Syndrome/Gut Microbiota | Increase in A. muciniphila abundance in the gut independently of body weight changes. | Endocannabinoid tone and microbiome functionality at baseline drives an individualized response to an MedDiet in ameliorating insulin sensitivity and inflammation. The MedDiet intervention lowered plasma AEA (p = 0.02) and increased plasma OEA/PEA (p = 0.009) and OEA/AEA (p = 0.006). |
| 25 | Asnicar et al., 2021 [52] | Microbiome connections with host metabolism and habitual diet from 1098 deeply phenotyped individuals. | 1098 | Metabolic Syndrome | Observational | Shotgun metagenomics | Metabolic Syndrome/Gut Microbiota | An unfavorable microbial cluster, mainly including Clostridium species (Clostridium innocuum, Clostridium symbiosum, Clostridium spiroforme, Clostridium leptum, Clostridium saccharolyticum), positively correlated with lipoproteins associated with an increased risk of CVD and T2D |
11. The Dietary Modulation of Gut Microbiota in Sleep Disorders
12. Gut Microbiota: The Mediator between Metabolic Homeostasis and Sleep Quality?
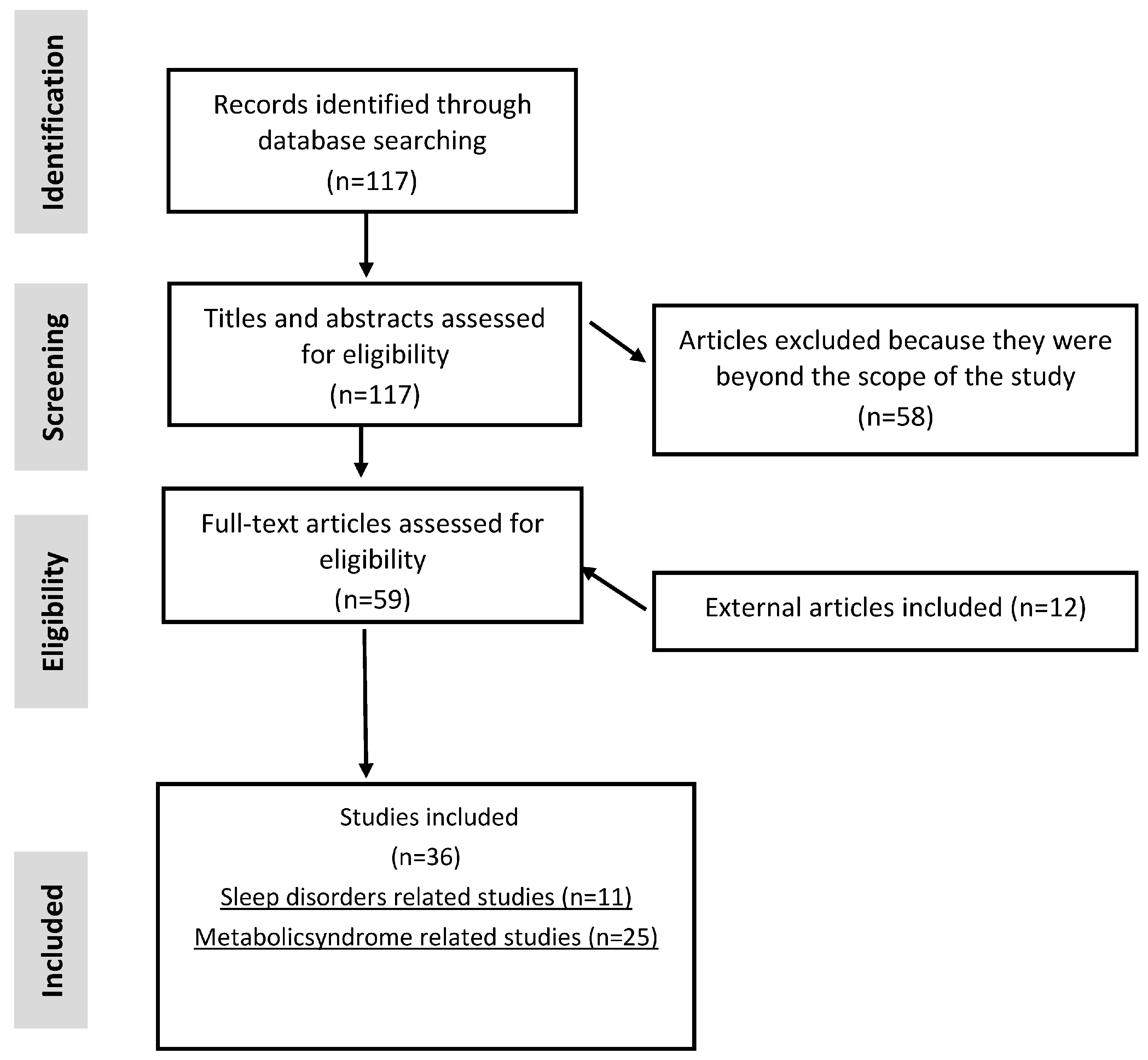
13. Methodology
14. Eligibility Criteria
- Dysbiosis and gut microbiome;
- Microbiome metabolites;
- Metabolic syndrome, sleep quality/efficiency, gut microbiome;
- Publication date range: 2013–August 2023;
- Language: English;
- Humans;
- RCTs and clinical trials.
15. Search Strategy
16. Data Extraction and Analysis
- Non-original articles (reviews, meta-analyses, protocols and letters);
- Child populations;
- Cognition problems, mental illness, and unrelated disorders;
- Unclear outcomes;
- Gut microbiota composition with qPCR/RFLP;
- Mechanistic studies.
17. Study Quality Assessment
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, Y.Y.; Chen, J.; Peng, M.; Zhou, J.; Chen, X.; Tan, X.; Wang, N.; Ma, H.; Guo, L.; Zhang, J.; et al. Association between sleep duration and metabolic syndrome: Linear and nonlinear Mendelian randomization analyses. J. Transl. Med. 2023, 21, 90. [Google Scholar] [CrossRef]
- Dubowy, C.; Sehgal, A. Circadian rhythms and sleep in Drosophila melanogaster. Genetics 2017, 205, 1373–1397. [Google Scholar] [CrossRef]
- Yao, Z.W.; Zhao, B.C.; Yang, X.; Lei, S.H.; Jiang, Y.M.; Liu, K.X. Relationships of sleep disturbance, intestinal microbiota, and postoperative pain in breast cancer patients: A prospective observational study. Sleep Breath. 2021, 25, 1655. [Google Scholar] [CrossRef] [PubMed]
- Carley, D.W.; Farabi, S.S. Physiology of sleep. Diabetes Spectr. 2016, 29, 5–9. [Google Scholar] [CrossRef]
- Hanson, J.A.; Huecker, M.R. Sleep Deprivation. StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547676/ (accessed on 21 October 2023).
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Fabbri, M.; Beracci, A.; Martoni, M.; Meneo, D.; Tonetti, L.; Natale, V. Measuring Subjective Sleep Quality: A Review. Int. J. Environ. Res. Public Health 2021, 18, 1082. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S. Overview of sleep disorders. Indian J. Med. Res. 2010, 131, 126–140. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. Hyperarousal and insomnia. Sleep Med. Rev. 1997, 1, 97–108. [Google Scholar] [CrossRef]
- Kay, D.B.; Buysse, D.J.; Bastien, H. Hyperarousal and Beyond: New Insights to the Pathophysiology of Insomnia Disorder through Functional Neuroimaging Studies. Brain Sci. 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Zoumakis, E.; Bixler, E.O.; Lin, H.M.; Follett, H.; Kales, A.; Chrousos, G.P. Adverse Effects of Modest Sleep Restriction on Sleepiness, Performance, and Inflammatory Cytokines. J. Clin. Endocrinol. Metab. 2004, 89, 2119–2126. [Google Scholar] [CrossRef]
- Moldofsky, H.; Lue, F.A.; Davidson, J.R.; Gorczynski, R. Effects of sleep deprivation on human immune functions. FASEB J. 1989, 3, 1972–1977. [Google Scholar] [CrossRef]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Reddy, V.; Sharma, S. Physiology, Circadian Rhythm. StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519507/ (accessed on 21 October 2023).
- Bechtel, W. Circadian rhythms and mood disorders: Are the phenomena and mechanisms causally related? Front. Psychiatry 2015, 6, 118. [Google Scholar] [CrossRef]
- Nutt, D.J.; Wilson, S.; Paterson, L. Sleep disorders as core symptoms of depression. Dialogues Clin. Neurosci. 2008, 10, 329–336. [Google Scholar] [CrossRef]
- Gulia, K.K.; Kumar, V.M. Sleep disorders in the elderly: A growing challenge. Psychogeriatrics 2018, 18, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-Analysis of Quantitative Sleep Parameters From Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values Across the Human Lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2019, 7, 1. [Google Scholar] [CrossRef]
- Yao, Y.; Silver, R. Mutual Shaping of Circadian Body-Wide Synchronization by the Suprachiasmatic Nucleus and Circulating Steroids. Front. Behav. Neurosci. 2022, 16, 877256. [Google Scholar] [CrossRef]
- Hwang, J.H.; Park, S.W. The relationship between poor sleep quality measured by the Pittsburgh Sleep Quality Index and smoking status according to sex and age: An analysis of the 2018 Korean Community Health Survey. Epidemiol. Health 2022, 44, e2022022. [Google Scholar] [CrossRef]
- Hinz, A.; Glaesmer, H.; Brähler, E.; Löffler, M.; Engel, C.; Enzenbach, C.; Hegerl, U.; Sander, C. Sleep quality in the general population: Psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Med. 2017, 30, 57–63. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Froy, O. Metabolism and Circadian Rhythms—Implications for Obesity. Endocr. Rev. 2010, 31, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.G. Sleep, circadian rhythms and health. Interface Focus 2020, 10, 20190098. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.G.; Kreitzman, L. The rhythms of life: What your body clock means to you! Exp. Physiol. 2014, 99, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.G.; Kreitzman, L. Rhythms of Life: The Biological Clocks That Control the Daily Lives of Every Living Thing; Profile Books: London, UK, 2004. [Google Scholar]
- Paschos, G.K.; FitzGerald, G.A. Circadian clocks and Metabolism: Implications for microbiome and aging. Trends Genet. 2017, 33, 760. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Molecular Analysis of Mammalian Circadian Rhythms. Annu. Rev. Physiol. 2003, 63, 647–676. [Google Scholar] [CrossRef]
- Van Der Horst, G.T.J.; Muijtjens, M.; Kobayashi, K.; Takano, R.; Kanno, S.I.; Takao, M.; De Wit, J.; Verkerk, A.; Eker, A.P.M.; Van Leenen, D.; et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 1999, 398, 627–630. [Google Scholar] [CrossRef]
- Schmitt, K.; Grimm, A.; Dallmann, R.; Oettinghaus, B.; Restelli, L.M.; Witzig, M.; Ishihara, N.; Mihara, K.; Ripperger, J.A.; Albrecht, U.; et al. Circadian Control of DRP1 Activity Regulates Mitochondrial Dynamics and Bioenergetics. Cell Metab. 2018, 27, 657–666.e5. [Google Scholar] [CrossRef]
- Lewy, A.J.; Bauer, V.K.; Cutler, N.L.; Sack, R.L.; Ahmed, S.; Thomas, K.H.; Blood, M.L.; Jackson, J.M.L. Morning vs Evening Light Treatment of Patients With Winter Depression. Arch. Gen. Psychiatry 1998, 55, 890–896. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef]
- Eban-Rothschild, A.; Appelbaum, L.; De Lecea, L. Neuronal Mechanisms for Sleep/Wake Regulation and Modulatory Drive. Neuropsychopharmacology 2018, 43, 937–952. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Diez-Gutiérrez, L.; Vicente, L.S.; Barrón, L.J.R.; del Carmen Villarán, M.; Chavarri, M. Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Kåhrström, C. Bacteria and the brain. Nat. Rev. Microbiol. 2014, 12, 725. [Google Scholar] [CrossRef]
- Sgro, M.; Kodila, Z.N.; Brady, R.D.; Reichelt, A.C.; Mychaisuk, R.; Yamakawa, G.R. Synchronizing our clocks as we age: The influence of the brain-gut-immune axis on the sleep-wake cycle across the lifespan. Sleep 2022, 45, zsab268. [Google Scholar] [CrossRef]
- Dogra, S.K.; Doré, J.; Damak, S. Gut Microbiota Resilience: Definition, Link to Health and Strategies for Intervention. Front. Microbiol. 2020, 11, 2014–2021. [Google Scholar] [CrossRef]
- Ma, D.; Forsythe, P.; Bienenstock, J. Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect. Immun. 2004, 72, 5308–5314. [Google Scholar] [CrossRef]
- Kubo, M. Diurnal Rhythmicity Programs of Microbiota and Transcriptional Oscillation of Circadian Regulator, NFIL3. Front. Immunol. 2020, 11, 552188. [Google Scholar] [CrossRef]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef]
- Oonk, M.; Krueger, J.M.; Davis, C.J. Voluntary sleep loss in rats. Sleep 2016, 39, 1467–1479. [Google Scholar] [CrossRef][Green Version]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Alexis, E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Cultured gut microbiota from twins discordant for obesity modulate adiposity and metabolic phenotypes in mice. Science 2014, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Hjorth, M.F.; Blædel, T.; Bendtsen, L.Q.; Lorenzen, J.K.; Holm, J.B.; Kiilerich, P.; Roager, H.M.; Kristiansen, K.; Larsen, L.H.; Astrup, A. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: Results from a post-hoc analysis. Int. J. Obes. 2018, 43, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Selber-Hnatiw, S.; Sultana, T.; Tse, W.; Abdollahi, N.; Abdullah, S.; Al Rahbani, J.; Alazar, D.; Alrumhein, N.J.; Aprikian, S.; Arshad, R.; et al. Metabolic networks of the human gut microbiota. Microbiology 2020, 166, 96–119. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.P.; Wang, B.; Jain, S.; Ding, J.; Rejeski, J.; Furdui, C.M.; Kitzman, D.W.; Taraphder, S.; Brechot, C.; Kumar, A.; et al. Gut microbiota A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 2023, 72, 1848–1865. [Google Scholar] [CrossRef] [PubMed]
- Cirujanos, C.Y.; León-Pedroza, J.I.; Alonso González-Tapia, L.; Del Olmo-Gil, E.; Castellanos-Rodríguez, D.; Escobedo, G.; González-Chávez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cirugía y Cir. 2015, 83, 543–551. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Hong, J.; Zhao, J.; Zhou, D.; Liu, Y.; Jiao, Z.; Song, J.; Zhang, Y.; Meng, L.; Yu, M. Overall Structural Alteration of Gut Microbiota and Relationships with Risk Factors in Patients with Metabolic Syndrome Treated with Inulin Alone and with Other Agents: An Open-Label Pilot Study. Mediat. Inflamm. 2022, 2022, 2078520. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Johansson-Boll, E.V.; Björck, I.M.E. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: A randomised cross-over study in healthy middle-aged subjects. Br. J. Nutr. 2015, 114, 899–907. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Schaafsma, A.; Mallee, L.; van den Belt, M.; Floris, E.; Kortman, G.; Veldman, J.; van den Ende, D.; Kardinaal, A. The effect of a whey-protein and galacto-oligosaccharides based product on parameters of sleep quality, stress, and gut microbiota in apparently healthy adults with moderate sleep disturbances: A randomized controlled cross-over study. Nutrients 2021, 13, 2204. [Google Scholar] [CrossRef]
- Müller, M.; Hernández, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.E.; van Eijk, H.; Canfora, E.E.; Blaak, E.E. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 2019, 9, 12515. [Google Scholar] [CrossRef]
- Li, D.; Mehta, J.L. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of coronary artery endothelial cells: Evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1116–1122. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Zhou, Y.; Wang, D.; Liu, X.; Li, L.; Wang, T.; Zhang, Y.; Jiang, M.; Tang, H.; et al. Gut microbiota changes and their relationship with inflammation in patients with acute and chronic insomnia. Nat. Sci. Sleep 2020, 12, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, C.; Qian, Y.; Zhang, S.; Zhang, C.; Zhao, W.; Zhang, T.; Zhang, B.; Chen, J.; Liu, S.; et al. Large-scale functional network connectivity mediate the associations of gut microbiota with sleep quality and executive functions. Hum. Brain Mapp. 2021, 42, 3088–3101. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; GarcíaGavilán, J.; CamachoBarcía, L.; Atzeni, A.; Muralidharan, J.; Papandreou, C.; Arcelin, P.; PalauGalindo, A.; Garcia, D.; Basora, J.; et al. Effects of the Mediterranean Diet or Nut Consumption on Gut Microbiota Composition and Fecal Metabolites and their Relationship with Cardiometabolic Risk Factors. Mol. Nutr. Food Res. 2021, 65, 2000982. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Yoon, M.-S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Soledad Sánchez, M.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- D’agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Zhang, Q.; Xu, L.; Xiao, X. Dietary Supplementation with Inulin Modulates the Gut Microbiota and Improves Insulin Sensitivity in Prediabetes. Int. J. Endocrinol. 2021, 2021, 5579369. [Google Scholar] [CrossRef]
- Kjølbæk, L.; Benítez-Páez, A.; Gómez del Pulgar, E.M.; Brahe, L.K.; Liebisch, G.; Matysik, S.; Rampelli, S.; Vermeiren, J.; Brigidi, P.; Larsen, L.H.; et al. Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial. Clin. Nutr. 2020, 39, 67–79. [Google Scholar] [CrossRef]
- Eriksen, A.K.; Brunius, C.; Mazidi, M.; Hellström, P.M.; Risérus, U.; Iversen, K.N.; Fristedt, R.; Sun, L.; Huang, Y.; Nørskov, N.P.; et al. Effects of whole-grain wheat, rye, and lignan supplementation on cardiometabolic risk factors in men with metabolic syndrome: A randomized crossover trial. Am. J. Clin. Nutr. 2020, 111, 864–876. [Google Scholar] [CrossRef]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Orel, R.; Avguštin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef]
- Akamine, Y.; Millman, J.F.; Uema, T.; Okamoto, S.; Yonamine, M.; Uehara, M.; Kozuka, C.; Kaname, T.; Shimabukuro, M.; Kinjo, K.; et al. Fermented brown rice beverage distinctively modulates the gut microbiota in Okinawans with metabolic syndrome: A randomized controlled trial. Nutr. Res. 2022, 103, 68–81. [Google Scholar] [CrossRef]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef]
- Galié, S.; García-Gavilán, J.; Papandreou, C.; Camacho-Barcía, L.; Arcelin, P.; Palau-Galindo, A.; Rabassa, A.; Bulló, M. Effects of Mediterranean Diet on plasma metabolites and their relationship with insulin resistance and gut microbiota composition in a crossover randomized clinical trial. Clin. Nutr. 2021, 40, 3798–3806. [Google Scholar] [CrossRef]
- Muralidharan, J.; Moreno-Indias, I.; Bulló, M.; Lopez, J.V.; Corella, D.; Castañer, O.; Vidal, J.; Atzeni, A.; Fernandez-García, J.C.; Torres-Collado, L.; et al. Effect on gut microbiota of a 1-y lifestyle intervention with Mediterranean diet compared with energy-reduced Mediterranean diet and physical activity promotion: PREDIMED-Plus Study. Am. J. Clin. Nutr. 2021, 114, 1148–1158. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Wang, D.; Huang, M.; Zhao, J.; Malik, V.; Liu, X.; Sun, L.; Lin, X.; Chen, Y. Gut Microbiota Composition is Associated with Responses to Peanut Intervention in Multiple Parameters Among Adults with Metabolic Syndrome Risk. Mol. Nutr. Food Res. 2021, 65, 2001051. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Perelman, D.; Topf, M.; Fragiadakis, G.K.; Robinson, J.L.; Sonnenburg, J.L.; Gardner, C.D.; Sonnenburg, E.D. Randomized controlled trial demonstrates response to a probiotic intervention for metabolic syndrome that may correspond to diet. Gut Microbes 2023, 15, 2178794. [Google Scholar] [CrossRef]
- Rabiei, S.; Hedayati, M.; Rashidkhani, B.; Saadat, N.; Shakerhossini, R. The Effects of Synbiotic Supplementation on Body Mass Index, Metabolic and Inflammatory Biomarkers, and Appetite in Patients with Metabolic Syndrome: A Triple-Blind Randomized Controlled Trial. J. Diet. Suppl. 2018, 16, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Gilijamse, P.W.; Hartstra, A.V.; Levin, E.; Wortelboer, K.; Serlie, M.J.; Ackermans, M.T.; Herrema, H.; Nederveen, A.J.; Imangaliyev, S.; Aalvink, S.; et al. Treatment with Anaerobutyricum soehngenii: A pilot study of safety and dose–response effects on glucose metabolism in human subjects with metabolic syndrome. NPJ Biofilms Microbiomes 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Bellikci-Koyu, E.; Pınar Sarer-Yurekli, B.; Akyon, Y.; Aydin-Kose, F.; Karagozlu, C.; Gokhan Ozgen, A.; Brinkmann, A.; Nitsche, A.; Ergunay, K.; Yilmaz, E.; et al. Effects of Regular Kefir Consumption on Gut Microbiota in Patients with Metabolic Syndrome: A Parallel-Group, Randomized, Controlled Study. Nutrients 2019, 11, 2089. [Google Scholar] [CrossRef]
- Ismael, S.; Silvestre, M.P.; Vasques, M.; Araújo, J.R.; Morais, J.; Duarte, M.I.; Pestana, D.; Faria, A.; Pereira-Leal, J.B.; Vaz, J.; et al. A Pilot Study on the Metabolic Impact of Mediterranean Diet in Type 2 Diabetes: Is Gut Microbiota the Key? Nutrients 2021, 13, 1228. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef]
- Marungruang, N.; Tovar, J.; Björck, I.; Hållenius, F.F. Improvement in cardiometabolic risk markers following a multifunctional diet is associated with gut microbial taxa in healthy overweight and obese subjects. Eur. J. Nutr. 2018, 57, 2927. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; García-Carpintero, S.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Landa, B.B.; Clemente, J.C.; Pérez-Martínez, P.; López-Miranda, J.; Pérez-Jiménez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61, 1700300. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Alizadeh, M.; Jamalzehi, A.; Hosseinzadeh, M.; Parastouei, K. Garlic supplementation improves intestinal transit time, lipid accumulation product and cardiometabolic indices in subjects with metabolic syndrome: A randomized controlled trial. Phytother. Res. 2023, 37, 2305–2314. [Google Scholar] [CrossRef]
- Xiao, S.; Fei, N.; Pang, X.; Shen, J.; Wang, L.; Zhang, B.; Zhang, M.; Zhang, X.; Zhang, C.; Li, M.; et al. A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. Fems Microbiol. Ecol. 2014, 87, 357. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Dibella, M.; Blesso, C.N.; Malysheva, O.; Caudill, M.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome. Nutrients 2022, 14, 1179. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Flores-Lopez, A.G.; Aguilar-Lopez, M.; Sanchez-Tapia, M.; Medina-Vera, I.; Dıaz, D.; Tovar, A.R.; Torres, N. Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects With Metabolic Syndrome. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2019, 8, e012401. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681. [Google Scholar] [CrossRef]
- Sutanto, C.N.; Loh, W.W.; Kim, J.E. The impact of tryptophan supplementation on sleep quality: A systematic review, meta-analysis, and meta-regression. Nutr. Rev. 2022, 80, 306–316. [Google Scholar] [CrossRef]
- Binks, H.; Vincent, G.E.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of Diet on Sleep: A Narrative Review. Nutrients 2020, 12, 936. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Butyrate Ameliorates Insufficient Sleep-Induced Intestinal Mucosal Damage in Humans and Mice. Microbiol. Spectr. 2023, 11, e0200022. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Riemann, B.L.; Flatt, A.A.; Valentino, T.; Lustgarten, M.S. Self-reported sleep quality is associated with gut microbiome composition in young, healthy individuals: A pilot study. Sleep Med. 2020, 73, 76–81. [Google Scholar] [CrossRef]
- Yang, X.; Xiao, H.; Zeng, Y.; Huang, L.; Ji, K.; Deng, D.; Yang, W.; Liu, L. Tianwang Buxin Granules Influence the Intestinal Flora in Perimenopausal Insomnia. BioMed Res. Int. 2021, 2021, 9979511. [Google Scholar] [CrossRef]
- Zhanfeng, N.; Liang, W.; Jing, K.; Jinbo, B.; Yanjun, C.; Hechun, X. Regulation of sleep disorders in patients with traumatic brain injury by intestinal flora based on the background of brain-gut axis. Front. Neurosci. 2022, 16, 934822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Bai, L.; Goel, N.; Bailey, A.; Jang, C.J.; Bushman, F.D.; Meerlo, P.; Dinges, D.F.; Sehgal, A. Human and rat gut microbiome composition is maintained following sleep restriction. Proc. Natl. Acad. Sci. USA 2017, 114, E1564–E1571. [Google Scholar] [CrossRef]
- Tang, S.-S.; Liang, C.-H.; Liu, Y.-L.; Wei, W.; Deng, X.-R.; Shi, X.-Y.; Wang, L.-M.; Zhang, L.-J.; Yuan, H.-J. Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome. World J. Gastroenterol. 2022, 28, 2320–2333. [Google Scholar] [CrossRef]
- Ho, Y.T.; Tsai, Y.C.; Kuo, T.B.J.; Yang, C.C.H. Effects of lactobacillus plantarum ps128 on depressive symptoms and sleep quality in self-reported insomniacs: A randomized, double-blind, placebo-controlled pilot trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef]
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front. Immunol. 2023, 14, 1158137. [Google Scholar] [CrossRef]
- Papandreou, C. ARTICLE Epidemiology and Population Health High sleep variability predicts a blunted weight loss response and short sleep duration a reduced decrease in waist circumference in the PREDIMED-Plus Trial. Int. J. Obes. 2020, 44, 330–339. [Google Scholar] [CrossRef]
- Gianfredi, V.; Nucci, D.; Tonzani, A.; Amodeo, R.; Benvenuti, A.L.; Villarini, M.; Moretti, M. Sleep disorder, Mediterranean Diet and learning performance among nursing students: InSOMNIA, a cross-sectional study. Ann. Ig. 2018, 30, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Proença, I.M.; Allegretti, J.R.; Bernardo, W.M.; de Moura, D.T.; Ponte Neto, A.M.; Matsubayashi, C.O.; Flor, M.M.; PST Kotinda, A.; de Moura, E.G. Fecal microbiota transplantation improves metabolic syndrome parameters: Systematic review with meta-analysis based on randomized clinical trials. Nutr. Res. 2020, 83, 1–14. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
| N | Authors | Title | Sample Size (n) | Disease Status | Exposure | Methods | Outcome | Microbiota-Related Outcome(s) | Other Outcome(s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Yang et al., 2021 [100] | Tianwang Buxin Granules Influence the Intestinal Flora in Perimenopausal Insomnia | 13 | Healthy | Tianwang Buxin Granules | Shotgun metagenomics | Insomnia/Gut Microbiota | Increase in Roseburia faecis, Ruminococcus, Prevotella copri, Fusicatenibacter saccharivorans, and Blautia obeum correlates with PSQI score. | Improvement in PSQI score: p < 0.05. |
| 2 | Zhanfeng et al., 2022 [101] | Regulation of sleep disorders in patients with traumatic brain injury by intestinal flora based on the background of brain-gut axis | 28 | TBI | Observational | 16S sequencing | Gut Microbiota | Increase in Lachnospiraceae, Bilophila, Odoribacter, Bacteroidales, Bacteroidia, and Bacteroidetes (p < 0.05). | |
| 3 | Zhang et al., 2017 [102] | Human and rat gut microbiome composition is maintained following sleep restriction | 11 | Healthy | Sleep Restriction | 16S sequencing | Gut Microbiota | No changes | |
| 4 | Gao et al., 2023 [98] | Sleep deprivation and sleep restriction resulted in downregulation of Faecalibacterium and butyrate abundance in the feces. Butyrate Ameliorates Insufficient Sleep-Induced Intestinal Mucosal Damage in Humans and Mice | 22 | Healthy | Sleep Restriction | 16S sequencing | Sleep/Gut Microbiota | Downregulation of Faecalibacterium and butyrate producers, increase in Anaerostipes, Fusicatenibacter, and Veillonaceae associated with sleep restriction. | |
| 5 | Tang et al., 2022 [103] | Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome | 27 | T2DM+OSAHS | Observational | 16S sequencing | Gut Microbiota | Decrease in Faecalibacterium, Eubacterium, and Lachnospiraceae. | The gut microbiota changes strongly correlated with the HCY, CRP, fasting plasma glucose, and hemoglobin A1c concentrations; AHI; mean oxygen saturation; and insulin resistance index in group T2DM + OSA (p < 0.05). |
| 6 | Grosicki et al., 2020 [99] | Self-reported sleep quality is associated with gut microbiome composition in young, healthy individuals: a pilot study | 28 | Healthy | Observational | 16S sequencing | Sleep/Gut Microbiota | Increases in Blautia and Ruminococcus and lower abundances of Prevotella correlate with sleep quality. | |
| 7 | Yao et al., 2021 [3] | Relationships of sleep disturbance, intestinal microbiota, and postoperative pain in breast cancer patients: a prospective observational study | 36 | Breast Cancer | Observational | 16S sequencing | Sleep/Gut Microbiota | At the phylum level, women with poor sleep quality had higher relative abundance of Firmicutes (p = 0.021) and lower relative abundance of Bacteroidetes (p = 0.013). At the genus level, women with poor sleep quality harbored a higher relative abundance of Acidaminococcus and a lower relative abundance of several genera. | |
| 8 | Schaafsma et al., 2021 [56] | The effect of a whey-protein and galacto-oligosaccharides based product on parameters of sleep quality, stress, and gut microbiota in apparently healthy adults with moderate sleep disturbances: A randomized controlled cross-over study | 47 | Healthy | Dairy-Based Product | 16S sequencing | Sleep/Gut Microbiota | Relative abundance of Bifidobacterium increased (p = 0.02). Redundancy analysis showed an inverse relationship between baseline microbiota composition and baseline PSQI (p = 0.046). | Compared to placebo (skimmed milk), PSQI was only lower at day 14 in the 2nd intervention period in intention-to-treat (ITT) (p = 0.017; n = 69) and per-protocol (PP) (p = 0.038; n = 64) analyses. Post hoc analysis (modified PP: n = 47, with baseline PSQI ≥ 9, and endline day 14) showed a decrease in PSQI (−1.60 ± 2.53; p = 0.034). |
| 9 | Ho et al., 2021 [104] | Effects of lactobacillus plantarum ps128 on depressive symptoms and sleep quality in self-reported insomniacs: A randomized, double-blind, placebo-controlled pilot trial | 40 | Healthy | Lactobacillus plantarum (PS128) | 16S sequencing | Sleep | Supplementation with Lactobacillus plantarum PS128 improved sleep quality (p < 0.05). | PS128 supplementation improved sleep quality. |
| 10 | Nishida et al., 2019 [97] | Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study | 60 | Healthy | Lactobacillus gasseri (CP2305) | 16S sequencing | Sleep/Gut Microbiota | Higher Bifidobacterium spp. and lower Streptococcus spp. | Intake of CP2305 improved sleep quality. |
| 11 | Zhu et al., 2023 [105] | Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism | 60 | Healthy | Lactobacillus plantarum JYLP-326 | 16S sequencing | Sleep/Gut Microbiota | Higher Bacteroides and Roseburia and lower Prevotella and Bifidobacterium associated with sleep disturbances. | JYLP-326 improved sleep quality. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, A.; Galiè, S. The Microbiota–Gut–Brain Axis in Metabolic Syndrome and Sleep Disorders: A Systematic Review. Nutrients 2024, 16, 390. https://doi.org/10.3390/nu16030390
dos Santos A, Galiè S. The Microbiota–Gut–Brain Axis in Metabolic Syndrome and Sleep Disorders: A Systematic Review. Nutrients. 2024; 16(3):390. https://doi.org/10.3390/nu16030390
Chicago/Turabian Styledos Santos, Adriano, and Serena Galiè. 2024. "The Microbiota–Gut–Brain Axis in Metabolic Syndrome and Sleep Disorders: A Systematic Review" Nutrients 16, no. 3: 390. https://doi.org/10.3390/nu16030390
APA Styledos Santos, A., & Galiè, S. (2024). The Microbiota–Gut–Brain Axis in Metabolic Syndrome and Sleep Disorders: A Systematic Review. Nutrients, 16(3), 390. https://doi.org/10.3390/nu16030390