Anti-Inflammatory Effect of Chestnut Honey and Cabbage Mixtures Alleviates Gastric Mucosal Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Preparation of Samples
2.3. High-Performance Liquid Chromatography-Variable Wavelength Detector Analysis
2.4. Calibration Curve
2.5. Cell Culture and Cell Viability Assay
2.6. Nitric Oxide (NO) Assay
2.7. Western Blot Analysis
2.8. Separation of Cytoplasmic and Nuclear Proteins
2.9. Quantitative Real-Time-Polymerase Chain Reaction (qRT-PCR)
2.10. Experimental Animals
2.11. Histological Analysis
2.12. ELISA Assay
2.13. Statistics
3. Results
3.1. Quantitative Analysis Results of MCHCB for KA and SA
3.2. CH and CB Mixtures Suppress the Expression of NO and iNOS in LPS-Stimulated RAW 264.7 Macrophages
3.3. CH and CB Mixtures Suppress the mRNA Expression of Pro-Inflammatory Cytokines by Modulating NF-κB Activity in LPS-Stimulated RAW 264.7 Macrophages
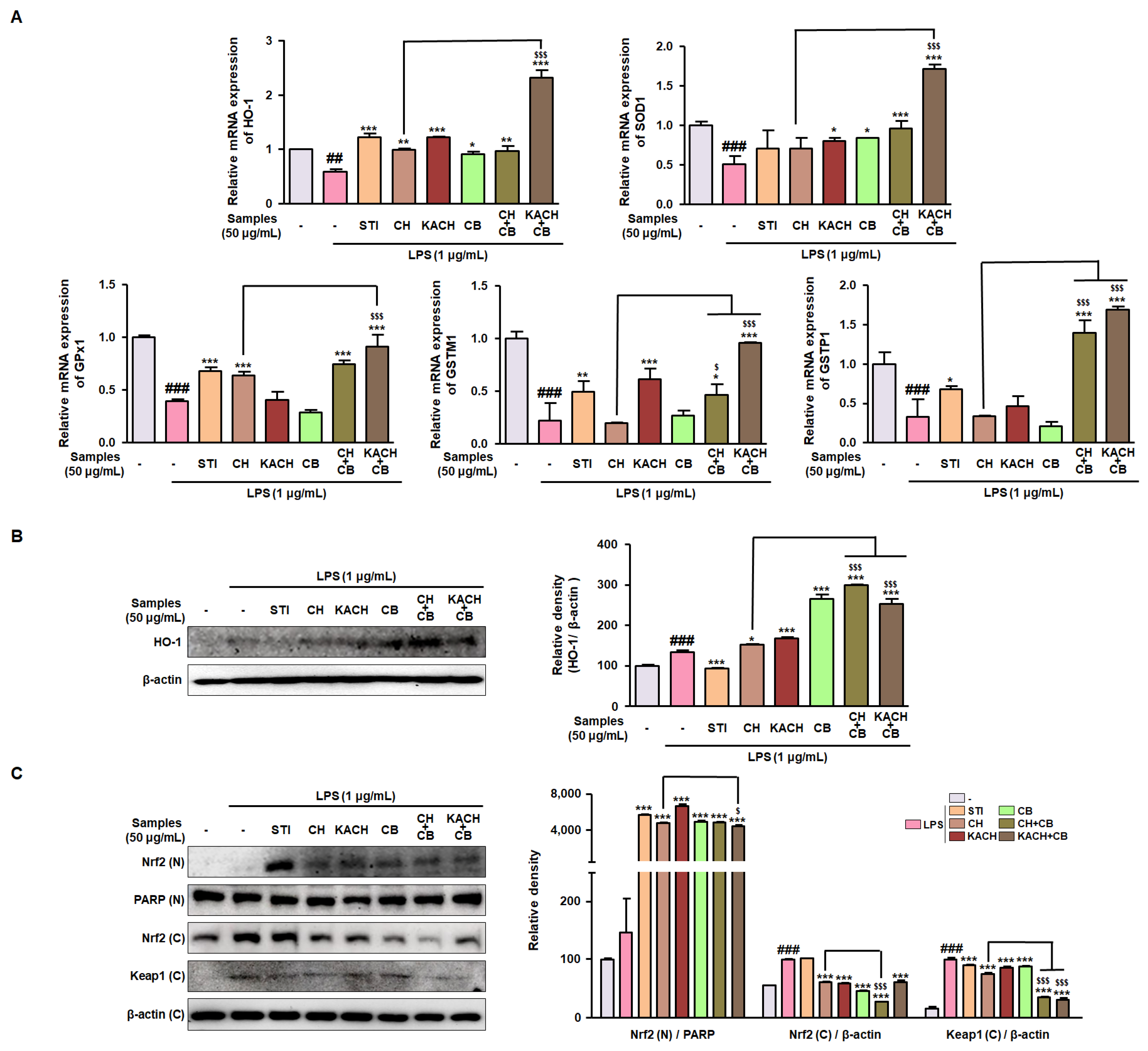
3.4. CH and CB Mixtures Enhance Antioxidant Enzymes by Modulating Nrf2 Activity in LPS-Stimulated Raw 264.7 Macrophages
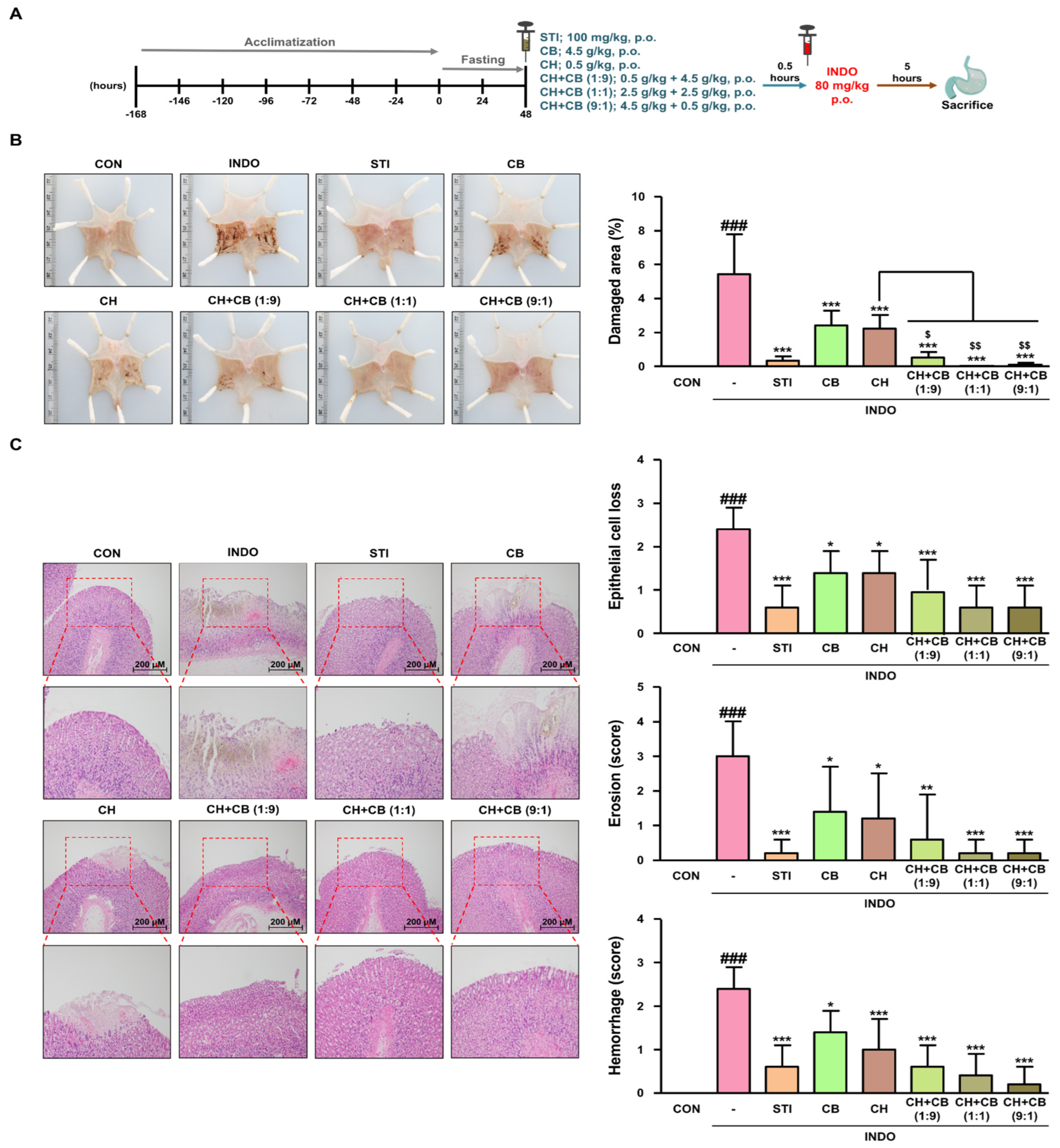
3.5. Various Ratios of CH and CB Mixtures Protect against Gastric Damage in INDO-Induced Rats
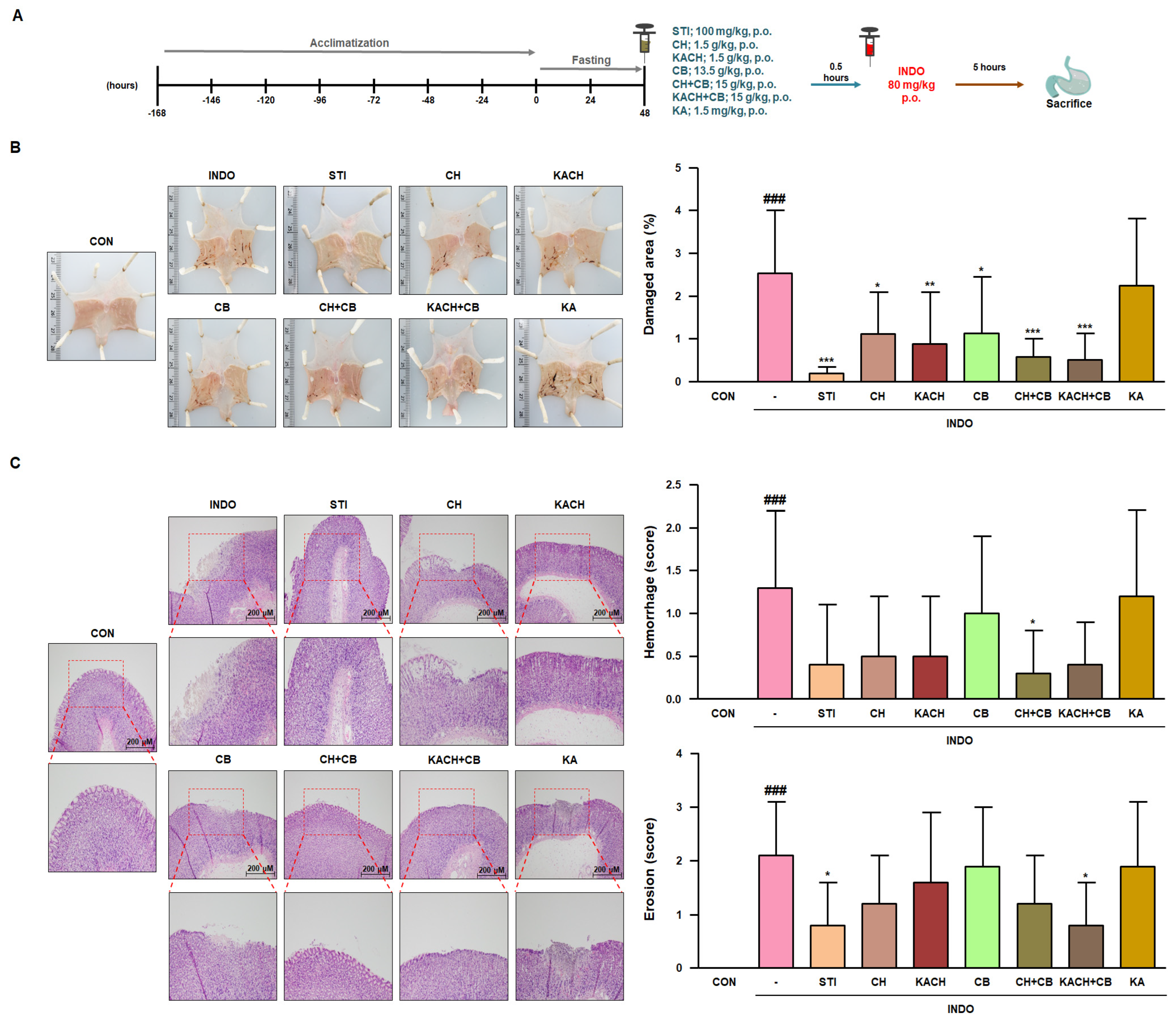
3.6. KACH and CB Mixtures Protect against Gastric Damage in INDO-Induced Rats
3.7. KACH and CB Mixtures Suppress the Inflammatory Response in INDO-Induced Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akanda, M.R.; Park, B.Y. Involvement of MAPK/NF-kappaB signal transduction pathways: Camellia japonica mitigates inflammation and gastric ulcer. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 95, 1139–1146. [Google Scholar] [CrossRef]
- Liu, S.; Shu, L.; Yang, J.; Liu, Y.; Zhang, S.; Wang, N.; Shi, Z. Rhein Exhibits Anti-Inflammatory Effects in Chronic Atrophic Gastritis via Nrf2 and MAPK Signaling. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2023, 34, 525–532. [Google Scholar] [CrossRef]
- Sohail, R.; Mathew, M.; Patel, K.K.; Reddy, S.A.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.U.; Razzaq Chaudhry, W.; Akbar, A. Effects of Non-steroidal Anti-inflammatory Drugs (NSAIDs) and Gastroprotective NSAIDs on the Gastrointestinal Tract: A Narrative Review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, N.; Hao, Y.; Liu, X.; Kang, J.; Mao, R.; Yu, X.; Li, Y. The Protective Effect of Walnut Oligopeptides against Indomethacin-Induced Gastric Ulcer in Rats. Nutrients 2023, 15, 1675. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, M.H.; Kim, H. Anti-Oxidant and Anti-Inflammatory Effects of Astaxanthin on Gastrointestinal Diseases. Int. J. Mol. Sci. 2022, 23, 15471. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.B.; Elzallat, M.; Mohammed, D.M.; Samir, S.; Hammam, O.A.; Abdel-Wareth, M.T.A. Cornu aspersum mucin attenuates indomethacins-induced gastric ulcers in mice via alleviating oxidative stress and inflammation. Heliyon 2023, 9, e15677. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.; Hwang, S.J.; Kim, W.J.; Youn, H.J.; Lee, H.J. The aqueous extract from Artemisia capillaris inhibits acute gastric mucosal injury by inhibition of ROS and NF-kB. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 99, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, H.; Wei, J.; Lin, S.; Zong, Z.; Gong, F.; Huang, X.; Sun, J.; Li, P.; Lin, H.; et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.H.; So, Y.K.; Han, S.N.; Kim, J.B. Isoegomaketone Upregulates Heme Oxygenase-1 in RAW264.7 Cells via ROS/p38 MAPK/Nrf2 Pathway. Biomol. Ther. 2016, 24, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, S.; Yang, B.; Lim, C.; Kim, J.H.; Kim, H.; Cho, S. Anti-inflammatory Effects of Brassica oleracea Var. capitata L. (Cabbage) Methanol Extract in Mice with Contact Dermatitis. Pharmacogn. Mag. 2018, 14, 174–179. [Google Scholar] [CrossRef]
- Melim, C.; Lauro, M.R.; Pires, I.M.; Oliveira, P.J.; Cabral, C. The Role of Glucosinolates from Cruciferous Vegetables (Brassicaceae) in Gastrointestinal Cancers: From Prevention to Therapeutics. Pharmaceutics 2022, 14, 190. [Google Scholar] [CrossRef]
- Ray, L.R.; Alam, M.S.; Junaid, M.; Ferdousy, S.; Akter, R.; Hosen, S.M.Z.; Mouri, N.J. Brassica oleracea var. capitata f. alba: A Review on its Botany, Traditional uses, Phytochemistry and Pharmacological Activities. Mini Rev. Med. Chem. 2021, 21, 2399–2417. [Google Scholar] [CrossRef]
- Moon, H.W.; Ku, K.M. Impact of an Agriphotovoltaic System on Metabolites and the Sensorial Quality of Cabbage (Brassica oleracea var. capitata) and Its High-Temperature-Extracted Juice. Foods 2022, 11, 498. [Google Scholar] [CrossRef]
- Khataybeh, B.; Jaradat, Z.; Ababneh, Q. Anti-bacterial, anti-biofilm and anti-quorum sensing activities of honey: A review. J. Ethnopharmacol. 2023, 317, 116830. [Google Scholar] [CrossRef] [PubMed]
- Tas-Kucukaydin, M.; Tel-Cayan, G.; Cayan, F.; Kucukaydin, S.; Hazar Ciftci, B.; Ceylan, O.; Emin Duru, M. Chemometric classification of chestnut honeys from different regions in Turkey based on their phenolic compositions and biological activities. Food Chem. 2023, 415, 135727. [Google Scholar] [CrossRef]
- Terzo, S.; Calvi, P.; Nuzzo, D.; Picone, P.; Allegra, M.; Mule, F.; Amato, A. Long-Term Ingestion of Sicilian Black Bee Chestnut Honey and/or D-Limonene Counteracts Brain Damage Induced by High Fat-Diet in Obese Mice. Int. J. Mol. Sci. 2023, 24, 3467. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, K.; Bao, L.; Chen, L.; Feng, L.; Liu, Z.; Wang, Y.; Fu, Y.; Zhang, N.; Hu, X. Kynurenic acid protects against mastitis in mice by ameliorating inflammatory responses and enhancing blood-milk barrier integrity. Mol. Immunol. 2021, 137, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, X.; Zhou, Y.; Du, J.; Lu, C.; Zhang, L.; Sun, S.; Wang, S.; Li, Y. Kynurenic acid inhibits macrophage pyroptosis by suppressing ROS production via activation of the NRF2 pathway. Mol. Med. Rep. 2023, 28, 211. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Moretti, R.M.; Nasti, R.; Cincinelli, R.; Dallavalle, S.; Montagnani Marelli, M. Apoptosis-mediated anticancer activity in prostate cancer cells of a chestnut honey (Castanea sativa L.) quinoline-pyrrolidine gamma-lactam alkaloid. Amino Acids 2021, 53, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Turska, M.; Paluszkiewicz, P.; Turski, W.A.; Parada-Turska, J. A Review of the Health Benefits of Food Enriched with Kynurenic Acid. Nutrients 2022, 14, 4182. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Lee, S.; Hwang, J.S.; Im, S.H.; Jun, C.D.; Lee, H.S.; Kim, S.H. DA-9601 suppresses 2, 4-dinitrochlorobenzene and dust mite extract-induced atopic dermatitis-like skin lesions. Int. Immunopharmacol. 2011, 11, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, B.; Lv, L.; Wang, Z.; He, J.; Chen, Z.; Wen, X.; Zhang, Y.; Sun, W.; Li, Y.; et al. Gastroprotective effect of aucubin against ethanol-induced gastric mucosal injury in mice. Life Sci. 2017, 189, 44–51. [Google Scholar] [CrossRef]
- Song, H.J.; Shin, C.Y.; Oh, T.Y.; Sohn, U.D. The protective effect of eupatilin on indomethacin-induced cell damage in cultured feline ileal smooth muscle cells: Involvement of HO-1 and ERK. J. Ethnopharmacol. 2008, 118, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Kim, J.S.; Lee, J.; Seo, Y.H.; Kim, H.S.; Ryu, S.M.; Choi, G.; Moon, B.C.; Lee, A.Y. Pharmacological Effects of Agastache rugosa against Gastritis Using a Network Pharmacology Approach. Biomolecules 2020, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Ugan, R.A.; Un, H. The Protective Roles of Butein on Indomethacin Induced Gastric Ulcer in Mice. Eurasian J. Med. 2020, 52, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.J.; Koh, D.J.; Kim, S.H.; Park, S.J.; Ryu, J.H.; Kim, D.G.; Lee, J.Y.; Lee, K.T. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-kappaB inactivation. J. Agric. Food Chem. 2008, 56, 10265–10272. [Google Scholar] [CrossRef] [PubMed]
- Lucic, D.; Pavlovic, I.; Brkljacic, L.; Bogdanovic, S.; Farkas, V.; Cedilak, A.; Nanic, L.; Rubelj, I.; Salopek-Sondi, B. Antioxidant and Antiproliferative Activities of Kale (Brassica oleracea L. Var. acephala DC.) and Wild Cabbage (Brassica incana Ten.) Polyphenolic Extracts. Molecules 2023, 28, 1840. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Motlhatlego, K.E.; Netshia, V. Traditionally used polyherbals in a southern African therapeutic context. J. Ethnopharmacol. 2022, 288, 114977. [Google Scholar] [CrossRef]
- Lee, T.; Park, H.S.; Jeong, J.H.; Jung, T.W. Kynurenic acid attenuates pro-inflammatory reactions in lipopolysaccharide-stimulated endothelial cells through the PPARdelta/HO-1-dependent pathway. Mol. Cell. Endocrinol. 2019, 495, 110510. [Google Scholar] [CrossRef]
- Raish, M.; Shahid, M.; Bin Jardan, Y.A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Abdelrahman, I.A.; Ahmad, A.; Al-Jenoobi, F.I. Gastroprotective Effect of Sinapic Acid on Ethanol-Induced Gastric Ulcers in Rats: Involvement of Nrf2/HO-1 and NF-kappaB Signaling and Antiapoptotic Role. Front. Pharmacol. 2021, 12, 622815. [Google Scholar] [CrossRef]
- Wang, R.; Luo, Y.; Lu, Y.; Wang, D.; Wang, T.; Pu, W.; Wang, Y. Maggot Extracts Alleviate Inflammation and Oxidative Stress in Acute Experimental Colitis via the Activation of Nrf2. Oxidative Med. Cell. Longev. 2019, 2019, 4703253. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Choi, E.; Hong, Y.H.; Kim, H.; Jang, Y.J.; Lee, J.S.; Choung, E.S.; Woo, B.Y.; Hong, Y.D.; Lee, S.; et al. Syk/NF-kappaB-targeted anti-inflammatory activity of Melicope accedens (Blume) T.G. Hartley methanol extract. J. Ethnopharmacol. 2021, 271, 113887. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.G.E.; Eissa, R.G. Amelioration of ethanol-induced gastric ulcer in rats by quercetin: Implication of Nrf2/HO1 and HMGB1/TLR4/NF-kappaB pathways. Heliyon 2022, 8, e11159. [Google Scholar] [CrossRef] [PubMed]
- Sadeghiani, G.; Khanehzad, M.; Sadighi Gilani, M.A.; Amidi, F.; Malekzadeh, M.; Rastegar, T. Evaluation of Nrf2/ARE Signaling Pathway in the Presence of Pentoxifylline as a Cryoprotectant in Mouse Spermatogonial Stem Cells. Biopreservation Biobanking 2023, 21, 294–307. [Google Scholar] [CrossRef]



| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| IL-6 | TTCCATCCAGTTGCCTTCTTG | GGGAGTGGTATCCTCTGTGAAGTC |
| TNF-α | ATGAGCACAGAAAGCATGAT | TACAGGCTTGTCACTCGAAT |
| HO-1 | GAATGAACACTCTGGAGATGACAC | TGTGAGGGACTCTGGTCTTTG |
| SOD1 | TGAAAGCGGTGTGCGTGCTGAAG | GGAATGCTCTCCTGAGAGTGAGA |
| GPx1 | ACACCGAGATGAACGATCTG | ATGTACTTGGGGTCGGTCAT |
| GSTM1 | CACAAGATCACCCAGAGCAA | TGGTTCTCCACAATGTCTGC |
| GSTP1 | GCCCAGATGGATATGGTGAA | ATGGGACGGTTCACATGTTC |
| β-actin | CCACACCTTCTACAATGAGC | CACAGGATTCCATACCCAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Jin, B.-R.; Lee, C.-D.; Kim, D.; Lee, A.Y.; Lee, S.; An, H.-J. Anti-Inflammatory Effect of Chestnut Honey and Cabbage Mixtures Alleviates Gastric Mucosal Damage. Nutrients 2024, 16, 389. https://doi.org/10.3390/nu16030389
Kim H-J, Jin B-R, Lee C-D, Kim D, Lee AY, Lee S, An H-J. Anti-Inflammatory Effect of Chestnut Honey and Cabbage Mixtures Alleviates Gastric Mucosal Damage. Nutrients. 2024; 16(3):389. https://doi.org/10.3390/nu16030389
Chicago/Turabian StyleKim, Hyo-Jung, Bo-Ram Jin, Chang-Dae Lee, Doyun Kim, Ah Young Lee, Sanghyun Lee, and Hyo-Jin An. 2024. "Anti-Inflammatory Effect of Chestnut Honey and Cabbage Mixtures Alleviates Gastric Mucosal Damage" Nutrients 16, no. 3: 389. https://doi.org/10.3390/nu16030389
APA StyleKim, H.-J., Jin, B.-R., Lee, C.-D., Kim, D., Lee, A. Y., Lee, S., & An, H.-J. (2024). Anti-Inflammatory Effect of Chestnut Honey and Cabbage Mixtures Alleviates Gastric Mucosal Damage. Nutrients, 16(3), 389. https://doi.org/10.3390/nu16030389







