A 14-Day Double-Blind, Randomized, Controlled Crossover Intervention Study with Anti-Bacterial Benzyl Isothiocyanate from Nasturtium (Tropaeolum majus) on Human Gut Microbiome and Host Defense
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Collection of Stool, Urine, and Serum Samples
2.3. Collection of Exhaled Breath Condensate (EBC)
2.4. Faecal Microbial DNA Extraction
2.5. High-Throughput 16S Ribosomal RNA (rRNA) Gene Sequencing
2.6. Bioinformatics Analysis
2.7. Metabolome Analysis
2.8. Quantification of hBD-1 Release by ELISA Assay
2.9. Broth Microdilution Assay
2.10. Statistical Analysis
3. Results
3.1. Microbiome Sequence Data
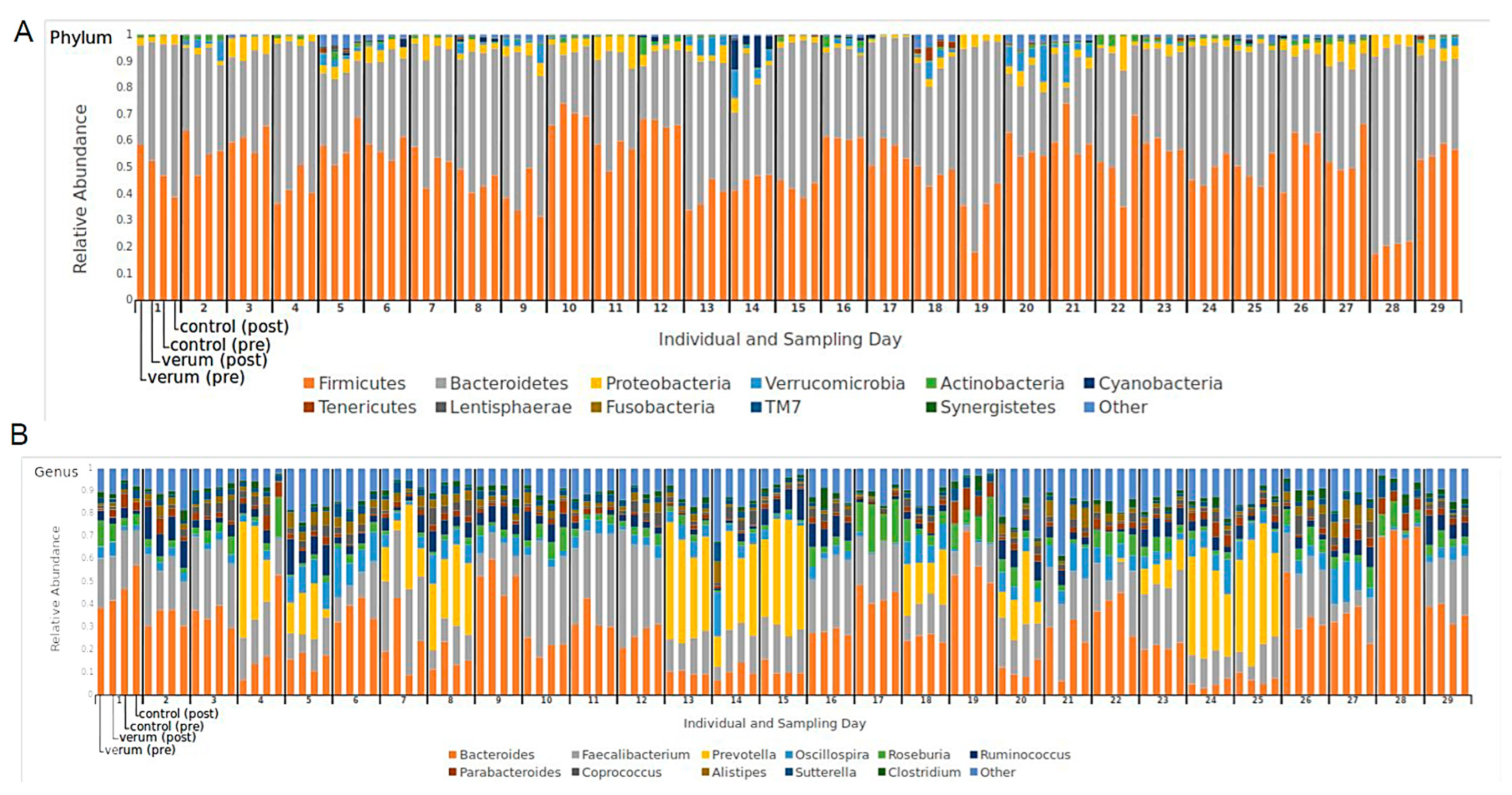
3.2. Microbial Composition
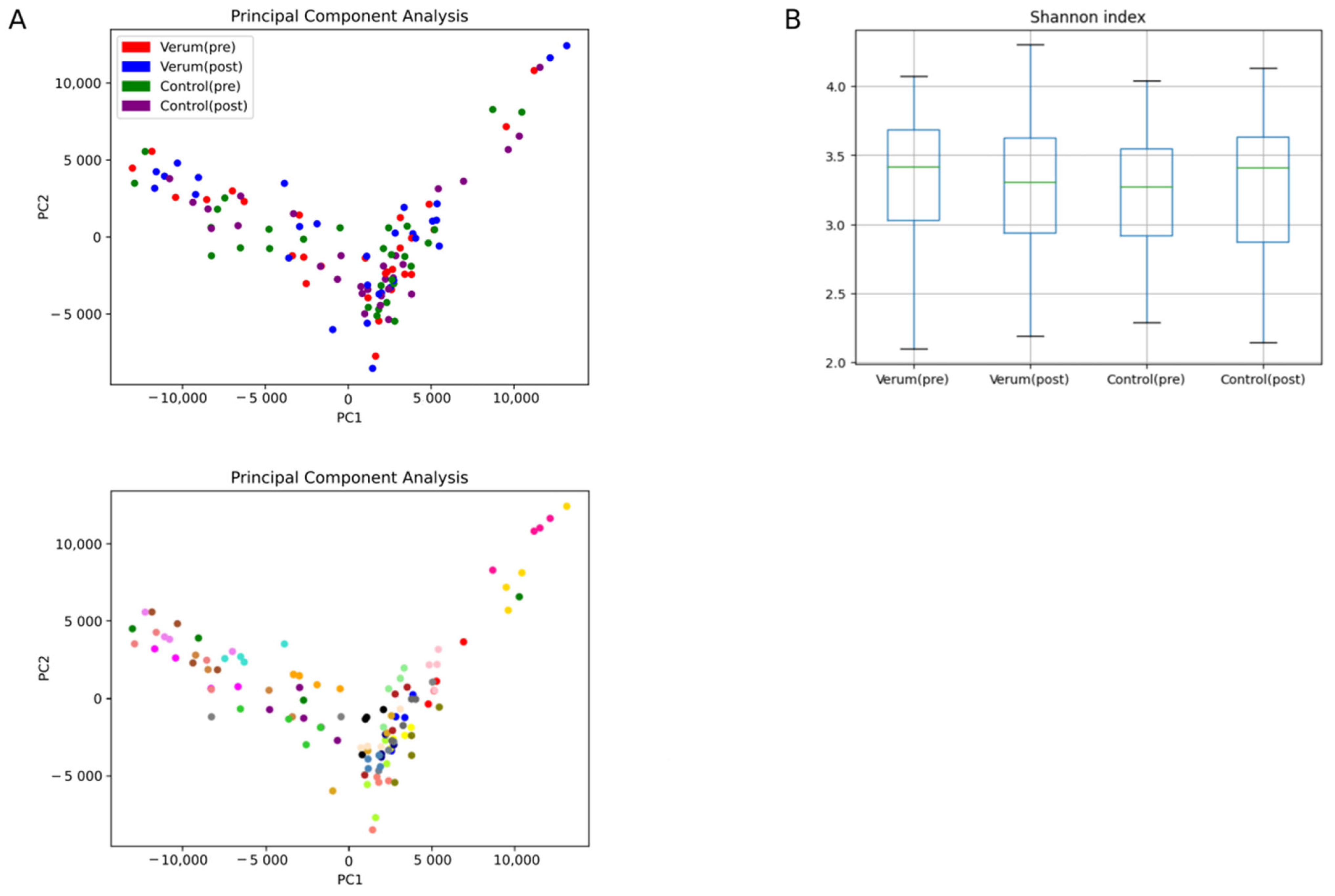
3.3. Bacterial Diversity Is Not Affected by Nasturtium Intervention
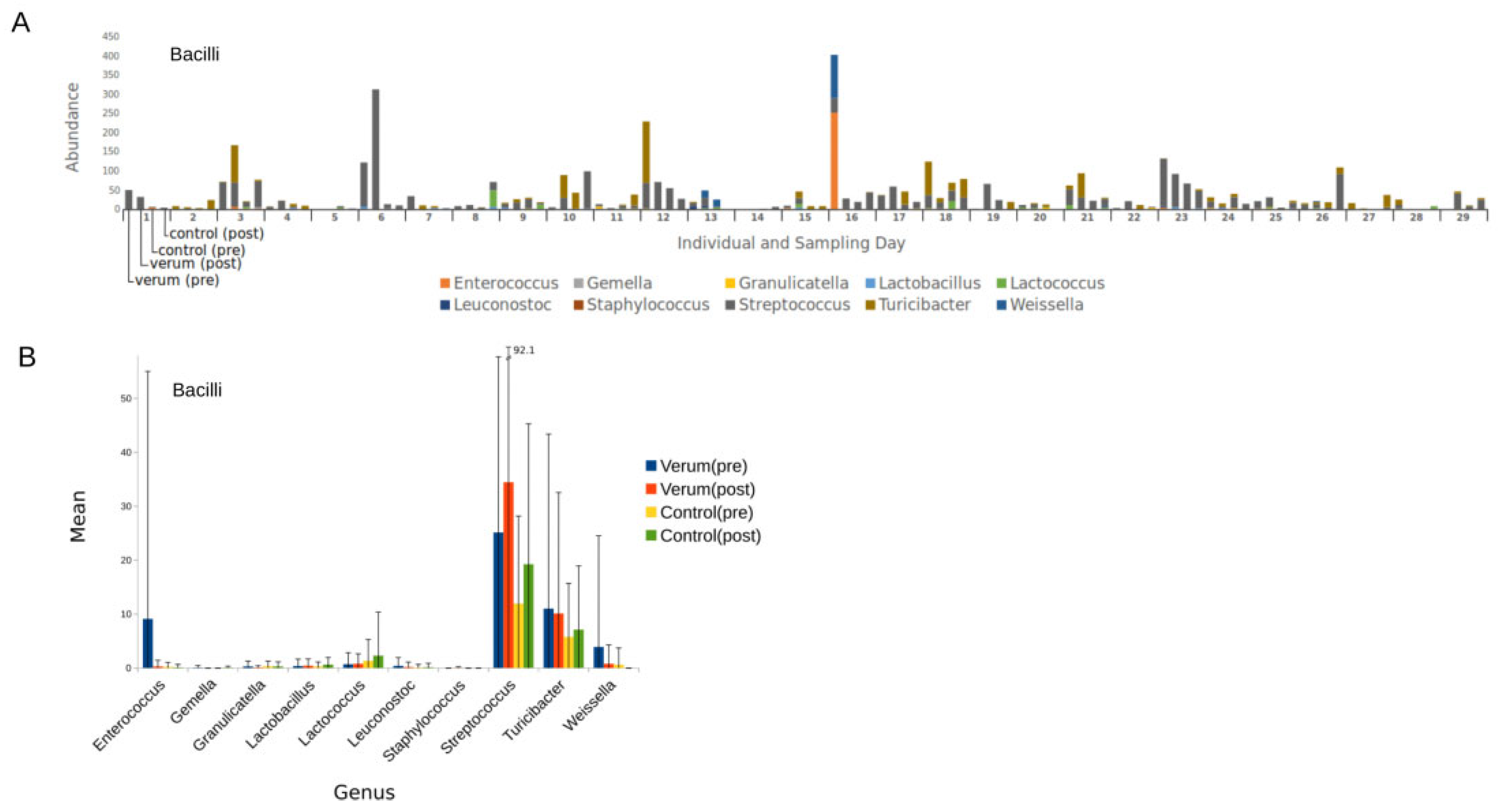
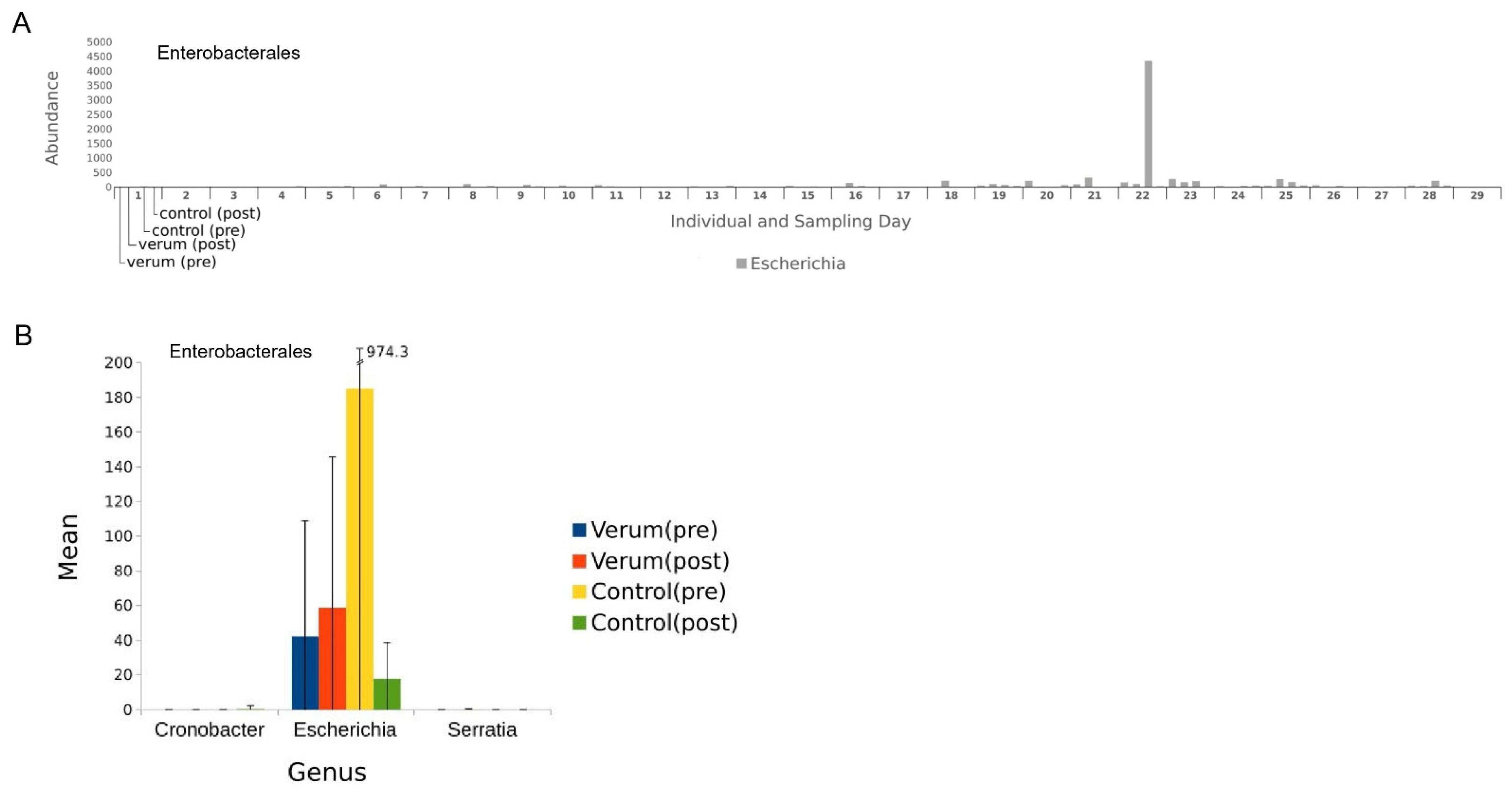
3.4. High-Level Phenotypes
3.5. Correlation of Gut Microbiome and Serum Metabolome
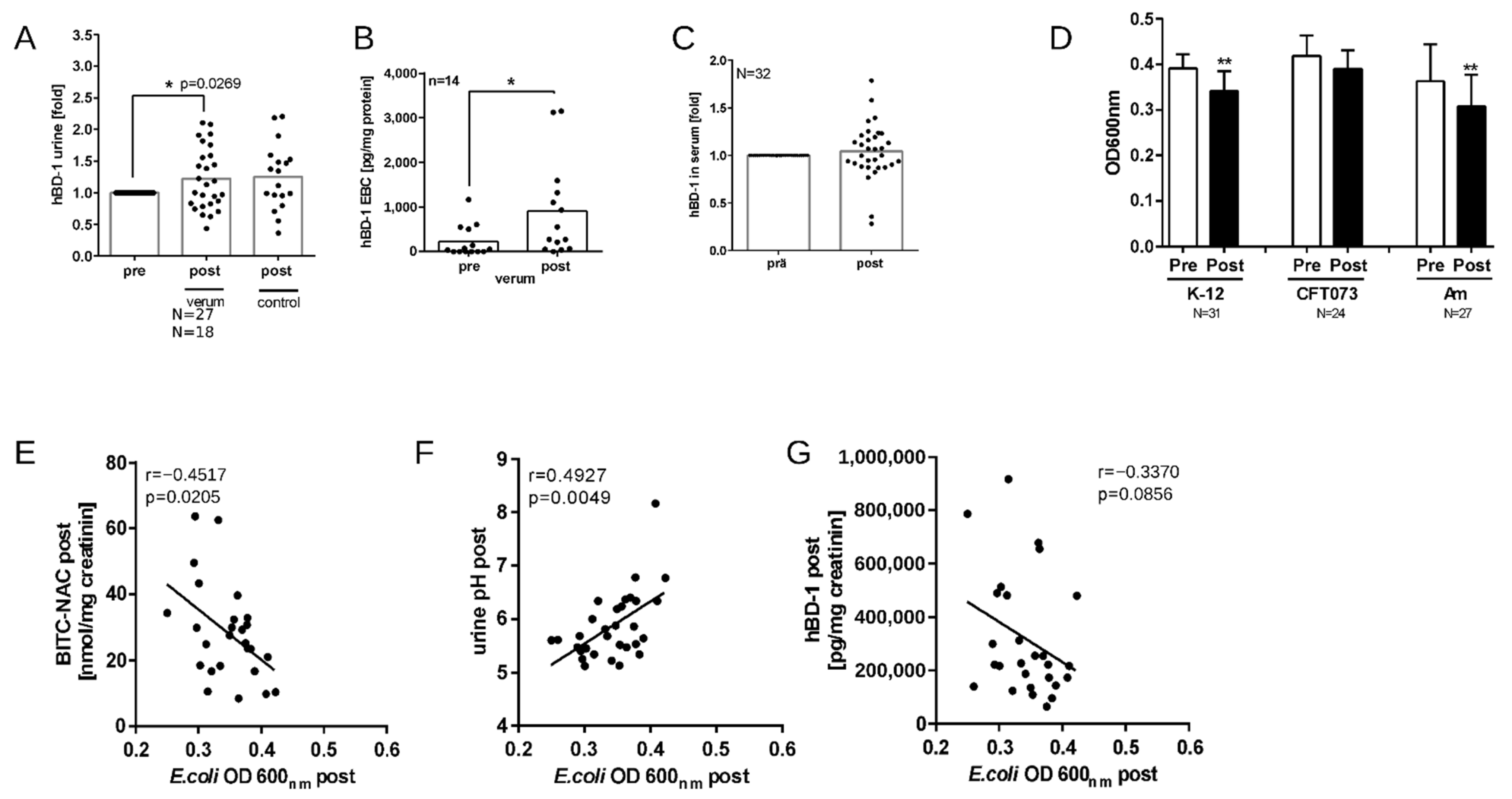
3.6. Nasturtium Intervention Increases hBD-1 in Urine and EBC
3.7. Nasturtium Intervention Increases the Antibacterial Activity of Urine Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2020, 11, 586548. [Google Scholar] [CrossRef] [PubMed]
- Nathan, M.; Scholten, R. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Ann. Intern. Med. 1999, 130, 459. [Google Scholar] [CrossRef]
- Kranz, J.; Schmidt, S.; Lebert, C.; Schneidewind, L.; Vahlensieck, W.; Sester, U.; Fünfstück, R.; Helbig, S.; Hofmann, W.; Hummers, E.; et al. Epidemiologie, Diagnostik, Therapie, Prävention und Management unkomplizierter, bakterieller, ambulant erworbener Harnwegsinfektionen bei erwachsenen Patienten: Aktualisierung 2017 der interdisziplinären AWMF S3-Leitlinie. Der Urologe. 2017, 56, 746–758. [Google Scholar] [CrossRef]
- Stange, R.; Schneider, B.; Albrecht, U.; Mueller, V.; Schnitker, J.; Michalsen, A. Results of a Randomized, Prospective, Double-Dummy, Double-Blind Trial to Compare Efficacy and Safety of a Herbal Combination Containing Tropaeoli Majoris Herba and Armoraciae Rusticanae Radix with Co-Trimoxazole in Patients with Acute and Uncomplicated Cystitis. Res. Rep. Urol. 2017, 9, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angew. Chem. Int. Ed. Engl. 2014, 53, 11430–11450. [Google Scholar] [CrossRef]
- Aires, A.; Mota, V.R.; Saavedra, M.J.; Rosa, E.a.S.; Bennett, R.N. The Antimicrobial Effects of Glucosinolates and Their Respective Enzymatic Hydrolysis Products on Bacteria Isolated from the Human Intestinal Tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef]
- Conrad, A.; Kolberg, T.; Engels, I.; Frank, U. In vitro study to evaluate the antibacterial activity of a combination of the haulm of nasturtium (Tropaeoli majoris herba) and of the roots of horseradish (Armoraciae rusticanae radix). Arzneimittelforschung 2006, 56, 842–849. [Google Scholar] [CrossRef]
- Zhang, Y. Allyl Isothiocyanate as a Cancer Chemopreventive Phytochemical. Mol. Nutr. Food Res. 2010, 54, 127–135. [Google Scholar] [CrossRef]
- Esteve, M. Mechanisms Underlying Biological Effects of Cruciferous Glucosinolate-Derived Isothiocyanates/Indoles: A Focus on Metabolic Syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A Critical Review of the Bioavailability of Glucosinolates and Related Compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular Basis for Chemoprevention by Sulforaphane: A Comprehensive Review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Rosenfeld, E.; Stintzi, A.; Baysse, C. Insights into the Mode of Action of Benzyl Isothiocyanate on Campylobacter Jejuni. Appl. Environ. Microbiol. 2013, 79, 6958–6968. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-Mediated Colonization Resistance against Intestinal Pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Zaura, E.; Brandt, B.W.; Teixeira de Mattos, M.J.; Buijs, M.J.; Caspers, M.P.M.; Rashid, M.-U.; Weintraub, A.; Nord, C.E.; Savell, A.; Hu, Y.; et al. Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome versus Long-Term Microbial Shifts in Feces. mBio 2015, 6, e01693-15. [Google Scholar] [CrossRef] [PubMed]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural History of the Infant Gut Microbiome and Impact of Antibiotic Treatment on Bacterial Strain Diversity and Stability. Sci. Transl. Med. 2016, 8, 343ra81. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Herz, C.; Frei, L.; Tran, H.T.T.; Claßen, S.; Spöttel, J.; Krell, M.; Hanschen, F.S.; Arvandi, M.; Binder, N.; Schreiner, M.; et al. A Monocentric, Randomized, Double-Blind, Controlled Crossover Trial of Nasturtium (Tropaeolum Majus) on the Lipid Regulator Prostaglandin E2. Front. Nutr. 2023, 10, 1223158. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, G.M.; Garey, K.W.; Robbins, R.A.; Danziger, L.H.; Rubinstein, I. Collection and Analysis of Exhaled Breath Condensate in Humans. Am. J. Respir. Crit. Care Med. 2001, 164, 731–737. [Google Scholar] [CrossRef]
- Haring, E.; Uhl, F.M.; Andrieux, G.; Proietti, M.; Bulashevska, A.; Sauer, B.; Braun, L.M.; de Vega Gomez, E.; Esser, P.R.; Martin, S.F.; et al. Bile Acids Regulate Intestinal Antigen Presentation and Reduce Graft-versus-Host Disease without Impairing the Graft-versus-Leukemia Effect. Haematologica 2021, 106, 2131–2146. [Google Scholar] [CrossRef]
- Schloss, P.D. Reintroducing Mothur: 10 Years Later. Appl. Environ. Microbiol. 2020, 86, e02343-19. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Bik, H.M.; Pitch Interactive Inc. Phinch: An Interactive, Exploratory Data Visualization Framework for –Omic Datasets. Genomics 2014, 009944. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S rRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for Integration and Interpretation of Large-Scale Molecular Data Sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Ward, T.; Larson, J.; Meulemans, J.; Hillmann, B.; Lynch, J.; Sidiropoulos, D.; Spear, J.R.; Caporaso, G.; Blekhman, R.; Knight, R.; et al. BugBase Predicts Organism-Level Microbiome Phenotypes. Bioinformatics 2017, 1–19. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An Isotope-Labeled Chemical Derivatization Method for the Quantitation of Short-Chain Fatty Acids in Human Feces by Liquid Chromatography–Tandem Mass Spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Just, S.; Mondot, S.; Ecker, J.; Wegner, K.; Rath, E.; Gau, L.; Streidl, T.; Hery-Arnaud, G.; Schmidt, S.; Lesker, T.R.; et al. The Gut Microbiota Drives the Impact of Bile Acids and Fat Source in Diet on Mouse Metabolism. Microbiome 2018, 6, 134. [Google Scholar] [CrossRef]
- Wudy, S.I.; Mittermeier-Klessinger, V.K.; Dunkel, A.; Kleigrewe, K.; Ensenauer, R.; Dawid, C.; Hofmann, T.F. High-Throughput Analysis of Underivatized Amino Acids and Acylcarnitines in Infant Serum: A Micromethod Based on Stable Isotope Dilution Targeted HILIC-ESI-MS/MS. J. Agric. Food Chem. 2023, 71, 8633–8647. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and Diet-Responsive Groups of Bacteria within the Human Colonic Microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sánchez, S.; Wang, D.; Augustijn, H.E.; Vich Vila, A.; Lifelines Cohort Study; Weersma, R.K.; Medema, M.H.; et al. Influence of the Microbiome, Diet and Genetics on Inter-Individual Variation in the Human Plasma Metabolome. Nat. Med. 2022, 28, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic Acid Improves Intestinal Barrier Function through Altering Gut Microbiota Composition in High-Fat Diet-Induced Mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef] [PubMed]
- Sreeramkumar, V.; Fresno, M.; Cuesta, N. Prostaglandin E 2 and T Cells: Friends or Foes? Immunol. Cell Biol. 2012, 90, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted Roles of PGE2 in Inflammation and Cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the Inter-Individual Variation in Response to Consumption of Plant Food Bioactives: Towards a Better Understanding of Their Role in Healthy Aging and Cardiometabolic Risk Reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Lehrer, R.I. Antimicrobial Peptides of Vertebrates. Curr. Opin. Immunol. 1998, 10, 41–44. [Google Scholar] [CrossRef]
- Becknell, B.; Spencer, J.D.; Carpenter, A.R.; Chen, X.; Singh, A.; Ploeger, S.; Kline, J.; Ellsworth, P.; Li, B.; Proksch, E.; et al. Expression and Antimicrobial Function of Beta-Defensin 1 in the Lower Urinary Tract. PLoS ONE 2013, 8, e77714. [Google Scholar] [CrossRef]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schröder, J.M. A Peptide Antibiotic from Human Skin. Nature 1997, 387, 861. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.E.; Bergaentzlé, M.; Bindler, F.; Marchioni, E.; Lintz, A.; Ennahar, S. In Vitro Efficacies of Various Isothiocyanates from Cruciferous Vegetables as Antimicrobial Agents against Foodborne Pathogens and Spoilage Bacteria. Food Control 2013, 30, 318–324. [Google Scholar] [CrossRef]
- Kellingray, L.; Tapp, H.S.; Saha, S.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Consumption of a Diet Rich in Brassica Vegetables Is Associated with a Reduced Abundance of Sulphate-Reducing Bacteria: A Randomised Crossover Study. Mol. Nutr. Food Res. 2017, 61, 1600992. [Google Scholar] [CrossRef] [PubMed]
- McElroy, K.E.; Luciani, F.; Thomas, T. GemSIM: General, Error-Model Based Simulator of next-Generation Sequencing Data. BMC Genom. 2012, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli Consumption Affects the Human Gastrointestinal Microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Klöpping-Ketelaars, I.W.A.A.; van den Berg, R.; Vaes, W.H.J. Bioavailability and Kinetics of Sulforaphane in Humans after Consumption of Cooked versus Raw Broccoli. J. Agric. Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Mota, V.R.; Saavedra, M.J.; Monteiro, A.A.; Simões, M.; Rosa, E.a.S.; Bennett, R.N. Initial in Vitro Evaluations of the Antibacterial Activities of Glucosinolate Enzymatic Hydrolysis Products against Plant Pathogenic Bacteria. J. Appl. Microbiol. 2009, 106, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.-O.; Kim, M.-B.; Lim, S.-B. Relationship between Chemical Structure and Antimicrobial Activities of Isothiocyanates from Cruciferous Vegetables against Oral Pathogens. J. Microbiol. Biotechnol. 2016, 26, 2036–2042. [Google Scholar] [CrossRef]
- Manges, A.R.; Johnson, J.R.; Riley, L.W. Intestinal Population Dynamics of UTI-Causing Escherichia Coli within Heterosexual Couples. Curr. Issues Intest. Microbiol. 2004, 5, 49–57. [Google Scholar] [PubMed]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia Coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-Antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Magruder, M.; Sholi, A.N.; Gong, C.; Zhang, L.; Edusei, E.; Huang, J.; Albakry, S.; Satlin, M.J.; Westblade, L.F.; Crawford, C.; et al. Gut Uropathogen Abundance Is a Risk Factor for Development of Bacteriuria and Urinary Tract Infection. Nat. Commun. 2019, 10, 5521. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Li, Y.; Geng, F.; Munday, R.; Zhang, Y. The Principal Urinary Metabolite of Allyl Isothiocyanate, N-Acetyl-S-(N-Allylthiocarbamoyl)Cysteine, Inhibits the Growth and Muscle Invasion of Bladder Cancer. Carcinogenesis 2012, 33, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Márton, M.-R.; Krumbein, A.; Platz, S.; Schreiner, M.; Rohn, S.; Rehmers, A.; Lavric, V.; Mersch-Sundermann, V.; Lamy, E. Determination of Bioactive, Free Isothiocyanates from a Glucosinolate-Containing Phytotherapeutic Agent: A Pilot Study with in Vitro Models and Human Intervention. Fitoterapia 2013, 85, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, Y.-M.; Wang, C.; Zhu, H.-P. Antibacterial Activity and Main Action Pathway of Benzyl Isothiocyanate Extracted from Papaya Seeds. J. Food Sci. 2021, 86, 169–176. [Google Scholar] [CrossRef]
- Platz, S.; Kühn, C.; Schiess, S.; Schreiner, M.; Kemper, M.; Pivovarova, O.; Pfeiffer, A.F.H.; Rohn, S. Bioavailability and Metabolism of Benzyl Glucosinolate in Humans Consuming Indian Cress (Tropaeolum majus L.). Mol. Nutr. Food Res. 2016, 60, 652–660. [Google Scholar] [CrossRef]
- Sun, J.; Charron, C.S.; Novotny, J.A.; Peng, B.; Yu, L.; Chen, P. Profiling Glucosinolate Metabolites in Human Urine and Plasma after Broccoli Consumption Using Non-Targeted and Targeted Metabolomic Analyses. Food Chem. 2020, 309, 125660. [Google Scholar] [CrossRef]
- Ioannou, Y.M.; Burka, L.T.; Matthews, H.B. Allyl Isothiocyanate: Comparative Disposition in Rats and Mice. Toxicol. Appl. Pharmacol. 1984, 75, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Petri, N.; Tannergren, C.; Holst, B.; Mellon, F.A.; Bao, Y.; Plumb, G.W.; Bacon, J.; O’Leary, K.A.; Kroon, P.A.; Knutson, L.; et al. Absorption/Metabolism of Sulforaphane and Quercetin, and Regulation of Phase II Enzymes, in Human Jejunum in Vivo. Drug Metab. Dispos. Biol. Fate Chem. 2003, 31, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Scholtes, C.; Herz, C.; Mersch-Sundermann, V. Pharmacokinetics and Pharmacodynamics of Isothiocyanates. Drug Metab. Rev. 2011, 43, 387–407. [Google Scholar] [CrossRef]
- Brüsewitz, G.; Cameron, B.D.; Chasseaud, L.F.; Görler, K.; Hawkins, D.R.; Koch, H.; Mennicke, W.H. The Metabolism of Benzyl Isothiocyanate and Its Cysteine Conjugate. Biochem. J. 1977, 162, 99–107. [Google Scholar] [CrossRef]
- Rouzaud, G.; Rabot, S.; Ratcliffe, B.; Duncan, A.J. Influence of Plant and Bacterial Myrosinase Activity on the Metabolic Fate of Glucosinolates in Gnotobiotic Rats. Br. J. Nutr. 2003, 90, 395–404. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Combourieu, B.; Elfoul, L.; Delort, A.M.; Rabot, S. Identification of New Derivatives of Sinigrin and Glucotropaeolin Produced by the Human Digestive Microflora Using 1H NMR Spectroscopy Analysis of in Vitro Incubations. Drug Metab. Dispos. Biol. Fate Chem. 2001, 29, 1440–1445. [Google Scholar] [PubMed]
- Cheng, D.-L.; Hashimoto, K.; Uda, Y. In Vitro Digestion of Sinigrin and Glucotropaeolin by Single Strains of Bifidobacterium and Identification of the Digestive Products. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2004, 42, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Zheng, J.J.; Kang, J.W.; Saboe, A.; Knights, D.; Zivkovic, A.M. A Guide to Diet-Microbiome Study Design. Front. Nutr. 2020, 7, 79. [Google Scholar] [CrossRef]
- Magliano, E.; Grazioli, V.; Deflorio, L.; Leuci, A.I.; Mattina, R.; Romano, P.; Cocuzza, C.E. Gender and Age-Dependent Etiology of Community-Acquired Urinary Tract Infections. Sci. World J. 2012, 2012, 349597. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex Differences in Gut Microbiota. World J. Mens Health 2020, 38, 48–60. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfäffle, S.P.; Herz, C.; Brombacher, E.; Proietti, M.; Gigl, M.; Hofstetter, C.K.; Mittermeier-Kleßinger, V.K.; Claßen, S.; Tran, H.T.T.; Rajguru, D.; et al. A 14-Day Double-Blind, Randomized, Controlled Crossover Intervention Study with Anti-Bacterial Benzyl Isothiocyanate from Nasturtium (Tropaeolum majus) on Human Gut Microbiome and Host Defense. Nutrients 2024, 16, 373. https://doi.org/10.3390/nu16030373
Pfäffle SP, Herz C, Brombacher E, Proietti M, Gigl M, Hofstetter CK, Mittermeier-Kleßinger VK, Claßen S, Tran HTT, Rajguru D, et al. A 14-Day Double-Blind, Randomized, Controlled Crossover Intervention Study with Anti-Bacterial Benzyl Isothiocyanate from Nasturtium (Tropaeolum majus) on Human Gut Microbiome and Host Defense. Nutrients. 2024; 16(3):373. https://doi.org/10.3390/nu16030373
Chicago/Turabian StylePfäffle, Simon P., Corinna Herz, Eva Brombacher, Michele Proietti, Michael Gigl, Christoph K. Hofstetter, Verena K. Mittermeier-Kleßinger, Sophie Claßen, Hoai T. T. Tran, Dhairya Rajguru, and et al. 2024. "A 14-Day Double-Blind, Randomized, Controlled Crossover Intervention Study with Anti-Bacterial Benzyl Isothiocyanate from Nasturtium (Tropaeolum majus) on Human Gut Microbiome and Host Defense" Nutrients 16, no. 3: 373. https://doi.org/10.3390/nu16030373
APA StylePfäffle, S. P., Herz, C., Brombacher, E., Proietti, M., Gigl, M., Hofstetter, C. K., Mittermeier-Kleßinger, V. K., Claßen, S., Tran, H. T. T., Rajguru, D., Dawid, C., Kreutz, C., Günther, S., & Lamy, E. (2024). A 14-Day Double-Blind, Randomized, Controlled Crossover Intervention Study with Anti-Bacterial Benzyl Isothiocyanate from Nasturtium (Tropaeolum majus) on Human Gut Microbiome and Host Defense. Nutrients, 16(3), 373. https://doi.org/10.3390/nu16030373






