Anti-Obesity Effect of Combining White Kidney Bean Extract, Propolis Ethanolic Extract and CrPi3 on Sprague-Dawley Rats Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of the α-Amylase Inhibitory Activity (α-AIE) in the White Kidney Bean Extract (WKBE)
2.3. Propolis Source and Extraction
2.4. Estimation of Total Concentration of Phenols in PEE
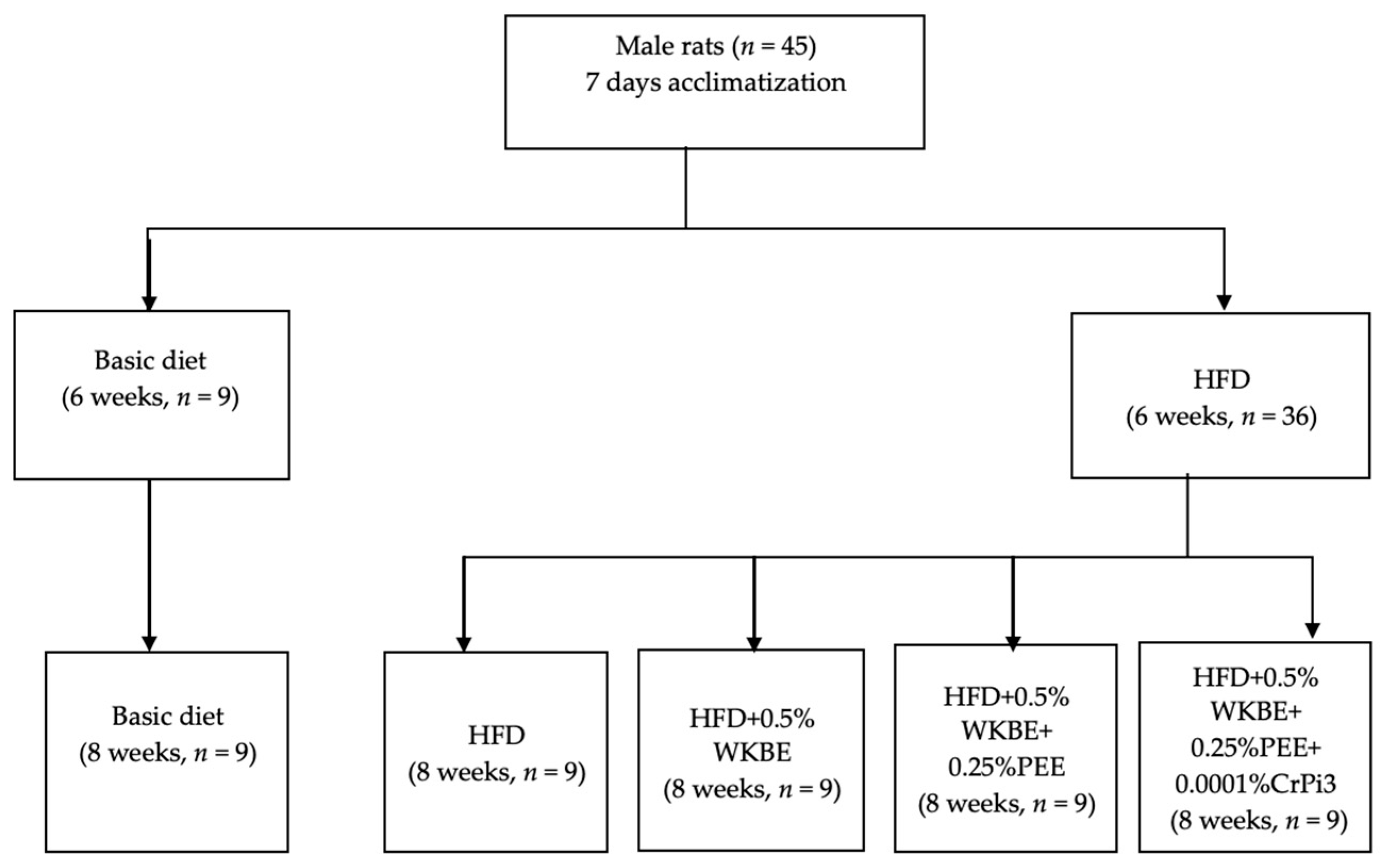
2.5. Animals and Experimental Diets
2.6. Measurement of Body Weight Gain, Food Consumption, and Food Utilization Rate
2.7. Collection of Blood Samples and Analysis
2.8. Weighing of Organs and Determination of Hepatic, Arterial, and Fecal Lipid Content
2.9. Determination of the Colonic Content pH, SCFA Concentration and Profile
2.10. Statistics
3. Results
3.1. Determining the α-Amylase Inhibitory Activity of White Kidney Bean Extract (WBE), and the Total Phenolic and Flavonoid Contents of the Propolis Ethanolic Extract (PEE)
3.2. Treatments and Measurement of the Body Weight Gain, Food Consumption, and Food Utilization Rate
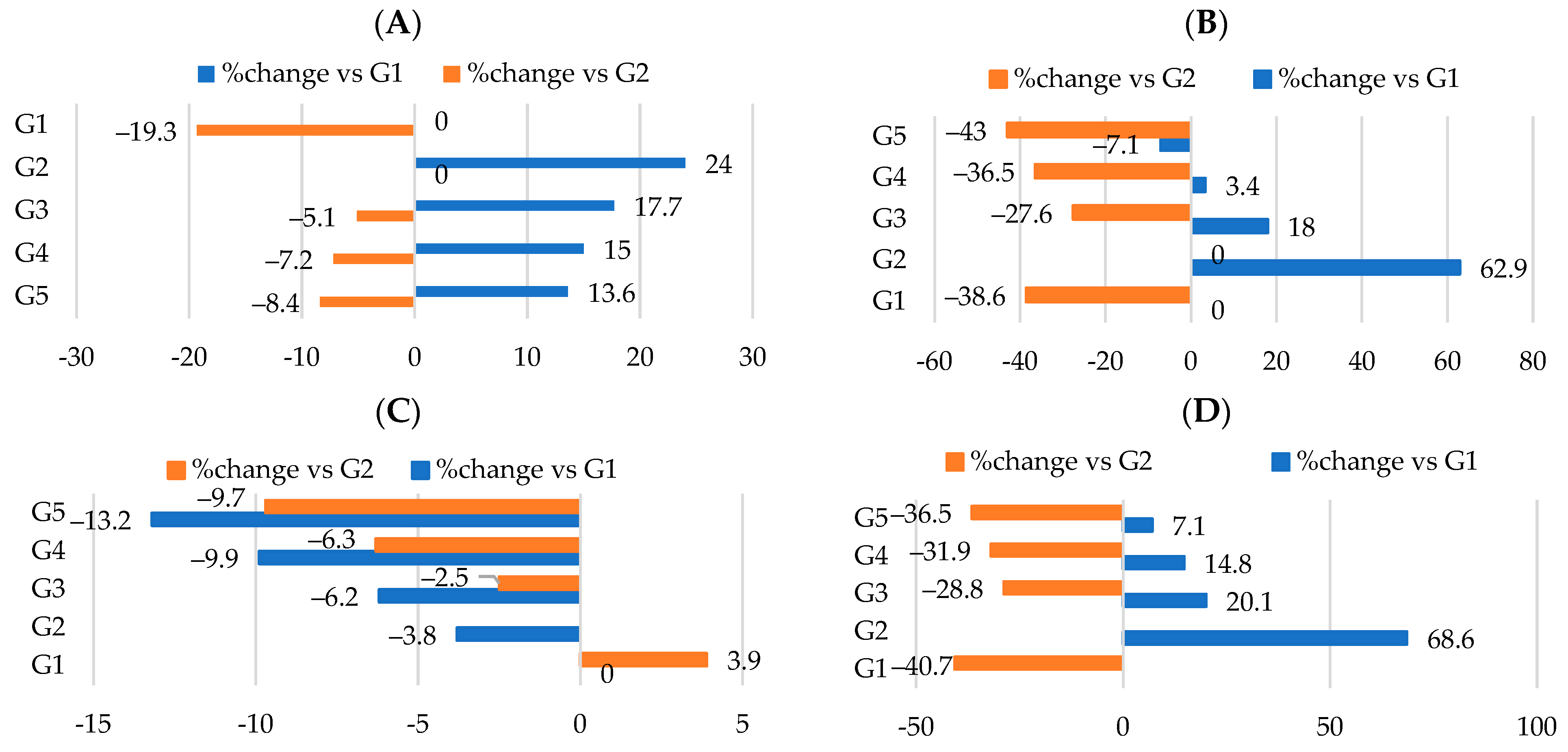
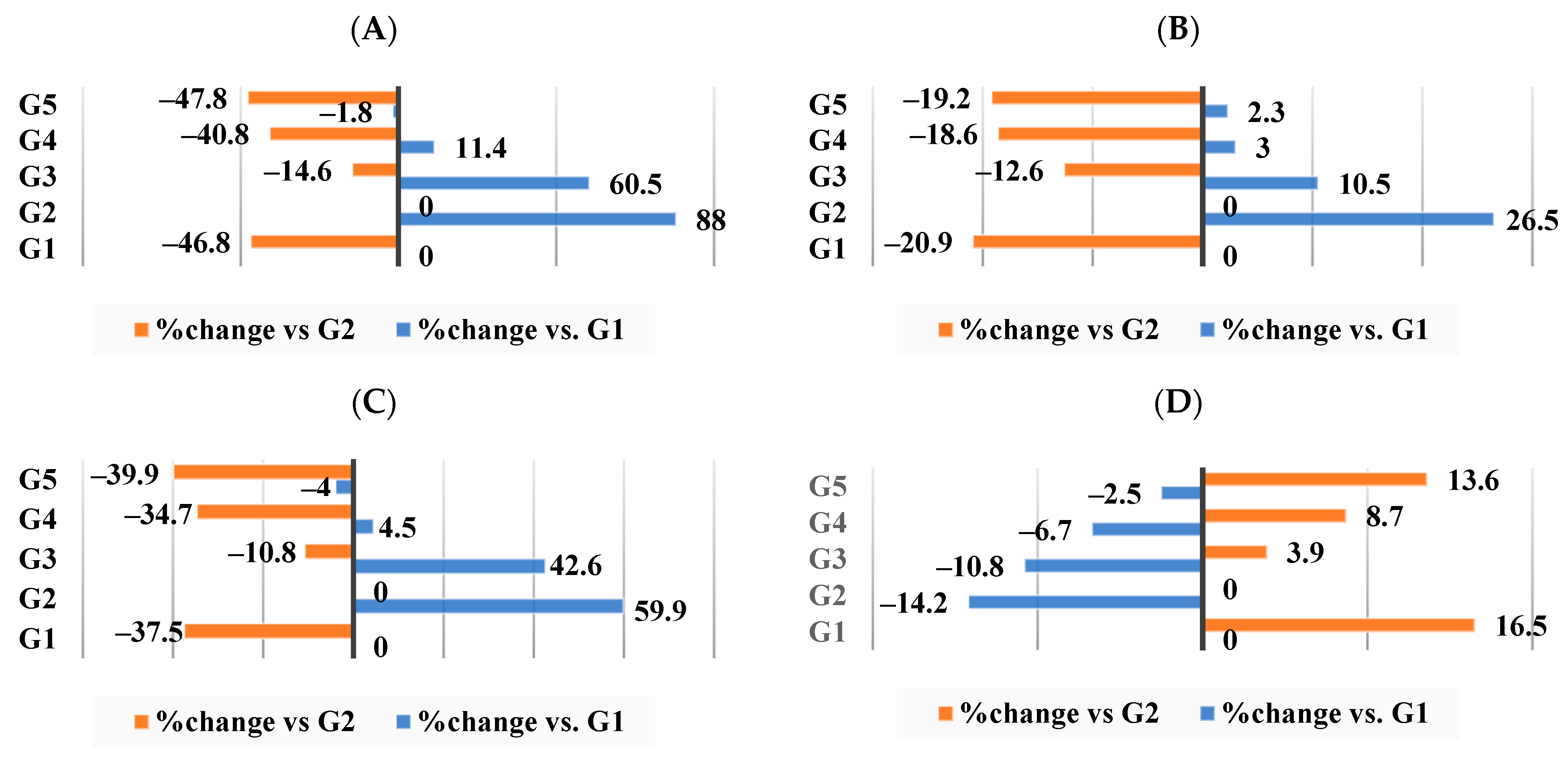
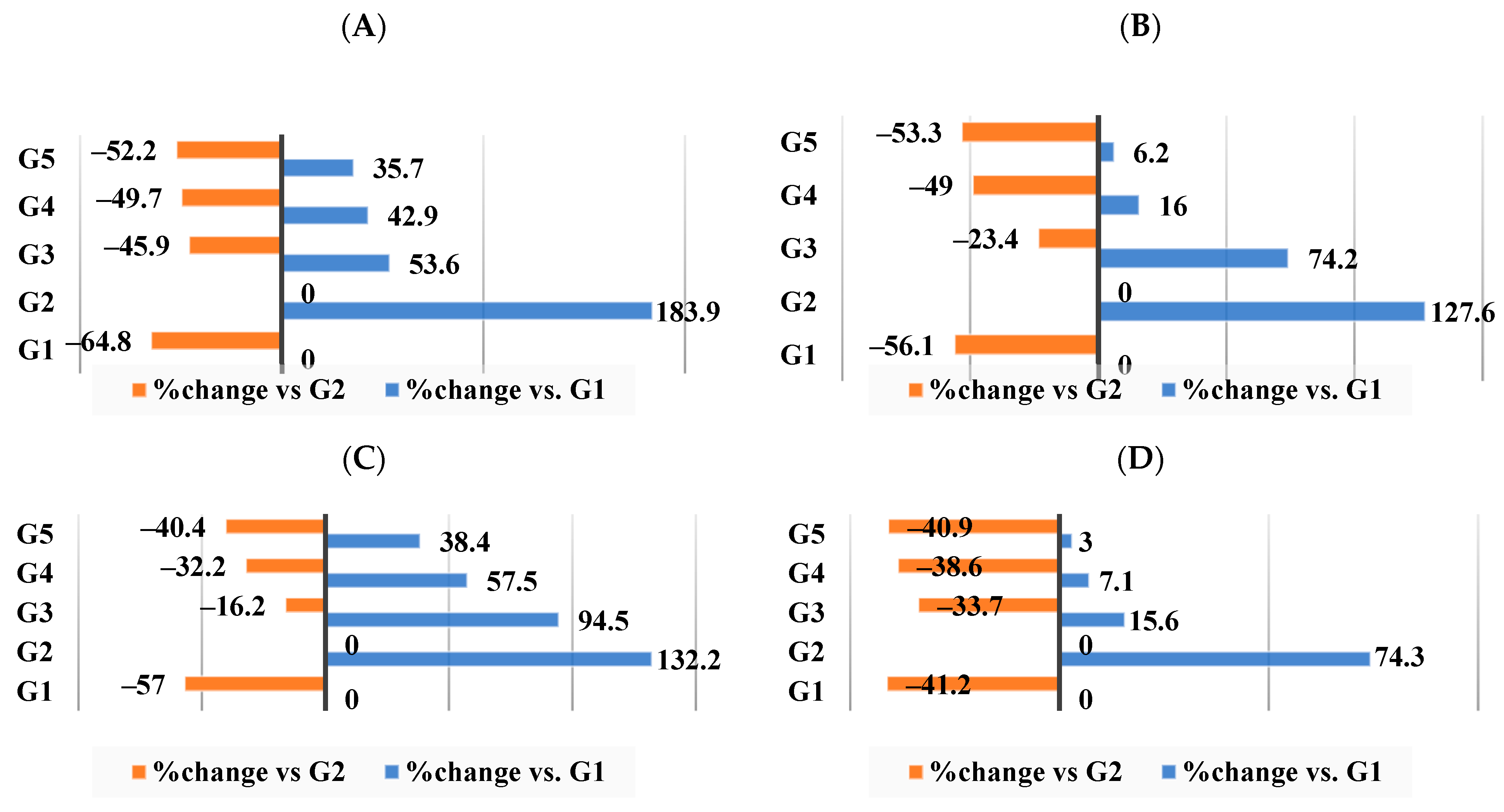
3.3. Effect of Different Nutraceutical Supplements on Relative Organs and Tissue Weight
3.4. Effect of Different Nutraceutical Supplements on Lipid Profiles
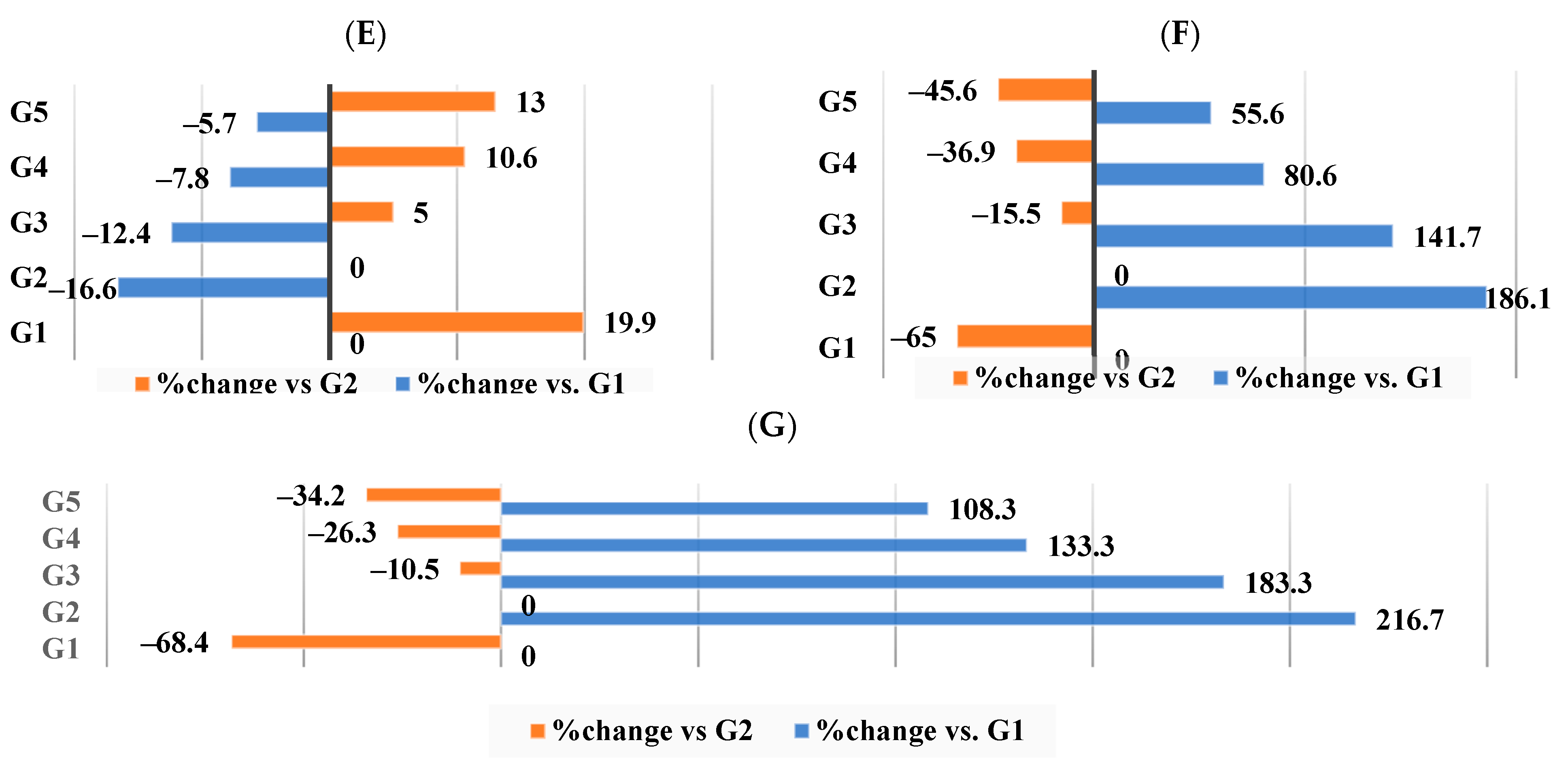
3.5. Effect of Different Nutraceutical Supplements on Total Artery Cholesterol, Triglycerides, Phospholipids, and Molar Ratio
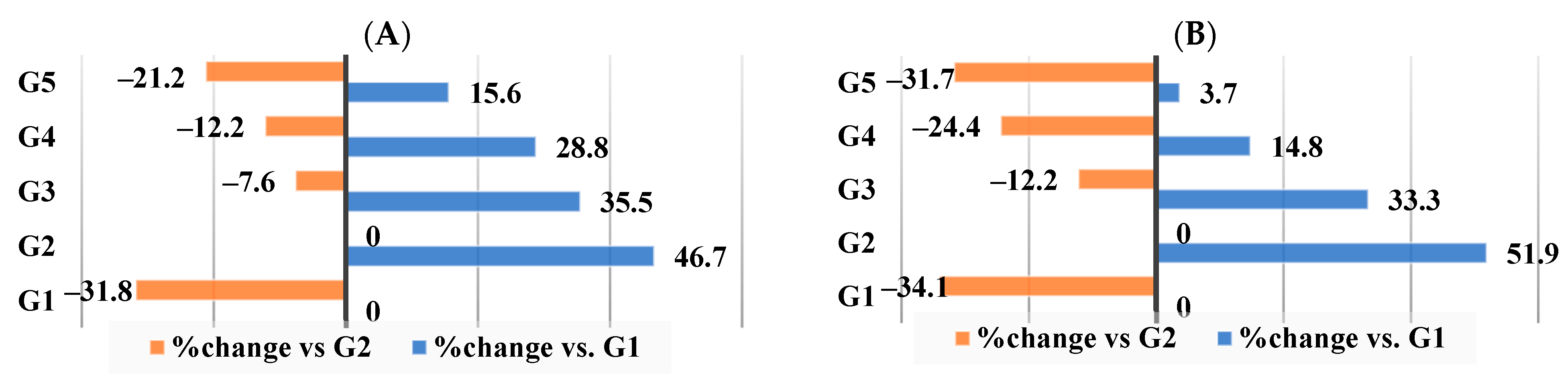
3.6. Effect of Different Nutraceutical Supplements on Total Liver Tissue Lipids, Total Cholesterol, and Triglyceride Levels
3.7. Effect of Different Nutraceutical Supplements on Liver Enzymes in Obese Rats
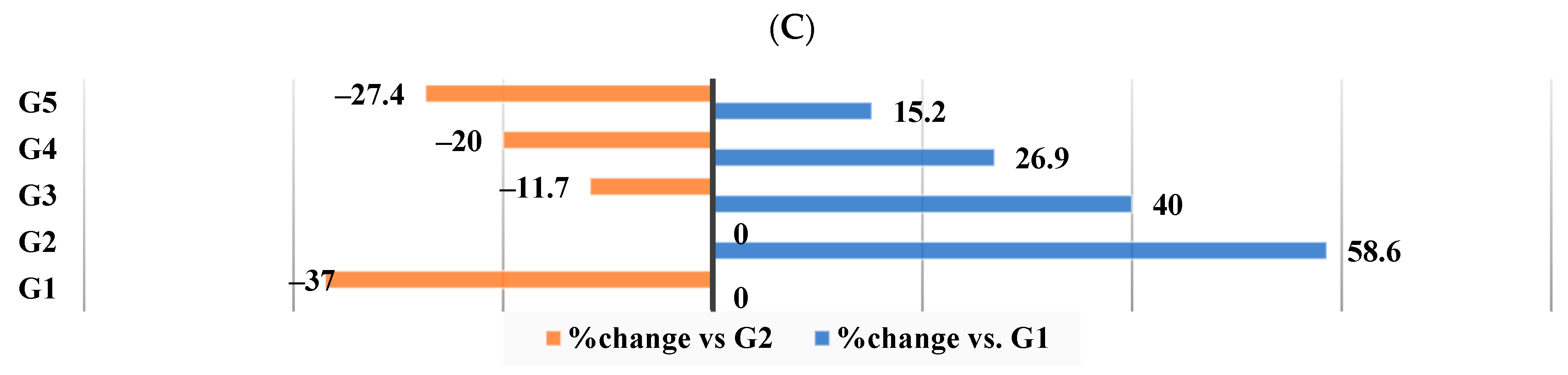
3.8. Effect of Different Nutraceutical Supplements on Kidney Functions and Glucose Levels
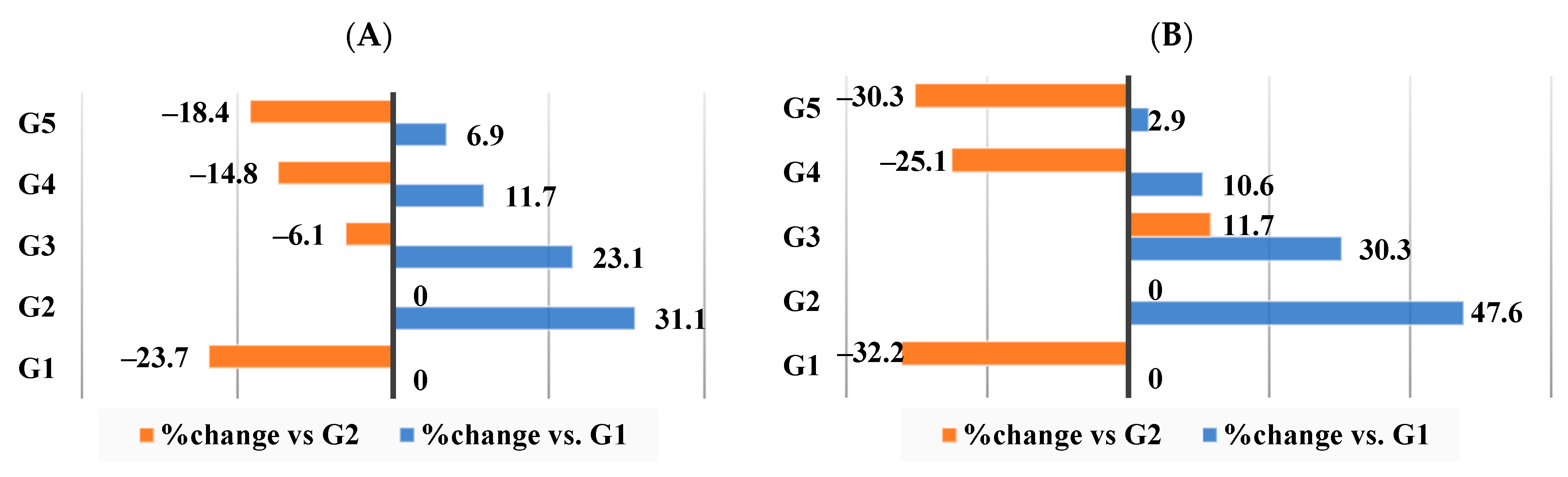
3.9. Effect of Different Nutraceutical Supplements on Concentration and Profile of the Short-Chain Fatty Acids (SCFAs) and Colonic pH
3.10. Effect of Different Nutraceutical Supplements on Total Fecal Lipids, Cholesterol, and Triglycerides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization: WHO. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 14 July 2023).
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, C. The global epidemic of obesity: An overview. Epidemiol. Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef]
- Garcés, C.; Gutierrez-Guisado, J.; Benavente, M.; Cano, B.; De Oya, M. Obesity in Spanish Schoolchildren: Relationship with Lipid Profile and Insulin Resistance. Obesity 2012, 13, 959–963. [Google Scholar] [CrossRef]
- Meigs, J.B.; Wilson, P.W.F.; Fox, C.S.; Vasan, R.S.; Nathan, D.M.; Sullivan, L.M.; D’agostino, R.B. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J. Clin. Endocrinol. Metab. 2006, 91, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Raud, B.; Gay, C.; Guiguet-Auclair, C.; Bonnin, A.; Gerbaud, L.; Pereira, B.; Duclos, M.; Boirie, Y.; Coudeyre, E. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci. Rep. 2020, 10, 3601. [Google Scholar] [CrossRef] [PubMed]
- Taroeno-Hariadi, K.W.; Hardianti, M.S.; Sinorita, H.; Aryandono, T. Obesity, leptin, and deregulation of microRNA in lipid metabolisms: Their contribution to breast cancer prognosis. Diabetol. Metab. Syndr. 2021, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Aziz, R.; Al Mahri, S.; Malik, S.S.; Haji, E.; Khan, A.H.; Khatlani, T.S.; Bouchama, A. Obesity and COVID-19: What makes obese host so vulnerable? Immun. Ageing 2021, 18, 1. [Google Scholar] [CrossRef]
- Friedman, J.M. Causes and Control of Excess Body Fat. Nature 2009, 459, 340–342. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metab. 2018, 28, 415–431. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Chen, H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2004, 112, 1821–1830. [Google Scholar] [CrossRef]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological management of obesity: An endocrine society clinical practice guideline. J. Clin. Endocr. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, C.V.; Vijayalakshmi, M.A.; Prakash, K.; Bansal, V.S.; Meenakshi, J.; Amit, A. Review article:Herbal approach for obesity Management. Am. J. Plant Sci. 2012, 3, 1003–1014. [Google Scholar] [CrossRef]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. 2021, 8, 67–83. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Layer, P.; Carlson, G.L.; DiMagno, E.P. Partially purified white bean amylase inhibitor reduces starch digestion in vitro and inactivates intraduodenal amylase in humans. Gastroenterology 1985, 88, 1895–1902. [Google Scholar] [CrossRef]
- Barrett, M.L.; Udani, J.K. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): A review of clinical studies on weight loss and glycemic control. Nutr. J. 2011, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. [Google Scholar] [CrossRef]
- Balica, G.; Vostinaru, O.; Stefanescu, C.; Mogosan, C.; Iaru, I.; Cristina, A.; Pop, C.E. Potential role of Propolis in the prevention and treatment of metabolic diseases. Plants 2021, 10, 883. [Google Scholar] [CrossRef]
- Abdl El Hady, F.K.; Hegazi, A.G. Gas chromatography–mass spectrometry (GC/MS) study of the Egyptian propolis 1- aliphatic, phenolic acids and their esters. J. Appl. Sci. 1994, 9, 749–760. [Google Scholar] [CrossRef]
- Koya-Miyata, S.; Arai, N.; Mizote, A.; Taniguchi, Y.; Ushio, S.; Iwaki, K.; Fukuda, S. Propolis prevents diet-induced hyperlipidemia and mitigates weight gain in diet-induced obesity in mice. Biol. Pharm. Bull. 2009, 32, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Chien, Y.-W.; Chang, M.-L.; Hou, C.-C.; Chan, C.-H.; Tang, H.-W.; Huang, H.-Y. Taiwanese Green Propolis Ethanol Extract Delays the Progression of Type 2 Diabetes Mellitus in Rats Treated with Streptozotocin/High-Fat Diet. Nutrients 2018, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Available online: https://ods.od.nih.gov/factsheets/Chromium-HealthProfessional/ (accessed on 19 July 2023).
- Pizzorno, J.E.; Murray, M.T. Textbook of Natural Medicine, 4th ed.; Churchill Livingstone: London, UK, 2013; Volume 25, pp. 1–1916. [Google Scholar]
- Albarracin, C.A.; Fuqua, B.C.; Evans, J.L.; Goldfine, I.D. Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2008, 24, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Drake, T.C.; Rudser, K.D.; Seaquist, E.R.; Saeed, A. Chromium infusion in hospitalised patients with severe insulin resistance: A retrospective analysis. Endod. Pract. 2012, 31, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.P.; Sack, D.A.; Roffman, M.; Finch, M.; Komorowski, J. A Double-Blind, Placebo-Controlled, Exploratory trial of chromium picolinate in atypical Depression: Effect on carbohydrate craving. J. Psychiatr. Pract. 2005, 11, 302–314. [Google Scholar] [CrossRef]
- Wang, H.H.; Chen, C.L.; Jeng, T.L.; Sung, J.M. Comparisons of α-Amylase Inhibitors from Seeds of Common Bean Mutants Extracted through Three Phase Partitioning. Food Chem. 2011, 128, 1066–1071. [Google Scholar] [CrossRef]
- Lagouri, V.; Prasianaki, D.; Krysta, F., III. Antioxidant Properties and Phenolic Composition of Greek Propolis Extracts. Int. J. Food Prop. 2014, 17, 511–522. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Takayama, M.; Itoh, S.; Nagasaki, T.; Tanimizu, I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin. Chim. Acta 1977, 79, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Virella, M.F.; Stone, P.; Ellis, S.; Colwell, J.A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem. 1997, 23, 882–884. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Glatter, T.R. Hyperlipidemia. What is ‘normal’, who should be treated and how. Postgrad. Med. 1984, 76, 49–55. [Google Scholar] [CrossRef]
- Dobiásová, M. AIP—Atherogenic index of plasma as a significant predictor of cardiovascular risk: Research to practice. Vnitr. Lek. 2006, 52, 64–71. [Google Scholar]
- Bergmeyer, H.U.; Scheibe, P.; Wahlefeld, A.W. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin. Chem. 1978, 24, 58–73. [Google Scholar] [CrossRef]
- Roy, A.V. Rapid method for determining alkaline phosphatase activity in serum with thymolphthalein monophosphate. Clin. Chem. 1970, 16, 431–436. [Google Scholar] [CrossRef]
- Patton, G.; Crouch, S. Colorimetric Method for the Determination of Serum Urea. Anal. Chem. 1977, 49, 464–469. [Google Scholar] [CrossRef]
- Husdan, H.; Rapoport, A. Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin. Chem. 1968, 14, 222–238. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L.; Berti, G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980, 26, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Nyman, M.; Jonsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall Int.: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Everard, A.; Cani, P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.; Zhu, Y.; Gao, Y.; Ren, G. Comparisons of phaseolin type and a-amylase inhibitor in common bean (Phaseolus vulgaris L.) in China. Crop J. 2016, 4, 68–72. [Google Scholar] [CrossRef][Green Version]
- Hua, Z.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Hernández Zarate, M.S.; AbrahamJuárez, M.R.; Cerón García, A.; Ozuna López, C.; Gutiérrez Chávez, A.J.; Segoviano Garfias, J.J.N.; Avila Ramos, F. Flavonoids, phenolic content, and antioxidant activity of propolis from various areas of Guanajuato, Mexico. Food Sci. Technol. 2018, 38, 210–215. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Abd El Hady, F.K. Egyptian propolis: 3. Antioxidant, antimicrobial activities and chemical composition of propolis from reclaimed lands. Z. Naturforsch. C J. Biosci. 2002, 57, 395–402. [Google Scholar] [CrossRef]
- Tormo, M.A.; Gil-Exojo, I.; de Tejada, A.R.; Campillo, J.E. Hypoglycaemic and anorexigenic activities of an alpha-amylase inhibitor from white kidney beans (Phaseolus vulgaris) in Wistar rats. Br. J. Nutr. 2004, 92, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Tormo, M.A.; Gil-Exojo, I.; Romero de Tejada, A.; Campillo, J.E. White bean amylase inhibitor administered orally reduces glycaemia in type 2 diabetic rats. Br. J. Nutr. 2006, 96, 539–544. [Google Scholar] [PubMed]
- Spadafranca, A.; Rinelli, S.; Riva, A.; Morazzoni, P.; Magni, P.; Bertoli, S.; Battezzati, A. Phaseolus vulgaris extract affects glycometabolic and appetite control in healthy human subjects. Br. J. Nutr. 2013, 109, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Shaban, E.E.; Elbakry, H.F.H.; Ibrahim, K.S.; El Sayed, E.M.; Salama, D.M.; Farrag, A.H. The effect of white kidney bean fertilized with nano-zinc on nutritional and biochemical aspects in rats. Biotechnol. Rep. 2019, 23, e00357. [Google Scholar] [CrossRef] [PubMed]
- Carai, M.A.; Fantini, N.; Loi, B.; Colombo, G.; Gessa, G.L.; Riva, A.; Bombardelli, E.; Morazzoni, P. Multiple cycles of repeated treatments with a Phaseolus vulgaris dry extract reduce food intake and body weight in obese rats. Br. J. Nutr. 2011, 106, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Han, W.; Yan, F.; Xu, D.; Chu, Q.; Zheng, X. Dietary Phaseolus vulgaris extract alleviated diet-induced obesity, insulin resistance and hepatic steatosis and alters gut microbiota composition in mice. J. Funct. Foods 2016, 20, 236–244. [Google Scholar] [CrossRef]
- Reverri, E.J.; Randolph, J.M.; Kappagoda, C.T.; Park, E.; Edirisinghe, I.; Burton-Freeman, B.M. Assessing beans as a source of intrinsic fiber on satiety in men and women with metabolic syndrome. Appetite 2017, 118, 75–81. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- Elsayed, M.; Salem, M.; Selem, S. Effect of feeding at different levels of chromium picolinate and magnesium sulfate onm diabetic rats. J. Food Dairy Sci. 2015, 6, 379–392. [Google Scholar] [CrossRef]
- Fotschki, B.; Ognik, K.; Cholewińska, E.; Grzelak-Błaszczyk, K.; Myszczyński, K.; Krauze, M.; Juśkiewicz, J. Effect of Chromium Nanoparticles and Switching from a High-Fat to a Low-Fat Diet on the Cecal Microenvironment in Obese Rats. Nutrients 2023, 15, 3118. [Google Scholar] [CrossRef]
- Nassar, A.M.K.; Salim, Y.M.M.; Eid, K.S.A.; Shaheen, H.M.; Saati, A.A.; Hetta, H.F.; Elmistekawy, A.; Batiha, G.E.-S. Ameliorative Effects of Honey, Propolis, Pollen, and Royal Jelly Mixture against Chronic Toxicity of Sumithion Insecticide in White Albino Rats. Molecules 2020, 25, 2633. [Google Scholar] [CrossRef] [PubMed]
- Hassan, L.; Bakour, M.; Ousaaid, D.; Ferreira-Santos, P.; Genisheva, Z.; El Ghouizi, A.; Aboulghazi, A.; Teixeira, J.A.; Lyoussi, B. Protective Effect of Honey and Propolis against Gentamicin-Induced Oxidative Stress and Hepatorenal Damages. Oxidative Med. Cell. Longev. 2021, 2021, 9719906. [Google Scholar] [CrossRef]
- Jahnabi, S.; Soma, C.; Dipayan, C. Effect of subchronic exposure to chromium on hematological and biochemical parameters of male albino rat. Asian J. Pharm. Clin. Res. 2017, 10, 345. [Google Scholar] [CrossRef]
- Matsuura, E.; Hughes, G.R.V.; Khamashta, M.A. Oxidation of LDL and its clinical implication. Autoimmun. Rev. 2008, 7, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Brunzell, J.D.; Davidson, M.; Furberg, C.D.; Goldberg, R.B.; Howard, B.V.; Stein, J.H.; Witztum, J.L. Lipoprotein management in patients with cardiometabolic risk: Consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J. Am. Coll. Cardiol. 2008, 15, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Assmann, G.; Gotto, A.M., Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation 2004, 109, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhao, T.; Mao, G.; Wang, W.; Feng, Y.; Li, F.; Zheng, D.; Wu, H.; Jin, D.; Yang, L.; et al. Type 2 Diabetic Rats on Diet Supplemented with Chromium Malate Show Improved Glycometabolism, Glycometabolism-Related Enzyme Levels and Lipid Metabolism. Edited by Marta Letizia Hribal. PLoS ONE 2015, 5, e0125952. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Rains, J.L.; Croad, J.L. Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-alpha, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free. Radic. Biol. Med. 2007, 43, 124–131. [Google Scholar] [CrossRef]
- Sahin, K.; Onderci, M.; Tuzcu, M.; Ustundag, B.; Cikim, G.; Ozercan, I.H.; Sriramoju, V.; Juturu, V.; Komorowski, J.R. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: The fat-fed, streptozotocin-treated rat. Metabolism 2007, 56, 1233–1240. [Google Scholar] [CrossRef]
- Seif, A.A. Chromium picolinate inhibits cholesterol-induced stimulation of platelet aggregation in hypercholesterolemic rats. Ir. J. Med. Sci. 2015, 184, 291–296. [Google Scholar] [CrossRef]
- Sakai, T.; Ohhata, M.; Fujii, M.; Oda, S.; Kusaka, Y.; Matsumoto, M.; Nakamoto, A.; Taki, T.; Nakamoto, M.; Shuto, E. Brazilian green propolis promotes weight loss and reduces fat accumulation in C57BL/6 mice fed a high-fat diet. Biol. Pharm. Bull. 2017, 40, 391. [Google Scholar] [CrossRef] [PubMed]
- Kilua, A.; Chihiro, H.; Han, K.-H.; Homma, K.; Fukuma, N.; Kamitani, T.; Suzuki, T.; Fukushima, M. Whole kidney bean (Phaseolus vulgaris) and bean hull reduce the total serum cholesterol, modulate the gut microbiota and affect the caecal fermentation in rats. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100232. [Google Scholar] [CrossRef]
- Ashraf, J.; Liu, L.; Awais, M.; Xiao, T.; Wang, L.; Zhou, X.; Tong, L.-T.; Zhou, S. Effect of thermosonication pre-treatment on mung bean (Vigna radiata) and white kidney bean (Phaseolus vulgaris) proteins: Enzymatic hydrolysis, cholesterol lowering activity and structural characterization. Ultrason. Sonochemistry 2020, 66, 105121. [Google Scholar] [CrossRef] [PubMed]
- De Lima, S.L.S.; Gomes, M.J.C.; da Silva, B.P.; Alves, N.E.G.; Toledo, R.C.L.; Theodoro, J.M.V.; Moreira, M.E.d.C.; Bento, J.A.C.; Bassinello, P.Z.; da Matta, S.L.P.; et al. Whole flour and protein hydrolysate from common beans reduce the inflammation in balb/c mice fed with high fat high cholesterol diet. Food Res. Int. 2019, 122, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Fuliang, H.; Hepburn, H.; Xuan, H.; Chen, M.; Daya, S.; Radloff, S. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol. Res. 2005, 51, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Prata, M.F.; de Carvalho, F.M.A.; Gonçalves-Júnior, W.D.; Santos, T.S.; Valois, R.B.V.; Borges, A.F.S.; Guimarães, A.O.; Araújo, A.A.S.; Pereira-Filho, R.N.; Santini, A.; et al. Hypolipidemic and anti-obesity effects of Hydroalcoholic extract of Brazilian red Propolis in a rodent model of dyslipidemia. Eur. J. Lipid Sci. Technol. 2022, 124, 2100017. [Google Scholar] [CrossRef]
- El Menyiy, N.; Al-Wali, N.; El Ghouizi, A.; El-Guendouz, S.; Salom, K.; Lyoussi, B. Potential therapeutic effect of Moroccan propolis in hyperglycemia, dyslipidemia, and hepatorenal dysfunction in diabetic rats. Iran. J. Basic. Med. Sci. 2019, 22, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Ichi, I.; Hori, H.; Takashima, Y.; Adachi, N.; Kataoka, R.; Okihara, K.; Hashimoto, K.; Kojo, S. The beneficial effect of propolis on fat accumulation and lipid metabolism in rats fed a high-fat diet. J. Food Sci. 2009, 74, H127–H131. [Google Scholar] [CrossRef]
- Ahmed, R.; Tanvir, E.M.; Hossen, S.; Afroz, R.; Ahmmed, I.; Rumpa, N.-E.; Paul, S.; Gan, S.H.; Sulaiman, S.A.; Khalil, I. Antioxidant properties and cardioprotective mechanism of Malaysian propolis in rats. Evid. Based Compl. Alternat. Med. 2017, 2017, 5370545–5370556. [Google Scholar] [CrossRef]
- Hegazy, A.M.; El-Sayed, E.M.; Ibrahim, K.S.; Abdel-Azeem, A.S. Dietary antioxidant for disease prevention corroborated by the Nrf2 pathway. J. Complement. Integr. Med. 2019, 16, 3. [Google Scholar] [CrossRef]
- Chien, Y.-H.; Yu, Y.-H.; Chen, Y.-W. Taiwanese green propolis ameliorates metabolic syndrome via remodeling of white adipose tissue and modulation of gut microbiota in diet-induced obese mice. Biomed. Pharmacother. 2023, 160, 114386. [Google Scholar] [CrossRef] [PubMed]
- Pattar, G.R.; Tackett, L.; Liu, P.; Elmendorf, J.S. Chromium picolinate positively influences the glucose transporter system via affecting cholesterol homeostasis in adipocytes cultured under hyperglycemic diabetic conditions. Mutat. Res. 2006, 610, 93–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimano, H. Sterol regulatory element-binding proteins (SREBPs): Transcriptional regulators of lipid synthetic genes. Prog. Lipid Res. 2001, 40, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef]
- Yurchenko, A.; Krenytska, D.; Savchuk, O.; Halenova, T.; Raksha, N.; Vovk, T.; Ostapchenko, L. The evaluation of lipid peroxidation and oxidative modification of proteins in blood serum under obesity development and the consumption of aqueous kidney beans Phaseolus vulgaris pods extract. Curr. Issues Pharm. Med. Sci. 2020, 33, 38–44. [Google Scholar] [CrossRef]
- Orhan, C.; Kucuk, O.; Tuzcu, M.; Sahin, N.; Komorowski, J.R.; Sahin, K. Effect of supplementing chromium histidinate and picolinate complexes along with biotin on insulin sensitivity and related metabolic indices in rats fed a high-fat diet. Food Sci. Nutr. 2018, 7, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Chen, C.J.; Liu, C.H.; Mao, F.C. Chromium attenuates high-fat diet-induced nonalcoholic fatty liver disease in KK/HlJ mice. Biochem. Biophys. Res. Commun. 2010, 397, 459–464. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.H.; Chen, M.L.; Hu, F.L. Protective effects of Chinese and Brazilian propolis treatment against hepatorenal lesion in diabetic rats. Hum. Exp. Toxicol. 2011, 30, 1246–1255. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Pathania, S.; Brites, C.; Rosa, E.A.S.; Barros, A.I. Potential of legumes: Nutritional value, bioactive properties, innovative food products, and application of eco-friendly tools for their assessment. Food Rev. Int. 2023, 39, 160–188. [Google Scholar] [CrossRef]
- Shi, Z.; Zhu, Y.; Teng, C.; Yao, Y.; Ren, G.; Richel, A. Anti-obesity effects of α-amylase inhibitor enriched-extract from white common beans (Phaseolus vulgaris L.) associated with the modulation of gut microbiota composition in high-fat diet-induced obese rats. Food Funct. 2020, 11, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Grau, R.; Díaz-Muñoz, M.D.; Cacheiro-Llaguno, C.; Fresno, M.; Iñiguez, M.A. Role of peroxisome proliferator-activated receptor alpha in the control of cyclooxygenase 2 and vascular endothelial growth factor: Involvement in tumor growth. PPAR Res. 2008, 2008, 352437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [PubMed]
- Holzner, L.M.W.; Murray, A.J. Hypoxia-Inducible Factors as Key Players in the Pathogenesis of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis. Front. Med. 2021, 8, 753268. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Feng, Q.; Niu, Z.; Zhang, S.; Wang, L.; Dong, L.; Hou, D.; Zhou, S. Protective Effects of White Kidney Bean (Phaseolus vulgaris L.) against Diet-Induced Hepatic Steatosis in Mice Are Linked to Modification of Gut Microbiota and Its Metabolites. Nutrients 2023, 15, 3033. [Google Scholar] [CrossRef]
- Hassan, L.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of Antioxidant-Rich Propolis and Bee Pollen Extracts against D-Glucose Induced Type 2 Diabetes in Rats. Food Res. Int. 2020, 138, 109802. [Google Scholar] [CrossRef]
- Qi, S.S.; Zheng, H.X.; Jiang, H.; Yuan, L.P.; Dong, L.C. Protective Effects of Chromium Picolinate Against Diabetic-Induced Renal Dysfunction and Renal Fibrosis in Streptozotocin-Induced Diabetic Rats. Biomolecules 2020, 10, 398. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chen, C.J.; Liu, C.H.; Mao, F.C. Chromium supplementation enhances insulin signalling in skeletal muscle of obese KK/HlJ diabetic mice. Diabetes Obes Metab. 2009, 11, 293–303. [Google Scholar] [CrossRef]
- Zuo, Z.; Pang, W.; Sun, W.; Lu, B.; Zou, L.; Zhang, D.; Wang, Y. Metallothionein–Kidney Bean Polyphenol Complexes Showed Antidiabetic Activity in Type 2 Diabetic Rats by Improving Insulin Resistance and Regulating Gut Microbiota. Foods 2023, 12, 3139. [Google Scholar] [CrossRef]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus Plantarum KLDS1.0344 and Lactobacillus AcidophilusKLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, Y.; Tao, L.; Chen, X.; Jones, T.J.; Wang, K.; Hu, F. Chinese Propolis Prevents Obesity and Metabolism Syndromes Induced by a High Fat Diet and Accompanied by an Altered Gut Microbiota Structure in Mice. Nutrients 2020, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Juśkiewicz, J.; Ognik, K.; Fotschki, J.; Napiórkowska, D.; Cholewińska, E.; Grzelak-Błaszczyk, K.; Krauze, M.; Fotschki, B. The Effects of Dietary Chromium Supplementation along with Discontinuing a High-Fat Diet on the Microbial Enzymatic Activity and the Production of SCFAs in the Faeces of Rats. Nutrients 2023, 15, 3962. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.; You, M.; Tian, W.; Le Leu, R.K.; Topping, D.L.; Conlon, M.A.; Wu, L.; Hu, F. Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet. Nutrients 2017, 9, 875. [Google Scholar] [CrossRef]
- Degen, L.; Oesch, S.; Casanova, M.; Graf, S.; Ketterer, S.; Drewe, J.; Beglinger, C. Effect of peptide YY3-36 on food intake in humans. Gastroenterology 2005, 129, 1430–1436. [Google Scholar] [CrossRef]
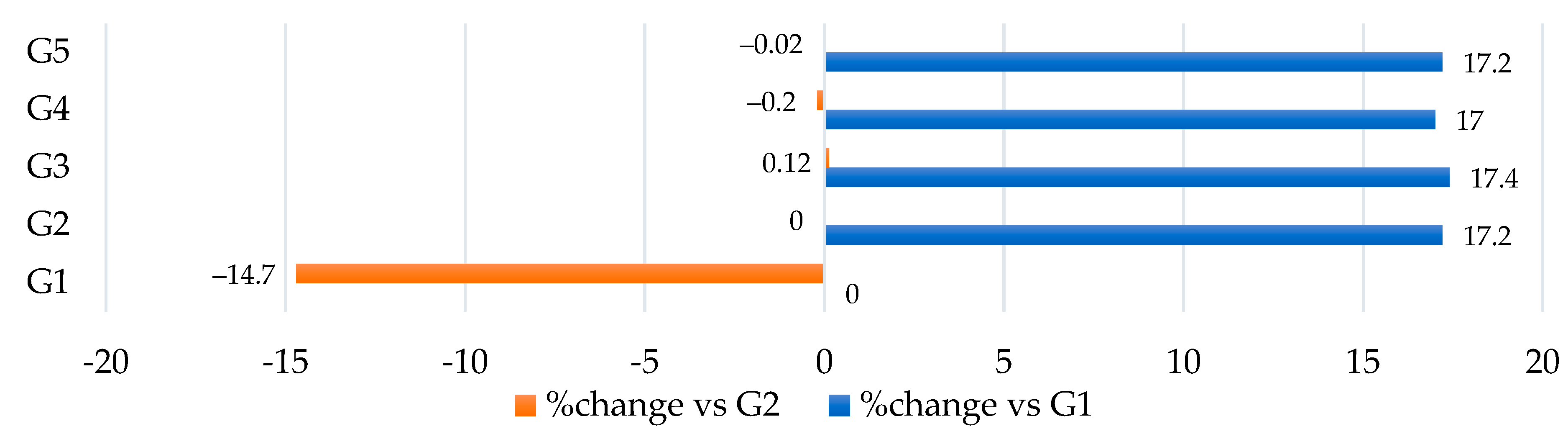





| Groups | G1 (−ve) Control Group | G2 (+ve) Control Group | G3 Supplement I | G4 Supplement II | G5 Supplement III |
|---|---|---|---|---|---|
| Corn Starch | 397.486 | 331.986 | 326.986 | 324.486 | 324.485 |
| Casein (≥85% protein) | 200 | 200 | 200 | 200 | 200 |
| Dextrinized corn starch | 132 | 70 | 70 | 70 | 70 |
| Sucrose | 100 | 50 | 50 | 50 | 50 |
| Soybean oil | 70 | 70 | 70 | 70 | 70 |
| Lard | ----- | 177.50 | 177.50 | 177.50 | 177.50 |
| Non-nutritive cellulose | 50 | 50 | 50 | 50 | 50 |
| Mineral mix | 35 | 35 | 35 | 35 | 35 |
| Vitamin mix | 10 | 10 | 10 | 10 | 10 |
| L-Cysteine | 3 | 3 | 3 | 3 | 3 |
| Choline bitartrates (41% choline) | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Tert-Butylhydroquinone | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
| White kidney bean (WKB) extract (3000 unit/g α-AI) | ----- | ----- | 5 | 5 | 5 |
| Propolis ethanolic extract (PEE) | ----- | ----- | ----- | 2.50 | 2.50 |
| Chromium picolinate (Chromium Eq. 120 ug) | ----- | ----- | ----- | ----- | 0.001 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 |
| Groups | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Initial weight (g) | 370.5 ± 5.9 b | 434.4 ± 4.9 a | 434.9 ± 4.9 a | 433.6 ± 4.9 a | 434.3 ± 4.9 a |
| Final weight (g) | 434.9 ± 6.3 c | 539.2 ± 6.1 a | 511.9 ± 6.2 b | 500.2 ± 5.7 b | 494.1 ± 5.6 b |
| Body weight gain (g) | 64.4 ± 0.7 c | 104.9 ± 1.2 a | 76.0 ± 0.9 b | 66.6 ± 0.8 c | 59.8 ± 0.7 d |
| Food consumption (g) | 380.6 ± 4.3 a | 366.2 ± 4.2 b | 357.1 ± 4.1 b | 343.0 ± 3.9 c | 330.5 ± 3.8 d |
| Food utilization rate (%) * | 16.9 ± 0.004 e | 28.5 ± 0.003 a | 20.3 ± 0.004 b | 19.4 ± 0.003 c | 18.1 ± 0.003 d |
| Groups | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Liver | 4.7 ± 0.05 d | 6.0 ± 40.07 a | 5.4 ± 0.06 b | 5.2 ± 0.06 c | 5.1 ± 0.06 c |
| Kidney | 1.15 ± 0.01 c | 1.3 ± 0.02 a | 1.21 ± 0.01 b | 1.18 ± 0.01 c | 1.16 ± 0.01 c |
| Spleen | 0.71 ± 0.01 c | 0.82 ± 0.01 a | 0.78 ± 0.01 b | 0.73 ± 0.01 c | 0.72 ± 0.01 c |
| Heart | 0.44 ± 0.005 e | 0.55 ± 0.007 a | 0.52 ± 0.007 b | 0.48 ± 0.005 c | 0.46 ± 0.005 d |
| Aorta | 0.06 ± 0.001 d | 0.13 ± 0.001 a | 0.09 ± 0.001 b | 0.071 ± 0.001 c | 0.07 ± 0.001 c |
| Intra-abdominal fat | 5.1 ± 0.06 d | 5.9 ± 0.07 a | 5.7 ± 0.07 b | 5.5 ± 0.06 c | 5.4 ± 0.06 c |
| Groups | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| TG (mmol/L) | 0.62 ± 0.002 e | 1.9 ± 0.02 a | 1.69 ± 0.02 b | 1.37 ± 0.02 c | 1.22 ± 0.01 d |
| Phospholipids (mmol/L) | 1.61 ± 0.02 b | 1.72 ± 0.02 a | 1.31 ± 0.02 c | 1.27 ± 0.01 c d | 1.22 ± 0.01 d |
| MDA (umol/L) | 2.32 ± 0.03 c | 4.39 ± 0.05 a | 3.44 ± 0.04 b | 2.46 ± 0.03 c d | 2.39 ± 0.03 d |
| TC (mmol/L) | 2.41 ± 0.03 e | 3.02 ± 0.03 a | 2.9 ± 0.03 b | 2.72 ± 0.03 c | 2.63 ± 0.03 d |
| HDL-C (mmol/L) | 1.93 ± 0.02 a | 1.61 ± 0.02 d | 1.69 ± 0.02 c | 1.78 ± 0.02 b | 1.82 ± 0.02 b |
| LDL-C (mmol/L) | 0.36 ± 0.004 e | 1.03 ± 0.01 a | 0.87 ± 0.01 b | 0.65 ± 0.007 c | 0.56 ± 0.007 d |
| VLDL-C (mmol/L) | 0.12 ± 0.001 e | 0.38 ± 0.005 a | 0.34 ± 0.004 b | 0.28 ± 0.003 c | 0.25 ± 0.003 d |
| Atherogenic index (AI) | 0.25 ± 0.003 e | 0.87 ± 0.01 a | 0.71 ± 0.008 b | 0.52 ± 0.007 c | 0.44 ± 0.005 d |
| Coronary risk ratio | 1.25 ± 0.01 d | 1.86 ± 0.02 a | 1.70 ± 0.02 b | 1.51 ± 0.02 c | 1.13 ± 1.01 e |
| HDL-C/LDL-C | 5.37 ± 0.06 a | 1.56 ± 0.02 e | 1.94 ± 0.02 d | 2.69 ± 0.03 c | 3.26 ± 0.04 b |
| LDL-C/HDL-C | 0.19 ± 0.003 e | 0.64 ± 0.007 a | 0.51 ± 0.007 b | 0.37 ± 0.004 c | 0.31 ± 0.004 d |
| Groups | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| TC (mg/g) | 1.67 ± 0.02 d | 3.14 ± 0.04 a | 2.68 ± 0.03 b | 1.86 ± 0.02 c | 1.64 ± 0.02 d |
| TG (mg/g) | 25.33 ± 0.29 c | 32.04 ± 0.36 a | 27.99 ± 0.32 b | 26.09 ± 0.3 c | 25.9 ± 0.29 c |
| Phospholipids (mg/g) | 2.02 ± 0.02 d | 3.23 ± 0.04 a | 2.88 ± 0.03 b | 2.11 ± 0.02 c | 1.94 ± 0.02 d |
| Molar ratio (Phospholipid/TC) | 1.20 ± 0.01 a | 1.03 ± 0.01 d | 1.07 ± 1.01 c | 1.12 ± 0.01 b | 1.17 ± 1.01 a |
| Groups | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Total lipids | 44.8 ± 0.51 b | 65.7 ± 0.75 a | 60.7 ± 0.69 b | 57.7 ± 0.66 c b | 51.8 ± 0.59 a d |
| Total cholesterol | 2.7 ± 0.03 e | 4.1 ± 0.05 a | 3.6 ± 0.04 b | 3.1 ± 0.03 c | 2.8 ± 0.03 d |
| Triglyceride | 14.5 ± 0.17 d | 23.0 ± 0.26 a | 20.3 ± 0.23 b | 18.4 ± 0.21 c | 16.7 ± 0.19 d |
| Groups | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| AST activity | 57.9 ± 0.66 e | 75.9 ± 0.86 a | 71.3 ± 0.81 b | 64.7 ± 0.74 c | 61.9 ± 0.7 d |
| ALT activity | 20.8 ± 0.24 d | 30.7 ± 0.35 a | 27.1 ± 0.31 b | 23.0 ± 0.26 c | 21.4 ± 0.24 d |
| ALP activity | 77.9 ± 0.89 d | 106.9 ± 1.2 a | 91.3 ± 1.04 b | 83.5 ± 0.95 c | 79.7 ± 0.91 d |
| Groups | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| BUN (Blood Urea Nitrogen) | 22.5 ± 0.26 e | 51.2 ± 0.58 a | 39.2 ± 0.45 b | 26.1 ± 0.3 c | 23.9 ± 0.27 d |
| Creatinine | 0.56 ± 0.01 e | 1.59 ± 0.02 a | 0.86 ± 0.01 b | 0.80 ± 0.01 c | 0.76 ± 0.01 d |
| Uric acid | 1.46 ± 0.02 e | 3.39 ± 0.04 a | 2.84 ± 0.03 b | 2.3 ± 0.03 c | 2.02 ± 0.02 d |
| Glucose | 80.2 ± 0.91 d | 139.8 ± 1.6 a | 92.7 ± 1.1 b | 85.9 ± 0.98 c | 82.6 ± 0.94 d |
| Groups | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Colonic content pH | 6.87 ± 0.08 a | 6.93 ± 0.08 a | 6.84 ± 0.08 a | 6.29 ± 0.07 b | 6.07 ± 0.07 c |
| Total SCFA concentration | 139.4 ± 1.58 d | 176.2 ± 2.0 b | 171.2 ± 1.95 c | 207.1 ± 2.35 a | 212.1 ± 2.41 a |
| Acetic acid | 122.5 ± 1.39 c | 163.3 ± 1.86 b | 156.3 ± 1.78 b | 185.2 ± 2.11 a | 190.2 ± 2.16 a |
| Propionic acid | 11.0 ± 0.12 c | 8.9 ± 0.1 d | 10.6 ± 0.12 d | 16.7 ± 0.19 a | 15.9 ± 0.18 b |
| Isobutyric acid | 1.56 ± 0.02 c | 1.48 ± 0.02 d | 1.76 ± 0.02 b | 1.74 ± 0.02 b | 2.3 ± 0.03 a |
| Butyric acid | 3.29 ± 0.04 c | 3.56 ± 0.04 b | 3.2 ± 0.04 c d | 3.39 ± 0.04 c | 3.61 ± 0.04 a b |
| Groups | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Total lipids | 22.7 ± 0.26 e | 21.0 ± 0.24 d | 27.9 ± 0.31 c | 30.6 ± 0.35 b | 32.1 ± 0.36 a |
| Total cholesterol | 1.67 ± 0.02 e | 1.15 ± 0.01 d | 3.49 ± 0.04 c | 5.35 ± 0.06 b | 5.78 ± 0.07 a |
| Triglyceride | 0.84 ± 0.01 e | 0.32 ± 0.004 d | 1.61 ± 0.01 c | 2.02 ± 0.02 b | 2.07 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelfattah, D.S.E.; Fouad, M.A.; Elmeshad, A.N.; El-Nabarawi, M.A.; Elhabal, S.F. Anti-Obesity Effect of Combining White Kidney Bean Extract, Propolis Ethanolic Extract and CrPi3 on Sprague-Dawley Rats Fed a High-Fat Diet. Nutrients 2024, 16, 310. https://doi.org/10.3390/nu16020310
Abdelfattah DSE, Fouad MA, Elmeshad AN, El-Nabarawi MA, Elhabal SF. Anti-Obesity Effect of Combining White Kidney Bean Extract, Propolis Ethanolic Extract and CrPi3 on Sprague-Dawley Rats Fed a High-Fat Diet. Nutrients. 2024; 16(2):310. https://doi.org/10.3390/nu16020310
Chicago/Turabian StyleAbdelfattah, Doaa Salah Eldin, Mervat A. Fouad, Aliaa N. Elmeshad, Mohamed A. El-Nabarawi, and Sammar Fathy Elhabal. 2024. "Anti-Obesity Effect of Combining White Kidney Bean Extract, Propolis Ethanolic Extract and CrPi3 on Sprague-Dawley Rats Fed a High-Fat Diet" Nutrients 16, no. 2: 310. https://doi.org/10.3390/nu16020310
APA StyleAbdelfattah, D. S. E., Fouad, M. A., Elmeshad, A. N., El-Nabarawi, M. A., & Elhabal, S. F. (2024). Anti-Obesity Effect of Combining White Kidney Bean Extract, Propolis Ethanolic Extract and CrPi3 on Sprague-Dawley Rats Fed a High-Fat Diet. Nutrients, 16(2), 310. https://doi.org/10.3390/nu16020310








