Defining “High-In” Saturated Fat, Sugar, and Sodium to Help Inform Front-of-Pack Labeling Efforts for Packaged Foods and Beverages in the United States
Abstract
1. Introduction
2. Materials and Methods
3. Results
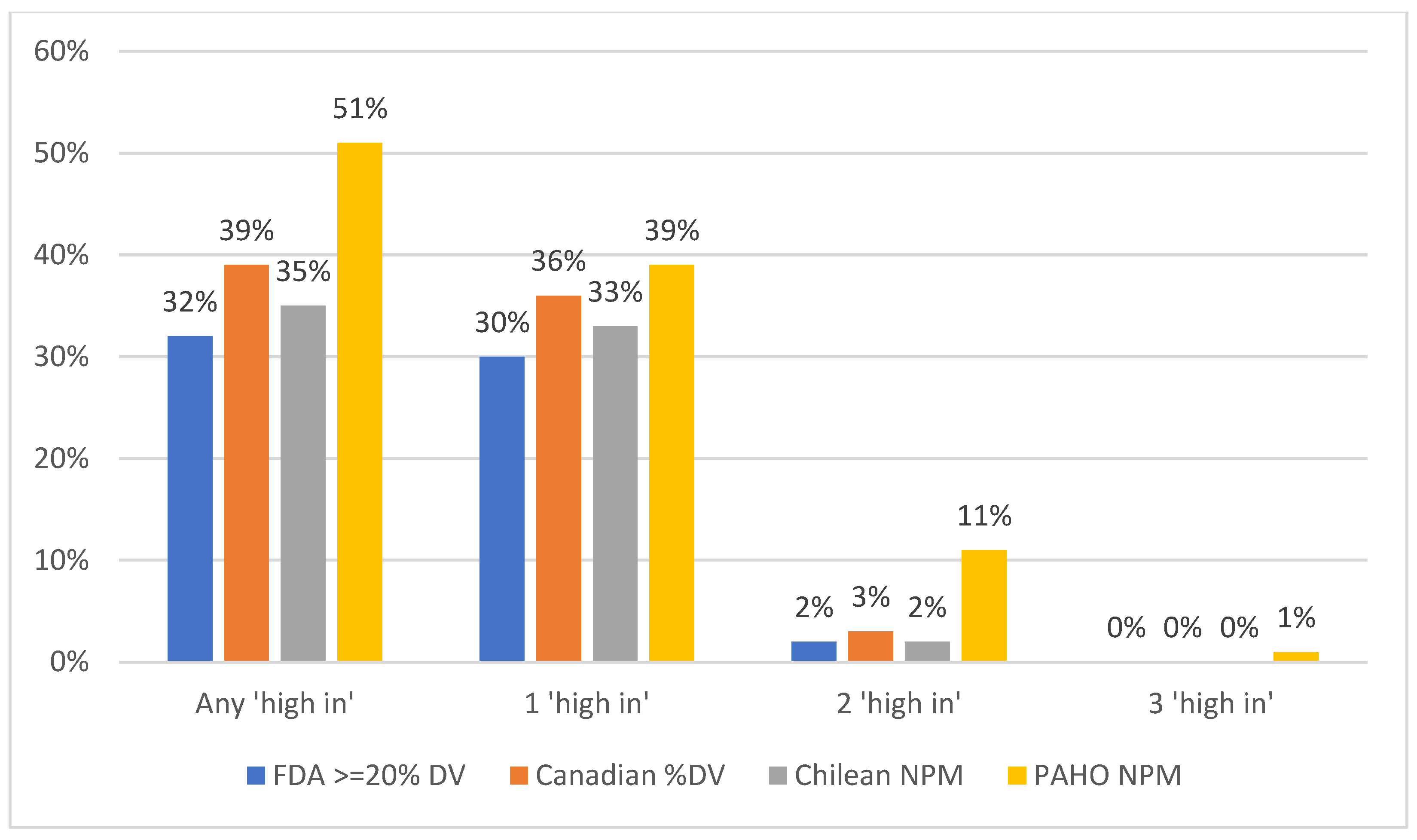
3.1. Proportion of Products That Met the “High-In” Criteria Under Each NPM, Overall
3.2. Proportion of Products That Met the “High-In” Criteria Under Each NPM, by Food Category
3.3. Proportion of Products That Met the “High-In” Criteria Under Each NPM, by Beverage Category
3.4. Proportion of Products That Met Each “High-In” Warning Labels’ Criteria Under the FDA’s Proposed NPM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martínez Steele, E.; Popkin, B.M.; Swinburn, B.; Monteiro, C.A. The share of ultra-processed foods and the overall nutritional quality of diets in the US: Evidence from a nationally representative cross-sectional study. Popul. Health Metr. 2017, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Crockett, R.A.; King, S.E.; Marteau, T.M.; Prevost, A.T.; Bignardi, G.; Roberts, N.W.; Stubbs, B.; Hollands, G.J.; Jebb, S.A. Nutritional labelling for healthier food or non-alcoholic drink purchasing and consumption. Cochrane Database Syst. Rev. 2018, 2, Cd009315. [Google Scholar] [CrossRef] [PubMed]
- The White House. Biden-Harris Administration National Strategy on Hunger, Nutrition, and Health. Available online: https://www.whitehouse.gov/wp-content/uploads/2022/09/White-House-National-Strategy-on-Hunger-Nutrition-and-Health-FINAL.pdf (accessed on 9 December 2024).
- Food and Drug Administration. Agency Information Collection Activities; Submission for Office of Management and Budget Review; Comment Request; Quantitative Research on Front of Package Labeling on Packaged Foods. Available online: https://www.federalregister.gov/documents/2023/06/15/2023-12820/agency-information-collection-activities-submission-for-office-of-management-and-budget-review#p-23 (accessed on 9 December 2024).
- Downs, S.M.; Thow, A.M.; Leeder, S.R. The effectiveness of policies for reducing dietary trans fat: A systematic review of the evidence. Bull. World Health Organ. 2013, 91, 262–269h. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Smith Taillie, L.; Popkin, B.; Kanter, R.; Vandevijvere, S.; Corvalán, C. Changes in the amount of nutrient of packaged foods and beverages after the initial implementation of the Chilean Law of Food Labelling and Advertising: A nonexperimental prospective study. PLoS Med. 2020, 17, e1003220. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Guidance for Industry: Food Labeling Guide. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-food-labeling-guide (accessed on 9 December 2024).
- US Food and Drug Administration. Nutrition Labeling and Education Act (NLEA) Requirements—Attachment 1. Available online: https://www.fda.gov/nutrition-labeling-and-education-act-nlea-requirements-attachment-1 (accessed on 9 December 2024).
- Pan American Health Organization. Pan American Health Organization Nutrient Profile Model. Available online: https://iris.paho.org/handle/10665.2/18621 (accessed on 27 February 2024).
- Instituto Brasileiro de Defesa do Consumidor. Understanding the Labels. Available online: https://alimentandopoliticas.org.br/wp-content/uploads/2022/10/Fact_sheet_Rotulagem_Ing.pdf (accessed on 9 December 2024).
- White, M.; Barquera, S. Mexico Adopts Food Warning Labels, Why Now? Health Syst. Reform 2020, 6, e1752063. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Front-Of-Package Nutrition Symbol Labelling Guide for Industry. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/guidance-documents/front-package-nutrition-symbol-labelling-industry.html (accessed on 27 February 2024).
- Mintel. Mintel Global New Products Database. Available online: https://www.mintel.com/products/gnpd/ (accessed on 9 December 2024).
- Corvalán, C.; Reyes, M.; Garmendia, M.L.; Uauy, R. Structural responses to the obesity and non-communicable diseases epidemic: The Chilean Law of Food Labeling and Advertising. Obes. Rev. 2013, 14 (Suppl. S2), 79–87. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.S.; Overwyk, K.; Bates, M.; Park, S.; Gillespie, C.; Cogswell, M.E. Temporal Trends in Dietary Sodium Intake Among Adults Aged ≥ 19 Years—United States, 2003–2016. Mmwr-Morbid. Mortal. Wkly. Rep. 2021, 70, 1478–1482. [Google Scholar] [CrossRef]
- Musicus, A.A.; Kraak, V.I.; Bleich, S.N. Policy Progress in Reducing Sodium in the American Diet, 2010–2019. Annu. Rev. Nutr. 2020, 40, 407–435. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.C.; Zhao, L.; Ahuja, J.K.C.; Gillespie, C.; Goldman, J.; Harris, D.M.; Jackson, S.L.; Moshfegh, A.; Rhodes, D.; Sebastian, R.S.; et al. Top Food Category Contributors to Sodium and Potassium Intake—United States, 2015–2016. Mmwr-Morb. Mortal. Wkly. Rep. 2020, 69, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Draft Guidance for Industry: Voluntary Sodium Reduction Goals, 2nd ed.; Food and Drug Administration: Rockville, MD, USA, 2024. [Google Scholar]
- Sebastian, R.S.; Hoy, M.K.; Goldman, J.D.; Moshfegh, A.J. Snack Consumption by U.S. Adults: What We Eat in America, NHANES 2017—March 2020. In FSRG Dietary Data Briefs; United States Department of Agriculture (USDA): Beltsville, MD, USA, 2010. [Google Scholar]
- Gearhardt, A.N.; DiFeliceantonio, A.G. Highly processed foods can be considered addictive substances based on established scientific criteria. Addiction 2023, 118, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Li, H. Which Front-of-Package Nutrition Label Is Better? The Influence of Front-of-Package Nutrition Label Type on Consumers’ Healthy Food Purchase Behavior. Nutrients 2023, 15, 2326. [Google Scholar] [CrossRef]
- Ganderats-Fuentes, M.; Morgan, S. Front-of-Package Nutrition Labeling and Its Impact on Food Industry Practices: A Systematic Review of the Evidence. Nutrients 2023, 15, 2630. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Garcia, L.; Meza-Hernández, M.; Diez-Canseco, F.; Taillie, L.S. Reformulation of Top-Selling Processed and Ultra-Processed Foods and Beverages in the Peruvian Food Supply after Front-of-Package Warning Label Policy. Int. J. Environ. Res. Public Health 2022, 20, 424. [Google Scholar] [CrossRef] [PubMed]
- Quintiliano Scarpelli, D.; Pinheiro Fernandes, A.C.; Rodriguez Osiac, L.; Pizarro Quevedo, T. Changes in Nutrient Declaration after the Food Labeling and Advertising Law in Chile: A Longitudinal Approach. Nutrients 2020, 12, 2371. [Google Scholar] [CrossRef]
- Lauber, K.; Rutter, H.; Gilmore, A.B. Big food and the World Health Organization: A qualitative study of industry attempts to influence global-level non-communicable disease policy. BMJ Glob. Health 2021, 6, e005216. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Tobias, A.; Crosbie, E.; Mialon, M.; Carriedo, A.; Schmidt, L.A. Food and beverage industry interference in science and policy: Efforts to block soda tax implementation in Mexico and prevent international diffusion. BMJ Glob. Health 2021, 6, e005662. [Google Scholar] [CrossRef]
- Barquera, S.; Rivera, J.A. Obesity in Mexico: Rapid epidemiological transition and food industry interference in health policies. Lancet Diabetes Endocrinol. 2020, 8, 746–747. [Google Scholar] [CrossRef]
- Pettigrew, S.; Coyle, D.; McKenzie, B.; Vu, D.; Lim, S.C.; Berasi, K.; Poowanasatien, A.; Suya, I.; Kowal, P. A review of front-of-pack nutrition labelling in Southeast Asia: Industry interference, lessons learned, and future directions. Lancet Reg. Health Southeast Asia 2022, 3, 100017. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Miles, D.R.; Taillie, L.S.; Dunford, E.K. A policy approach to identifying food and beverage products that are ultra-processed and high in added salt, sugar and saturated fat in the United States: A cross-sectional analysis of packaged foods. Lancet Reg. Health Am. 2024, 32, 100713. [Google Scholar] [CrossRef] [PubMed]
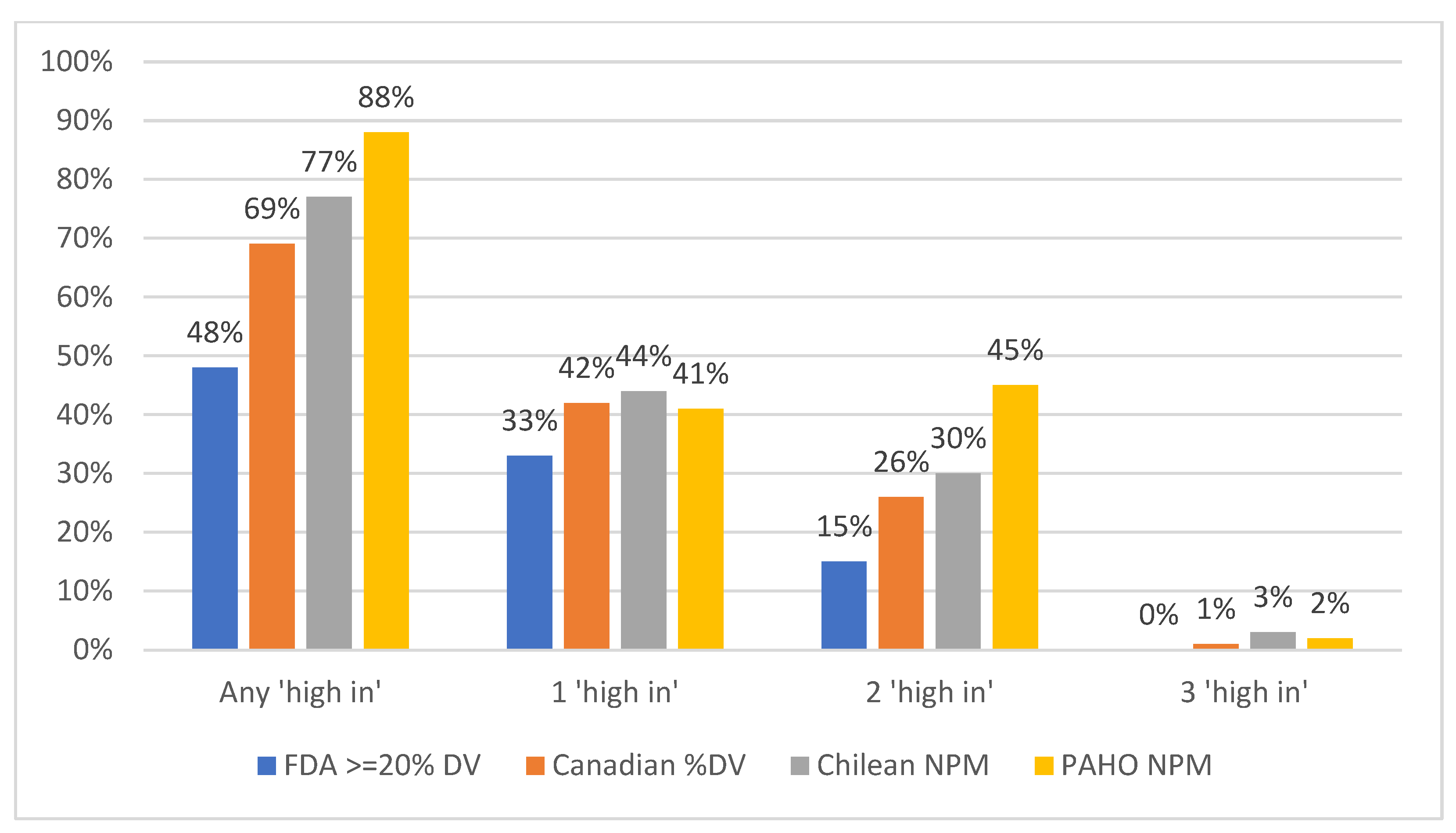
| Food Group | n | FDA ≥ 20% DV | Canadian %DV | Chilean NPM | PAHO NPM | % Agreement Between All 4 NPMs | Kappa Canadian %DV | Kappa Chilean NPM | Kappa PAHO NPM |
|---|---|---|---|---|---|---|---|---|---|
| All food products | 47,503 | 48 | 69 | 77 | 88 | 51 | 0.56 | 0.22 | 0.20 |
| Bars | 1506 | 39 | 70 | 90 | 88 | 45 | 0.43 | 0.10 | 0.14 |
| Breakfast cereals | 1377 | 56 | 77 | 91 | 91 | 61 | 0.55 | 0.18 | 0.21 |
| Hot cereals | 289 | 42 | 62 | 72 | 80 | 52 | 0.61 | 0.18 | 0.30 |
| Bread products | 1493 | 10 | 25 | 78 | 95 | 14 | 0.49 | 0.05 | 0.01 |
| Cheese, butter, and other dairy | 3312 | 72 | 92 | 85 | 96 | 63 | 0.35 | 0.09 | 0.02 |
| Desserts | 6025 | 74 | 92 | 96 | 97 | 76 | 0.41 | 0.19 | 0.14 |
| Main dish—frozen | 1589 | 92 | 85 | 56 | 99 | 55 | 0.32 | 0.13 | 0.20 |
| Main dish—non-frozen | 1582 | 71 | 79 | 55 | 97 | 50 | 0.55 | 0.38 | 0.08 |
| Fruit and vegetables | 1863 | 13 | 24 | 13 | 63 | 45 | 0.66 | 0.54 | 0.16 |
| Proteins | 4449 | 56 | 79 | 72 | 92 | 55 | 0.50 | 0.40 | 0.12 |
| Side dishes | 2267 | 29 | 42 | 29 | 52 | 68 | 0.72 | 0.61 | 0.52 |
| Salty snacks | 7260 | 22 | 59 | 89 | 84 | 29 | 0.33 | 0.05 | 0.10 |
| Sweet snacks | 5571 | 77 | 87 | 89 | 89 | 86 | 0.65 | 0.53 | 0.54 |
| Sauces, dips, and seasonings | 6313 | 18 | 49 | 81 | 89 | 20 | 0.38 | 0.02 | 0.03 |
| Sweet spreads and syrups | 1173 | 31 | 71 | 86 | 83 | 39 | 0.31 | 0.10 | 0.17 |
| Soup | 640 | 79 | 86 | 33 | 96 | 32 | 0.74 | 0.09 | 0.24 |
| Yogurt | 794 | 53 | 66 | 32 | 74 | 49 | 0.74 | 0.52 | 0.32 |
| Beverage Group | n | FDA ≥ 20% DV | Canadian %DV | Chilean NPM | PAHO NPM | % Agreement Between All 4 NPMs | Kappa Canadian %DV | Kappa Chilean NPM | Kappa PAHO NPM |
|---|---|---|---|---|---|---|---|---|---|
| All beverage products | 4306 | 32 | 39 | 35 | 51 | 72 | 0.84 | 0.68 | 0.59 |
| Dairy drinks and flavored milks | 511 | 35 | 46 | 41 | 83 | 36 | 0.77 | 0.43 | 0.06 |
| Other dairy liquid | 381 | 9 | 58 | 69 | 71 | 18 | 0.13 | 0.09 | 0.08 |
| Fermented drinks | 83 | 39 | 48 | 8 | 65 | 40 | 0.81 | 0.08 | 0.50 |
| Flavored water | 681 | 1 | 1 | 0 | 6 | 94 | 0.93 | 0.24 | |
| Juice and juice drinks | 915 | 35 | 37 | 34 | 43 | 88 | 0.96 | 0.87 | 0.83 |
| Ready-to-drink coffee and tea | 509 | 51 | 55 | 41 | 65 | 74 | 0.93 | 0.69 | 0.73 |
| Sodas—regular | 481 | 84 | 87 | 83 | 93 | 87 | 0.91 | 0.77 | 0.56 |
| Sodas—diet | 244 | 0 | 0 | 0 | 18 | 82 | |||
| Sports and energy drinks | 412 | 33 | 38 | 23 | 50 | 69 | 0.89 | 0.75 | 0.61 |
| Water | 89 | 0 | 0 | 0 | 0 | 100 |
| Food Group | n | FDA ≥ 20% DV Added Sugar | FDA ≥ 20% DV Sodium | FDA ≥ 20% DV Saturated Fat |
|---|---|---|---|---|
| All food products | 47,503 | 22 | 15 | 27 |
| Bars | 1506 | 19 | 0 | 24 |
| Breakfast cereals | 1377 | 48 | 0 | 10 |
| Hot cereals | 289 | 35 | 2 | 7 |
| Bread products | 1493 | 1 | 6 | 4 |
| Cheese, butter, and other dairy | 3312 | 0 | 2 | 71 |
| Desserts | 6025 | 62 | 1 | 49 |
| Main dish—frozen | 1589 | 5 | 85 | 65 |
| Main dish—non-frozen | 1582 | 6 | 65 | 32 |
| Fruit and vegetables | 1863 | 5 | 8 | 2 |
| Proteins | 4449 | 2 | 45 | 25 |
| Side dishes | 2267 | 0 | 24 | 9 |
| Salty snacks | 7260 | 6 | 7 | 14 |
| Sweet snacks | 5571 | 67 | 0 | 42 |
| Sauces, dips, and seasonings | 6313 | 4 | 12 | 4 |
| Sweet spreads and syrups | 1173 | 24 | 0 | 9 |
| Soup | 640 | 2 | 77 | 20 |
| Yogurt | 794 | 37 | 0 | 28 |
| Beverage Group * | n | FDA ≥ 20% DV Added Sugar | FDA ≥ 20% DV Sodium | FDA ≥ 20% DV Saturated Fat |
|---|---|---|---|---|
| All beverage products | 4306 | 30 | 1 | 3 |
| Dairy drinks and flavored milks | 511 | 25 | 0 | 14 |
| Other dairy liquid | 381 | 7 | 0 | 7 |
| Fermented drinks | 83 | 37 | 0 | 1 |
| Flavored water | 681 | 1 | 0 | 0 |
| Juice and juice drinks | 915 | 34 | 2 | 0 |
| Ready-to-drink coffee and tea | 509 | 50 | 0 | 4 |
| Sodas—regular | 481 | 84 | 1 | 0 |
| Sodas—diet | 244 | 0 | 0 | 0 |
| Sports and energy drinks | 412 | 30 | 5 | 0 |
| Water | 89 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunford, E.K.; Miles, D.R.; Hollingsworth, B.A.; Heller, S.; Popkin, B.M.; Ng, S.W.; Taillie, L.S. Defining “High-In” Saturated Fat, Sugar, and Sodium to Help Inform Front-of-Pack Labeling Efforts for Packaged Foods and Beverages in the United States. Nutrients 2024, 16, 4345. https://doi.org/10.3390/nu16244345
Dunford EK, Miles DR, Hollingsworth BA, Heller S, Popkin BM, Ng SW, Taillie LS. Defining “High-In” Saturated Fat, Sugar, and Sodium to Help Inform Front-of-Pack Labeling Efforts for Packaged Foods and Beverages in the United States. Nutrients. 2024; 16(24):4345. https://doi.org/10.3390/nu16244345
Chicago/Turabian StyleDunford, Elizabeth K., Donna R. Miles, Bridget A. Hollingsworth, Samantha Heller, Barry M. Popkin, Shu Wen Ng, and Lindsey Smith Taillie. 2024. "Defining “High-In” Saturated Fat, Sugar, and Sodium to Help Inform Front-of-Pack Labeling Efforts for Packaged Foods and Beverages in the United States" Nutrients 16, no. 24: 4345. https://doi.org/10.3390/nu16244345
APA StyleDunford, E. K., Miles, D. R., Hollingsworth, B. A., Heller, S., Popkin, B. M., Ng, S. W., & Taillie, L. S. (2024). Defining “High-In” Saturated Fat, Sugar, and Sodium to Help Inform Front-of-Pack Labeling Efforts for Packaged Foods and Beverages in the United States. Nutrients, 16(24), 4345. https://doi.org/10.3390/nu16244345






