Limosilactobacillus fermentum MG5368 and Lactiplantibacillus plantarum MG989 Regulates Skin Health in UVB-Induced HaCaT Cells and Hairless Mice Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Samples
2.2. In Vitro Test
2.2.1. Cell Culture
2.2.2. Ultraviolet B (UVB) Exposure and Cell Viability
2.3. In Vivo Test
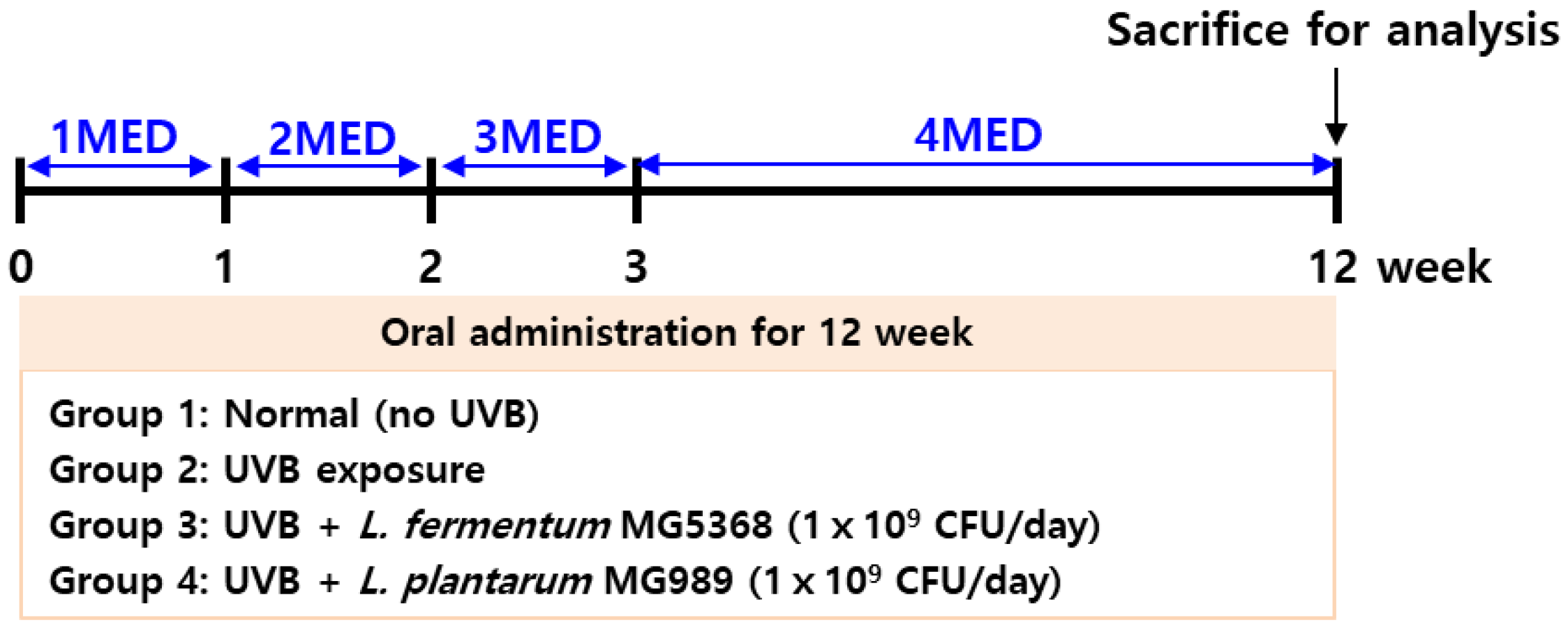
2.3.1. Animal Experiment and UVB Exposure
2.3.2. Evaluation of Skin Wrinkle and Skin Elasticity
2.3.3. Analysis of Histopathology and Collagen Content
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
3.1. Cell Protective Effects of CFS from Bacterial Strains in UVB-Exposed HaCaT Keratinocytes Including MMPs, HA, and Collagen Production
3.2. CFS of L. fermentum MG5368 and L. plantarum MG989 Regulates the AP-1/Smad Signaling Pathway in UVB-Exposed HaCaT Keratinocytes
3.3. Oral Administration of L. fermentum MG5368 and L. plantarum MG989 Decreases Wrinkle and Elasticity in the Dorsal Skin of the UVB-Exposed SKH-1 Hairless Mice
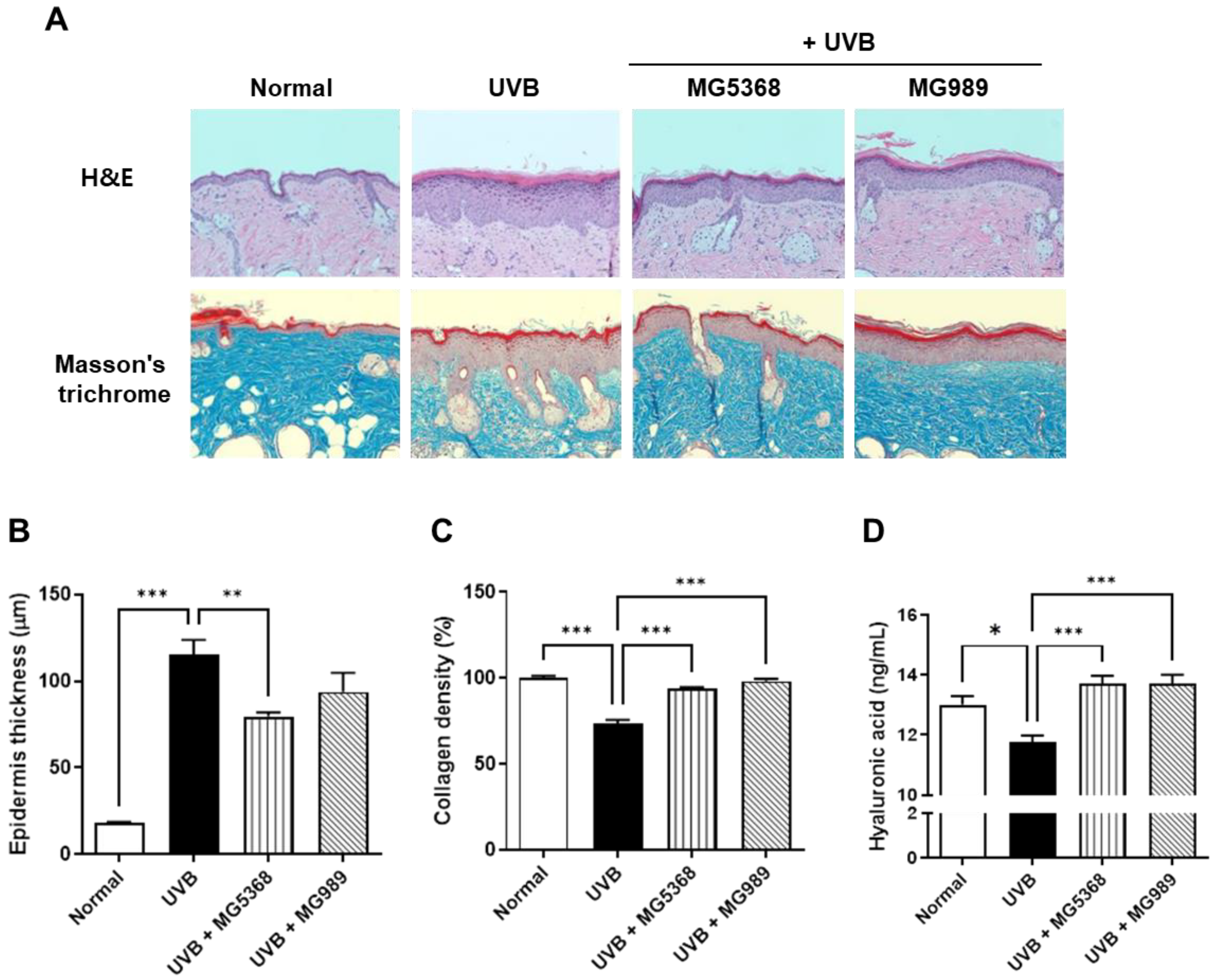
3.4. Oral Administration of L. fermentum MG5368 and L. plantarum MG989 Reduces Epidermis Thickness, Collagen, and HA in the Dorsal Skin of the UVB-Exposed SKH-1 Hairless Mice
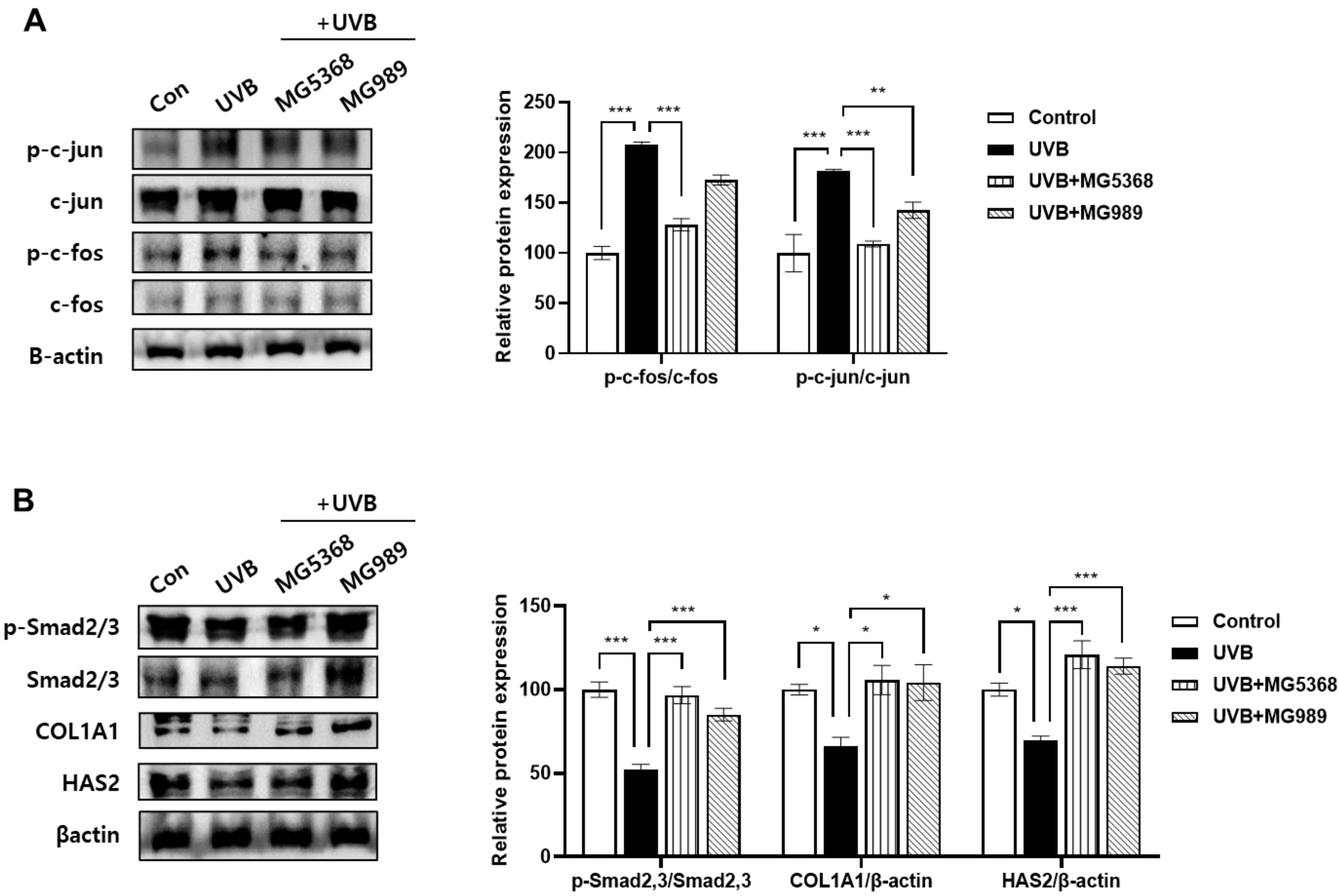
3.5. Oral Administration of L. fermentum MG5368 and L. plantarum MG989 Regulated AP-1/Smad Signaling Pathway in the Dorsal Skin of the UVB-Exposed SKH-1 Hairless Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.Y.; Park, J.-Y.; Kim, Y.; Kang, C.-H. Protective effect of Bifidobacterium animalis subs. lactis MG741 as probiotics against UVB-exposed fibroblasts and hairless mice. Microorganisms 2022, 10, 2343. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kang, C.-H. Latilactobacillus sakei Wikim0066 protects skin through MMP regulation on UVB-irradiated in vitro and in vivo model. Nutrients 2023, 15, 726. [Google Scholar] [CrossRef]
- Ichihashi, M.; Ando, H.; Yoshida, M.; Niki, Y.; Matsui, M. Photoaging of the skin. Anti-Aging Med. 2009, 6, 46–59. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Han, H.-S.; Shin, J.-S.; Myung, D.-B.; Ahn, H.S.; Lee, S.H.; Kim, H.J.; Lee, K.-T. Hydrangea serrata (Thunb.) Ser. extract attenuate UVB-induced photoaging through MAPK/AP-1 inactivation in human skin fibroblasts and hairless mice. Nutrients 2019, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Lee, J.Y.; Kim, Y.; Kang, C.-H. Lactic acid bacteria improve the photoprotective effect via MAPK/AP-1/MMP signaling pathway on skin fibroblasts. Microorganisms 2022, 10, 2481. [Google Scholar] [CrossRef]
- Yu, Y.; Dunaway, S.; Champer, J.; Kim, J.; Alikhan, A. Changing our microbiome: Probiotics in dermatology. Br. J. Dermatol. 2020, 182, 39–46. [Google Scholar] [CrossRef]
- Jwo, J.Y.; Chang, Y.T.; Huang, Y.C. Effects of probiotics supplementation on skin photoaging and skin barrier function: A systematic review and meta-analysis. Photodermatol. Photoimmunol. Photomed. 2023, 39, 122–131. [Google Scholar] [CrossRef]
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A systematic review. J. Neuroinflammation 2019, 16, 231. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Kim, J.-W.; Kim, S.-I.; Kim, S.; Nguyen, T.H.; Kang, C.-H. Antioxidant and anti-inflammatory effect and probiotic properties of lactic acid bacteria isolated from canine and feline feces. Microorganisms 2021, 9, 1971. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.J.; Navarro, C.; Durán, P.; Galan-Freyle, N.J.; Parra Hernández, L.A.; Pacheco-Londoño, L.C.; Castelanich, D.; Bermúdez, V.; Chacin, M. Antioxidants in Photoaging: From Molecular Insights to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 2403. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant activity and probiotic properties of lactic acid bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, Y.; Jeong, Y.; Kang, H. Anti-Inflammatory Response in TNFα/IFNγ-Induced HaCaT Keratinocytes and Probiotic Properties of Lacticaseibacillus rhamnosus MG4644, Lacticaseibacillus paracasei MG4693, and Lactococcus lactis MG5474. J. Microbiol. Biotechnol. 2023, 33, 1039. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Z.; Pu, C.; Kong, C.; Chen, S.; Lu, W.; Zhang, J. The structural characterization and UV-protective properties of an exopolysaccharide from a Paenibacillus isolate. Front. Pharmacol. 2024, 15, 1434136. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.; Kim, J.-I.; Lee, H.-Y.; Moon, G.-S.; Kang, C.-H. Improvements in human keratinocytes and antimicrobial effect mediated by cell-free supernatants derived from probiotics. Fermentation 2022, 8, 332. [Google Scholar] [CrossRef]
- Kim, J.-O.; An, G.; Choi, J.-H. Protective effect of mixture of Acanthopanax sessiliflorum and Chaenomeles sinensis against ultraviolet B-induced photodamage in human fibroblast and hairless mouse. Food Sci. Biotechnol. 2024, 33, 1715–1725. [Google Scholar] [CrossRef]
- Carlos da Silva, G.; Barbosa, M.B.; Júnior, F.B.C.; Moreira, P.L.; Werka, R.; Martin, A.A. Detection of skin wrinkles and quantification of roughness using a novel image processing technique from a dermatoscope device. Ski. Res. Technol. 2023, 29, e13335. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.O.; Kim, S.Y.; Lee, J.M.; Lee, E.; Na, J.; Yoo, K.H.; Park, S.J.; Kim, B.J. Effect of A. polygama APEE (Actinidia polygama ethanol extract) or APWE (Actinidia polygama water extract) on wrinkle formation in UVB-irradiated hairless mice. J. Cosmet. Dermatol. 2023, 22, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Arnal-Forné, M.; Molina-García, T.; Ortega, M.; Marcos-Garcés, V.; Molina, P.; Ferrández-Izquierdo, A.; Sepulveda, P.; Bodí, V.; Ríos-Navarro, C.; Ruiz-Saurí, A. Changes in human skin composition due to intrinsic aging: A histologic and morphometric study. Histochem. Cell Biol. 2024, 162, 259–271. [Google Scholar] [CrossRef]
- Lolou, V.; Panayiotidis, M.I. Functional role of probiotics and prebiotics on skin health and disease. Fermentation 2019, 5, 41. [Google Scholar] [CrossRef]
- Ra, J.; Lee, D.E.; Kim, S.H.; Jeong, J.-W.; Ku, H.K.; Kim, T.-Y.; Choi, I.-D.; Jeung, W.; Sim, J.-H.; Ahn, Y.-T. Effect of oral administration of Lactobacillus plantarum HY7714 on epidermal hydration in ultraviolet B-irradiated hairless mice. J. Microbiol. Biotechnol. 2014, 24, 1736–1743. [Google Scholar] [CrossRef]
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.-H. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Kim, J.-S.; Park, H.M.; Kim, S.; Paek, N.-S. Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 Biotech 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Sparavigna, A. Role of the extracellular matrix in skin aging and dedicated treatment-state of the art. Plast. Aesthet. Res 2020, 7, 14. [Google Scholar] [CrossRef]
- Hachiya, A.; Sriwiriyanont, P.; Fujimura, T.; Ohuchi, A.; Kitahara, T.; Takema, Y.; Kitzmiller, W.J.; Visscher, M.O.; Tsuboi, R.; Boissy, R.E. Mechanistic effects of long-term ultraviolet B irradiation induce epidermal and dermal changes in human skin xenografts. Am. J. Pathol. 2009, 174, 401–413. [Google Scholar] [CrossRef]
- Yuksel Egrilmez, M.; Kocturk, S.; Aktan, S.; Oktay, G.; Resmi, H.; Simsek Keskin, H.; Guner Akdogan, G.; Ozkan, S. Melatonin prevents UVB-induced skin photoaging by inhibiting oxidative damage and MMP expression through JNK/AP-1 signaling pathway in human dermal fibroblasts. Life 2022, 12, 950. [Google Scholar] [CrossRef]
- Kim, J.-M.; Chung, K.-S.; Yoon, Y.-S.; Jang, S.-Y.; Heo, S.-W.; Park, G.; Jang, Y.-P.; Ahn, H.-S.; Shin, Y.-K.; Lee, S.-H. Dieckol isolated from Eisenia bicyclis ameliorates wrinkling and improves skin hydration via MAPK/AP-1 and TGF-β/Smad signaling pathways in UVB-irradiated hairless mice. Mar. Drugs 2022, 20, 779. [Google Scholar] [CrossRef]
- Kim, M.-J.; Woo, S.W.; Kim, M.-S.; Park, J.-E.; Hwang, J.-K. Anti-photoaging effect of aaptamine in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes. J. Asian Nat. Prod. Res. 2014, 16, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Noh, E.-M.; Lee, G.; Lim, C.-H.; Kwon, K.B.; Kim, J.-M.; Song, H.-K.; Yang, H.J.; Kim, M.J.; Kim, M.-s.; Lee, Y.-R. Protective effects of Evodiae Fructus extract against ultraviolet-induced MMP-1 and MMP-3 expression in human dermal fibroblasts. J. Herb. Med. 2022, 35, 100586. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Lee, J.-J.; Ng, S.-C.; Ni, Y.-T.; Liu, J.-S.; Chen, C.-J.; Padma, V.V.; Huang, C.-Y.; Kuo, W.-W. Protective effects of galangin against H2O2/UVB-induced dermal fibroblast collagen degradation via hsa-microRNA-4535-mediated TGFβ/Smad signaling. Aging 2021, 13, 25342. [Google Scholar] [CrossRef]
- Devos, H.; Zoidakis, J.; Roubelakis, M.G.; Latosinska, A.; Vlahou, A. Reviewing the regulators of COL1A1. Int. J. Mol. Sci. 2023, 24, 10004. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef]
- Abe, M.; Masuda, M.; Mizukami, Y.; Inoue, S.; Mizutani, Y. Epidermal keratinocytes regulate hyaluronan metabolism via extracellularly secreted hyaluronidase 1 and hyaluronan synthase 3. J. Biol. Chem. 2024, 300, 107449. [Google Scholar] [CrossRef]



| Spp. | Strain | Origin | NCBI Accession No. |
|---|---|---|---|
| Limosilactobacillus fermentum | MG5368 | Food | ON631268.1 |
| MG4294 | Human | MW404502.1 | |
| MG5159 | Food | MN435579.1 | |
| MG4538 | Human | MN368558.1 | |
| MG5091 | Food | OP102518.1 | |
| Lactiplantibacillus plantarum | MG989 | Human | MN061270.1 |
| MG5530 | Food | ON668221.1 | |
| MG5023 | Food | OP102478.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Lee, J.Y.; Hong, S.; Heo, H.; Lee, H.; Kim, Y.G.; Kim, B.-K.; Choi, S.-I.; Lee, J. Limosilactobacillus fermentum MG5368 and Lactiplantibacillus plantarum MG989 Regulates Skin Health in UVB-Induced HaCaT Cells and Hairless Mice Model. Nutrients 2024, 16, 4083. https://doi.org/10.3390/nu16234083
Park J-Y, Lee JY, Hong S, Heo H, Lee H, Kim YG, Kim B-K, Choi S-I, Lee J. Limosilactobacillus fermentum MG5368 and Lactiplantibacillus plantarum MG989 Regulates Skin Health in UVB-Induced HaCaT Cells and Hairless Mice Model. Nutrients. 2024; 16(23):4083. https://doi.org/10.3390/nu16234083
Chicago/Turabian StylePark, Jeong-Yong, Ji Yeon Lee, Seonghwa Hong, Huijin Heo, Hana Lee, Yong Gyeong Kim, Byoung-Kook Kim, Soo-Im Choi, and Junsoo Lee. 2024. "Limosilactobacillus fermentum MG5368 and Lactiplantibacillus plantarum MG989 Regulates Skin Health in UVB-Induced HaCaT Cells and Hairless Mice Model" Nutrients 16, no. 23: 4083. https://doi.org/10.3390/nu16234083
APA StylePark, J.-Y., Lee, J. Y., Hong, S., Heo, H., Lee, H., Kim, Y. G., Kim, B.-K., Choi, S.-I., & Lee, J. (2024). Limosilactobacillus fermentum MG5368 and Lactiplantibacillus plantarum MG989 Regulates Skin Health in UVB-Induced HaCaT Cells and Hairless Mice Model. Nutrients, 16(23), 4083. https://doi.org/10.3390/nu16234083






