Anti-Obesity Effects of a Collagen with Low Digestibility and High Swelling Capacity: A Human Randomized Control Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Technological Treatment of Collagen
2.2. In Vitro Studies
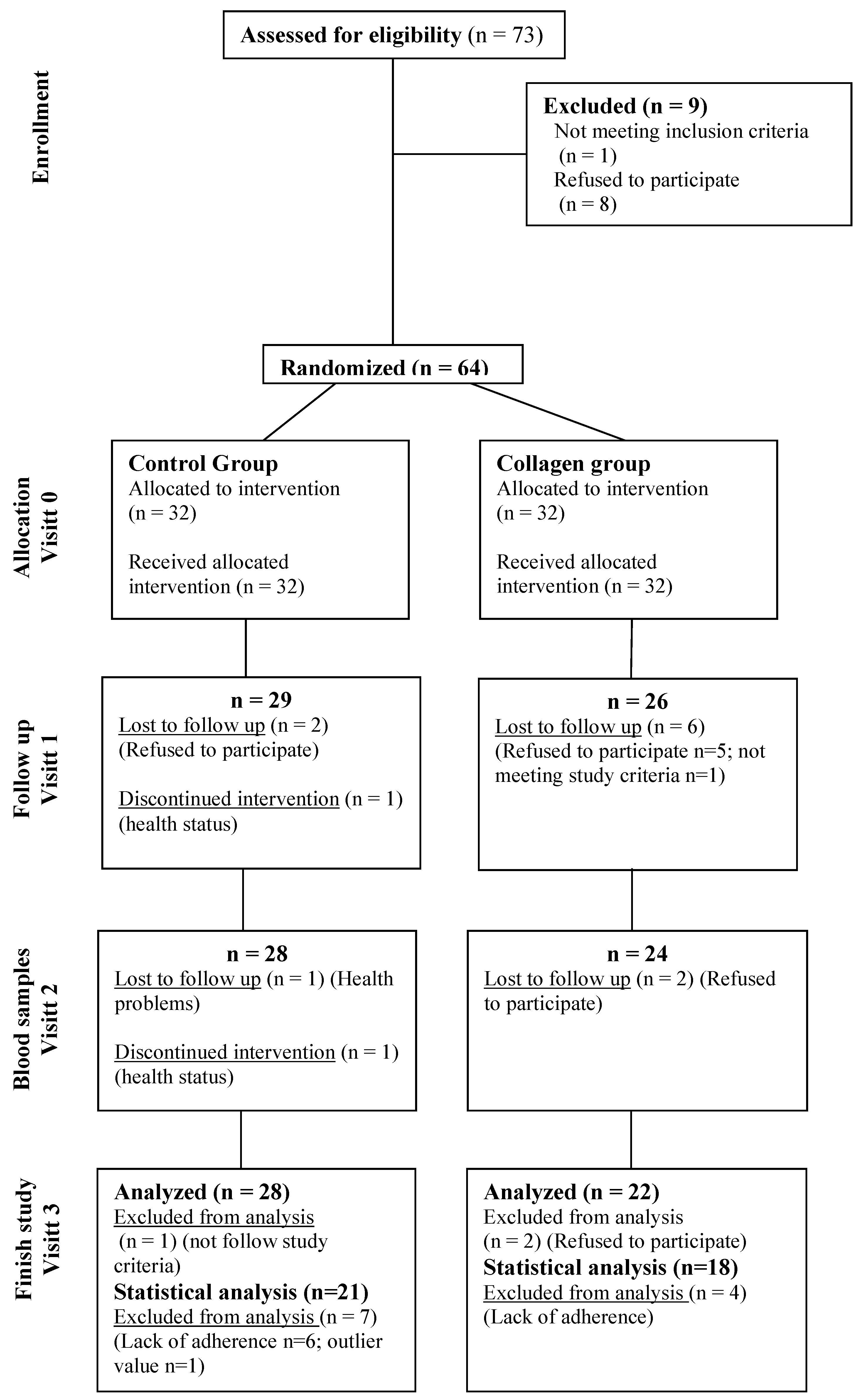
2.3. Human Intervention Trial: Subjects and Design
- Control group: healthy dietary recommendations based on the “Guía de la alimentación saludable para la atención primaria y colectivos de ciudadanos de la Sociedad Española de Nutrición Comunitaria” [11].
- Collagen group: the same healthy dietary recommendations plus 2 protein bars/day.
2.4. Anthropometric, Body Composition and Biochemical Analysis
2.5. Postprandial Blood Levels of Ghrelin in Rats
2.6. Statistical Analyses
3. Results
3.1. In Vitro Studies
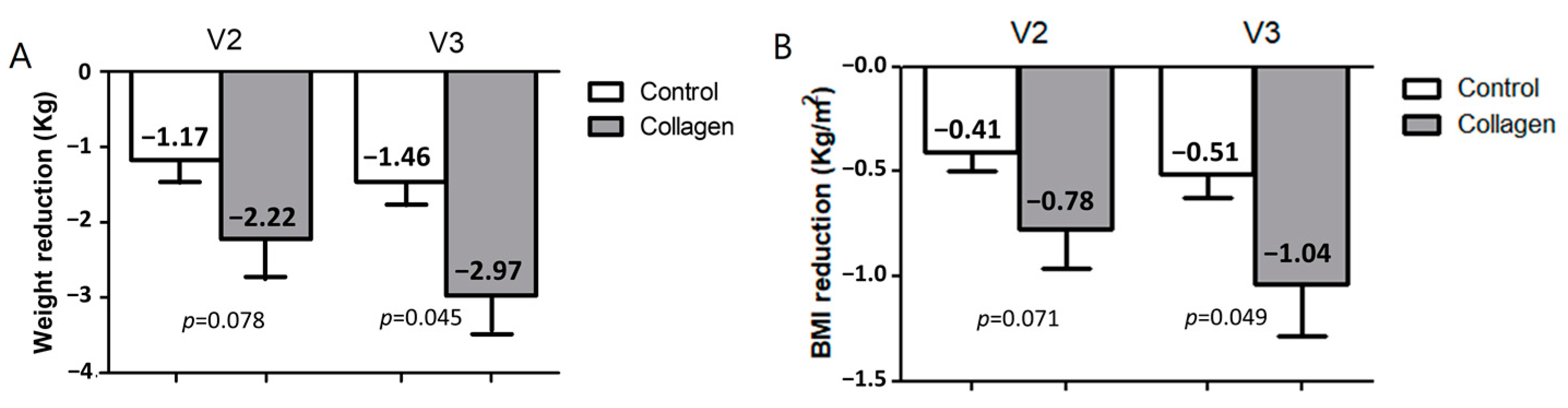
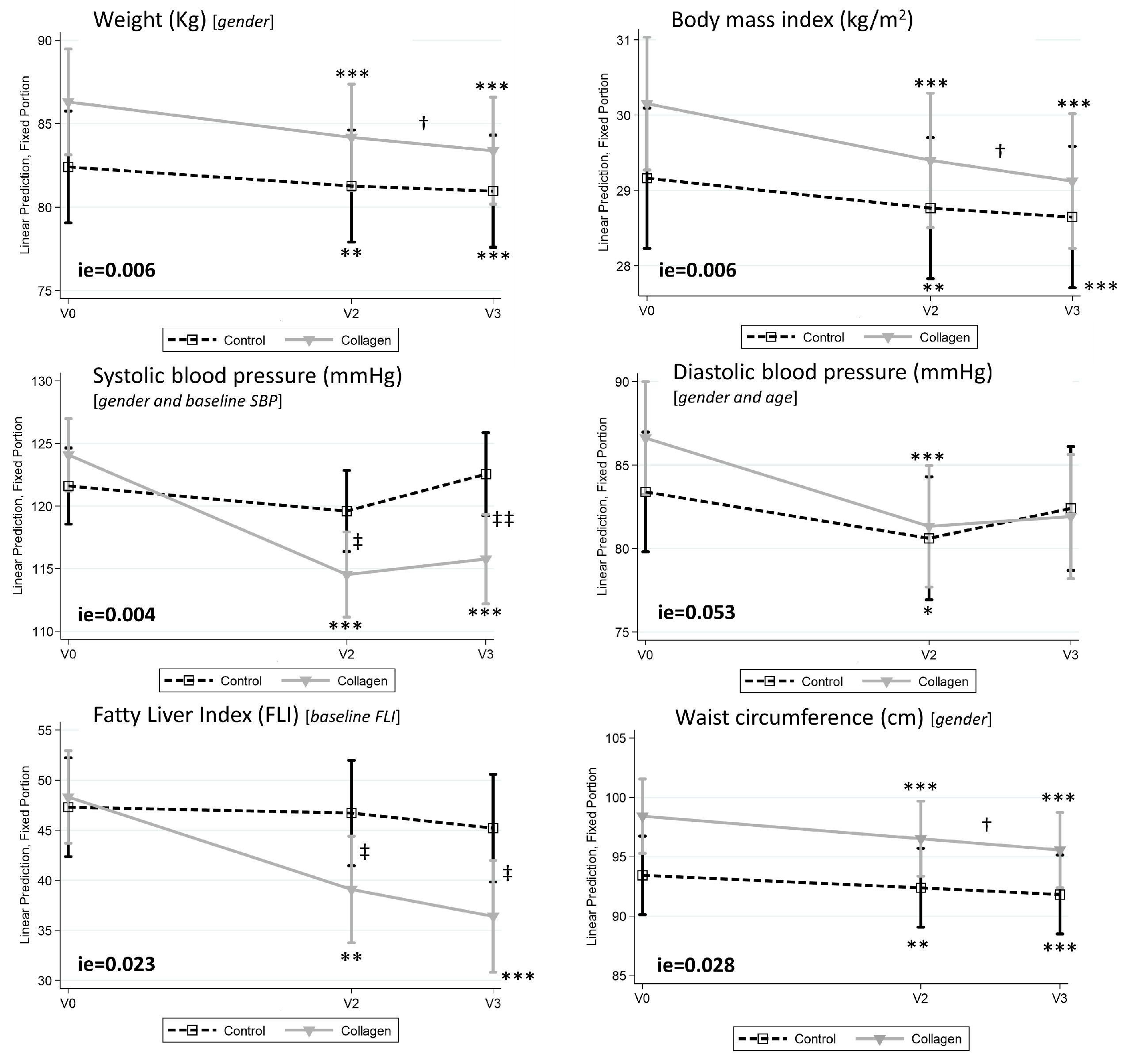
3.2. Collagen Reduces Body Weight, Body Mass Index, and Fatty Liver Index in Humans with Overweight/Obesity
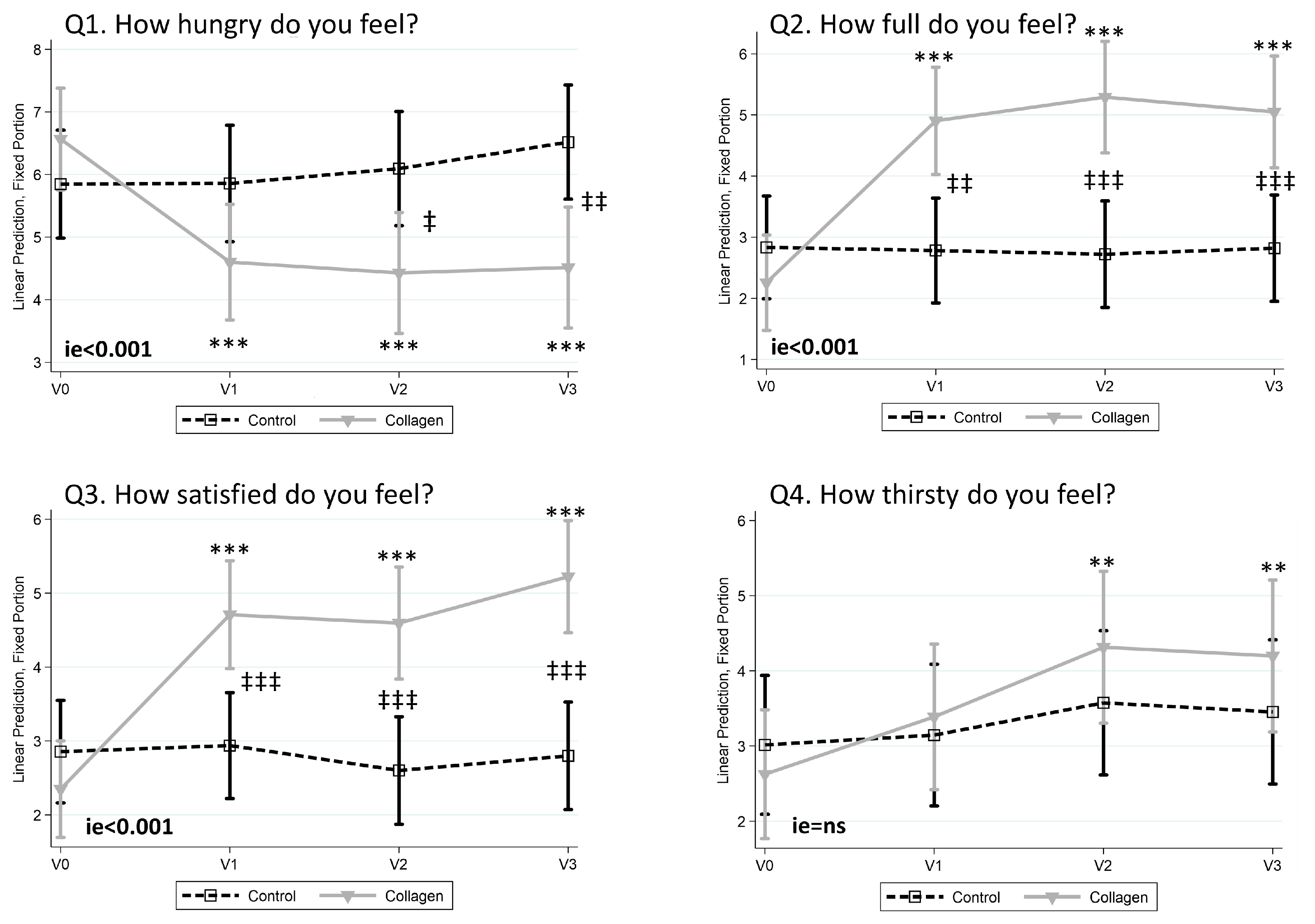
3.3. Collagen Reduces the Sensation of Hunger and Increases Fullness and Satisfaction
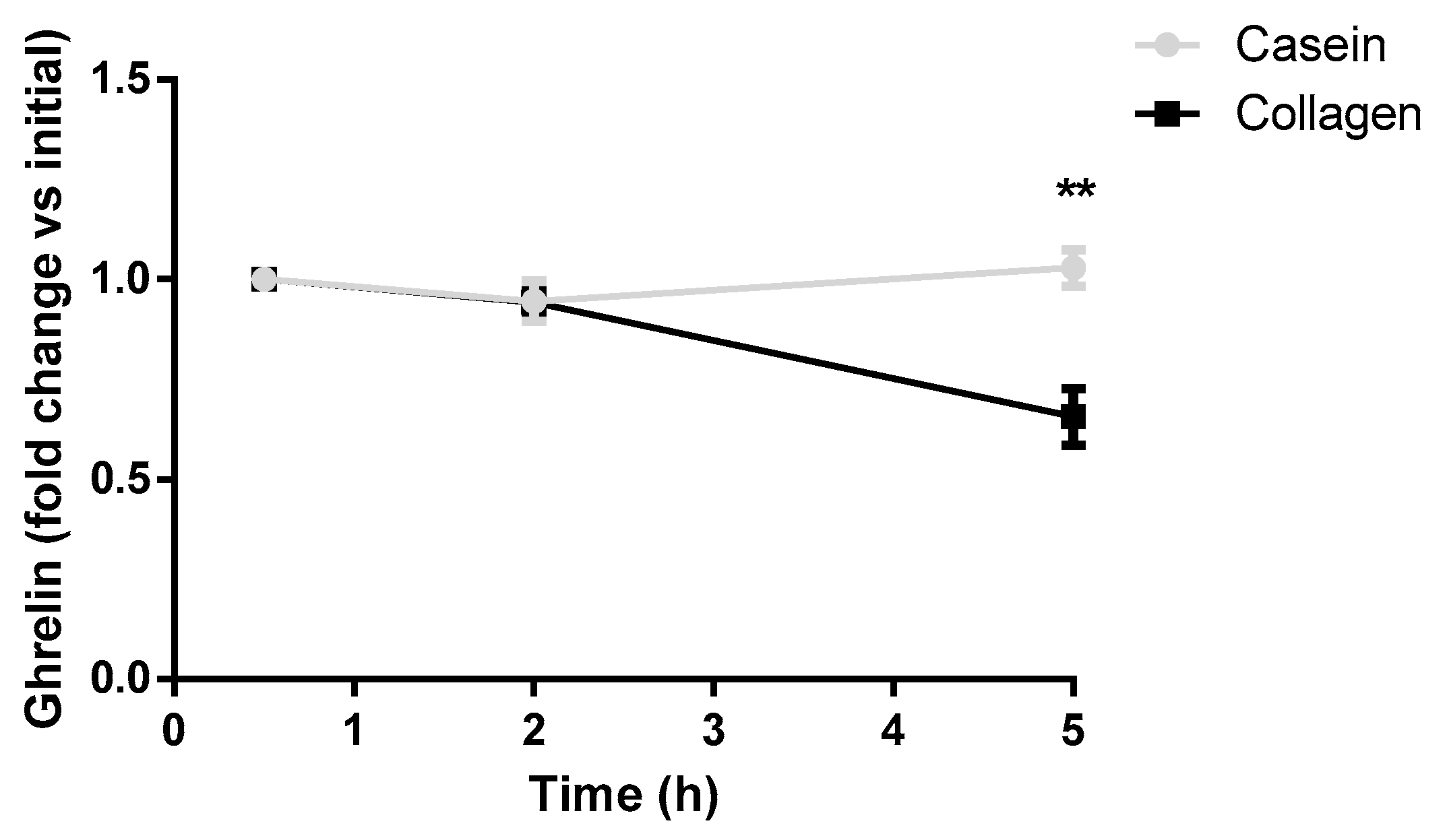
3.4. Collagen Decreases Postprandial Blood Ghrelin Levels in Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| CCK | cholecystokinin |
| DEXA | dual-energy X-ray absorptiometry |
| FFQ | food frequency questionnaire |
| FLI | fatty liver index |
| GLP-1 | glucagon-like peptide-1 |
| GGT | gamma-glutamyltransferase |
| ie | interaction effect |
| Mets | metabolic equivalents |
| Psi | initial collagen dry weight |
| Pcol | collagen sample weight |
| Psf | final solid dry weight |
| Ps | dry weight |
| Pp | plate weight |
| Pf | filter weight |
| PYY | peptide YY |
| QSA | questionnaire of subjective appetite |
| REE | resting energy expenditure |
| SBP | systolic blood pressure |
| SUN | Seguimiento Universidad de Navarra |
| SW | swelling |
| TG | triglycerides |
| TyG | Triglyceride–Glucose |
| VAS | visual analog scale |
References
- The GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13. [Google Scholar] [CrossRef] [PubMed]
- Boix-Castejón, M.; Roche, E.; Olivares-Vicente, M.; Álvarez-Martínez, F.J.; Herranz-López, M.; Micol, V. Plant Compounds for Obesity Treatment through Neuroendocrine Regulation of Hunger: A Systematic Review. Phytomedicine 2023, 113, 154735. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.D.; de Almeida Santos Junior, V.; Pimentel, J.D.; Carregã, G.L.F.; Cazarin, C.B.B. Collagen Supplementation in Skin and Orthopedic Diseases: A Review of the Literature. Heliyon 2023, 9, e14961. [Google Scholar] [CrossRef] [PubMed]
- Kviatkovsky, S.A.; Hickner, R.C.; Ormsbee, M.J. Collagen Peptide Supplementation for Pain and Function: Is It Effective? Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.T.; Andersen, S.V.; Astrup, A.; Blundell, J.; Sjödin, A. Is Reducing Appetite Beneficial for Body Weight Management in the Context of Overweight and Obesity? A Systematic Review and Meta-Analysis from Clinical Trials Assessing Body Weight Management after Exposure to Satiety Enhancing and/or Hunger Reducing Products. Obes. Rev. 2019, 20, 983–997. [Google Scholar] [CrossRef]
- Said, M.I. Role and Function of Gelatin in the Development of the Food and Non-Food Industry: A Review. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012086. [Google Scholar] [CrossRef]
- Au-Yeung, F.; Jovanovski, E.; Jenkins, A.L.; Zurbau, A.; Ho, H.V.T.; Vuksan, V. The Effects of Gelled Konjac Glucomannan Fibre on Appetite and Energy Intake in Healthy Individuals: A Randomised Cross-over Trial. Br. J. Nutr. 2018, 119, 109–116. [Google Scholar] [CrossRef]
- Turnbull, W.H.; Thomas, H.G. The Effect of a Plantago ovata Seed Containing Preparation on Appetite Variables, Nutrient and Energy Intake. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 338–342. [Google Scholar]
- Berthold, H.K.; Unverdorben, S.; Degenhardt, R.; Unverdorben, M.; Gouni-Berthold, I. Effect of a Cellulose-Containing Weight-Loss Supplement on Gastric Emptying and Sensory Functions. Obesity 2008, 16, 2272–2280. [Google Scholar] [CrossRef]
- Harkness, M.L.R.; Harkness, R.D.; Venn, M.F. Digestion of Native Collagen in the Gut. Gut 1978, 19, 240–243. [Google Scholar] [CrossRef]
- Aranceta-Bartrina, J.; Partearroyo, T.; López-Sobaler, A.M.; Ortega, R.M.; Varela-Moreiras, G.; Serra-Majem, L.; Pérez-Rodrigo, C. Updating the Food-Based Dietary Guidelines for the Spanish Population: The Spanish Society of Community Nutrition (SENC) Proposal. Nutrients 2019, 11, 2675. [Google Scholar] [CrossRef] [PubMed]
- De La Iglesia, R.; Lopez-Legarrea, P.; Abete, I.; Bondia-Pons, I.; Navas-Carretero, S.; Forga, L.; Martinez, J.A.; Zulet, M.A. A New Dietary Strategy for Long-Term Treatment of the Metabolic Syndrome Is Compared with the American Heart Association (AHA) Guidelines: The MEtabolic Syndrome REduction in NAvarra (RESMENA) Project. Br. J. Nutr. 2014, 111, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The Product of Triglycerides and Glucose, a Simple Measure of Insulin Sensitivity. Comparison with the Euglycemic-Hyperinsulinemic Clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A Simple and Accurate Predictor of Hepatic Steatosis in the General Population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Jang, B.Y.; Bu, S.Y. Total Energy Intake According to the Level of Skeletal Muscle Mass in Korean Adults Aged 30 Years and Older: An Analysis of the Korean National Health and Nutrition Examination Surveys (KNHANES) 2008-2011. Nutr. Res. Pract. 2018, 12, 222–232. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Method 925.10; The Association of Official Analytical Chemists: Rockville, MD, USA, 2000. [Google Scholar]
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L.; Covas, M.I.; Marrugat, J. Validation of the Minnesota Leisure Time Physical Activity Questionnaire In Spanish Women. Investigators of the MARATDON Group. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef]
- De La Fuente-Arrillaga, C.; Vzquez Ruiz, Z.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ Validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, Power and Validity of Visual Analogue Scales in Assessment of Appetite Sensations in Single Test Meal Studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Vargas-Alvarez, M.A.; Al-Sehaim, H.; Brunstrom, J.M.; Castelnuovo, G.; Navas-Carretero, S.; Martínez, J.A.; Almiron-Roig, E. Development and Validation of a New Methodological Platform to Measure Behavioral, Cognitive, and Physiological Responses to Food Interventions in Real Time. Behav. Res. Methods 2022, 54, 2777–2801. [Google Scholar] [CrossRef]
- Birketvedt, G.S.; Shimshi, M.; Thom, E.; Florholmen, J. Experiences with Three Different Fiber Supplements in Weight Reduction. Med. Sci. Monit. 2005, 11, PI5–PI8. [Google Scholar] [PubMed]
- Lin, D.; Peters, B.A.; Friedlander, C.; Freiman, H.J.; Goedert, J.J.; Sinha, R.; Miller, G.; Bernstein, M.A.; Hayes, R.B.; Ahn, J. Association of Dietary Fibre Intake and Gut Microbiota in Adults. Br. J. Nutr. 2018, 120, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cheng, K.; Kang, Y.; Cheng, C.; Zhang, C.; Shang, L. Deacetylated Konjac Glucomannan with a Slower Hydration Rate Delays Rice Digestion and Weakens Appetite Response. Molecules 2024, 29, 1681. [Google Scholar] [CrossRef] [PubMed]
- Madaghiele, M.; Demitri, C.; Surano, I.; Silvestri, A.; Vitale, M.; Panteca, E.; Zohar, Y.; Rescigno, M.; Sannino, A. Biomimetic Cellulose-Based Superabsorbent Hydrogels for Treating Obesity. Sci. Rep. 2021, 11, 21394. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L.; Aronne, L.J.; Raben, A.; Astrup, A.; Apovian, C.M.; Hill, J.O.; Kaplan, L.M.; Fujioka, K.; Matejkova, E.; Svacina, S.; et al. A Randomized, Double-Blind, Placebo-Controlled Study of Gelesis100: A Novel Nonsystemic Oral Hydrogel for Weight Loss. Obesity 2019, 27, 205. [Google Scholar] [CrossRef]
- Abdalla, M.M.I. Ghrelin—Physiological Functions and Regulation. Eur. Endocrinol. 2015, 11, 90–95. [Google Scholar] [CrossRef]
- Perez-Tilve, D.; Heppner, K.; Kirchner, H.; Lockie, S.H.; Woods, S.C.; Smiley, D.L.; Tschöp, M.; Pfluger, P. Ghrelin-Induced Adiposity Is Independent of Orexigenic Effects. FASEB J. 2011, 25, 2814. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of Energy-Restricted High-Protein, Low-Fat Compared with Standard-Protein, Low-Fat Diets: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Rolland, V.; Wilson, S.A.J.; Westerterp, K.R. Satiety Related to 24 h Diet-Induced Thermogenesis during High Protein/Carbohydrate vs High Fat Diets Measured in a Respiration Chamber. Eur. J. Clin. Nutr. 1999, 53, 495–502. [Google Scholar] [CrossRef]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2020, 29, 166. [Google Scholar] [CrossRef]
- Yamamoto, S.; Deguchi, K.; Onuma, M.; Numata, N.; Sakai, Y. Absorption and Urinary Excretion of Peptides after Collagen Tripeptide Ingestion in Humans. Biol. Pharm. Bull. 2016, 39, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Shin, H.; Ahn, H.; Park, Y.K. Low-Molecular Collagen Peptide Supplementation and Body Fat Mass in Adults Aged ≥ 50 Years: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. Res. 2023, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Astre, G.; Deleruyelle, S.; Dortignac, A.; Bonnet, C.; Valet, P.; Dray, C. Diet-Induced Obesity and Associated Disorders Are Prevented by Natural Bioactive Type 1 Fish Collagen Peptides (Naticol®) Treatment. J. Physiol. Biochem. 2018, 74, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, T.; Sakamoto, T.; Kitano, T.; Inoue, N.; Sugihara, F.; Harada, N.; Yamaji, R. The Collagen Derived Dipeptide Hydroxyprolyl-Glycine Promotes C2C12 Myoblast Differentiation and Myotube Hypertrophy. Biochem. Biophys. Res. Commun. 2016, 478, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Kimira, Y.; Osawa, K.; Osawa, Y.; Mano, H. Preventive Effects of Collagen-Derived Dipeptide Prolyl-Hydroxyproline against Dexamethasone-Induced Muscle Atrophy in Mouse C2C12 Skeletal Myotubes. Biomolecules 2023, 13, 1617. [Google Scholar] [CrossRef]
- Bullón-Vela, V.; Abete, I.; Tur, J.A.; Konieczna, J.; Romaguera, D.; Pintó, X.; Corbella, E.; Martínez-González, M.A.; Sayón-Orea, C.; Toledo, E.; et al. Relationship of Visceral Adipose Tissue with Surrogate Insulin Resistance and Liver Markers in Individuals with Metabolic Syndrome Chronic Complications. Ther. Adv. Endocrinol. Metab. 2020, 11. [Google Scholar] [CrossRef]
- Garcia-Caraballo, S.C.; Comhair, T.M.; Verheyen, F.; Gaemers, I.; Schaap, F.G.; Houten, S.M.; Hakvoort, T.B.M.; Dejong, C.H.C.; Lamers, W.H.; Koehler, S.E. Prevention and Reversal of Hepatic Steatosis with a High-Protein Diet in Mice. Biochim. Biophys. Acta 2013, 1832, 685–695. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Axarlis, K.; Tsoureki, A.; Michailidou, S.; Efraimoglou, C.; Lapi, I.; Kolliniati, O.; Dermitzaki, E.; Venihaki, M.; Kousoulaki, K.; et al. Fish-Derived Protein Hydrolysates Increase Insulin Sensitivity and Alter Intestinal Microbiome in High-Fat-Induced Obese Mice. Mar. Drugs 2023, 21, 343. [Google Scholar] [CrossRef]
- Jendricke, P.; Centner, C.; Zdzieblik, D.; Gollhofer, A.; König, D. Specific Collagen Peptides in Combination with Resistance Training Improve Body Composition and Regional Muscle Strength in Premenopausal Women: A Randomized Controlled Trial. Nutrients 2019, 11, 892. [Google Scholar] [CrossRef]
| Control | Collagen | Interaction Effect 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (V0) n = 25 | 2 Months (V2) n = 22 | 3 Months (V3) n = 21 | p-Value V0 vs. V2 | p-Value V0 vs. V3 | p-Value V2 vs. V3 | Baseline (V0) n = 28 | 2 Months (V2) n = 20 | 3 Months (V3) n = 18 | p-Value V0 vs. V2 | p-Value V0 vs. V3 | p-Value V2 vs. V3 | p-Value | |
| Anthropometry | |||||||||||||
| Weight (Kg) [gender 2] | 82.43 ± 2.43 | 81.70 ± 2.58 | 80.12 ± 2.41 | <0.01 | <0.001 | ns | 85.76 ± 1.62 | 83.34 ± 2.08 | 84.00 ± 1.91 | <0.001 | <0.001 | <0.05 | 0.006 |
| Body mass index (kg/m2) | 29.16 ± 0.49 | 28.79 ± 0.55 | 28.38 ± 0.52 | <0.01 | <0.001 | ns | 30.15 ± 0.44 | 29.08 ± 0.55 | 28.95 ± 0.54 | <0.001 | <0.001 | <0.05 | 0.006 |
| Waist circumference (cm) [gender 2] | 93.5 ± 2.0 | 92.2 ± 2.1 | 91.0 ± 2.2 | <0.01 | <0.001 | ns | 98.0 ± 1.8 | 94.9 ± 2.2 | 95.2 ± 2.3 | <0.001 | <0.001 | <0.05 | 0.028 |
| Hip circumference (cm) [gender and Mets 2] | 106.7 ± 1.0 | 106.3 ± 1.1 | 105.3 ± 1.0 | <0.01 | <0.001 | ns | 109.1 * ± 1.0 | 107.7 ± 1.2 | 107.1 ± 1.3 | <0.001 | <0.001 | ns | ns (0.064) |
| Body composition (DXA 3) | |||||||||||||
| Total fat mass (%) [g 2] | 40.4 ± 1.3 | 39.8 ± 1.4 | 38.8 ± 1.5 | <0.01 | <0.001 | <0.05 | 40.3 ± 1.6 | 38.2 ± 1.9 | 37.1 ± 2.0 | <0.001 | <0.001 | ns | ns |
| Visceral adipose tissue (Kg) [g and a 2] | 1.39 ± 0.13 | 1.28 ± 0.12 | 1.20 ± 0.12 | <0.01 | <0.001 | <0.05 | 1.53 ± 0.13 | 1.35 ± 0.16 | 1.44 ± 0.18 | <0.001 | <0.001 | ns | ns |
| Total bone mineral density (g/cm2) [g and a 2] | 1.16 ± 0.02 | 1.15 ± 0.03 | 1.15 ± 0.02 | ns | ns | ns | 1.20 ± 0.03 | 1.20 ± 0.03 | 1.22 ± 0.04 | ns | ns | ns | ns |
| Fat-free mass (Kg) [g 2] | 47.8 ± 1.9 | 47.7 ± 2.0 | 47.7 ± 2.1 | ns | ns | ns | 49.8 ± 2.0 | 50.0 ± 2.2 | 51.2 ± 2.2 | ns | <0.001 | ns | 0.004 |
| Sarcopenic index [g 2] | 27.04 ± 0.67 | 27.29 ± 0.76 | 27.54 ± 0.79 | ns | <0.05 | ns | 26.96 ± 0.80 | 27.82 ± 0.92 | 28.28 ± 1.02 | <0.05 | <0.05 | ns | ns |
| Glycemic profile (Blood) | |||||||||||||
| Glucose (mg/dL) [age 2] | 98.85 ± 2.48 | 96.52 ± 2.13 | 101.23 ± 3.02 | ns | ns | ns | 103.21 ± 4.08 | 99.33 ± 4.24 | 98.94 ± 1.96 | ns | ns | ns | ns |
| Insulin (µU/mL) | 12.53 ± 2.75 | 8.39 * ± 0.69 | 10.97 ± 1.46 | ns | ns | ns | 13.52 ± 1.23 | 10.21 ± 0.75 | 10.28 ± 1.55 | ns | ns | ns | ns |
| HOMA-IR | 60.21 ± 17.61 | 36.71 ± 3.48 | 51.25 ± 7.68 | ns | ns | ns | 63.53 ± 7.27 | 45.89 ± 4.48 | 45.67 ± 7.01 | ns | ns | ns | ns |
| Blood pressure | |||||||||||||
| Systolic blood pressure (mmHg) [g and SBPbasal 2] | 118.6 ± 3.1 | 115.6 ± 3.4 | 119.0 ± 3.0 | ns | ns | ns | 127.3 ± 3.4 | 118.7 * ± 2.5 | 119.3 ** ± 3.1 | <0.001 | <0.001 | ns | 0.004 |
| Diastolic blood pressure (mm Hg) [g and a 2] | 83.0 ± 2.2 | 78.8 ± 1.9 | 80.6 ± 2.0 | <0.05 | ns | ns | 86.5 ± 1.9 | 81.6 ± 1.8 | 81.9 ± 2.2 | <0.001 | ns | ns | ns (0.053) |
| Lipid profile (Blood) | |||||||||||||
| Total cholesterol (mg/dL) [g, a, M, and e 2] | 220.8 ± 7.5 | 208.9 ± 6.8 | 209.5 ± 6.3 | <0.05 | ns | ns | 222.5 ± 6.0 | 219.1 ± 7.9 | 218.0 ± 7.6 | ns | ns | ns | ns |
| HDL cholesterol (mg/dL) [g 2] | 57.2 ± 3.1 | 54.8 ± 3.1 | 56.7 ± 2.9 | ns | ns | ns | 54.3 ± 2.6 | 52.8 ± 3.2 | 51.6 ± 3.6 | ns | ns | ns | ns |
| LDL cholesterol (mg/dL) [g, a, M, and e 2] | 144.9 ± 7.0 | 138.1 ± 5.8 | 135.6 ± 5.8 | <0.05 | <0.01 | ns | 146.8 ± 5.5 | 147.5 ± 6.7 | 147.2 ± 5.4 | ns | ns | ns | ns |
| Total cholesterol/HDL cholesterol Ratio [g and e 2] | 4.23 ± 0.24 | 4.09 ± 0.22 | 3.98 ± 0.22 | ns | ns | ns | 4.32 ± 0.19 | 4.39 ± 0.21 | 4.40 ± 0.19 | ns | ns | ns | ns |
| Triglycerides (mg/dL) [g 2] | 93.7 ± 11.4 | 80.7 ± 6.9 | 86.3 ± 9.9 | ns | ns | ns | 106.9 ± 11.7 | 94.2 ± 9.7 | 95.9 ± 135 | ns | ns | ns | ns |
| TyG Index [g 2] | 4.49 ± 0.06 | 4.43 ± 0.05 | 4.47 ± 0.07 | ns | ns | ns | 4.57 ± 0.06 | 4.52 ± 0.06 | 4.51 ± 0.06 | ns | ns | ns | ns |
| FLI Index [FLIbsal 2] | 43.1 ± 5.5 | 39.5 ± 5.6 | 35.8 ± 6.0 | ns | ns | ns | 55.1 ± 4.4 | 43.3 * ± 5.3 | 43.6 * ± 5.4 | <0.01 | <0.001 | ns | 0.023 |
| Transaminase profile (Blood) | |||||||||||||
| GGT (U/L) | 27.7 ± 6.0 | 27.0 ± 5.6 | 25.6 ± 5.4 | ns | ns | ns | 27.2 ± 2.9 | 19.8 ± 2.1 | 21.7 ± 3.0 | ns | ns | ns | ns |
| AST (U/dL) [M 2] | 22.7 ± 1.9 | 22.3 ± 1.5 | 23.0 ± 2.0 | ns | ns | ns | 25.9 ± 3.1 | 20.6 ± 1.4 | 24.4 ± 3.4 | ns | ns | ns | ns |
| ALT (U/dL) | 26.9 ± 3.4 | 26.0 ± 2.4 | 26.6 ± 2.8 | ns | ns | ns | 27.0 ± 2.3 | 20.2 ± 2.0 | 23.3 ± 2.8 | <0.01 | ns | ns | ns |
| Other biochemical parameters (blood) | |||||||||||||
| C-reactive protein (mg/dL) | 2.90 ± 0.62 | 2.84 ± 0.62 | 2.78 ± 0.62 | ns | ns | ns | 2.87 ± 0.58 | 2.65 ± 0.66 | 2.27 ± 0.48 | ns | ns | ns | ns |
| Leptin (mg/dL) [s 2] | 9.12 ± 1.68 | 5.32 ± 1.12 | 4.29 ± 0.91 | <0.01 | <0.001 | ns | 10.87 ± 2.64 | 6.48 ± 1.57 | 7.37 * ± 2.20 | <0.01 | <0.01 | ns | ns |
| Homocysteine (umol/L) [s 2] | 10.33 ± 0.62 | 10.95 ± 0.60 | 11.96 ± 0.93 | Ns | <0.001 | <0.05 | 11.42 ± 0.82 | 12.52 ± 0.91 | 12.71 ± 0.81 | <0.05 | <0.01 | ns | ns |
| Renal function (Urine) | |||||||||||||
| Albumin (mg/dL) | 0.45 ± 0.09 | 0.52 ± 0.14 | 0.60 ± 0.21 | ns | ns | ns | 0.64 ± 0.20 | 0.59 ± 0.20 | 0.53 ± 0.19 | ns | ns | ns | ns |
| Creatinine (mg(dL) [s 2] | 74.05 ± 6.26 | 86.87 ± 11.32 | 85.86 ± 11–98 | ns | ns | ns | 84.28 ± 8.57 | 74.17 ± 8.33 | 90.77 ± 8.07 | ns | ns | ns | ns |
| Albumin-to-creatinine ratio (mg/g) | 6.99 ± 1.52 | 7.40 ± 2.56 | 6.91 ± 1.43 | ns | ns | ns | 7.96 ± 2.85 | 8.36 ± 3.21 | 4.76 ± 1.06 | ns | ns | ns | ns |
| Collagen content | |||||||||||||
| Collagen in stools (%) | 0.91 ± 0.19 | 0.76 ± 0.14 | 1.08 ± 0.25 | ns | ns | ns | 0.67 ± 0.12 | 2.75 *** ± 0.34 | 3.32 *** ± 0.62 | <0.001 | <0.001 | <0.05 | <0.001 |
| Collagen in urine (mg/20 mL of urine) | 0.92 ± 0.07 | ND | 0.76 ± 0.18 | ND | ns | ns | 0.90 ± 0.08 | ND | 1.58 *** ± 0.17 | ND | <0.001 | ns | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Yoldi, M.; Riezu-Boj, J.I.; Abete, I.; Ibero-Baraibar, I.; Aranaz, P.; González-Salazar, I.; Izco, J.M.; Recalde, J.I.; González-Navarro, C.J.; Milagro, F.I.; et al. Anti-Obesity Effects of a Collagen with Low Digestibility and High Swelling Capacity: A Human Randomized Control Trial. Nutrients 2024, 16, 3550. https://doi.org/10.3390/nu16203550
López-Yoldi M, Riezu-Boj JI, Abete I, Ibero-Baraibar I, Aranaz P, González-Salazar I, Izco JM, Recalde JI, González-Navarro CJ, Milagro FI, et al. Anti-Obesity Effects of a Collagen with Low Digestibility and High Swelling Capacity: A Human Randomized Control Trial. Nutrients. 2024; 16(20):3550. https://doi.org/10.3390/nu16203550
Chicago/Turabian StyleLópez-Yoldi, Miguel, José I. Riezu-Boj, Itziar Abete, Idoia Ibero-Baraibar, Paula Aranaz, Itxaso González-Salazar, Jesús M. Izco, José I. Recalde, Carlos J. González-Navarro, Fermín I. Milagro, and et al. 2024. "Anti-Obesity Effects of a Collagen with Low Digestibility and High Swelling Capacity: A Human Randomized Control Trial" Nutrients 16, no. 20: 3550. https://doi.org/10.3390/nu16203550
APA StyleLópez-Yoldi, M., Riezu-Boj, J. I., Abete, I., Ibero-Baraibar, I., Aranaz, P., González-Salazar, I., Izco, J. M., Recalde, J. I., González-Navarro, C. J., Milagro, F. I., & Zulet, M. A. (2024). Anti-Obesity Effects of a Collagen with Low Digestibility and High Swelling Capacity: A Human Randomized Control Trial. Nutrients, 16(20), 3550. https://doi.org/10.3390/nu16203550








