Higher Adherence to the Mediterranean Diet Is Associated with a Lower Risk of Steatotic, Alcohol-Related, and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Retrospective Analysis
Highlights
- Greater adherence to the Mediterranean diet significantly reduces the risk of steatotic liver disease, metabolic dysfunction-associated steatotic liver disease, and alcohol-related liver disease.
- Individuals with higher adherence to the diet showed lower triglyceride levels, insulin resistance markers (TyG index), and atherogenic indices compared to those with lower adherence.
- These findings suggest that following a Mediterranean diet may act as a protective factor against liver diseases and cardiovascular risks in the Korean population.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Anthropometric and Laboratory Measurements
2.3. Definition of SLD, MASLD, and ALD
2.4. Cardiovascular Risk Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Adherence to the Mediterranean Diet and Liver Disease
4.2. Potential Mechanisms
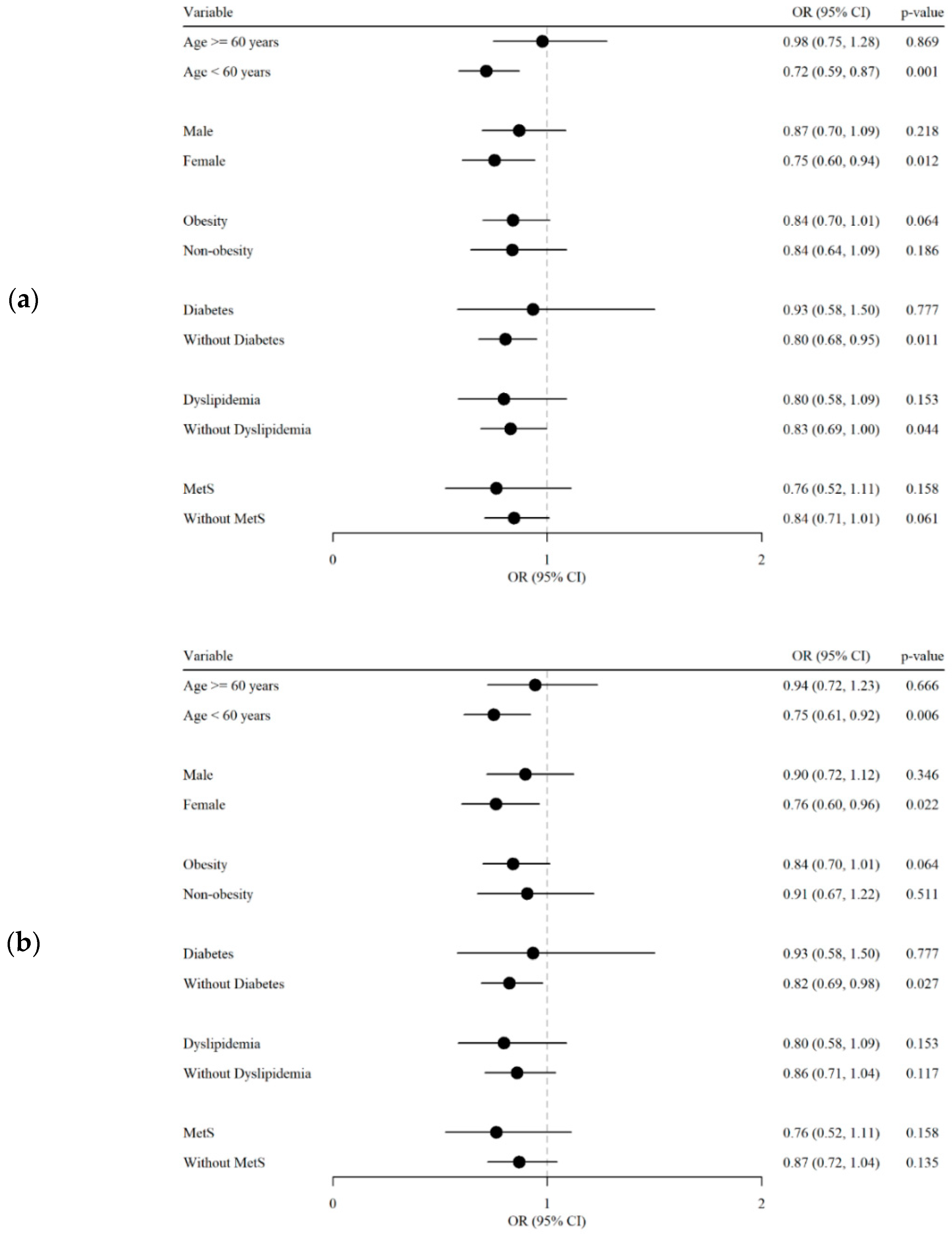
4.3. Subgroup Analysis
4.4. Study Limitations
4.5. Study Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouad, Y. Metabolic-associated fatty liver disease: New nomenclature and approach with hot debate. World J. Hepatol. 2023, 15, 123–128. [Google Scholar] [CrossRef]
- Israelsen, M.; Torp, N.; Johansen, S.; Hansen, C.D.; Hansen, E.D.; Thorhauge, K.; Hansen, J.K.; Villesen, I.; Bech, K.; Wernberg, C.; et al. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: An analysis of data from a prospective cohort study. Lancet Gastroenterol. Hepatol. 2024, 9, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhao, L.; Dong, J.; Chen, H.; Li, D.; Zhang, X.; Hassan, M.M.; Steck, S.E.; Li, X.; Xiang, Y.B.; et al. The role of dietary factors in nonalcoholic fatty liver disease to hepatocellular carcinoma progression: A systematic review. Clin. Nutr. 2022, 41, 2295–2307. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Day, C.P.; Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010, 363, 1341–1350. [Google Scholar] [CrossRef]
- American Heart Association; American Stroke Association. Cardiovascular Disease: A Costly Burden for America Projections Through 2035; American Heart Association: Dallas, TX, USA, 2017; Available online: https://www.heart.org/-/media/Files/About-Us/Policy-Research/Fact-Sheets/Public-Health-Advocacy-and-Research/CVD-A-Costly-Burden-for-America-Projections-Through-2035.pdf (accessed on 2 July 2024).
- Magkos, F.; Tetens, I.; Bügel, S.G.; Felby, C.; Schacht, S.R.; Hill, J.O.; Ravussin, E.; Astrup, A. A Perspective on the Transition to Plant-Based Diets: A Diet Change May Attenuate Climate Change, but Can It Also Attenuate Obesity and Chronic Disease Risk? Adv. Nutr. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Bellavia, A.; Tektonidis, T.G.; Orsini, N.; Wolk, A.; Larsson, S.C. Quantifying the benefits of Mediterranean diet in terms of survival. Eur. J. Epidemiol. 2016, 31, 527–530. [Google Scholar] [CrossRef]
- deKoning, L.; Anand, S.S. Adherence to a Mediterranean diet and survival in a Greek population. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. N Engl J Med 2003, 348, 2599–608. Vasc. Med. 2004, 9, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Lee, H.; Yoon, Y.; Kim, H.M.; Chu, S.H.; Lee, J.W. Development and Validation of a Questionnaire to Measure Adherence to the Mediterranean Diet in Korean Adults. Nutrients 2020, 12, 1102. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- W. H. O. Consultation on Obesity; World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Jahangiry, L.; Farhangi, M.A.; Rezaei, F. Framingham risk score for estimation of 10-years of cardiovascular diseases risk in patients with metabolic syndrome. J. Health Popul. Nutr. 2017, 36, 36. [Google Scholar] [CrossRef]
- Dobiásová, M. [AIP—Atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice]. Vnitr. Lek. 2006, 52, 64–71. [Google Scholar] [PubMed]
- Tao, L.C.; Xu, J.N.; Wang, T.T.; Hua, F.; Li, J.J. Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc. Diabetol. 2022, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Pastori, D.; Polimeni, L.; Bucci, T.; Ceci, F.; Calabrese, C.; Ernesti, I.; Pannitteri, G.; Violi, F.; Angelico, F.; et al. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am. J. Gastroenterol. 2017, 112, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Page, J. Nonalcoholic fatty liver disease: The hepatic metabolic syndrome. J. Am. Acad. Nurse Pract. 2012, 24, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, M.; Kontogianni, M.D.; Margariti, A.; Tiniakos, D.; Fragopoulou, E.; Zafiropoulou, R.; Papatheodoridis, G. Associations between dietary intake and the presence of the metabolic syndrome in patients with non-alcoholic fatty liver disease. J. Hum. Nutr. Diet. 2015, 28, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Properzi, C.; O’Sullivan, T.A.; Sherriff, J.L.; Ching, H.L.; Jeffrey, G.P.; Buckley, R.F.; Tibballs, J.; MacQuillan, G.C.; Garas, G.; Adams, L.A. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology 2018, 68, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. NAFLD and increased risk of cardiovascular disease: Clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 2020, 69, 1691–1705. [Google Scholar] [CrossRef]
- Argyrakopoulou, G.; Fountouli, N.; Dalamaga, M.; Kokkinos, A. Revisiting Resting Metabolic Rate: What is the Relation to Weight Fluctuations? Curr. Obes. Rep. 2023, 12, 502–513. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef] [PubMed]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef] [PubMed]
- Algabbani, F.M.; Algabbani, A.M. Treatment adherence among patients with hypertension: Findings from a cross-sectional study. Clin. Hypertens. 2020, 26, 18. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-X.; Ma, X.-N.; Guan, C.-H.; Li, Y.-D.; Mauricio, D.; Fu, S.-B. Cardiovascular disease in type 2 diabetes mellitus: Progress toward personalized management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, D.; Li, H.; Xu, Y. The imaging techniques and diagnostic performance of ultrasound, CT, and MRI in detecting liver steatosis and fat quantification: A systematic review. J. Radiat. Res. Appl. Sci. 2023, 16, 100658. [Google Scholar] [CrossRef]
- Hetland, L.E.; Kronborg, T.M.; Thing, M.; Werge, M.P.; Junker, A.E.; Rashu, E.B.; O’Connell, M.B.; Olsen, B.H.; Jensen, A.H.; Wewer Albrechtsen, N.J.; et al. Suboptimal diagnostic accuracy of ultrasound and CT for compensated cirrhosis: Evidence from prospective cohort studies. Hepatol. Commun. 2023, 7, e0231. [Google Scholar] [CrossRef]
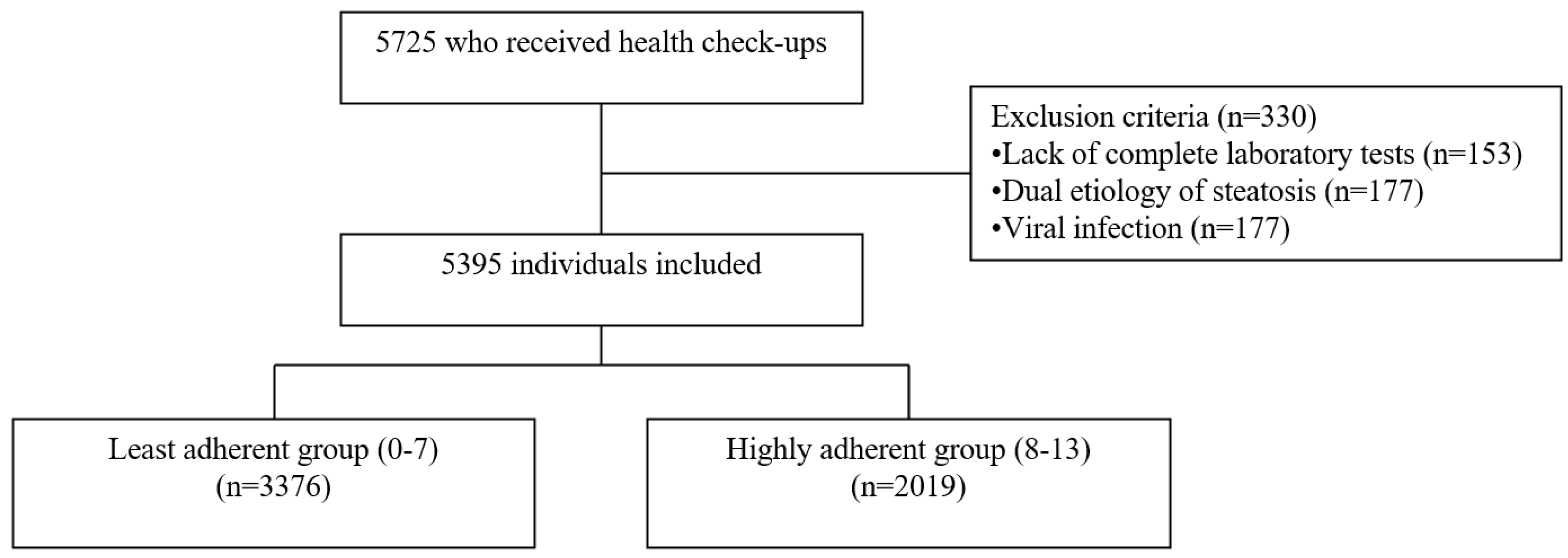
| Characteristics | Total (n = 5395) | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|---|
| Least Adherent Group (0–7) | Highly Adherent Group (8–13) | p | Least Adherent Group (0–7) | Highly Adherent Group (8–13) | p | ||
| (n = 3376) | (n = 2019) | (n = 1716) | (n = 1716) | ||||
| Age, years | 50.2 ± 13.3 | 47.0 ± 13.0 | 55.5 ± 12.0 | <0.001 | 53.8 ± 11.7 | 53.8 ± 11.7 | >0.999 |
| Male Sex, n (%) | 2825 (52.4%) | 1897 (56.2%) | 928 (46.0%) | <0.001 | 857 (49.9%) | 857 (49.9%) | >0.999 |
| BMI, kg/m2 | 24.2 ± 3.5 | 24.2 ± 3.6 | 24.3 ± 3.3 | 0.743 | 24.2 ± 3.4 | 24.3 ± 3.3 | 0.435 |
| SBP, mmHg | 122.9 ± 14.1 | 122.2 ± 14.2 | 124.3 ± 13.9 | <0.001 | 124.2 ± 13.9 | 123.7 ± 13.9 | 0.327 |
| DBP, mmHg | 74.3 ± 11.3 | 74.2 ± 11.5 | 74.5 ± 11.1 | 0.227 | 75.0 ± 11.0 | 74.5 ± 11.3 | 0.143 |
| Current smoker, n (%) | 1001 (18.6%) | 774 (22.9%) | 227 (11.2%) | <0.001 | 334 (19.5%) | 217 (12.6%) | <0.001 |
| Exercise, n (%) | 3320 (61.6%) | 1897 (56.3%) | 1423 (70.5%) | <0.001 | 994 (58.0%) | 1200 (69.9%) | <0.001 |
| Alcohol, n (%) | 2494 (46.2%) | 1719 (50.9%) | 775 (38.4%) | <0.001 | 792 (46.2%) | 711 (41.5%) | 0.006 |
| History of hypertension, n (%) | 1157 (21.4%) | 616 (18.2%) | 541 (26.8%) | <0.001 | 432 (25.2%) | 426 (24.8%) | 0.844 |
| History of DM, n (%) | 700 (13.0%) | 375 (11.1%) | 325 (16.1%) | <0.001 | 254 (14.8%) | 269 (15.7%) | 0.506 |
| History of dyslipidemia, n (%) | 1143 (21.2%) | 595 (17.6%) | 548 (27.1%) | <0.001 | 403 (23.5%) | 430 (25.1%) | 0.301 |
| History of CVD, n (%) | 282 (5.2%) | 156 (4.6%) | 126 (6.2%) | 0.012 | 101 (5.9%) | 98 (5.7%) | 0.884 |
| History of cancer, n (%) | 207 (3.8%) | 103 (3.1%) | 104 (5.2%) | <0.001 | 77 (4.5%) | 74 (4.3%) | 0.868 |
| Glucose, mg/dL | 101.4 ± 20.6 | 100.5 ± 21.3 | 102.9 ± 19.2 | <0.001 | 102.9 ± 22.7 | 102.6 ± 19.1 | 0.642 |
| Total Cholesterol, mg/dL | 189.2 ± 38.9 | 190.7 ± 37.8 | 186.8 ± 40.6 | <0.001 | 189.5 ± 39.5 | 186.9 ± 39.9 | 0.053 |
| LDL, mg/dL | 125.3 ± 37.4 | 126.5 ± 36.7 | 123.3 ± 38.6 | 0.003 | 125.5 ± 37.8 | 123.5 ± 38.1 | 0.127 |
| HDL, mg/dL | 57.8 ± 15.2 | 57.6 ± 15.3 | 58.1 ± 14.9 | 0.229 | 58.0 ± 15.4 | 57.7 ± 14.9 | 0.589 |
| Triglyceride, mg/dL | 118.1 ± 78.7 | 121.3 ± 81.6 | 112.8 ± 73.1 | <0.001 | 119.0 ± 78.9 | 113.7 ± 75.3 | 0.046 |
| TyG Index | 8.5 ± 0.6 | 8.5 ± 0.6 | 8.5 ± 0.6 | 0.025 | 8.6 ± 0.6 | 8.5 ± 0.6 | 0.013 |
| AIP | 0.6 ± 0.7 | 0.6 ± 0.7 | 0.5 ± 0.7 | <0.001 | 0.6 ± 0.7 | 0.6 ± 0.7 | 0.046 |
| AST, IU/L | 27.2 ± 13.4 | 26.5 ± 13.4 | 28.2 ± 13.5 | <0.001 | 27.4 ± 13.4 | 27.8 ± 13.2 | 0.413 |
| ALT, IU/L | 25.7 ± 17.9 | 25.7 ± 18.4 | 25.8 ± 16.9 | 0.796 | 25.3 ± 17.4 | 25.9 ± 17.2 | 0.373 |
| GGT, IU/L | 32.7 ± 42.5 | 34.6 ± 45.9 | 29.6 ± 35.9 | <0.001 | 33.3 ± 40.4 | 30.5 ± 37.7 | 0.034 |
| CRP, mg/L | 1.3 ± 3.0 | 1.3 ± 3.1 | 1.3 ± 2.7 | 0.574 | 1.4 ± 3.8 | 1.3 ± 2.9 | 0.594 |
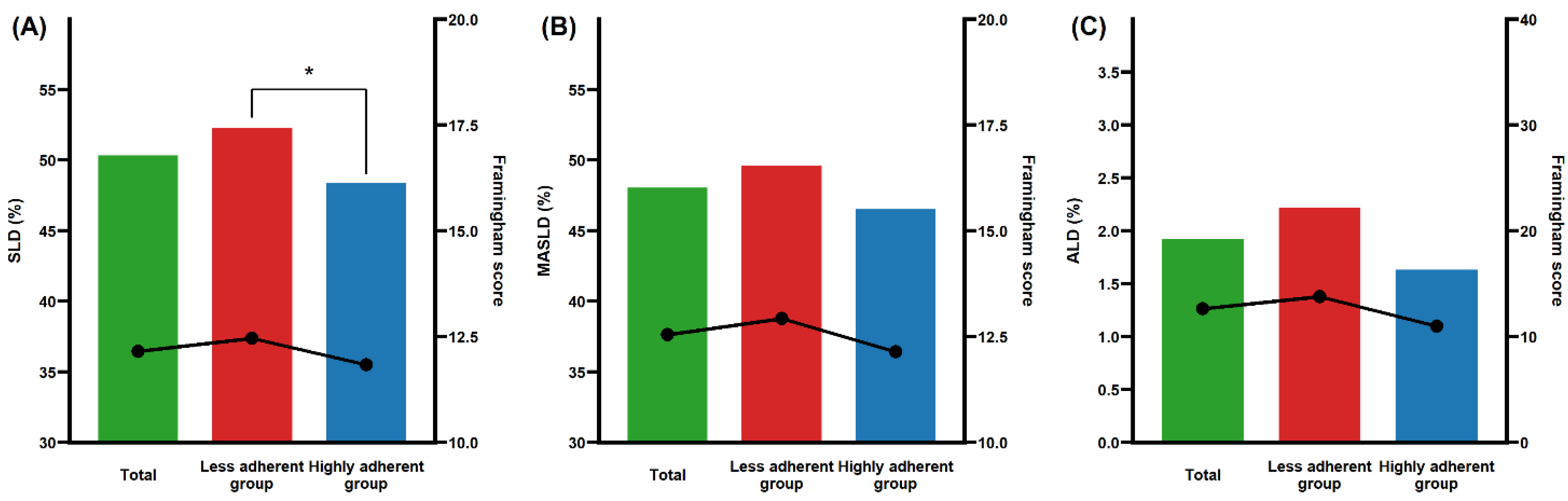
| SLD, n (%) | 2605 (48.3%) | 1614 (47.8%) | 991 (49.1%) | 0.379 | 897 (52.3%) | 830 (48.4%) | 0.024 |
| MASLD, n (%) | 2479 (45.9%) | 1525 (45.2%) | 954 (47.3%) | 0.146 | 851 (49.6%) | 798 (46.5%) | 0.076 |
| ALD, n (%) | 109 (2.0%) | 77 (2.3%) | 32 (1.6%) | 0.097 | 38 (2.2%) | 28 (1.6%) | 0.263 |
| Framingham risk score | 8.7 ± 8.0 | 8.3 ± 8.1 | 9.3 ± 8.0 | <0.001 | 10.1 ± 9.0 | 9.3 ± 8.1 | 0.009 |
| SLD | MASLD | ALD | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Model 1 a | Less adherent group | Ref | Ref | Ref | |||
| Highly adherent group | 0.842 (0.732–0.969) | 0.017 | 0.871 (0.756–1.004) | 0.056 | 0.686 (0.683–0.689) | <0.001 | |
| Model 2 b | Less adherent group | Ref | Ref | Ref | |||
| Highly adherent group | 0.834 (0.714–0.973) | 0.021 | 0.856 (0.730–1.003) | 0.055 | 0.330 (0.103–1.054) | 0.061 | |
| Model 3 c | Less adherent group | Ref | Ref | Ref | |||
| Highly adherent group | 0.822 (0.703–0.960) | 0.014 | 0.842 (0.717–0.989) | 0.037 | 0.382 (0.111–1.317) | 0.128 | |
| Model 4 d | Less adherent group | Ref | Ref | Ref | |||
| Highly adherent group | 0.818 (0.700–0.957) | 0.012 | 0.839 (0.714–0.986) | 0.033 | 0.677 (0.671–0.683) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Kim, S.; Lee, Y.; Kwon, Y.-J.; Lee, J.-W. Higher Adherence to the Mediterranean Diet Is Associated with a Lower Risk of Steatotic, Alcohol-Related, and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Retrospective Analysis. Nutrients 2024, 16, 3551. https://doi.org/10.3390/nu16203551
Lee JY, Kim S, Lee Y, Kwon Y-J, Lee J-W. Higher Adherence to the Mediterranean Diet Is Associated with a Lower Risk of Steatotic, Alcohol-Related, and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Retrospective Analysis. Nutrients. 2024; 16(20):3551. https://doi.org/10.3390/nu16203551
Chicago/Turabian StyleLee, Ji Yae, Sue Kim, Yaeji Lee, Yu-Jin Kwon, and Ji-Won Lee. 2024. "Higher Adherence to the Mediterranean Diet Is Associated with a Lower Risk of Steatotic, Alcohol-Related, and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Retrospective Analysis" Nutrients 16, no. 20: 3551. https://doi.org/10.3390/nu16203551
APA StyleLee, J. Y., Kim, S., Lee, Y., Kwon, Y.-J., & Lee, J.-W. (2024). Higher Adherence to the Mediterranean Diet Is Associated with a Lower Risk of Steatotic, Alcohol-Related, and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Retrospective Analysis. Nutrients, 16(20), 3551. https://doi.org/10.3390/nu16203551






