Interaction of Cooking-Generated Aerosols on the Human Nervous System and the Impact of Caloric Restriction Post-Exposure
Highlights
- Exposure to cooking-generated aerosols led to a 12.82% increase in alpha-band activity in the non-zero-calorie group two hours post-exposure.
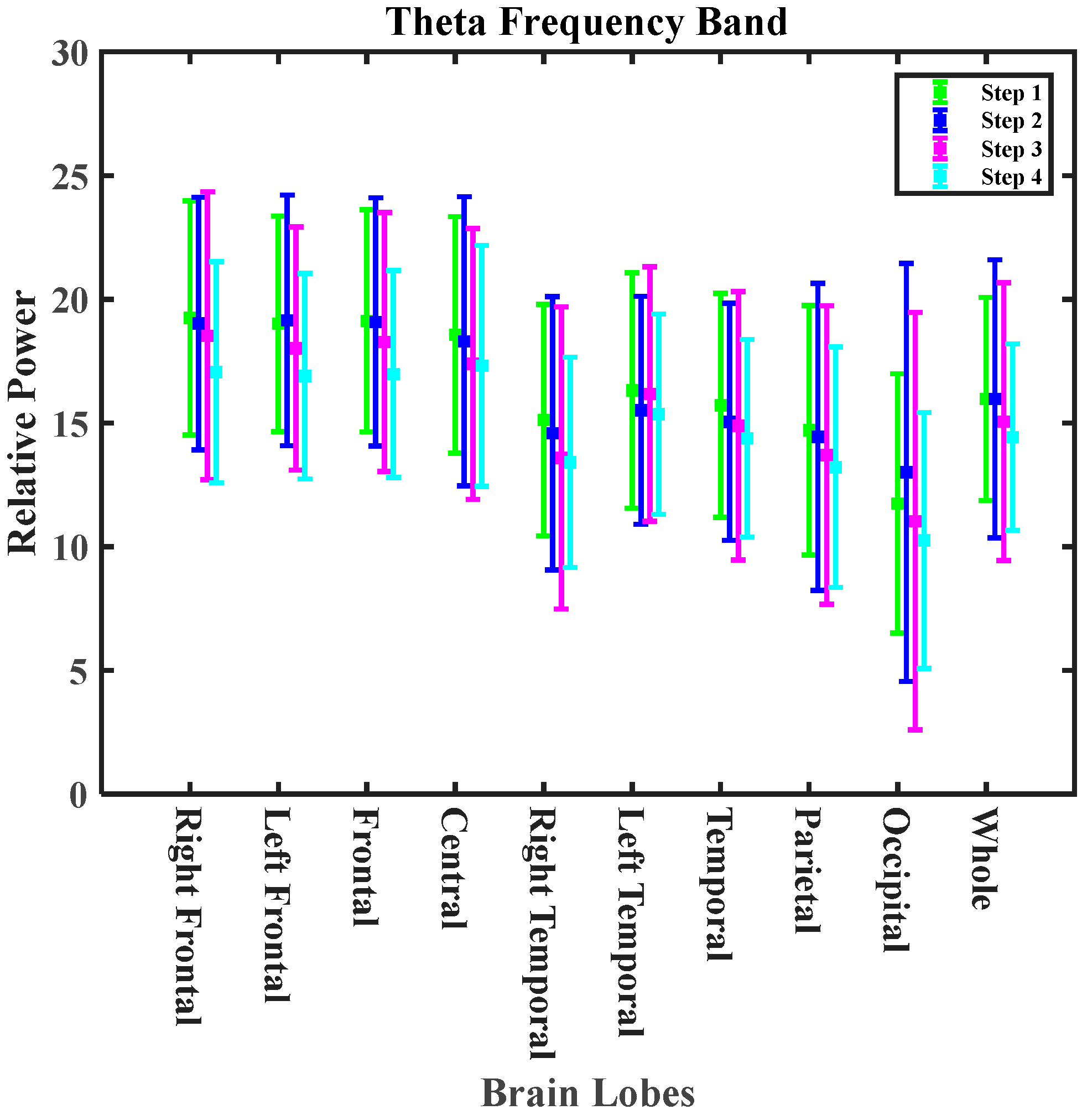
- Zero-calorie intake after exposure mitigated the impacts of cooking-generated aerosols on the alpha, beta3, theta, and delta bands.
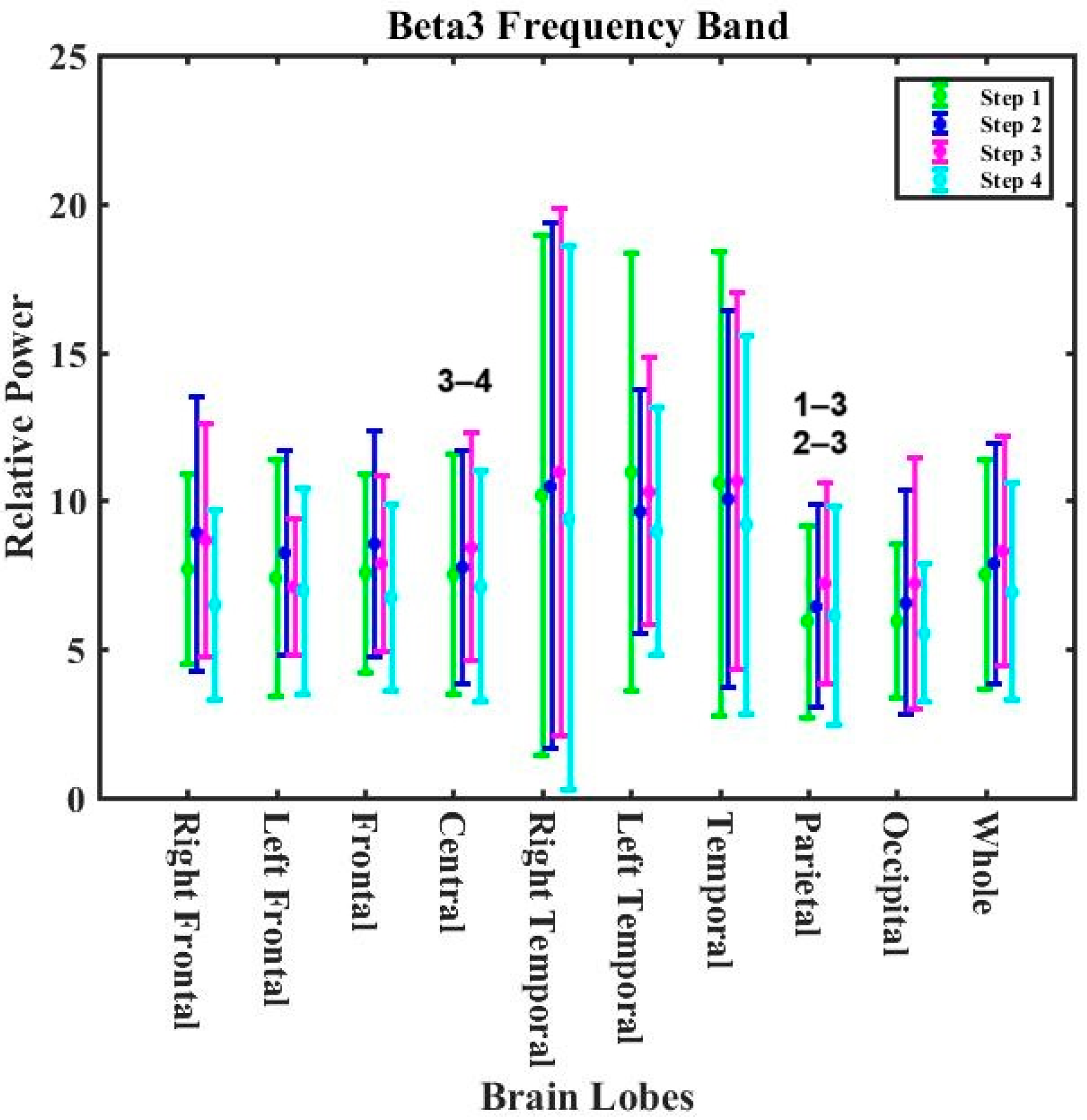
- Zero-calorie intake, however, exacerbated the impacts of cooking-generated aerosols on overall brain activity in the beta1 and beta2 bands.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Indoor Air Quality During the Experiment
2.3. Effect Assessments
2.4. Experimental Design
2.5. Control Experiments
2.6. Brain Wave Pattern Percentage Calculation
2.7. Statistical Analysis Method
- H0 = µ1 = µ2 = µ3= µ4;
- H0 = mean RP is the same at all times;
- H1 = mean RP is significantly different at one or more time points;
- µ is the population mean of RP, and the related groups are the subjects before, during, and after cooking.
3. Results
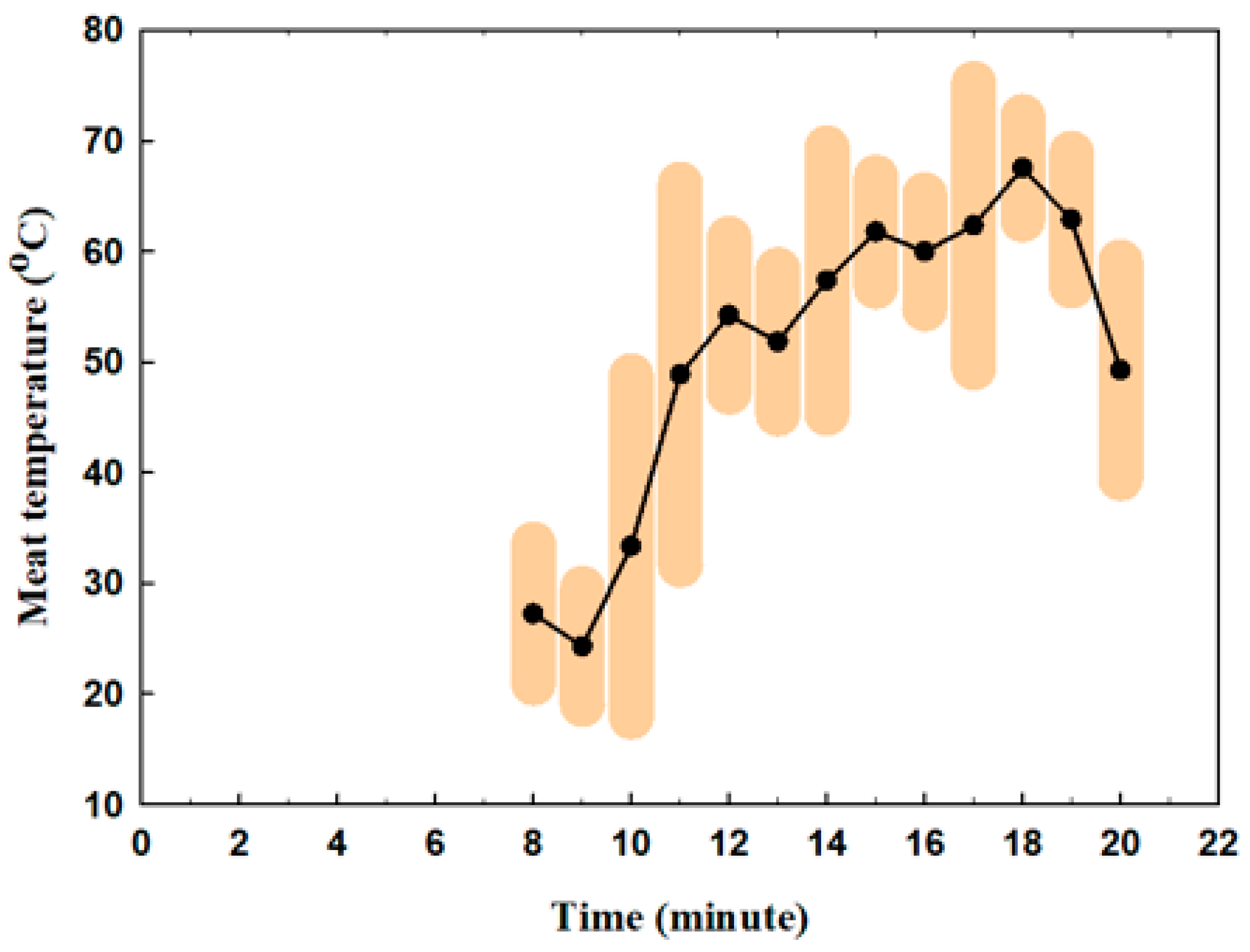
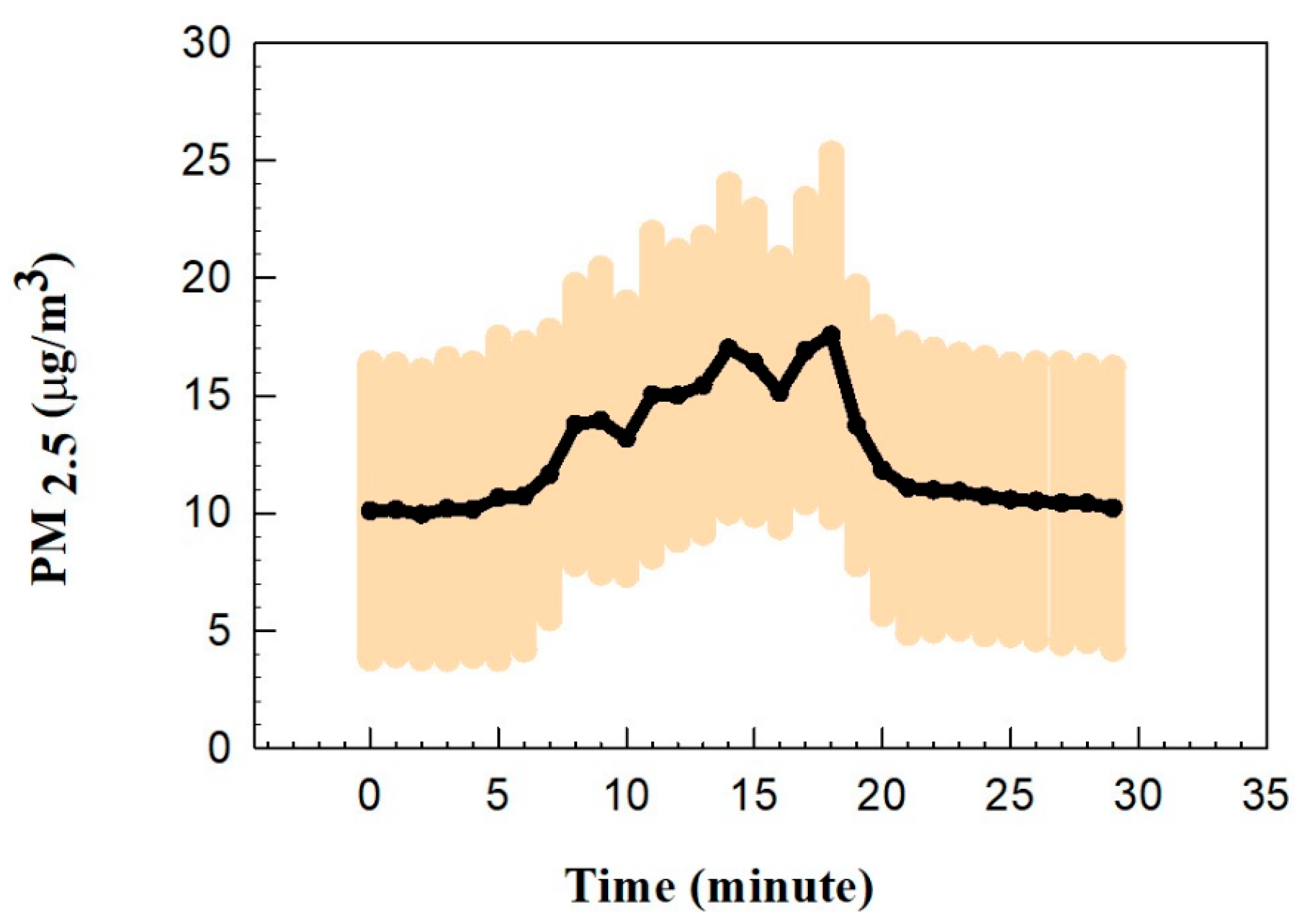
3.1. Indoor Air Quality During the Experiment
3.2. Brain Response
3.2.1. Control Experiments Results
3.2.2. Electrode Analysis for Exposure Experiments
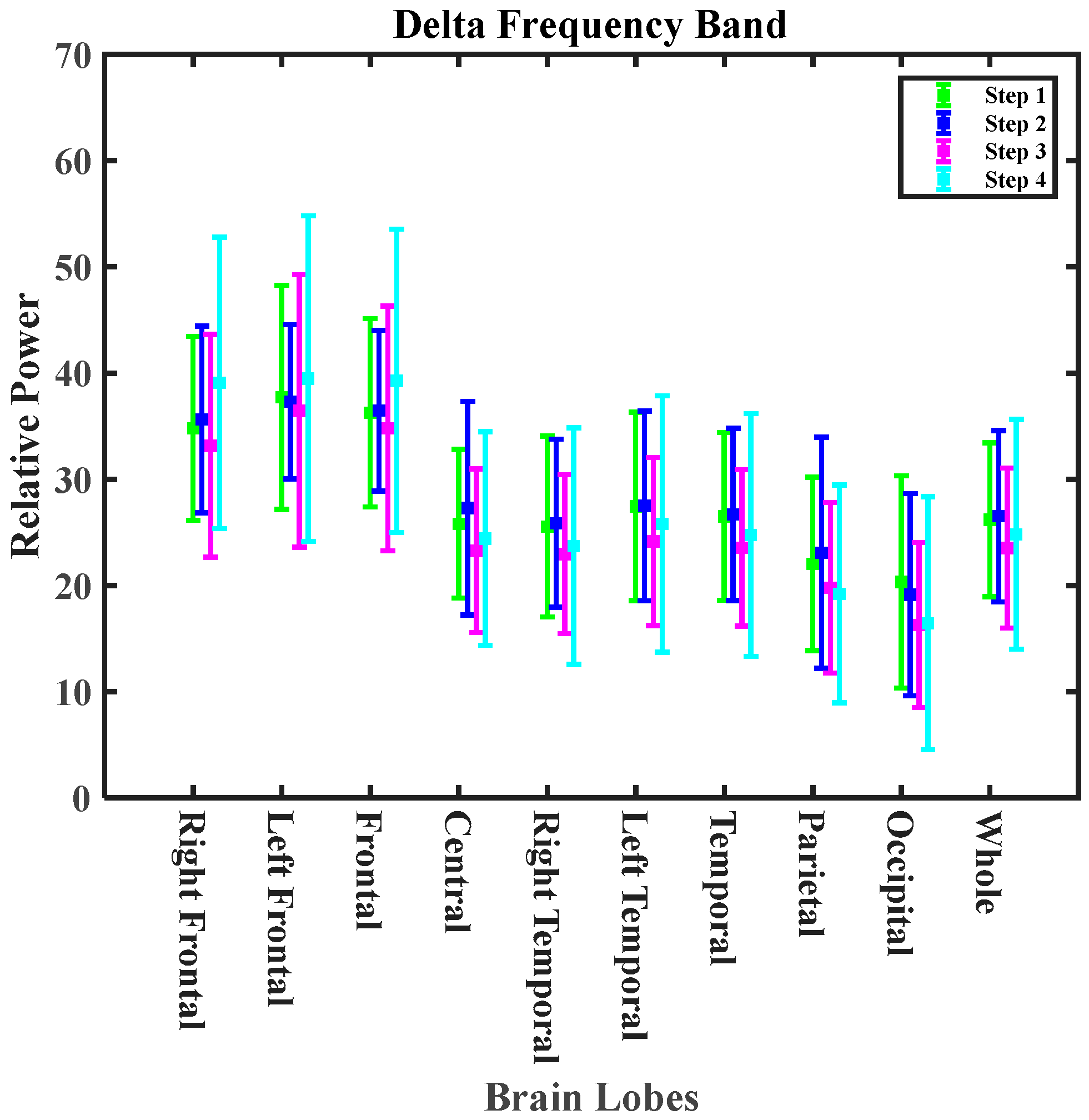
3.2.3. Lobe Analysis for Exposure Experiments
4. Discussion
5. Limitations of This Study and Future Work
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baeza_Romero, M.T.; Dudzinska, M.R.; Torkmahalleh, M.A.; Barros, N.; Coggins, A.M.; Ruzgar, D.G.; Kildsgaard, I.; Naseri, M.; Rong, L.; Saffell, J.; et al. A review of critical residential buildings parameters and activities when investigating indoor air quality and pollutants. Indoor Air 2022, 32, e13144. [Google Scholar] [CrossRef]
- Brasche, S.; Bischof, W. Daily time spent indoors in German homes–baseline data for the assessment of indoor ex-posure of German occupants. Int. J. Hyg. Environ. Health 2005, 208, 247–253. [Google Scholar] [CrossRef]
- Badida, P.; Krishnamurthy, A.; Jayaprakash, J. Meta analysis of health effects of ambient air pollution exposure in low-and middle-income countries. Environ. Res. 2023, 216, 114604. [Google Scholar] [CrossRef]
- Bălă, G.-P.; Râjnoveanu, R.-M.; Tudorache, E.; Motișan, R.; Oancea, C. Air pollution exposure—The (in)visible risk factor for respiratory diseases. Environ. Sci. Pollut. Res. 2021, 28, 19615–19628. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Chen, J.; You, C.; Ma, L.; Zhang, W.; Li, D. Household air pollution, adherence to a healthy lifestyle, and risk of cardiometabolic multimorbidity: Results from the China health and retirement longitudinal study. Sci. Total Environ. 2023, 855, 158896. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.B.; Arora, T.; Singh, S.; Singh, R. Critical review on emerging health effects associated with the indoor air quality and its sustainable management. Sci. Total Environ. 2023, 872, 162163. [Google Scholar] [CrossRef]
- Wallace, L. Indoor sources of ultrafine and accumulation mode particles: Size distributions, size-resolved concentrations, and source strengths. Aerosol Sci. Technol. 2006, 40, 348–360. [Google Scholar] [CrossRef]
- Wallace, L.; Wang, F.; Howard-Reed, C.; Persily, A. Contribution of gas and electric stoves to residential ultrafine particle concentrations between 2 and 64 nm: Size distributions and emission and coagulation rates. Environ. Sci. Technol. 2008, 42, 8641–8647. [Google Scholar] [CrossRef]
- Brauer, M.; Hirtle, R.; Lang, B.; Ott, W. Assessment of indoor fine aerosol contributions from environmental tobacco smoke and cooking with a portable nephelometer. J. Expo. Sci. Environ. Epidemiol. 2000, 10, 136–144. [Google Scholar] [CrossRef]
- DeCarlo, P.F.; Avery, A.M.; Waring, M.S. Thirdhand smoke uptake to aerosol particles in the indoor environment. Sci. Adv. 2018, 4, eaap8368. [Google Scholar] [CrossRef]
- Singer, B.C.; Coleman, B.K.; Destaillats, H.; Hodgson, A.T.; Lunden, M.M.; Weschler, C.J.; Nazaroff, W.W. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos. Environ. 2006, 40, 6696–6710. [Google Scholar] [CrossRef]
- Wong, J.P.S.; Carslaw, N.; Zhao, R.; Zhou, S.; Abbatt, J.P.D. Observations and impacts of bleach washing on indoor chlorine chemistry. Indoor Air 2017, 27, 1082–1090. [Google Scholar] [CrossRef]
- Abdullahi, K.L.; Delgado-Saborit, J.M.; Harrison, R.M. Emissions and indoor concentrations of particulate matter and its specific chemical components from fast: A review. Atmos. Environ. 2013, 71, 260–294. [Google Scholar] [CrossRef]
- Torkmahalleh, M.A.; Gorjinezhad, S.; Unluevcek, H.S.; Hopke, P.K. Review of factors impacting emission/concentration of cooking generated par-ticulate matter. Sci. Total Environ. 2017, 586, 1046–1056. [Google Scholar] [CrossRef]
- Schiavon, M.; Rada, E.C.; Ragazzi, M.; Antognoni, S.; Zanoni, S. Domestic activities and PM generation: A contribution to the understanding of indoor sources of air pollution. Int. J. Sustain. Dev. Plan. 2015, 2015, 347–360. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Liu, L.; Tao, P.; Zhang, B.; Huan, C.; Zhang, X.; Wang, M. Review of effluents and health effects of cooking and the performance of kitchen ventilation. Aerosol Air Qual. Res. 2019, 19, 1937–1959. [Google Scholar] [CrossRef]
- Kang, K.; Kim, H.; Kim, D.D.; Lee, Y.G.; Kim, T. Characteristics of cooking-generated PM10 and PM2.5 in residential buildings with different cooking and ventilation types. Sci. Total Environ. 2019, 668, 56–66. [Google Scholar] [CrossRef]
- Klein, F.; Baltensperger, U.; Prévôt, A.S.H.; El Haddad, I. Quantification of the impact of cooking processes on indoor concentrations of volatile organic species and primary and secondary organic aerosols. Indoor Air 2019, 29, 926–942. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Z.; Wen, W.; Son, J.H.; Li, L.; Wang, L.; Chen, J. Spatial distributions of particle number size distributions generated during cooking processes and the impacts of range hoods. Sci. Total Environ. 2023, 881, 163243. [Google Scholar] [CrossRef]
- Allen, J.L.; Oberdorster, G.; Morris-Schaffer, K.; Wong, C.; Klocke, C.; Sobolewski, M.; Conrad, K.; Mayer-Proschel, M.; Cory-Slechta, D.A. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology 2017, 59, 140–154. [Google Scholar] [CrossRef]
- Bhargava, A.; Tamrakar, S.; Aglawe, A.; Lad, H.; Srivastava, R.K.; Mishra, D.K.; Tiwari, R.; Chaudhury, K.; Goryacheva, I.Y.; Mishra, P.K. Ultrafine particulate matter impairs mitochondrial redox homeostasis and activates phosphatidylinositol 3-kinase mediated DNA damage responses in lymphocytes. Environ. Pollut. 2018, 234, 406–419. [Google Scholar] [CrossRef]
- Clifford, S.; Mazaheri, M.; Salimi, F.; Ezz, W.N.; Yeganeh, B.; Low-Choy, S.; Walker, K.; Mengersen, K.; Marks, G.B.; Morawska, L. Effects of exposure to ambient ultrafine particles on respiratory health and systemic inflammation in children. Environ. Int. 2018, 114, 167–180. [Google Scholar] [CrossRef]
- Guo, L.; Johnson, G.R.; Hofmann, W.; Wang, H.; Morawska, L. Deposition of ambient ultrafine particles in the respiratory tract of children: A novel experimental method and its application. J. Aerosol Sci. 2020, 139, 105465. [Google Scholar] [CrossRef]
- Kreyling, W.G. Discovery of unique and ENM—Specific pathophysiologic pathways: Comparison of the translocation of inhaled iridium nanoparticles from nasal epithelium versus alveolar epithelium towards the brain of rats. Toxicol. Appl. Pharmacol. 2016, 299, 41–46. [Google Scholar] [CrossRef]
- Simko, M.; Mattsson, M.-O. Interactions between nanosized materials and the brain. Curr. Med. Chem. 2014, 21, 4200–4214. [Google Scholar] [CrossRef]
- Domino, E.F.; Ni, L.; Thompson, M.; Zhang, H.; Shikata, H.; Fukai, H.; Sakaki, T.; Ohya, I. Tobacco smoking produces widespread dominant brain wave alpha frequency increases. Int. J. Psychophysiol. 2009, 74, 192–198. [Google Scholar] [CrossRef]
- Golding, J. Effects of cigarette smoking on resting EEG, visual evoked potentials and photic driving. Pharmacol. Biochem. Behav. 1988, 29, 23–32. [Google Scholar] [CrossRef]
- Rass, O.; Ahn, W.-Y.; O’donnell, B.F. Resting-state EEG, impulsiveness, and personality in daily and nondaily smokers. Clin. Neurophysiol. 2016, 127, 409–418. [Google Scholar] [CrossRef]
- Crüts, B.; van Etten, L.; Törnqvist, H.; Blomberg, A.; Sandström, T.; Mills, N.L.; Borm, P.J. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part. Fibre Toxicol. 2008, 5, 1–6. [Google Scholar] [CrossRef]
- Gawryluk, J.R.; Palombo, D.J.; Curran, J.; Parker, A.; Carlsten, C. Brief diesel exhaust exposure acutely impairs functional brain connectivity in humans: A randomized controlled crossover study. Environ. Health 2023, 22, 7. [Google Scholar] [CrossRef]
- Arnaldi, D.; Donniaquio, A.; Mattioli, P.; Massa, F.; Grazzini, M.; Meli, R.; Filippi, L.; Grisanti, S.; Famà, F.; Terzaghi, M.; et al. Epilepsy in neurodegenerative dementias: A clinical, epidemiological, and EEG study. J. Alzheimer’s Dis. 2020, 74, 865–874. [Google Scholar] [CrossRef]
- Bhat, S.; Acharya, U.R.; Dadmehr, N.; Adeli, H. Clinical neurophysiological and automated EEG-based diagnosis of the Alzheimer’s disease. Eur. Neurol. 2015, 74, 202–210. [Google Scholar] [CrossRef]
- Torkmahalleh, M.A.; Naseri, M.; Nurzhan, S.; Gabdrashova, R.; Bekezhankyzy, Z.; Gimnkhan, A.; Malekipirbazari, M.; Jouzizadeh, M.; Tabesh, M.; Farrokhi, H.; et al. Human exposure to aerosol from indoor gas stove cooking and the resulting nervous system responses. Indoor Air 2022, 32, e12983. [Google Scholar] [CrossRef]
- Naseri, M.; Jouzizadeh, M.; Tabesh, M.; Malekipirbazari, M.; Gabdrashova, R.; Nurzhan, S.; Farrokhi, H.; Khanbabaie, R.; Mehri-Dehnavi, H.; Bekezhankyzy, Z.; et al. The impact of frying aerosol on human brain activity. Neurotoxicology 2019, 74, 149–161. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Mattson, M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Me-Tabolism 2012, 16, 706–722. [Google Scholar] [CrossRef]
- Ooi, T.C.; Meramat, A.; Rajab, N.F.; Shahar, S.; Ismail, I.S.; Azam, A.A.; Sharif, R. Intermittent fasting enhanced the cognitive function in older adults with mild cognitive impairment by in-ducing biochemical and metabolic changes: A 3-year progressive study. Nutrients 2020, 12, 2644. [Google Scholar] [CrossRef]
- Currenti, W.; Godos, J.; Castellano, S.; Caruso, G.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Association between time restricted feeding and cognitive status in older Italian adults. Nutrients 2021, 13, 191. [Google Scholar] [CrossRef]
- Currenti, W.; Godos, J.; Castellano, S.; Caruso, G.; Ferri, R.; Caraci, F.; Grosso, G.; Galvano, F. Time-restricted feeding is associated with mental health in elderly Italian adults. Chronobiol. Internatl. 2021, 38, 1507–1516. [Google Scholar] [CrossRef]
- Höhn, S.; Dozières-Puyravel, B.; Auvin, S. History of dietary treatment: Guelpa & Marie first report of intermittent fasting for epilepsy in 1911. Epilepsy Behav. 2019, 94, 277–280. [Google Scholar]
- Brocchi, A.; Rebelos, E.; Dardano, A.; Mantuano, M.; Daniele, G. Effects of intermittent fasting on brain metabolism. Nutrients 2022, 14, 1275. [Google Scholar] [CrossRef]
- Landgrave-Gómez, J.; Mercado-Gómez, O.F.; Vázquez-García, M.; Rodríguez-Molina, V.; Córdova-Dávalos, L.; Arriaga-Ávila, V.; Miranda-Martínez, A.; Guevara-Guzmán, R. Anticonvulsant effect of time-restricted feeding in a pilocarpine-induced seizure model: Metabolic and epigenetic implications. Front. Cell Neurosci. 2016, 10, 7. [Google Scholar] [CrossRef]
- Friedman, M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J. Am. Stat. Assoc. 1937, 32, 675–701. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. Biom Bull 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Torkmahalleh, M.A.; Ospanova, S.; Baibatyrova, A.; Nurbay, S.; Zhanakhmet, G.; Shah, D. Contributions of burner, pan, meat and salt to PM emission during grilling. Environ. Res. 2018, 164, 11–17. [Google Scholar] [CrossRef]
- Wallace, L.A.; Ott, W.R.; Weschler, C.J. Ultrafine particles from electric appliances and cooking pans: Experiments suggesting desorp-tion/nucleation of sorbed organics as the primary source. Indoor Air 2015, 25, 536–546. [Google Scholar] [CrossRef]
- Buonanno, G.; Johnson, G.; Morawska, L.; Stabile, L. Volatility Characterization of Cooking-Generated Aerosol Particles. Aerosol Sci. Technol. 2011, 45, 1069–1077. [Google Scholar] [CrossRef]
- Cacot, P.; Tesolin, B.; Sebban, C. Diurnal variations of EEG power in healthy adults. Electroencephalogr. Clin. Neurophysiol. 1995, 94, 305–312. [Google Scholar] [CrossRef]
- Kaiser, D.A. Ultradian and circadian effects in electroencephalography activity. Biofeedback 2008, 36, 148. [Google Scholar]
- Preti, M.G.; Bolton, T.A.; Van De Ville, D. The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage 2017, 160, 41–54. [Google Scholar] [CrossRef]
- Cummings, L.; Dane, A.; Rhodes, J.; Lynch, P.; Hughes, A.M. Diurnal variation in the quantitative EEG in healthy adult volunteers. Br. J. Clin. Pharmacol. 2000, 50, 21–26. [Google Scholar] [CrossRef]
- Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef]
- Malek, N.; Baker, M.R.; Mann, C.; Greene, J. Electroencephalographic markers in dementia. Acta Neurol. Scand. 2017, 135, 388–393. [Google Scholar] [CrossRef]
- Micanovic, C.; Pal, S. The diagnostic utility of EEG in early-onset dementia: A systematic review of the literature with narrative analysis. J. Neural Transm. 2014, 121, 59–69. [Google Scholar] [CrossRef]
- Mientus, S.; Gallinat, J.; Wuebben, Y.; Pascual-Marqui, R.D.; Mulert, C.; Frick, K.; Dorn, H.; Herrmann, W.M.; Winterer, G. Cortical hypoactivation during resting EEG in schizophrenics but not in depressives and schizotypal subjects as revealed by low resolution electromagnetic tomography (LORETA). Psychiatry Res. Neuroimaging 2002, 116, 95–111. [Google Scholar] [CrossRef]
- Rosén, I. Electroencephalography as a diagnostic tool in dementia. Dement. Geriatr. Cogn. Disord. 1997, 8, 110–116. [Google Scholar] [CrossRef]
- Soikkeli, R.; Partanen, J.; Soininen, H.; Pääkkönen, A.; Riekkinen, P. Slowing of EEG in Parkinson’s disease. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 159–165. [Google Scholar] [CrossRef]
- Almeneessier, A.S.; BaHammam, A.A.; Olaish, A.H.; Pandi-Perumal, S.R.; Manzar, D.; BaHammam, A.S. Effects of diurnal intermittent fasting on daytime sleepiness reflected by EEG absolute power. J. Clin. Neurophysiol. 2019, 36, 213–219. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69. [Google Scholar]
- Calderón-Garcidueñas, L.; Azzarelli, B.; Acuna, H.; Garcia, R.; Gambling, T.M.; Osnaya, N.; Monroy, S.; Tizapantzi, M.D.R.; Carson, J.L.; Villarreal-Calderon, A.; et al. Air pollution and brain damage. Toxicol. Pathol. 2002, 30, 373–389. [Google Scholar] [CrossRef]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J.; et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef]
- Lin, D.M.; Ngai, J. Development of the vertebrate main olfactory system. Curr. Opin. Neurobiol. 1999, 9, 74–78. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Franco-Lira, M.; Torres-Jardón, R.; Henriquez-Roldán, C.; Barragán-Mejía, G.; Valencia-Salazar, G.; Gonzaléz-Maciel, A.; Reynoso-Robles, R.; Villarreal-Calderón, R.; Reed, W. Pediatric respiratory and systemic effects of chronic air pollution exposure: Nose, lung, heart, and brain pathology. Toxicol. Pathol. 2007, 35, 154–162. [Google Scholar] [CrossRef]
- Sohal, R.S.; Ku, H.-H.; Agarwal, S.; Forster, M.J.; Lal, H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994, 74, 121–133. [Google Scholar] [CrossRef]
- Wachsman, J.T. The beneficial effects of dietary restriction: Reduced oxidative damage and enhanced apoptosis. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1996, 350, 25–34. [Google Scholar] [CrossRef]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Sci. Total Environ. 1996, 273, 59–63. [Google Scholar] [CrossRef]
- Fusco, S.; Pani, G. Brain response to calorie restriction. Cell Mol. Life Sci. 2013, 70, 3157–3170. [Google Scholar] [CrossRef]
- Kaptan, Z.; Akgün-Dar, K.; Kapucu, A.; Dedeakayoğulları, H.; Batu.; Üzüm, G. Long term consequences on spatial learning-memory of low-calorie diet during adolescence in female rats; hippocampal and prefrontal cortex BDNF level, expression of NeuN and cell proliferation in dentate gyrus. Brain Res. 2015, 1618, 194–204. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. 2014, 25, 89–98. [Google Scholar] [CrossRef]
- Duan, W.; Guo, Z.; Jiang, H.; Ware, M.; Mattson, M.P. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology 2003, 144, 2446–2453. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Martin, B.; Maudsley, S.; Golden, E.; Cutler, R.G.; Mattson, M.P. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 2009, 19, 951–961. [Google Scholar] [CrossRef]
- Johnson, D.A.; Johnson, J.A. Nrf2—A therapeutic target for the treatment of neurodegenerative diseases. Free. Radic. Biol. Med. 2015, 88, 253–267. [Google Scholar] [CrossRef]
- Singh, R.; Lakhanpal, D.; Kumar, S.; Sharma, S.; Kataria, H.; Kaur, M.; Kaur, G. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age 2012, 34, 917–933. [Google Scholar] [CrossRef]
- Cerqueira, F.M.; Cunha, F.M.; Laurindo, F.R.; Kowaltowski, A.J. Calorie restriction increases cerebral mitochondrial respiratory capacity in a NO•-mediated mecha-nism: Impact on neuronal survival. Free Radic. Biol. Med. 2012, 52, 1236–1241. [Google Scholar] [CrossRef]





| Frequency Band | Control Group (Diurnal Effect) | Non-Zero Calorie Intake Group (Diurnal and Exposure Effects) | Effect of Exposure | 2 h-Zero Calorie Intake Group (Diurnal and Exposure and Zero Calorie Intake Effects) | Effect of Zero Calorie Intake on the Exposure Effect |
|---|---|---|---|---|---|
| Alpha | decreasing trend 2.54% | increasing trend 10.28% | 12.82% increases | increasing trend 2.13% | 8.15% mitigating effect |
| Beta1 | increasing trend 26.24% | decreasing trend 3.10% | 29.34% reductions | decreasing trend 4.63% | 1.53% exacerbating effect |
| Beta2 | increasing trend 17.80% | decreasing trend 1.69% | 19.49% reductions | decreasing trend 4.47% | 2.78% exacerbating effect |
| Beta3 | increasing trend 12.73% | decreasing trend 5.80% | 18.53% reductions | decreasing trend 4.28% | 1.52% mitigating effect |
| Delta | decreasing trend 12.49% | decreasing trend 7.69% | 6.40% increases | decreasing trend 6.40% | 1.29% mitigating effect |
| Theta | increasing trend 0.31% | decreasing trend 4.98% | 5.29% reductions | increasing trend 7.79% | 12.77% mitigating effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseri, M.; Sadeghi, S.; Malekipirbazari, M.; Nurzhan, S.; Gabdrashova, R.; Bekezhankyzy, Z.; Khanbabaie, R.; Crape, B.; Shah, D.; Amouei Torkmahalleh, M. Interaction of Cooking-Generated Aerosols on the Human Nervous System and the Impact of Caloric Restriction Post-Exposure. Nutrients 2024, 16, 3525. https://doi.org/10.3390/nu16203525
Naseri M, Sadeghi S, Malekipirbazari M, Nurzhan S, Gabdrashova R, Bekezhankyzy Z, Khanbabaie R, Crape B, Shah D, Amouei Torkmahalleh M. Interaction of Cooking-Generated Aerosols on the Human Nervous System and the Impact of Caloric Restriction Post-Exposure. Nutrients. 2024; 16(20):3525. https://doi.org/10.3390/nu16203525
Chicago/Turabian StyleNaseri, Motahareh, Sahar Sadeghi, Milad Malekipirbazari, Sholpan Nurzhan, Raikhangul Gabdrashova, Zhibek Bekezhankyzy, Reza Khanbabaie, Byron Crape, Dhawal Shah, and Mehdi Amouei Torkmahalleh. 2024. "Interaction of Cooking-Generated Aerosols on the Human Nervous System and the Impact of Caloric Restriction Post-Exposure" Nutrients 16, no. 20: 3525. https://doi.org/10.3390/nu16203525
APA StyleNaseri, M., Sadeghi, S., Malekipirbazari, M., Nurzhan, S., Gabdrashova, R., Bekezhankyzy, Z., Khanbabaie, R., Crape, B., Shah, D., & Amouei Torkmahalleh, M. (2024). Interaction of Cooking-Generated Aerosols on the Human Nervous System and the Impact of Caloric Restriction Post-Exposure. Nutrients, 16(20), 3525. https://doi.org/10.3390/nu16203525







