Association Between Ultraprocessed Food Consumption and Metabolic Disorders in Children and Adolescents with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. UPF Consumption
2.2. Anthropometric Measurements
2.3. Biochemical Assessment
2.4. Hepatic Steatosis Data
2.5. Other Variables
2.6. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Diet and UPF Consumption
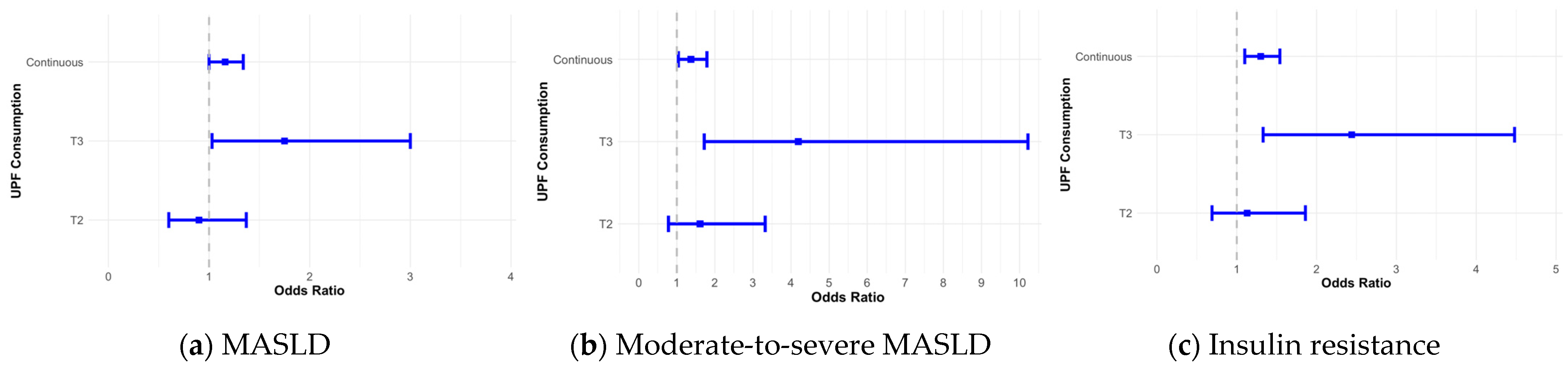
3.3. UPF Consumption and Metabolic Risk Disorders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlos Augusto Monteiro: Nutrition and obesity. Bull. World Health Organ. 2024, 102, 560–561. [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef]
- Fardet, A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: A preliminary study with 98 ready-to-eat foods. Food Funct. 2016, 7, 2338–2346. [Google Scholar] [CrossRef]
- Moubarac, J.C.; Parra, D.C.; Cannon, G.; Monteiro, C.A. Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr. Obes. Rep. 2014, 3, 256–272. [Google Scholar] [CrossRef]
- McArdle, M.A.; Finucane, O.M.; Connaughton, R.M.; McMorrow, A.M.; Roche, H.M. Mechanisms of obesity-induced inflammation and insulin resistance: Insights into the emerging role of nutritional strategies. Front. Endocrinol. 2013, 4, 52. [Google Scholar] [CrossRef]
- Orliaguet, L.; Dalmas, E.; Drareni, K.; Venteclef, N.; Alzaid, F. Mechanisms of Macrophage Polarization in Insulin Signaling and Sensitivity. Front. Endocrinol. 2020, 11, 62. [Google Scholar] [CrossRef]
- Wieser, V.; Moschen, A.R.; Tilg, H. Inflammation, cytokines and insulin resistance: A clinical perspective. Arch. Immunol. Ther. Exp. 2013, 61, 119–125. [Google Scholar] [CrossRef]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Mendonça, R.D.; Pimenta, A.M.; Gea, A.; de la Fuente-Arrillaga, C.; Martinez-Gonzalez, M.A.; Lopes, A.C.; Bes-Rastrollo, M. Ultraprocessed food consumption and risk of overweight and obesity: The University of Navarra Follow-Up (SUN) cohort study. Am. J. Clin. Nutr. 2016, 104, 1433–1440. [Google Scholar] [CrossRef]
- Vandevijvere, S.; Jaacks, L.M.; Monteiro, C.A.; Moubarac, J.C.; Girling-Butcher, M.; Lee, A.C.; Pan, A.; Bentham, J.; Swinburn, B. Global trends in ultraprocessed food and drink product sales and their association with adult body mass index trajectories. Obes. Rev. 2019, 20, 10–19. [Google Scholar] [CrossRef]
- Sung, H.; Park, J.M.; Oh, S.U.; Ha, K.; Joung, H. Consumption of Ultra-Processed Foods Increases the Likelihood of Having Obesity in Korean Women. Nutrients 2021, 13, 698. [Google Scholar] [CrossRef]
- Shim, J.S.; Ha, K.H.; Kim, D.J.; Kim, H.C. Ultra-Processed Food Consumption and Obesity in Korean Adults. Diabetes Metab. J. 2023, 47, 547–558. [Google Scholar] [CrossRef]
- Park, H.; Lee, Y.; Hwang, J.; Lee, Y. Ultra-processed food consumption and increased risk of metabolic syndrome in Korean adults: A cross-sectional analysis of the KNHANES 2016–2020. Nutrition 2024, 122, 112374. [Google Scholar] [CrossRef]
- Kim, L.; Choi, Y.H.; Huh, D.A.; Moon, K.W. Associations of minimally processed and ultra-processed food intakes with cardiovascular health in Korean adults: The Korea National Health and Nutrition Examination Survey (KNHANES VI), 2013–2015. J. Expo. Sci. Environ. Epidemiol. 2024. [Google Scholar] [CrossRef]
- Cho, Y.; Ryu, S.; Kim, R.; Shin, M.J.; Oh, H. Ultra-processed Food Intake and Risk of Type 2 Diabetes in Korean Adults. J. Nutr. 2024, 154, 243–251. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Z.; Wang, H.; Zhang, J.; Su, C.; Jia, X.; Du, W.; Jiang, H.; Li, W.; Wang, L.; et al. Association between Ultra-Processed Food Consumption and Metabolic Syndrome among Adults in China-Results from the China Health and Nutrition Survey. Nutrients 2023, 15, 752. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, T.; Mao, W.; Zhao, F.; Luan, D.; Li, J. Ultra-Processed Food Consumption and Risk of Overweight or Obesity in Chinese Adults: Chinese Food Consumption Survey 2017–2020. Nutrients 2023, 15, 4005. [Google Scholar] [CrossRef]
- Li, M.; Shi, Z. Ultra-Processed Food Consumption Associated with Incident Hypertension among Chinese Adults-Results from China Health and Nutrition Survey 1997–2015. Nutrients 2022, 14, 4783. [Google Scholar] [CrossRef]
- Li, M.; Shi, Z. Association between Ultra-Processed Food Consumption and Diabetes in Chinese Adults-Results from the China Health and Nutrition Survey. Nutrients 2022, 14, 4241. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Le Garf, S.; Nègre, V.; Anty, R.; Gual, P. Metabolic Fatty Liver Disease in Children: A Growing Public Health Problem. Biomedicines 2021, 9, 1915. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Newton, K.P.; Awai, H.I.; Choi, L.J.; Garcia, M.A.; Ellis, L.L.; Vanderwall, K.; Fontanesi, J. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013, 38, 1267–1277. [Google Scholar] [CrossRef]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef]
- Hartmann, P.; Zhang, X.; Loomba, R.; Schnabl, B. Global and national prevalence of nonalcoholic fatty liver disease in adolescents: An. analysis of the global burden of disease study 2019. Hepatology 2023, 78, 1168–1181. [Google Scholar] [CrossRef]
- Yu, E.L.; Golshan, S.; Harlow, K.E.; Angeles, J.E.; Durelle, J.; Goyal, N.P.; Newton, K.P.; Sawh, M.C.; Hooker, J.; Sy, E.Z.; et al. Prevalence of Nonalcoholic Fatty Liver Disease in Children with Obesity. J. Pediatr. 2019, 207, 64–70. [Google Scholar] [CrossRef]
- EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [CrossRef]
- Vesković, M.; Šutulović, N.; Hrnčić, D.; Stanojlović, O.; Macut, D.; Mladenović, D. The Interconnection between Hepatic Insulin Resistance and Metabolic Dysfunction-Associated Steatotic Liver Disease-The Transition from an Adipocentric to Liver-Centric Approach. Curr. Issues Mol. Biol. 2023, 45, 9084–9102. [Google Scholar] [CrossRef]
- Bo, T.; Gao, L.; Yao, Z.; Shao, S.; Wang, X.; Proud, C.G.; Zhao, J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024, 36, 947–968. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Park, H.J.; Park, S.J.; Kim, J.Y. Development of Korean NOVA food classification and estimation of ultra-processed food intake among adults: Using data from the 2018 Korea National Health and Nutrition Examination Survey. Korean J. Community Nutr. 2022, 27, 455–467. [Google Scholar] [CrossRef]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 October 2024).
- Kurtoğlu, S.; Hatipoğlu, N.; Mazıcıoğlu, M.; Kendirici, M.; Keskin, M.; Kondolot, M. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 100–106. [Google Scholar] [CrossRef]
- Tang, A.; Desai, A.; Hamilton, G.; Wolfson, T.; Gamst, A.; Lam, J.; Clark, L.; Hooker, J.; Chavez, T.; Ang, B.D.; et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 2015, 274, 416–425. [Google Scholar] [CrossRef]
- Shao, C.X.; Ye, J.; Dong, Z.; Li, F.; Lin, Y.; Liao, B.; Feng, S.; Zhong, B. Steatosis grading consistency between controlled attenuation parameter and MRI-PDFF in monitoring metabolic associated fatty liver disease. Ther. Adv. Chronic Dis. 2021, 12, 20406223211033119. [Google Scholar] [CrossRef]
- WHO. Physical Activity Surveillance. Available online: https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/physical-activity-surveillance (accessed on 7 October 2024).
- Hubbard, A.E.; Ahern, J.; Fleischer, N.L.; Van der Laan, M.; Lippman, S.A.; Jewell, N.; Bruckner, T.; Satariano, W.A. To GEE or not to GEE: Comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 2010, 21, 467–474. [Google Scholar] [CrossRef]
- Ziegler, A.; Vens, M. Generalized estimating equations. Notes on the choice of the working correlation matrix. Methods Inf. Med. 2010, 49, 421–425; discussion 426–432. [Google Scholar] [CrossRef]
- Rauber, F.; da Costa Louzada, M.L.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Martinez Steele, E.; Lo, C.H.; Zhang, F.F.; Zhang, X. Higher ultra-processed food intake was positively associated with odds of NAFLD in both US adolescents and adults: A national survey. Hepatol. Commun. 2023, 7. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, H.; Zeng, Y.; Chen, Y.; Xu, C. Association between ultra-processed foods consumption and risk of non-alcoholic fatty liver disease: A population-based analysis of NHANES 2011–2018. Br. J. Nutr. 2023, 130, 996–1004. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, S.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; Gu, Y.; Wang, Y.; Zhang, T.; et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study. Int. J. Epidemiol. 2022, 51, 237–249. [Google Scholar] [CrossRef]
- Fridén, M.; Kullberg, J.; Ahlström, H.; Lind, L.; Rosqvist, F. Intake of Ultra-Processed Food and Ectopic-, Visceral- and Other Fat Depots: A Cross-Sectional Study. Front. Nutr. 2022, 9, 774718. [Google Scholar] [CrossRef]
- Targher, G.; Tilg, H.; Byrne, C.D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588. [Google Scholar] [CrossRef]
- Gassaway, B.M.; Petersen, M.C.; Surovtseva, Y.V.; Barber, K.W.; Sheetz, J.B.; Aerni, H.R.; Merkel, J.S.; Samuel, V.T.; Shulman, G.I.; Rinehart, J. PKCε contributes to lipid-induced insulin resistance through cross talk with p70S6K and through previously unknown regulators of insulin signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E8996–E9005. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Wang, A.; Beddow, S.A.; Geisler, J.G.; Kahn, M.; Zhang, X.M.; Monia, B.P.; Bhanot, S.; Shulman, G.I. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Investig. 2007, 117, 739–745. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14, 21–28. [Google Scholar] [CrossRef]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef]
- Martinez Steele, E.; Marrón Ponce, J.A.; Cediel, G.; Louzada, M.L.C.; Khandpur, N.; Machado, P.; Moubarac, J.C.; Rauber, F.; Corvalán, C.; Levy, R.B.; et al. Potential reductions in ultra-processed food consumption substantially improve population cardiometabolic-related dietary nutrient profiles in eight countries. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2739–2750. [Google Scholar] [CrossRef]
- Hernández, E.; Kahl, S.; Seelig, A.; Begovatz, P.; Irmler, M.; Kupriyanova, Y.; Nowotny, B.; Nowotny, P.; Herder, C.; Barosa, C.; et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J. Clin. Investig. 2017, 127, 695–708. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Blendis, L.; Halpern, Z.; Oren, R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 2007, 47, 711–717. [Google Scholar] [CrossRef]
- Teymoori, F.; Farhadnejad, H.; Moslehi, N.; Mirmiran, P.; Mokhtari, E.; Azizi, F. The association of dietary insulin and glycemic indices with the risk of type 2 diabetes. Clin. Nutr. 2021, 40, 2138–2144. [Google Scholar] [CrossRef]
- Costa Louzada, M.L.; Martins, A.P.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.C.; Cannon, G.; Monteiro, C.A. Ultra-processed foods and the nutritional dietary profile in Brazil. Rev. Saude Publica 2015, 49, 38. [Google Scholar] [CrossRef]
- Hall, K.D. A review of the carbohydrate-insulin model of obesity. Eur. J. Clin. Nutr. 2017, 71, 323–326. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Deretzi, G.; Zavos, C.; Mantzoros, C.S. The emerging role of endocrine disruptors in pathogenesis of insulin resistance: A concept implicating nonalcoholic fatty liver disease. Curr. Mol. Med. 2012, 12, 68–82. [Google Scholar] [CrossRef]
- Bertoli, S.; Leone, A.; Battezzati, A. Human Bisphenol A Exposure and the “Diabesity Phenotype”. Dose Response 2015, 13, 1559325815599173. [Google Scholar] [CrossRef]
- Rolfo, A.; Nuzzo, A.M.; De Amicis, R.; Moretti, L.; Bertoli, S.; Leone, A. Fetal-Maternal Exposure to Endocrine Disruptors: Correlation with Diet Intake and Pregnancy Outcomes. Nutrients 2020, 12, 1744. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Borthakur, A.; Tobacman, J.K. Common food additive carrageenan inhibits proglucagon expression and GLP-1 secretion by human enteroendocrine L-cells. Nutr. Diabetes 2024, 14, 28. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef]
- Cano, R.; Pérez, J.L.; Dávila, L.A.; Ortega, Á.; Gómez, Y.; Valero-Cedeño, N.J.; Parra, H.; Manzano, A.; Véliz Castro, T.I.; Albornoz, M.P.D.; et al. Role of Endocrine-Disrupting Chemicals in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4807. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Sonestedt, E.; Borné, Y. Associations of ultra-processed food consumption, circulating protein biomarkers, and risk of cardiovascular disease. BMC Med. 2023, 21, 415. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-processed food exposure and adverse health outcomes: Umbrella review of epidemiological meta-analyses. Bmj 2024, 384, e077310. [Google Scholar] [CrossRef]
- Vadiveloo, M.; Parekh, N.; Mattei, J. Greater healthful food variety as measured by the US Healthy Food Diversity index is associated with lower odds of metabolic syndrome and its components in US adults. J. Nutr. 2015, 145, 564–571. [Google Scholar] [CrossRef]
- Mozaffari, H.; Hosseini, Z.; Lafrenière, J.; Conklin, A.I. The role of dietary diversity in preventing metabolic-related outcomes: Findings from a systematic review. Obes. Rev. 2021, 22, e13174. [Google Scholar] [CrossRef]
- Jahromi, M.K.; Daftari, G.; Tehrani, A.N.; Amirshekari, G.; Farhadnejad, H.; Teymoori, F.; Salehi-Sahlabadi, A.; Mirmiran, P. The association of the healthy food diversity index with the risk of non-alcoholic fatty liver disease among the adult population. Clin. Nutr. ESPEN 2024, 59, 404–411. [Google Scholar] [CrossRef]
- Ebrahimi Mousavi, S.; Dehghanseresht, N.; Dashti, F.; Khazaei, Y.; Salamat, S.; Asbaghi, O.; Mansoori, A. The association between Dietary Diversity Score and odds of nonalcoholic fatty liver disease: A case-control study. Eur. J. Gastroenterol. Hepatol. 2022, 34, 678–685. [Google Scholar] [CrossRef]
- Hayes, K.C.; Khosla, P.; Hajri, T.; Pronczuk, A. Saturated fatty acids and LDL receptor modulation in humans and monkeys. Prostaglandins Leukot. Essent. Fatty Acids 1997, 57, 411–418. [Google Scholar] [CrossRef]
- Grundy, S.M.; Denke, M.A. Dietary influences on serum lipids and lipoproteins. J. Lipid Res. 1990, 31, 1149–1172. [Google Scholar] [CrossRef]
- Dietschy, J.M. Dietary fatty acids and the regulation of plasma low density lipoprotein cholesterol concentrations. J. Nutr. 1998, 128, 444s–448s. [Google Scholar] [CrossRef]
- Shin, W.-K.; Shin, S.; Lee, J.-K.; Kang, D.; Lee, J.E. Carbohydrate Intake and Hyperlipidemia among Population with High-Carbohydrate Diets: The Health Examinees Gem Study. Mol. Nutr. Food Res. 2021, 65, 2000379. [Google Scholar] [CrossRef]
- Ochoa-Avilés, A.; Verstraeten, R.; Lachat, C.; Andrade, S.; Van Camp, J.; Donoso, S.; Kolsteren, P. Dietary intake practices associated with cardiovascular risk in urban and rural Ecuadorian adolescents: A cross-sectional study. BMC Public. Health 2014, 14, 939. [Google Scholar] [CrossRef][Green Version]
- Nouri, M.; Eskandarzadeh, S.; Makhtoomi, M.; Rajabzadeh-Dehkordi, M.; Omidbeigi, N.; Najafi, M.; Faghih, S. Association between ultra-processed foods intake with lipid profile: A cross-sectional study. Sci. Rep. 2023, 13, 7258. [Google Scholar] [CrossRef]
- Nogueira, J.P.; Cusi, K. Role of Insulin Resistance in the Development of Nonalcoholic Fatty Liver Disease in People With Type 2 Diabetes: From Bench to Patient Care. Diabetes Spectr. 2024, 37, 20–28. [Google Scholar] [CrossRef]
- Al-Mansoori, L.; Al-Jaber, H.; Prince, M.S.; Elrayess, M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation 2022, 45, 31–44. [Google Scholar] [CrossRef]
- Contreras-Rodriguez, O.; Solanas, M.; Escorihuela, R.M. Dissecting ultra-processed foods and drinks: Do they have a potential to impact the brain? Rev. Endocr. Metab. Disord. 2022, 23, 697–717. [Google Scholar] [CrossRef]
- Wang, D.D.; Hu, F.B. Dietary Fat and Risk of Cardiovascular Disease: Recent Controversies and Advances. Annu. Rev. Nutr. 2017, 37, 423–446. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; Takakuwa, H.; Hamaguchi, M.; et al. Trans Fatty Acid Intake Induces Intestinal Inflammation and Impaired Glucose Tolerance. Front. Immunol. 2021, 12, 669672. [Google Scholar] [CrossRef]
- Shinozaki, N.; Murakami, K.; Kimoto, N.; Masayasu, S.; Sasaki, S. Highly Processed Food Consumption and its Association With Overall Diet Quality in a Nationwide Sample of 1,318 Japanese Children and Adolescents: A Cross-Sectional Analysis Based on 8-Day Weighed Dietary Records. J. Acad. Nutr. Diet. 2024. [Google Scholar] [CrossRef]
- Costa, C.D.S.; Assunção, M.C.F.; Loret de Mola, C.; Cardoso, J.S.; Matijasevich, A.; Barros, A.J.D.; Santos, I.S. Role of ultra-processed food in fat mass index between 6 and 11 years of age: A cohort study. Int. J. Epidemiol. 2021, 50, 256–265. [Google Scholar] [CrossRef]
- Vilela, S.; Magalhães, V.; Severo, M.; Oliveira, A.; Torres, D.; Lopes, C. Effect of the food processing degree on cardiometabolic health outcomes: A prospective approach in childhood. Clin. Nutr. 2022, 41, 2235–2243. [Google Scholar] [CrossRef]
- García-Blanco, L.; de la O, V.; Santiago, S.; Pouso, A.; Martínez-González, M.; Martín-Calvo, N. High consumption of ultra-processed foods is associated with increased risk of micronutrient inadequacy in children: The SENDO project. Eur. J. Pediatr. 2023, 182, 3537–3547. [Google Scholar] [CrossRef]
- Enes, C.C.; Camargo, C.M.D.; Justino, M.I.C. Ultra-processed food consumption and obesity in adolescents. Rev. Nutr. 2019, 32, e180170. [Google Scholar] [CrossRef]
- Chavez-Ugalde, I.Y.; de Vocht, F.; Jago, R.; Adams, J.; Ong, K.K.; Forouhi, N.G.; Colombet, Z.; Ricardo, L.I.C.; van Sluijs, E.; Toumpakari, Z. Ultra-processed food consumption in UK adolescents: Distribution, trends, and sociodemographic correlates using the National Diet and Nutrition Survey 2008/09 to 2018/19. Eur. J. Nutr. 2024, 1–15. [Google Scholar] [CrossRef]
- Sparrenberger, K.; Friedrich, R.R.; Schiffner, M.D.; Schuch, I.; Wagner, M.B. Ultra-processed food consumption in children from a Basic Health Unit. J. Pediatr. 2015, 91, 535–542. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Middleton, M.S.; Behling, C.; Newton, K.P.; Awai, H.I.; Paiz, M.N.; Lam, J.; Hooker, J.C.; Hamilton, G.; Fontanesi, J.; et al. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology 2015, 61, 1887–1895. [Google Scholar] [CrossRef]
- Shin, J.; Kim, M.J.; Shin, H.J.; Yoon, H.; Kim, S.; Koh, H.; Lee, M.J. Quick assessment with controlled attenuation parameter for hepatic steatosis in children based on MRI-PDFF as the gold standard. BMC Pediatr. 2019, 19, 112. [Google Scholar] [CrossRef]
- Saravia, L.; Moliterno, P.; Skapino, E. Food Diary, Food Frequency Questionnaire, and 24-Hour Dietary Recall. In Basic Protocols in Foods and Nutrition; Betim Cazarin, C.B., Ed.; Springer: New York, NY, USA, 2022; pp. 223–247. [Google Scholar]
- Gazan, R.; Vieux, F.; Mora, S.; Havard, S.; Dubuisson, C. Potential of existing online 24-h dietary recall tools for national dietary surveys. Public. Health Nutr. 2021, 24, 5361–5386. [Google Scholar] [CrossRef]
- Hernández-Cordero, S.; López-Olmedo, N.; Rodríguez-Ramírez, S.; Barquera-Cervera, S.; Rivera-Dommarco, J.; Popkin, B. Comparing a 7-day diary vs. 24 h-recall for estimating fluid consumption in overweight and obese Mexican women. BMC Public. Health 2015, 15, 1031. [Google Scholar] [CrossRef]
| Overall Population | Tertiles of Ultraprocessed Food Consumption 1,2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | (n = 298) | T1 (n = 98) | T2 (n = 99) | T3 (n = 101) | p-Value 3 | ||||
| Age, months, median (IQR) | 146.5 | (133.0, 162.0) | 145 | (133.0, 157.0) | 145.0 | (129.0, 160.0) | 154.0 | (135.0, 167.0) | 0.072 |
| Gender, N (%) | |||||||||
| Girl | 86 | (28.9) | 28 | (25.6) | 30 | (30.3) | 28 | (27.7) | 0.919 |
| Boy | 212 | (71.1) | 70 | (71.4) | 69 | (69.7) | 73 | (72.3) | |
| Screen time, N (%) | |||||||||
| <3 h | 101 | (33.9) | 39 | (39.8) | 36 | (36.4) | 26 | (25.7) | 0.086 |
| <4 h | 70 | (23.5) | 25 | (25.5) | 24 | (24.2) | 21 | (20.8) | |
| ≥4 h | 127 | (42.6) | 34 | (34.7) | 39 | (39.4) | 54 | (53.5) | |
| Activity level, N (%) | |||||||||
| Low | 106 | (35.6) | 32 | (32.7) | 41 | (41.4) | 33 | (32.7) | 0.045 |
| Moderate | 96 | (32.2) | 29 | (29.6) | 24 | (24.2) | 43 | (42.6) | |
| High | 96 | (32.0) | 37 | (37.8) | 34 | (34.3) | 25 | (24.7) | |
| Birth weight, kg, median (IQR) | 3.4 | (3.1, 3.6) | 3.4 | (3.2, 3.6) | 3.3 | (3.0, 3.6) | 3.4 | (3.2, 3.7) | 0.587 |
| Maternal education, N (%) | |||||||||
| ≤High school | 60 | (20.1) | 18 | (18.4) | 17 | (17.2) | 25 | (24.8) | 0.274 |
| University | 182 | (61.1) | 56 | (57.1) | 64 | (64.6) | 62 | (61.4) | |
| ≥Graduate school | 56 | (18.8) | 24 | (24.0) | 18 | (18.2) | 14 | (13.9) | |
| Hepatic fat, %, median (IQR) 4 | 12.0 | (5.5, 20.6) | 8.4 | (5.1, 19.5) | 12.5 | (5.5, 20.5) | 13.9 | (6.4, 22.0) | 0.023 |
| Total cholesterol, mg/dL, median (IQR) 5 | 164.0 | (146.0, 183.0) | 167.5 | (147.0, 187.0) | 164.0 | (146.0, 184.0) | 162.0 | (145.0, 179.0) | 0.549 |
| Triglyceride, g/dL, median (IQR) 5 | 105.0 | (77.0, 148.0) | 96.0 | (72.0, 139.0) | 106.0 | (77.0, 148.0) | 113.0 | (79.0, 155.0) | 0.220 |
| HDL-C, mg/dL, median (IQR) 5 | 48.0 | (42.0, 55.0) | 50.0 | (42.5, 55.0) | 48.0 | (44.0, 57.0) | 48.0 | (42.0, 54.0) | 0.468 |
| LDL-C, mg/dL, median (IQR) 5 | 100.0 | (85.0, 118.0) | 101.0 | (83.0, 118.0) | 102 | (88.0, 119.0) | 97.0 | (84.0, 118.0) | 0.424 |
| Glucose, mg/dL, median (IQR) 5 | 91.0 | (86.0, 96.0) | 91.0 | (85.0, 97.0) | 91.0 | (86.0, 96.0) | 91.0 | (88.0, 97.0) | 0.772 |
| Overall Population | Tertiles of Ultraprocessed Food Consumption 1,2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | (n = 298) | T1 (n = 98) | T2 (n = 99) | T3 (n = 101) | p-Value 3 | ||||
| % Grams, %g/d | |||||||||
| Unprocessed or minimally processed foods | 63.3 | (51.7, 72.2) | 74.0 | (69.5, 80.1) | 64.6 | (57.7, 68.8) | 49.1 | (41.4, 54.3) | <0.001 |
| Processed culinary ingredients | 2.5 | (1.7, 3.2) | 3.0 | (2.2, 3.9) | 2.6 | (1.9, 3.2) | 1.8 | (1.2, 2.7) | <0.001 |
| Processed foods | 10.9 | (7.0, 16.1) | 12.9 | (8.5, 18.2) | 12.4 | (8.5, 16.3) | 8.3 | (4.6, 13.6) | <0.001 |
| Ultraprocessed foods | 20.4 | (12.4, 32.4) | 8.4 | (5.2, 12.4) | 20.3 | (17.8, 23.5) | 38.0 | (32.3, 46.8) | <0.001 |
| Other source 4 | 79.6 | (67.4, 87.5) | 91.3 | (87.6, 94.8) | 79.7 | (76.5, 82.2) | 62.0 | (53.2, 67.7) | <0.001 |
| % Calories, %kcal/d | |||||||||
| Unprocessed or minimally processed foods | 58.7 | (47.1, 69.1) | 71.9 | (66.5, 77.8) | 58.3 | (51.8, 64.4) | 44.1 | (37.7, 53.7) | <0.001 |
| Processed culinary ingredients | 6.2 | (4.2, 8.3) | 8.0 | (6.0, 10.2) | 6.0 | (4.6, 7.7) | 4.6 | (2.9, 6.7) | <0.001 |
| Processed foods | 5.9 | (2.4, 10.9) | 6.4 | (3.2, 11.8) | 7.7 | (3.8, 13.0) | 3.7 | (1.6, 9.1) | <0.001 |
| Ultraprocessed foods | 25.6 | (14.4, 39.8) | 11.1 | (7.2, 16.8) | 25.9 | (20.7, 33.3) | 44.8 | (36.0, 51.3) | <0.001 |
| Other source 4 | 74.4 | (60.2, 85.6) | 88.9 | (83.2, 92.8) | 74.1 | (66.7, 79.3) | 55.2 | (48.7, 64.0) | <0.001 |
| Overall Population | Tertiles of Ultraprocessed Food Consumption 1,2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | (n = 298) | T1 (n = 98) | T2 (n = 99) | T3 (n = 101) | p-Value 3 | ||||
| Total energy, kcal | 1916.6 | (1547.5, 2263.4) | 1738.5 | (1455.7, 2086.1) | 1966.7 | (1612.8, 2255.0) | 2111.6 | (1606.1, 2405.0) | 0.002 |
| Contributions to energy, % | |||||||||
| Carbohydrates | 52.8 | (47.8, 58.7) | 55.9 | (51.1, 59.8) | 52.8 | (45.2, 58.4) | 51.3 | (46.7, 56.4) | 0.006 |
| Proteins | 15.4 | (14.1, 17.6) | 15.4 | (14.2, 17.2) | 15.4 | (13.9, 17.5) | 15.4 | (14.1, 18.4) | 0.801 |
| Total fat | 29.8 | (25.2, 34.4) | 27.4 | (23.7, 31.4) | 29.8 | (25.5, 35.1) | 31.7 | (27.3, 35.3) | <0.001 |
| SFAs | 5.2 | (3.6, 6.7) | 5.9 | (4.4, 7.9) | 5.2 | (3.5, 6.3) | 4.6 | (3.2, 6.0) | <0.001 |
| MUFAs | 6.3 | (4.4, 8.0) | 7.5 | (5.6, 9.1) | 5.6 | (4.0, 7.6) | 5.6 | (4.0, 7.2) | <0.001 |
| PUFAs | 5.5 | (3.9, 6.9) | 6.2 | (5.2, 8.0) | 5.1 | (3.6, 6.6) | 4.6 | (3.0, 6.1) | <0.001 |
| Protein intake per body weight, g/kg | 1.1 | (0.8, 1.4) | 1.0 | (0.8, 1.3) | 1.1 | (0.8, 1.4) | 1.1 | (0.8, 1.3) | 0.410 |
| Fiber, g | 16.2 | (12.7, 20.6) | 17.1 | (13.9, 21.8) | 16.6 | (13.3, 20.2) | 14.6 | (10.7, 19.1) | 0.006 |
| Sodium, g | 3.1 | (2.4, 3.9) | 3.0 | (2.2, 3.8) | 3.1 | (2.5, 3.8) | 3.4 | (2.5, 4.2) | 0.116 |
| Tertiles of Ultraprocessed Food Consumption 1 β Coefficient (95% CI) | Continuous 2 β Coefficient (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables 3,4 | T1 (n = 98) | T2 (n = 99) | T3 (n = 101) | |||||
| FMI, kg/m2 | 0 | (reference) | 0.006 | (−0.022, 0.034) | 0.004 | (−0.027, 0.035) | 0.003 | (−0.005, 0.010) |
| Body fat mass, kg | 0 | (reference) | 0.002 | (−0.026, 0.030) | 0.003 | (−0.028, 0.035) | 0.002 | (−0.005, 0.010) |
| Body fat percentage, % | 0 | (reference) | 0.033 | (−0.584, 0.649) | −0.100 | (−0.804, 0.605) | −0.014 | (−0.186, 0.158) |
| Trunk fat mass, kg | 0 | (reference) | 0.001 | (−0.031, 0.032) | 0.004 | (−0.031, 0.038) | 0.002 | (−0.006, 0.010) |
| Trunk fat percentage, % | 0 | (reference) | 0.246 | (−0.527, 1.019) | −0.035 | (−0.895, 0.825) | −0.001 | (−0.210, 0.208) |
| Lean mass, kg | 0 | (reference) | 0.003 | (−0.010, 0.015) | 0.009 | (−0.005, 0.023) | 0.004 | (0.0003, 0.007) |
| Hepatic fat, % 5 | 0 | (reference) | 0.043 | (−0.063, 0.148) | 0.127 | (−0.010, 0.263) | 0.028 | (−0.008, 0.063) |
| AST, U/L 6 | 0 | (reference) | −0.005 | (−0.081, 0.072) | 0.043 | (−0.052, 0.139) | 0.007 | (−0.020, 0.035) |
| ALT, U/L 6 | 0 | (reference) | 0.036 | (−0.084, 0.156) | 0.082 | (−0.064, 0.228) | 0.012 | (−0.032, 0.056) |
| γ-GTP, U/L 6 | 0 | (reference) | 0.026 | (−0.037, 0.090) | 0.068 | (−0.008, 0.145) | 0.014 | (−0.010, 0.038) |
| Total cholesterol, mg/dL 6 | 0 | (reference) | −0.013 | (−0.046, 0.020) | −0.034 | (−0.065, −0.003) | −0.010 | (−0.018, −0.002) |
| Triglyceride, g/dL 6 | 0 | (reference) | 0.011 | (−0.088, 0.109) | 0.055 | (−0.048, 0.159) | 0.008 | (−0.021, 0.036) |
| HDL cholesterol, mg/dL 6 | 0 | (reference) | −0.001 | (−0.035, 0.033) | −0.010 | (−0.046, 0.026) | −0.003 | (−0.012, 0.007) |
| LDL cholesterol, mg/dL 6 | 0 | (reference) | −0.015 | (−0.060, 0.030) | −0.056 | (−0.103, −0.010) | −0.016 | (−0.028, −0.004) |
| Glucose, mg/dL 6 | 0 | (reference) | −0.013 | (−0.039, 0.012) | 0.01 | (−0.02, 0.04) | −0.0004 | (−0.01, 0.01) |
| Insulin, μU/mL 6 | 0 | (reference) | −0.001 | (−0.122, 0.121) | 0.133 | (0.005, 0.262) | 0.032 | (−0.001, 0.065) |
| HOMA-IR 6,7 | 0 | (reference) | −0.020 | (−0.155, 0.115) | 0.133 | (−0.005, 0.271) | 0.029 | (−0.008, 0.066) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-y.; Lim, J.H.; Joung, H.; Yoon, D. Association Between Ultraprocessed Food Consumption and Metabolic Disorders in Children and Adolescents with Obesity. Nutrients 2024, 16, 3524. https://doi.org/10.3390/nu16203524
Lee G-y, Lim JH, Joung H, Yoon D. Association Between Ultraprocessed Food Consumption and Metabolic Disorders in Children and Adolescents with Obesity. Nutrients. 2024; 16(20):3524. https://doi.org/10.3390/nu16203524
Chicago/Turabian StyleLee, Gyeong-yoon, Joo Hyun Lim, Hyojee Joung, and Dankyu Yoon. 2024. "Association Between Ultraprocessed Food Consumption and Metabolic Disorders in Children and Adolescents with Obesity" Nutrients 16, no. 20: 3524. https://doi.org/10.3390/nu16203524
APA StyleLee, G.-y., Lim, J. H., Joung, H., & Yoon, D. (2024). Association Between Ultraprocessed Food Consumption and Metabolic Disorders in Children and Adolescents with Obesity. Nutrients, 16(20), 3524. https://doi.org/10.3390/nu16203524






